For the purposes of this article, the main pleural diseases are divided into pleural effusion, pneumothorax, and focal solid pleural lesions.
Pleural effusion
The capability of TUS in detecting pleural effusion and differentiating pleural fluid and pleural thickening has been well established in recent years[1,3,4,6,10-14]. Moreover, TUS has been proved to be more accurate and preferable to radiographic measurement in the quantification of pleural effusion[11,15]. Consequently, according to its ready availability, TUS has become a major imaging modality in determining the presence and nature of pleural effusion, and in guiding the aspiration of pleural fluid or the placement of chest tubes[3,16].
Pleural effusion can be divided into transudates (protein concentration < 3 g/100 mL) caused by systemic factors, and exudates (protein concentration > 3 g/100 mL) caused by inflammatory or neoplastic diseases[3]. To determine ultrasonographically the nature of pleural effusion, Yang et al[13] have suggested to classify pleural effusion into anechoic, complex non-septated, complex septated, and homogeneously echogenic effusion. According to this classification, the effusion is defined as anechoic if echo-free spaces are present between the visceral and parietal pleura (Figure 5); complex non-septated if heterogeneous echogenic material is inside the anechoic pleural effusion (Figure 6); complex septated if fibrin strands or septa are floating inside the anechoic pleural effusion (Figure 7A and B); and homogeneously echogenic if homogeneously echogenic spaces are present between visceral and parietal pleura (Figure 8A and B). Transudates usually appear anechoic with an echo-free pattern, although partially treated transudates of congestive heart failure may occasionally be echogenic[17]. However, anechoic effusion can be either a transudate or an exudate. Moreover, although hemorrhagic effusion, empyema, and chylothorax usually present as homogeneously echogenic effusion, differential diagnosis among these entities is not possible with TUS, and explorative thoracentesis with macroscopic and physicochemical examination of the fluid may be required to clarify the nature of the effusion.
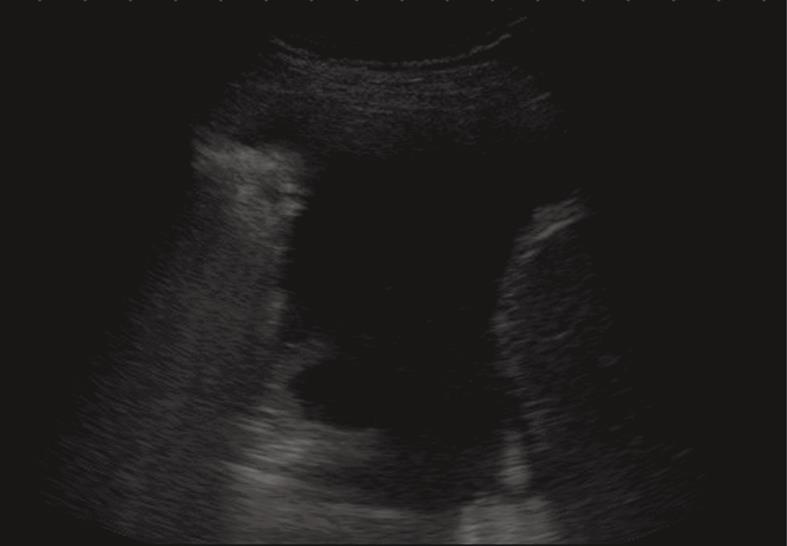
Figure 5 Anechoic echo-free pleural effusion.
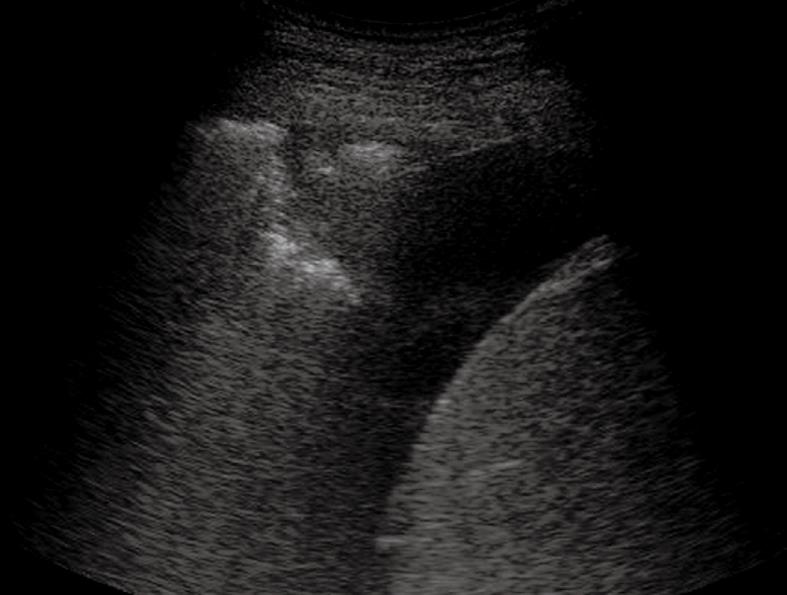
Figure 6 Heterogeneous echogenic material inside the anechoic pleural effusion.
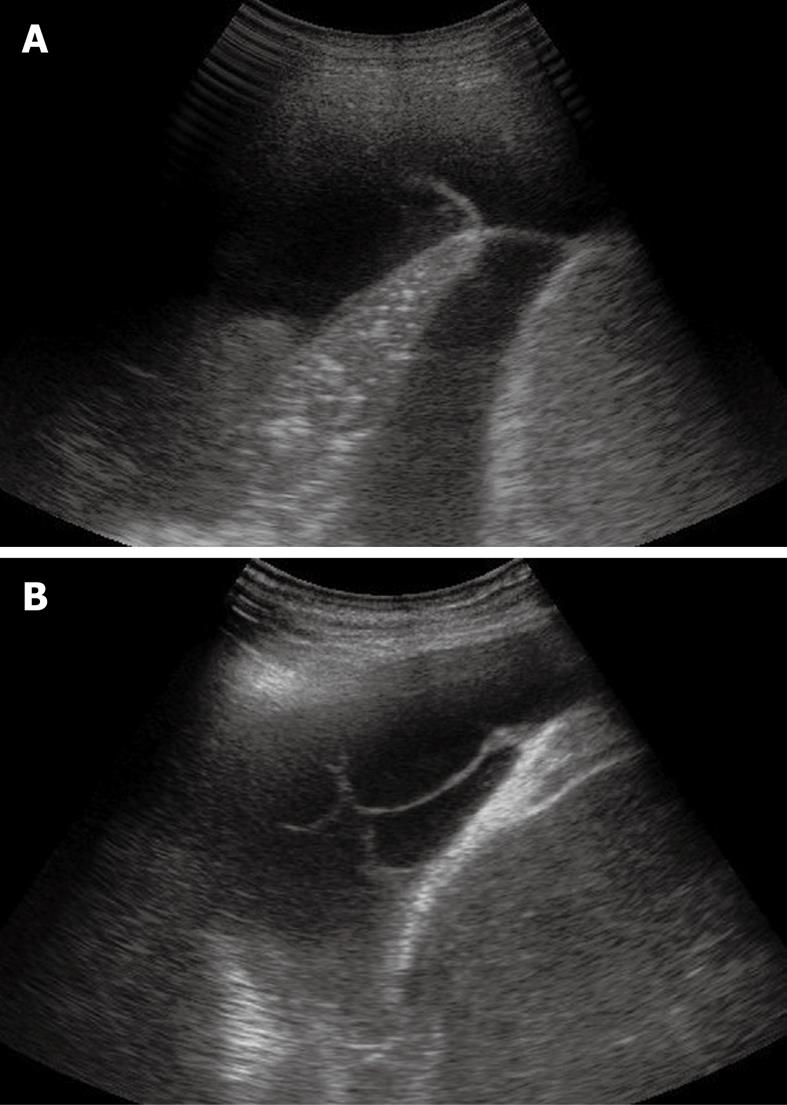
Figure 7 Sporadic (A) and multiple (B) fibrin strands or septa floating inside the anechoic pleural effusion.
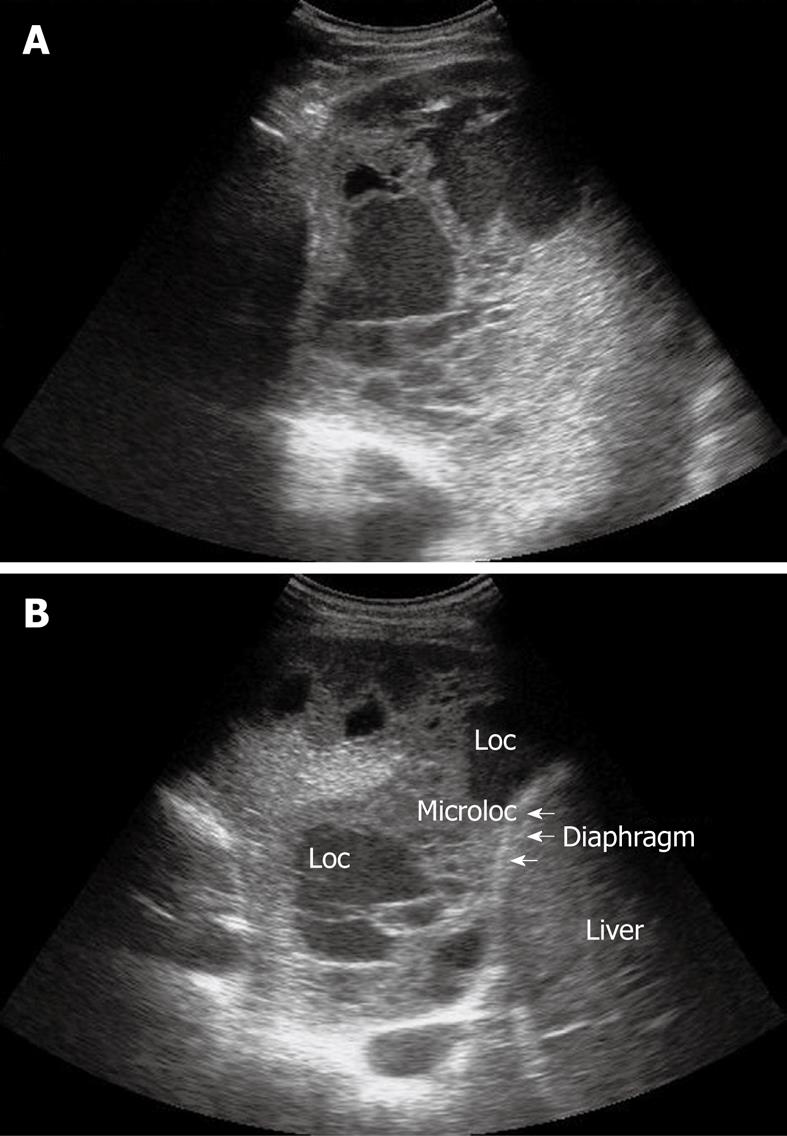
Figure 8 Homogeneous echogenic material inside the pleural space (A and B).
Loc: Loculation; Microloc: Microloculation.
Several formulas have been proposed to quantify pleural effusion, but their usefulness in clinical practice is questionable. However, TUS is considered preferable to radiographic measurement to obtain an empirical estimate of the volume. Since the distribution of pleural fluid in the pleural space is dependent on the position of the patient, the use of a standard sonographic method of evaluation with the patient in the sitting position is recommended[3].
Pneumothorax
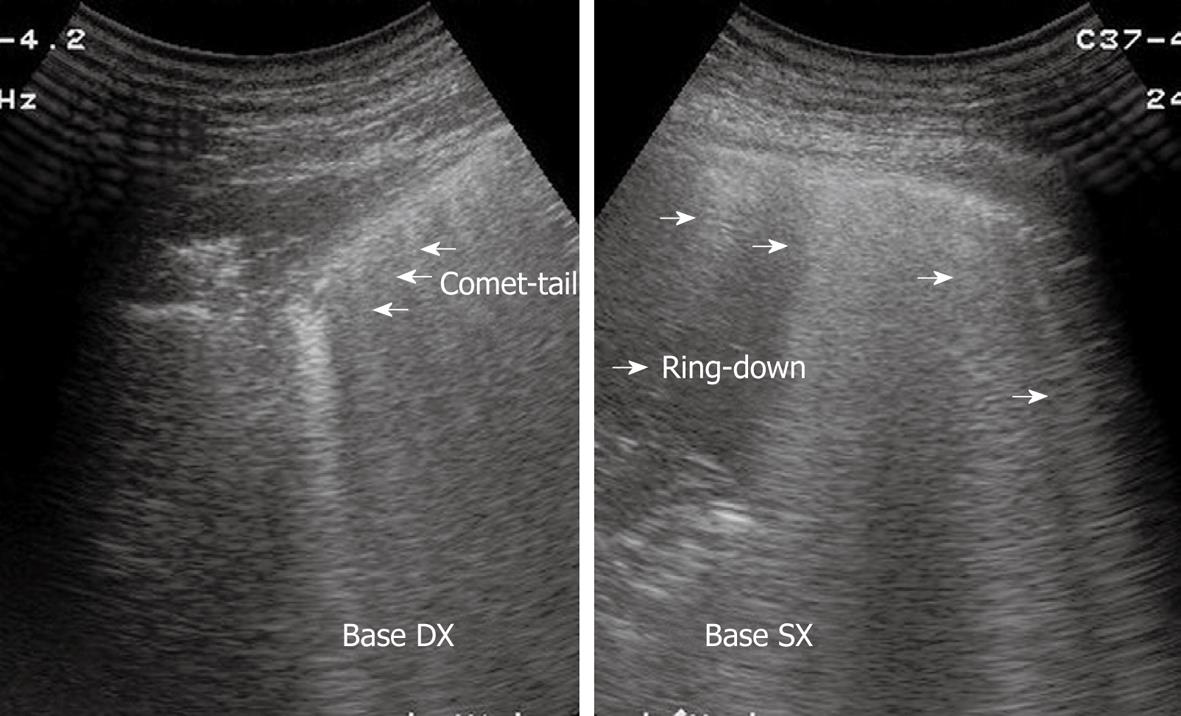
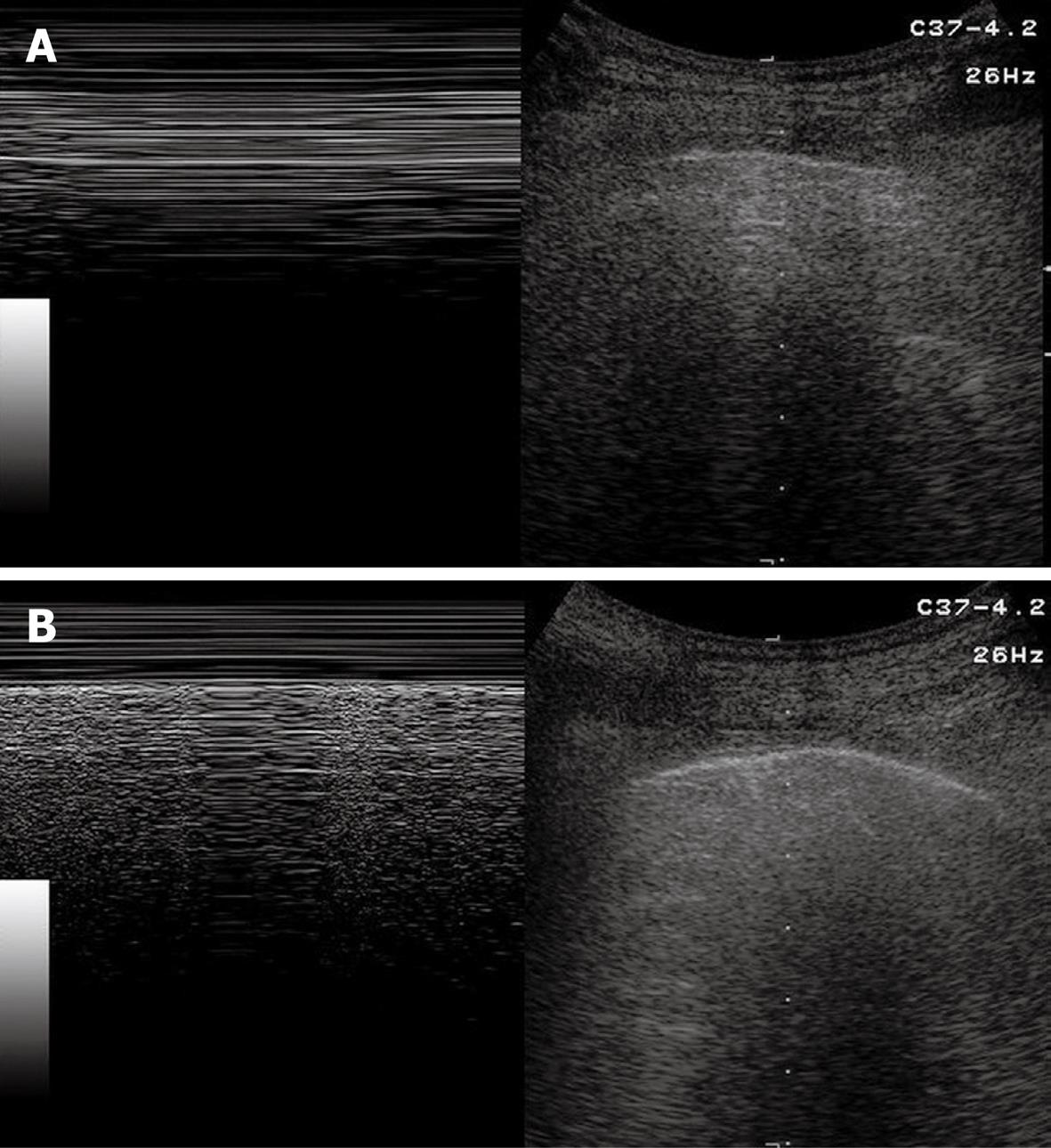
Sonographic images of the lung are composed exclusively of artefacts because air prevents transmission of the ultrasound beam[1-4]. Consequently, it would seem theoretically inconceivable that air in the lungs can be distinguished ultrasonographically from air in the pleural space. However, TUS is able to depict a pneumothorax, as the artefacts in air-containing lungs differ from those induced by air in the pleural space. Indeed, the presence of air in the pleural space prevents sonographic visualization of visceral pleural movements during breathing with disappearance of the gliding sign and comet-tail artefacts, which can be evoked only at the boundary between the visceral pleura and ventilated pulmonary alveoli. Moreover, the presence of air in the pleural space generates reverberation artefacts that form parallel horizontal echoic lines characterized by artefactual immobility during breathing movements. These artefacts (the so-called “frozen echoes”) can be well documented with M-mode imaging (Figure 9A), and can be easily distinguished from the normal “frosted glass-like” artefacts due to the breath-dependent movements (Figure 9B)[18-24].
Figure 9 Pneumothorax.
A: B-mode image (right image) shows horizontal reverberation artefacts corresponding to frozen echoes in M-mode image (left image), due to loss of breathing-dependent motion of pleural line; B: After resolution of pneumothorax, B-mode image (right image) shows pleural line and a single comet-tail artefact; in M-mode image (left image), breathing-dependent movements appear as “frosted glass” artefacts, quite different from frozen echoes seen in A.
Based on these findings, some studies have demonstrated that TUS enables us to detect even very small amounts of air when parasternal anterior scans with supine patients are performed. Such ability plays a very important role particularly in severely ill or traumatized patient. Chest radiography in standing patients is commonly considered the method of choice for the diagnosis of pneumothorax. However, as a rule, the severely ill or traumatized patients can only be subjected to radiography in the recumbent position, which makes diagnosis of a small pneumothorax much more difficult. As a consequence of this limit of chest radiography, in recent years, bedside TUS has gained increasing consideration as a first-line approach in emergency departments and intensive care units to evaluate unstable patients[25-30]. However, TUS cannot replace CT in the diagnostic work-up of critically ill patients, especially if they are traumatized, and CT should always be performed when the patients have become stable.
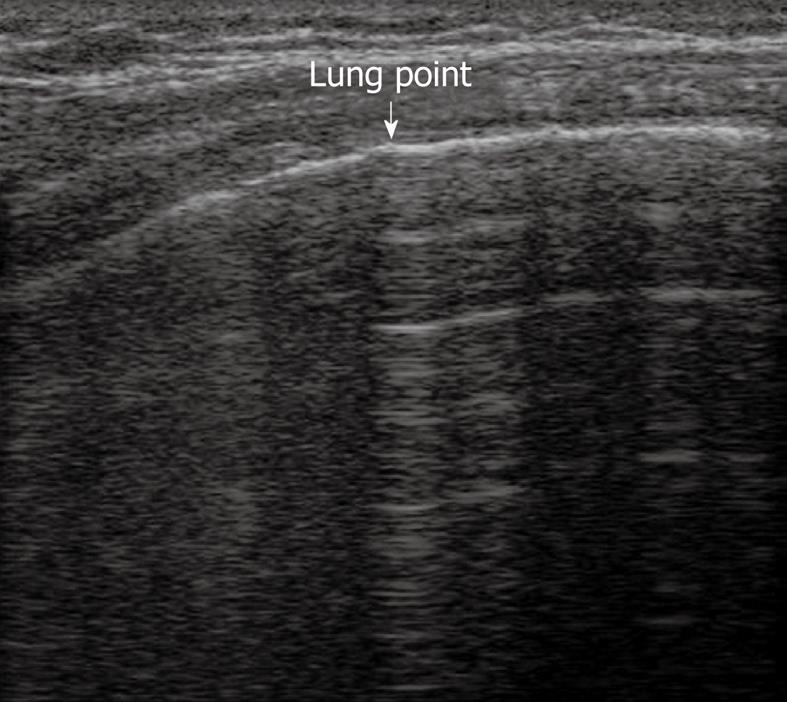
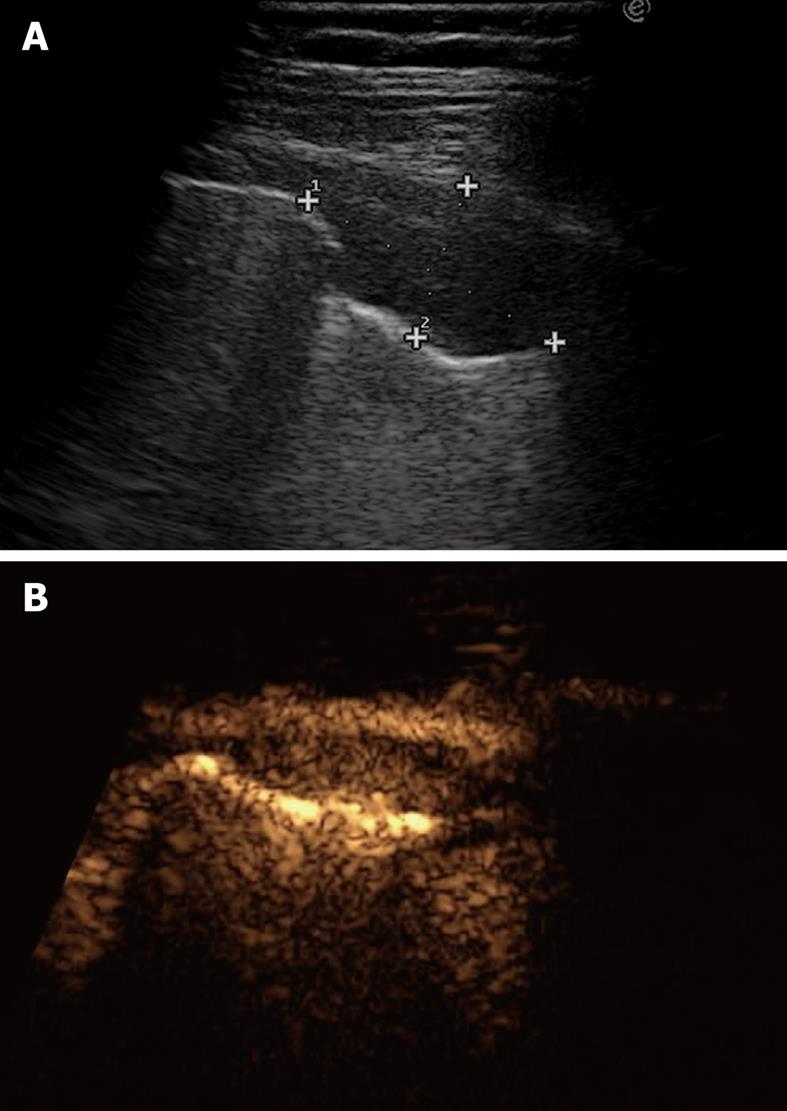
TUS has been proven to be at least as effective as chest radiography in detecting or excluding pneumothorax after interventional thoracic procedures[24,31,32]. In a recent prospective comparison with chest radiography, Sartori et al[24] have reported a sensitivity and specificity of 100% of TUS for detection of pneumothorax after sonographically guided lung biopsy, versus a sensitivity of 87.5% and specificity of 100% for chest radiography. In another study performed in patients undergoing a miscellany of interventional thoracic procedures, Reißig et al[32] have observed a sensitivity and specificity of 100% for TUS in excluding post-interventional pneumothorax, versus a sensitivity of 98% and specificity of 100% for chest radiography. However, despite its high accuracy in detecting pneumothorax, TUS is not yet considered a reliable tool for estimating the volume of pneumothorax. The size of pneumothorax can be roughly approximated by assessment of the lung point, defined as the boundary between the pleural air sickle and the reappearance of normally moving visceral pleura (Figure 10)[33]. However, the depth of pulmonary collapse cannot be evaluated, and chest radiography is necessary to quantify pneumothorax when the lung point is identified far from the site of biopsy needle entry or drainage placement[34].
Figure 10 Lung point.
Horizontal reverberation artefacts are interrupted by reappearance of irregular, fragmented, thickened pleural line with comet-tail artefacts.
Focal pleural lesions
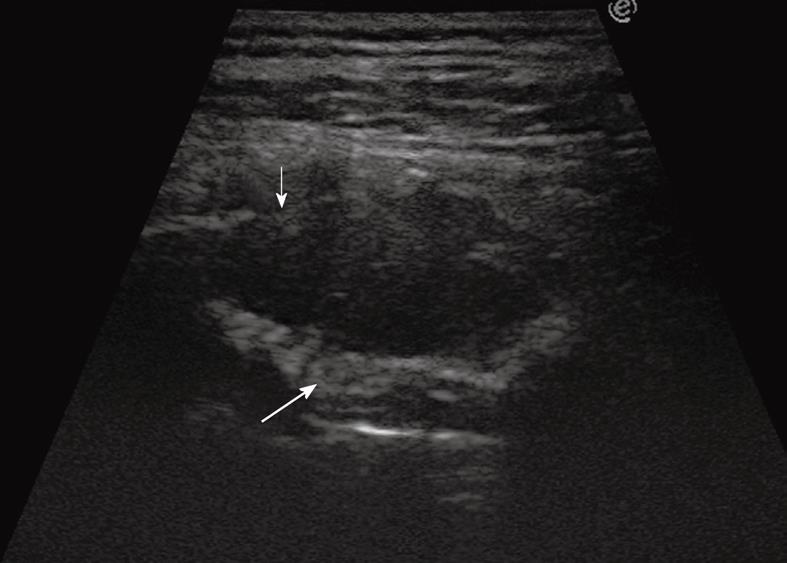
TUS has been proved to be far superior to chest radiography in classifying pleural-based lesions into extrapleural, pleural, and parenchymal lesions[3]. Extrapleural masses usually displace the overlying parietal and visceral pleura, which results in an obtuse angle between the tumor and the chest wall. If rib destruction or muscle infiltration can also be demonstrated, the extrapleural site of origin of the lesion is further confirmed (Figure 11). Pleural lesions arising from the visceral or parietal pleura are usually confined to the pleural space and show an ovoid or trapezoidal shape with irregular, knotty, or polypoid profiles (Figures 12 and 13), sometimes similar to that of extrapleural lesions. Peripheral pulmonary lesions abutting the pleura typically show an acute angle between the lesion and the chest wall (Figure 14). However, there may be some overlap in the appearance of extrapleural, pleural, and peripheral subpleural lung lesions. Large extrapleural tumors may invade the pleural space and pulmonary parenchyma, pedunculated pleural tumors may prolapse into the adjacent lung parenchyma, which results in acute angles between the lesion and the chest wall, and large pulmonary tumors that broadly infiltrate the pleura may show an obtuse angle with the chest wall. However, the role of TUS in the detection and differential diagnosis of focal pleural lesions is limited. Indeed, unless the tumor is large enough to allow clear depiction and imaging-guided biopsy, the diagnosis of focal pleural lesions is challenging for all imaging techniques. In this regard, some reports have recently suggested a good diagnostic yield of 18F-fluoro-2-deoxy-D-glucose positron emission tomography (PET) and PET/CT[35,36], but at present, thoracoscopy is often needed to achieve a reliable diagnosis of focal pleural lesions.
Figure 11 Extrapleural mass disrupting a rib (thin arrow) and displacing and disrupting the pleural line (large arrow).
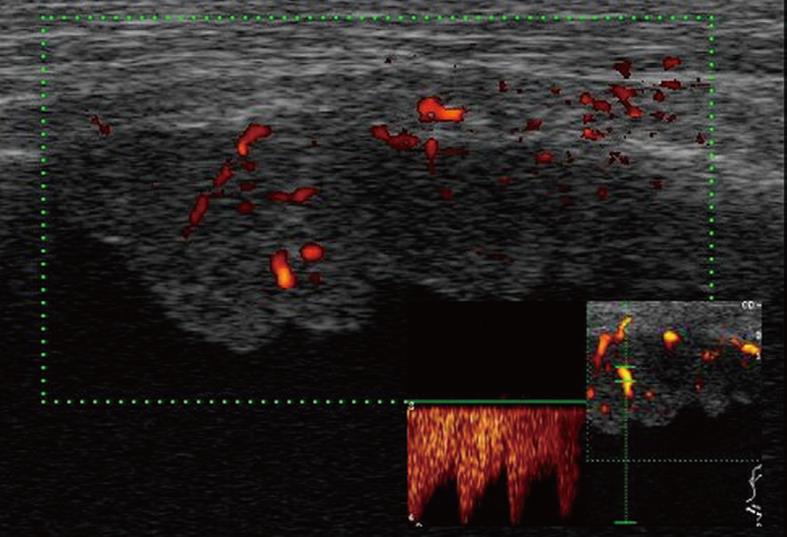
Figure 12 Pleural mesothelioma.
Solid pleural mass with irregular lobulated borders.
Figure 13 Color Doppler sonography shows flow signals inside the lesion, with mainly arterial vascularization.
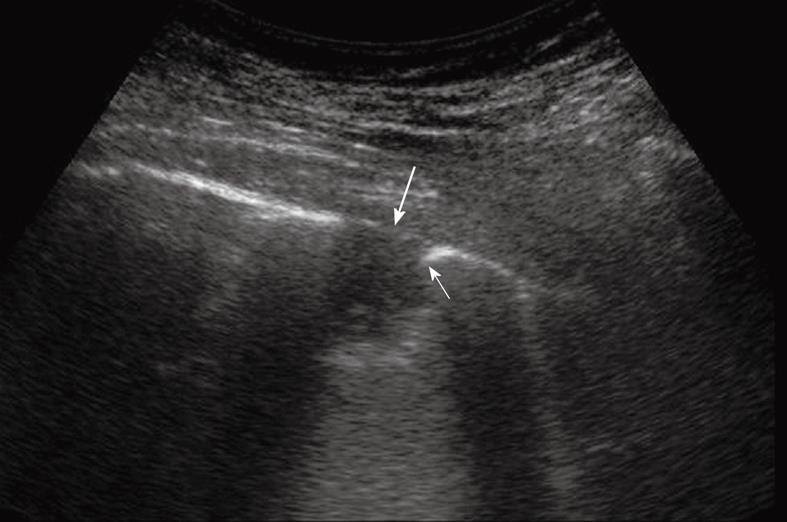
Figure 14 Peripheral pulmonary nodule abutting and infiltrating the pleura.
Note the acute angle between the nodule and the pleural line (thin arrow) and the disruption of the pleural line (large arrow).
One of the most interesting advantages of TUS is its ability to assess the presence of pleural tumor infiltration by real-time imaging. If the subpleural parenchymal tumor shows breathing-dependent up and down movements, infiltration of the pleura can be excluded. Conversely, if the pulmonary tumor does not move during breathing and the pleural line appears disrupted, fixation to the parietal pleura due to neoplastic infiltration or desmoplastic reaction has occurred. Moreover, in extrapleural lesions infiltration of the visceral pleura can be demonstrated when the comet tail artefacts, which normally move up and down during breathing, become fixed. TUS is also superior to chest radiography in differentiating loculated effusion from solid pleural tumors, and in defining the internal structure of a pleural mass. Color Doppler sonography can help in differentiating homogeneously echogenic loculated effusion from solid lesions, although it cannot depict very slow flow signals. In these cases, low mechanical index, contrast-enhanced sonography with second generation contrast agents could represent a reliable tool to differentiate vascularized tissue lesions (Figure 15A and B) from non-vascularized echogenic effusion (Figure 16). However, at present, there are no definitive data in the literature to support such an assumption.
Figure 15 Pleural metastasis.
A: B-mode sonogram shows a hypoechoic lesion resembling either a solid mass or a homogeneously echoic saccate effusion; B: Contrast-enhanced sonography shows contrast enhancement of the lesion 20 s after bolus injection of ultrasound contrast agent. Sonographically-guided biopsy confirmed metastasis from breast carcinoma.
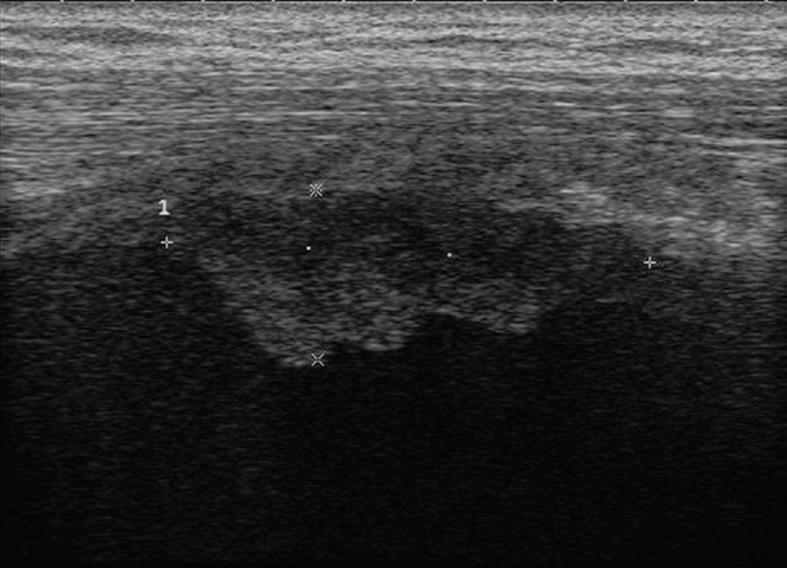
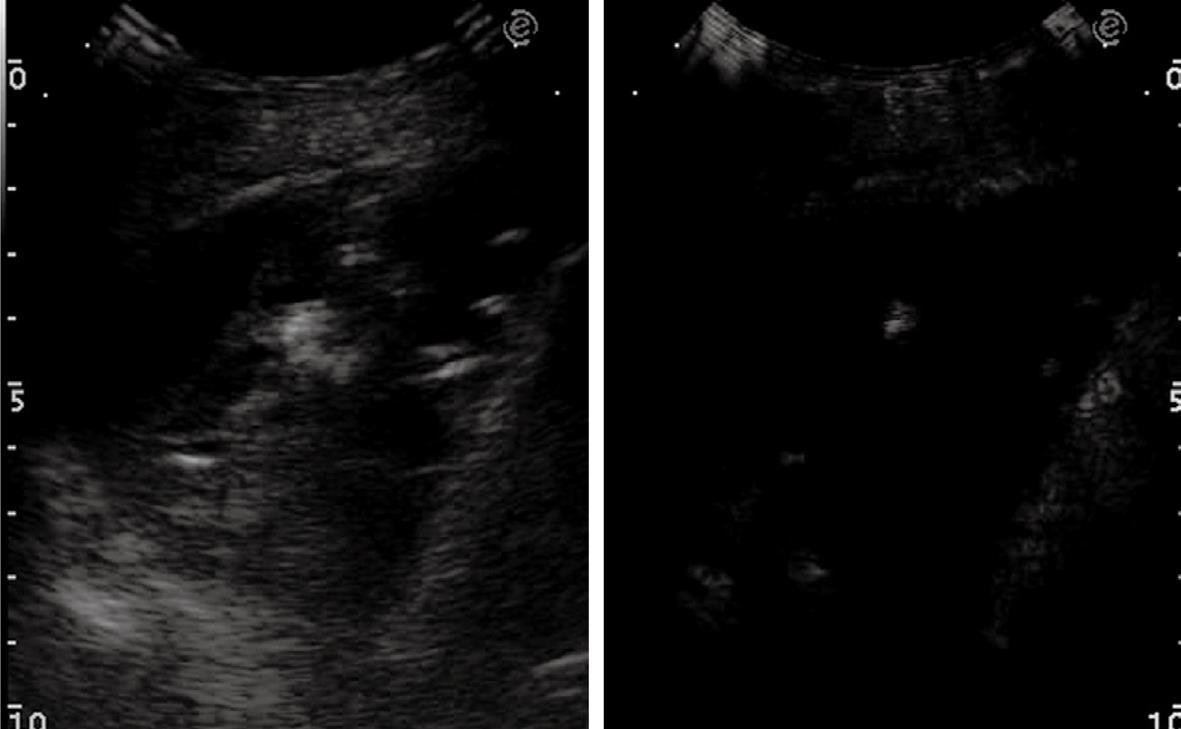
Figure 16 Pleural effusion with broad echoic debris mimicking a solid lesion (left image).
Contrast-enhanced sonography shows no enhancement within the effusion (right image).
Benign pleural lesions are quite rare, and include pleural thickening or plaques, fibroma, and lipoma. They usually present as hyperechoic, well-defined pleural lesions that form an obtuse angle with the chest wall. Tumors of the peripheral nerves (neurofibroma, schwannoma) are extrapleural masses of the thoracic wall that may not infrequently mimic a pleural tumor. They are generally benign, but sometimes they can also be malignant, are usually round, sharply marginated masses of high, mixed or low echogenicity. However, in most cases, sonographic features of benign pleural lesions and neurogenic tumors cannot provide a definitive diagnosis, and pathological confirmation is often needed[3,37,38].
Malignant pleural lesions include pleural mesothelioma, pleural metastasis, pleural infiltration of bronchogenic carcinoma, and pleural lymphoma (very infrequent). Pleural mesothelioma usually appears as a diffuse pleural thickening or as a hypoechoic or isoechoic vascularized mass with irregular shape or lobulated borders (Figures 12 and 13). Pleural effusion, frequently hemorrhagic, is often present. Pleural metastases constitute the majority of malignant neoplasms that involve the pleura. Bronchogenic carcinoma is the first cause of pleural metastasis, but primary neoplasms of breast, gastrointestinal tract, kidney and ovaries are also frequent sources. Usually, pleural metastases become manifest as pleural effusion, as they are frequently too small (< 1-2 mm) to be detected by the imaging techniques (TUS or CT). However, TUS plays an important role as the method of choice to guide thoracentesis for cytological confirmation. Pleural metastases that can be detected by TUS usually appear as relatively small hypoechoic lesions with obtuse margins with the chest wall (Figure 15A), or as large masses with complex echogenicity. Conversely, pleural involvement from bronchogenic carcinoma typically appears as a hypoechoic mass with acute angulation between the lesion and the chest wall. As previously described, TUS can easily exclude or document infiltration of the parietal pleura by assessing the mobility of the tumor during breathing movements. However, desmoplastic reaction and inflammation can fix the tumor to the adjacent pleura and disrupt the pleural line, thus simulating an invasive lesion. Although in some cases the sonographic features of the lesions can help to establish the nature of the tumor, cyto-histological confirmation is often needed, and TUS is the method of choice to guide pleural biopsy. TUS-guided biopsy of pleural lesions is a safe and effective technique, and when the lesions can be well identified, it represents a feasible tool to obtain tissue samples with a very low risk of pneumothorax, as the procedure can be monitored in real-time and aerated lung tissue is usually not penetrated by the biopsy needle[16,24,39].