Published online Dec 28, 2010. doi: 10.4329/wjr.v2.i12.455
Revised: December 20, 2010
Accepted: December 26, 2010
Published online: December 28, 2010
The extensive use of imaging techniques in differential diagnosis of abdominal conditions and screening of hepatocellular carcinoma in patients with chronic hepatic diseases, has led to an important increase in identification of focal liver lesions. The development of contrast-enhanced ultrasound (CEUS) opens a new window in the diagnosis and follow-up of these lesions. This technique offers obvious advantages over the computed tomography and magnetic resonance, without a decrease in its sensitivity and specificity. The new second generation contrast agents, due to their intravascular distribution, allow a continuous evaluation of the enhancement pattern, which is crucial in characterization of liver lesions. The dual blood supply in the liver shows three different phases, namely arterial, portal and late phases. The enhancement during portal and late phases can give important information about the lesion’s behavior. Each liver lesion has a different enhancement pattern that makes possible an accurate approach to their diagnosis. The role of emerging techniques as a contrast-enhanced three-dimensional US is also discussed. In this article, the advantages, indications and technique employed during CEUS and the different enhancement patterns of most benign and malignant focal liver lesions are discussed.
- Citation: Molins IG, Font JMF, Álvaro JC, Navarro JLL, Gil MF, Rodríguez CMF. Contrast-enhanced ultrasound in diagnosis and characterization of focal hepatic lesions. World J Radiol 2010; 2(12): 455-462
- URL: https://www.wjgnet.com/1949-8470/full/v2/i12/455.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i12.455
The widespread availability of imaging modalities such as ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI), to screen for liver lesions and in the study of nonspecific abdominal complaints, has greatly increased the diagnosis of liver lesions in asymptomatic patients[1].
The precise diagnosis of liver lesions should not only be based on imaging techniques but also must be taken into acco-unt the clinical history and laboratory data. For example, the use of oral contraception in young women, history of cirrhosis, positive serologic test for hepatitis viruses, elevation of α fetoprotein (AFP) or excessive alcohol consumption may provide important clues. Therefore, a comprehensive clinical history and physical examination are as important as the imaging technique for the characterization of liver masses. In some cases, histology is needed for the final diagnosis.
Ultrasound is usually the first line of investigation in the detection of focal liver lesions due to its relatively low cost, safety and wide availability, but the differentiation between benign and malignant lesions is difficult in many cases with this technique[2,3]. The introduction of contrast media in imaging technique can better characterize the non-invasive focal liver lesions based on their enhancement pattern than the surrounding liver parenchyma. Contrast-enhanced CT and MRI are most commonly used, but the introduction of ultrasound contrast has expanded the role of sonography in the characterization of focal liver masses[4].
The ultrasound contrast agents are microbubbles with an approximate size of red blood cells that circulate into vessels but not through the vascular endothelium into the intersticium. This property helps to provide accurate information about the vascularity of the lesion[5]. There are different types of contrast but all of them consist of a gas microbubble stabilized with a phospholipid-membrane.
The contrast works as a signal enhancer. The interface between microbubble and aquatic medium reflects the ultrasonic wave improving the contrast between the blood and hepatic tissue around.
Under low mechanical index imaging, the microbubbles have a much higher nonlinear behavior than the native tissue, resulting in detectable echoes[2]. Pulse inversion imaging suppresses echoes from tissues in favor of those from bubbles. When used at a low mechanical index, pulse-inversion imaging does not cause disruption of the microbubbles, thus allowing a continuous assessment of the vessels as the contrast agent traverses the imaging field[6].
Contrast agents are safe and produce very few adverse effects. Severe anaphylactoid reactions have been described in 0.001% of abdominal explorations, similar to those described in MRI contrasts (gadolinium) and fewer than allergic reactions to CT iodized contrasts.
Before ultrasound contrast is injected, a complete sonographic study in B-mode must be performed, with or without the use of Doppler. After the lesion is identified, the transducer has to be placed in a fixed position in order to visualize the mass along the whole exploration. The mechanical index ought to be changed to a low index to prevent a fast destruction of microbubbles, thereby allowing a complete multiphase imaging. The principal vascular structures and other anatomic references like the diaphragm must remain visualized. Some sonographers have a double screen with one of them under a low index state and the other one in B-mode, which facilitates the visualization under both mode conditions at the same time. The contrast is usually administered in bolus through a peripheral vein followed by a saline flush. The chronometer must be started as the same time as the initial injection. It is recommended that the exploration be performed continuously in the first 90 s to evaluate correctly the arterial and portal phase, but can be explored discontinuously in the late phase. If a second lesion has to be studied, the previous microbubbles can be washed out sweeping with the transducer by using a high mechanical index. It is recommended to record the exploration[7].
The liver, with its dual blood supply, shows first enhancement in the arterial phase as the contrast agent fills the hepatic artery, with progressively enhancement as it arrives to the portal vein. The arterial phase (10-35 s after the injection) gives information about the amount and type of the lesion’s microvascularization. The portal (30-120 s after the injection) and late phases (over 120 s after the injection) give more information about the elimination of the contrast in the lesion than about that of the rest liver parenchyma. It is believed that the contrast is uptaken by Kupffer cells or remains in the hepatic sinusoids in the late phase. There are new contrast agents that provide the additional post-vascular or Kupffer phase that allows an assumption of the degree of malignancy based on Kupffer function[8].
The portal and late phase enhancement can give important information about the lesion’s behavior. The majority of malignant lesions present a lower enhancement than the rest liver parenchyma, while the majority of benign solid lesions present a higher than or the same enhancement as the rest liver parenchyma[2]. The differentiation between benign and malign tumors is possible in 95% of cases in the late phase, between 120 s and 5 min after contrast injection[9].
The use of ultrasounds provides several advantages over CT and MRI, such as lack of exposure to radiation, more availability and less expensive contrast-enhanced US (CEUS)[10]. Furthermore, the contrast media used in sonography are not nephrotoxic, do not influence the thyroidal metabolism while the microbubbles remain in the intravascular space, allowing a continuous assessment of the lesions’ vascularity and enhancement with a high temporal resolution, not limited to the pre-defined time points[11,12]. In CT and MRI, the very early contrast period can be missed and the enhancement during the first seconds gives important information, especially in highly arterialized lesions. Another advantage is that contrast injection can be repeated if necessary, due to its excellent tolerance[7]. CEUS has a sensitivity of 90%, a specificity of 99% and an accuracy of 89% in diagnosis of malignant liver lesions, as shown in Von Herbay’s study[13].
The limits of enhanced-sonography for detection of liver lesions are the same as conventional ultrasonography. Enhanced-sonography is an operator-dependent technique, due to the evaluation of some liver segments like VIII segment with difficult accessibility, cirrhotic or fatty livers which limit the ultrasound penetration, patients with low collaboration during exploration, and the need of additional injection of contrast for patients with multiple lesions.
The indications for the use of CEUS are summarized in Table 1.
| Patients for whom the ultrasonography without contrast, computed tomography, magnetic resonance imaging or cytology is not conclusive |
| Evaluation of lesions before percutaneous treatment to have a first image to compare after the procedure if necessary |
| Guidance of the needle or the probe during percutaneous treatment |
| Immediate evaluation of the lesion after percutaneous treatment to detect viable areas[24] |
| Follow-up of patients with liver lesions treated by percutaneous ethanol injection or radiofrequency ablation |
| Detection of liver tumors and study of its microcirculation |
| Evaluation of organ perfusion |
| Study of macrocirculation |
| Improvement of sensibility and specificity during intraoperative sonography |
CEUS is contraindicated in patients with acute cardiac failure, class III/IV cardiac failure, cardiac rhythm disorders, recent coronary arterial intervention or factors suggestive of clinical instability, right-to-left shunts, severe pulmonary hypertension and uncontrolled systemic hypertension. Ultrasonographic contrast media have not been used in patients under the age of 18 years, pregnant or breastfeeding women. They must be used with caution in patients with severe chronic obstructive pulmonary disease[3]. It is recommended that patients stay at hospital under medical supervision for at least 30 min after contrast administration.
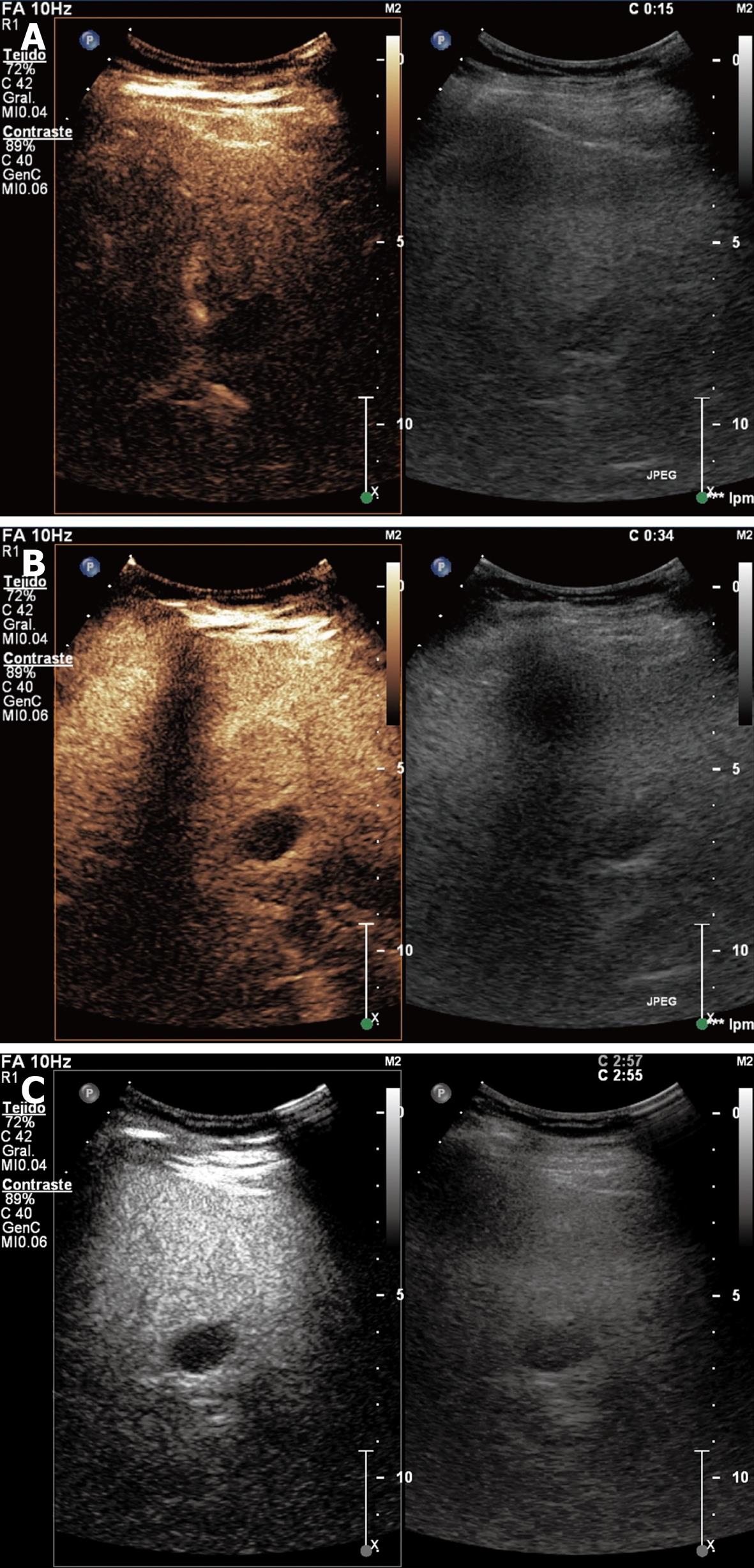
Simple cyst: It is a congenital lesion surrounded by biliary-type epithelium with serous content. It is usually asymptomatic but may produce abdominal pain if it reaches large dimensions. It is anechoic with posterior enhancement in conventional sonography and hypoenhanced in all phases with contrast. Simple cysts do not need surveying or treatment, unless they accompany hemorrhage or infection (Figure 1).
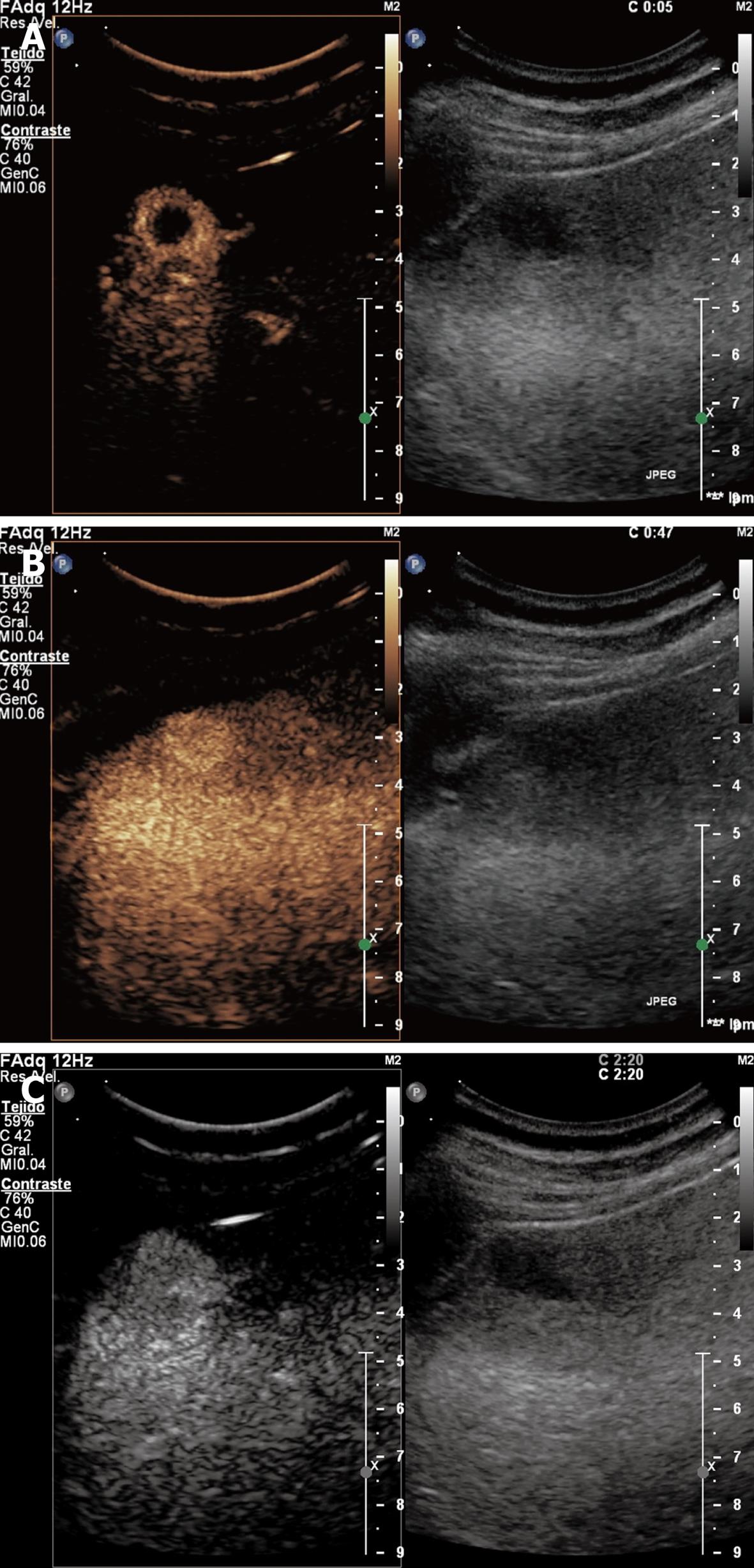
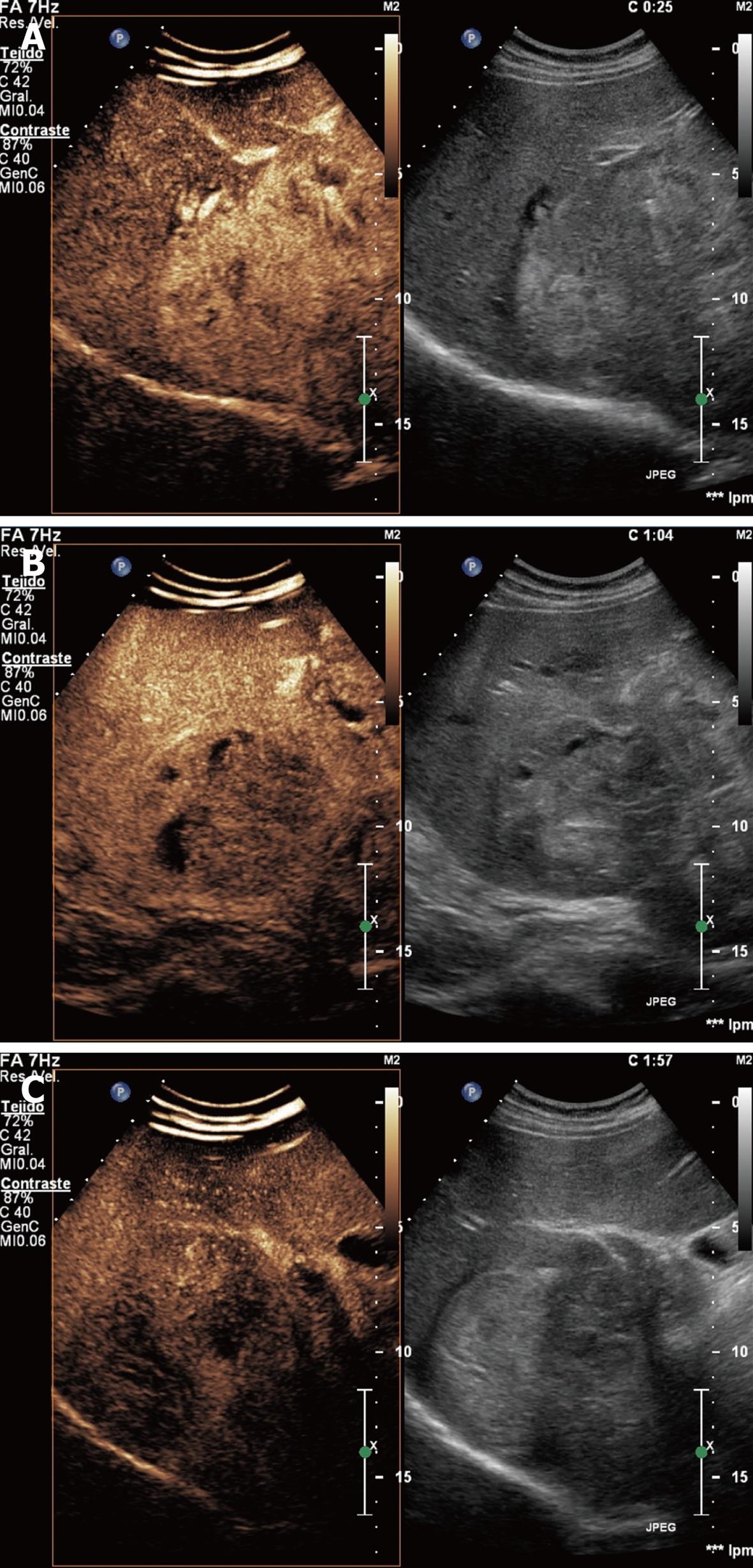
Hemangioma: It is the commonest tumor in the liver with a prevalence of 0.4%-7.4%[14]. It is more frequent in women at the age of 30-50 years. This lesion consists of multiple vessels supported by fibrous interstitium. Usually asymptomatic, it may cause abdominal pain. Only in exceptional cases, it is associated with thrombopenia, consumption coagulopathy and microangiopathic anemia (Kassabach-Merritt syndrome)[15]. It may grow during pregnancy or estrogenic therapies.
Ultrasonography shows a hyperechogenic, well defined lesion. CEUS shows that hemangiomas present peripheral nodular enhancement with centripetal filling during the portal phase and remaining isoenhanced in the late phase (Figure 2).
Labeled red blood cell scintigraphy is the best and least expensive modality for lesions over 2.5 cm in diameter and MRI for lesions under 2 cm in diameter[1].
Asymptomatic patients with hepatic hemangiomas do not need follow-up as there is no risk of malignant transformation, but when a homogeneous hyperechoic lesion is discovered in a patient with a history of cirrhosis or known malignancy, further characterization is recommended[4].
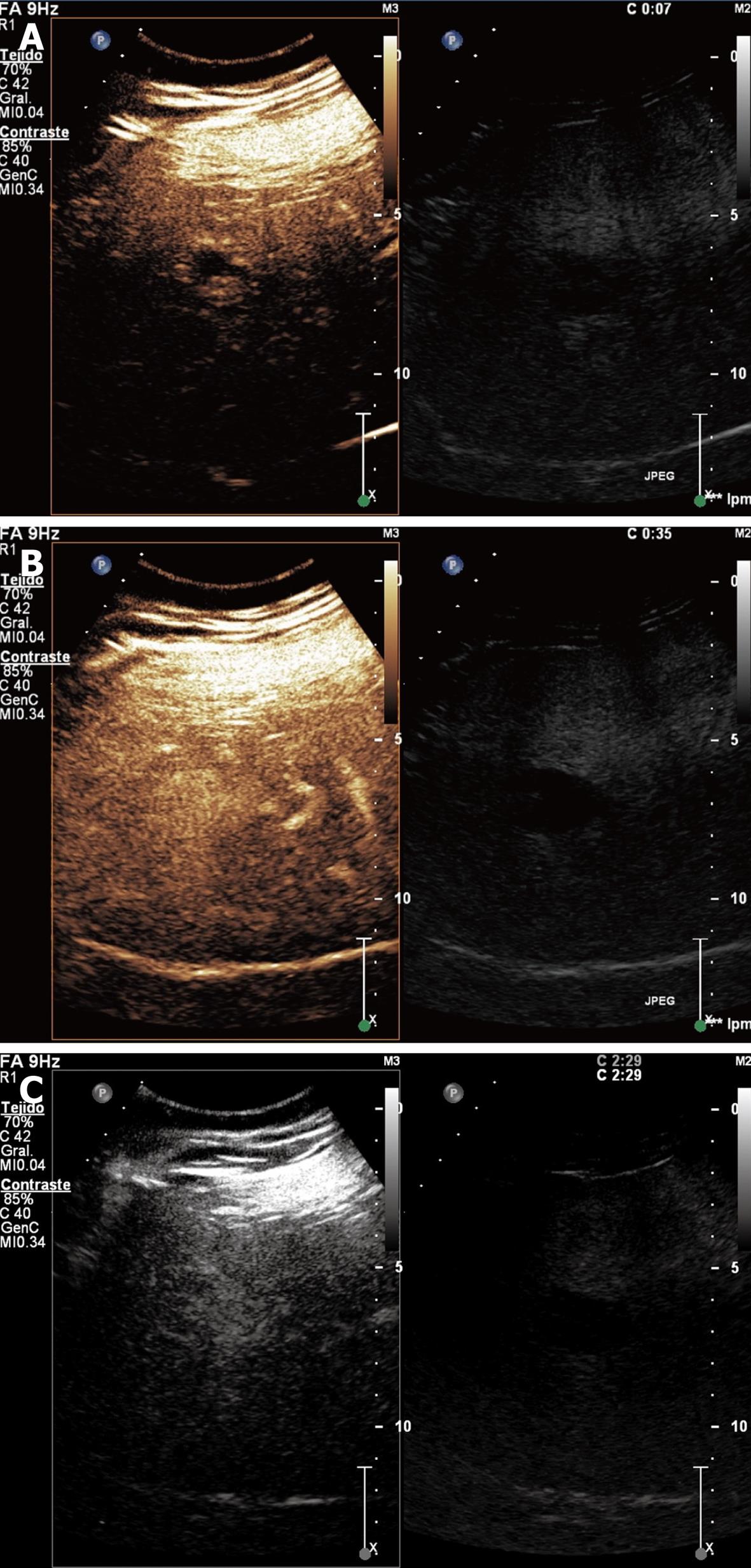
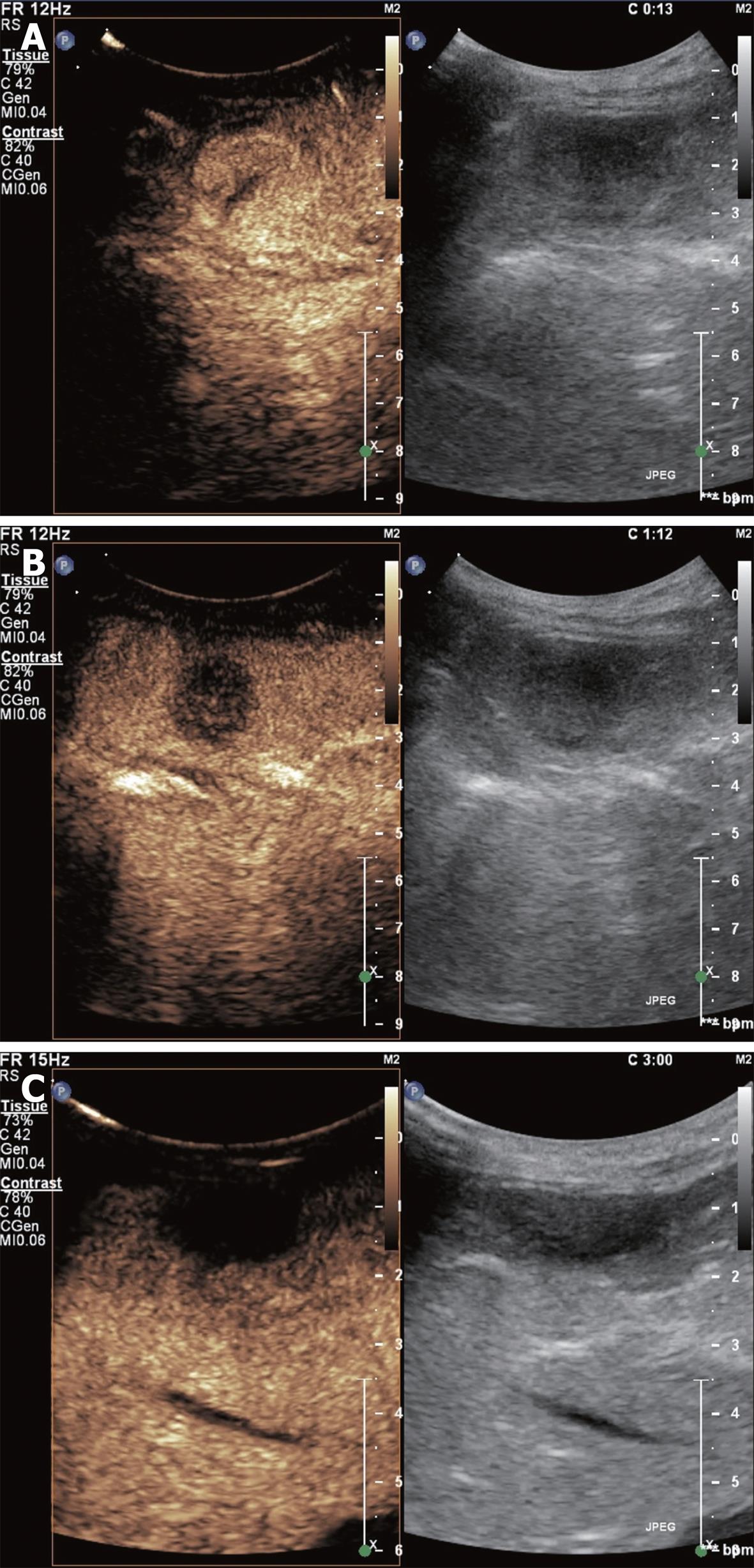
Focal nodular hyperplasia: It is a pseudotumor, which is considered a hyperplastic proliferation of normal liver cells in response to a preexisting arterial malformation. Large lesions commonly have a central scar made up from fibrous stroma with a supply artery and hyperplastic bile ducts[15]. It is more frequent in young women and usually asymptomatic.
Enhanced sonography demonstrates that the lesion typically presents a central enhancement in the arterial phase, with a centrifugal filling through radial vascular branches (wheel sign). This image occurs in 95% of cases if the lesion measures at least 3 cm in diameter and in 20%-30% of cases if the lesion is smaller. In the portal phase, the lesion usually remains enhanced with a non-enhancing central scar and becomes isoenhanced in the late phase (Figure 3). Nowadays, MRI is the technique of choice for the diagnosis of focal nodular hyperplasia (FNH), but the characteristic image of the wheel sign is better visualized with CEUS due to its dynamic facet[9].
No cases of malignization are reported. Such cases do not need treatment or follow-up. The interruption of oral contraception is recommended as it may reduce the size of the lesion.
Adenoma: It is related with the use of anabolic androgens, type-1 glycogenosis and oral contraceptives with an incidence of 0.03%. It is histologically made up of normal or atypical hepatocytes with none or few bile ducts and Kupffer cells. Up to 25% of liver adenomas cause abdominal pain in the right hypochondrium or in the epigastrium[16]. In very large adenomas, intratumoral hemorrhage is frequent, provoking hemoperitoneum in exceptional cases.
During CEUS, adenomas show a fast centripetal hyperenhancement during arterial phase, but in the majority of cases, the enhancement is irregular in the presence of necrosis or hemorrhages. In portal phase, the adenoma begins to wash out contrast becoming iso or hypoenhanced with the liver parenchyma.
The management is surgical due to its high bleeding risk and potential malignant degeneration [5% of hepatic adenomas transform to hepatocellular carcinoma (HCC)][1].
Regenerative nodule: Regenerative nodules often appear in cirrhotic livers or after a massive hepatic necrosis. These nodules are isoenhanced during the three phases of contrast sonography. Progression to HCC is possible.
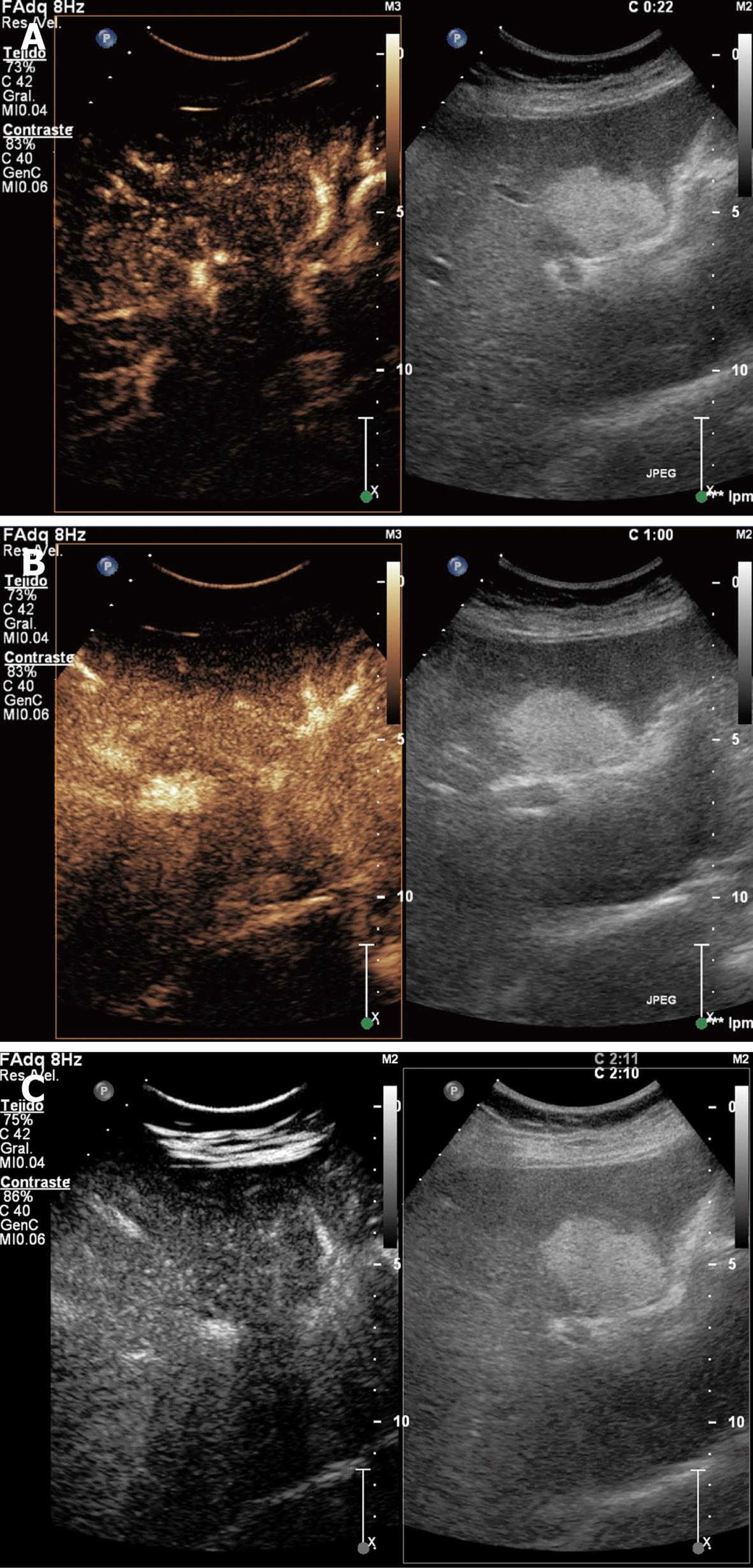
Focal fat accumulation: It is present in 10% of patients with liver esteatosis. These fat accumulations are usually located next to the gallbladder, portal veins or the falciform ligament. It is isoenhanced during the arterial, portal and late phases (Figure 4).
The characteristics of hepatic benign lesions according to their vascular pattern on CEUS scan are summarized in Table 2.
| Arterial phase | Portal phase | Late phase | |
| Hemangioma | Peripheral nodular hyperenhancement with centripetal progression | Slow centripetal filling. The lesion becomes iso or hyperenhanced | Iso or hyperenhanced |
| Focal nodular hyperplasia | Hyperenhanced in the center of the lesion (central vessel) with fast centrifugal filling due to radial vascular branches: “wheel sign” | Iso or hyperenhanced | Iso or hyperenhanced. Sometimes the central scar can be seen |
| Adenoma | Highly hyperenhanced with complete filling and fast wash-out. No radial branches | Iso or hyperenhanced | Iso or hyperenhanced |
| Simple cist | No enhancement | No enhancement | No enhancement |
| Regenerative nodule | Isoenhanced | Isoenhanced | Isoenhanced |
| Focal fatty accumulation | Isoenhanced | Isoenhanced | Isoenhanced |
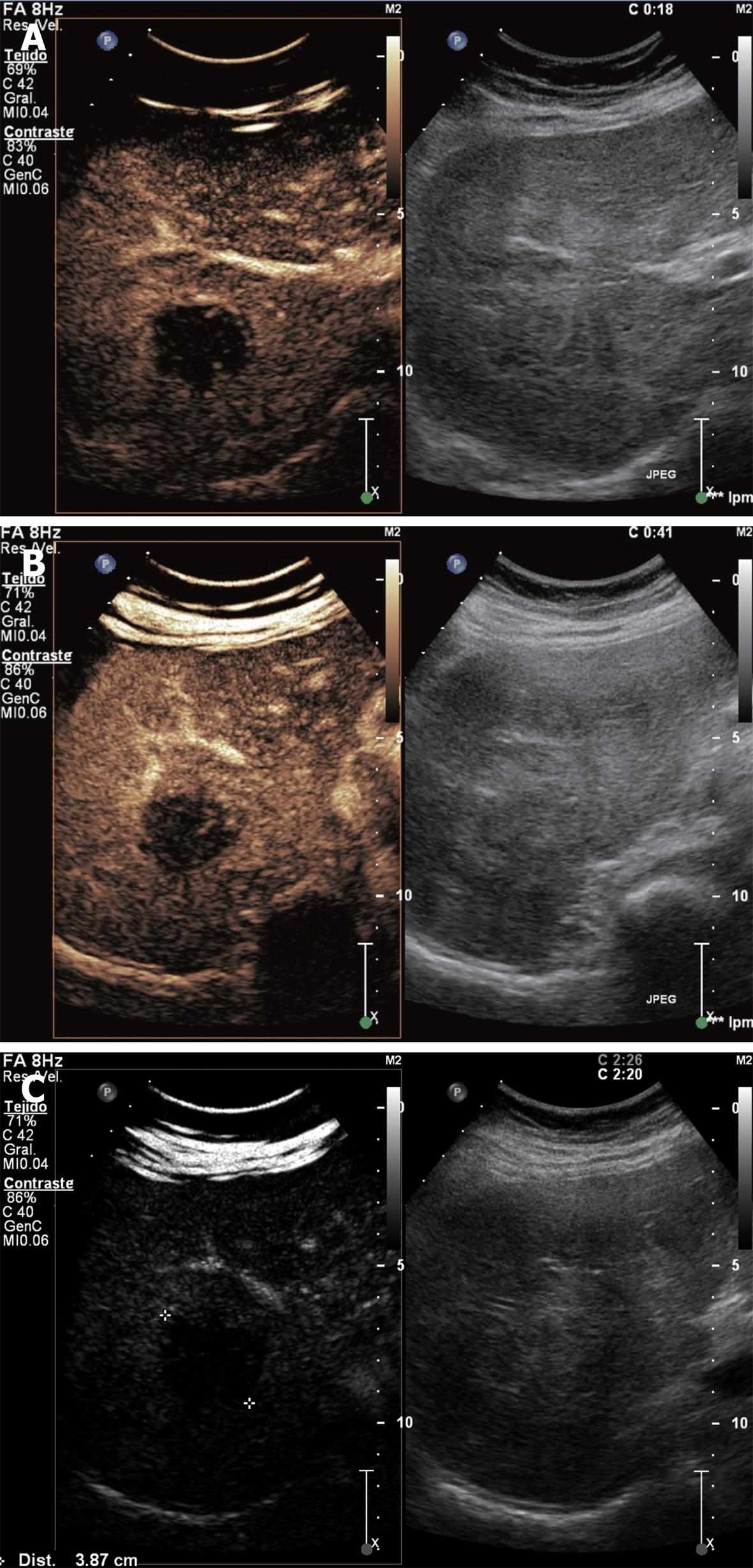
HCC: It is the most frequent primary tumor of the liver and the fifth more common malignancy worldwide. It represents the third cause of cancer-related death[17]. About 80% of HCCs appear in cirrhotic population. A liver mass in a cirrhotic patient should be considered a HCC until proven otherwise[1]. Screening of HCC is recommended in these patients through the determination of AFP and a conventional ultrasound every 6 mo. This makes possible a curative or palliative treatment in its early phases.
HCC may be silent but is usually associated with weight loss, abdominal pain, hepatomegaly or ascites. Laboratory data include elevation of serum alkaline phosphatase, persistent leukocytosis and increased ratio of serum AST/ALT.
Two thirds of HCC are hyperechoic in conventional ultrasonography and the other third of HCC are heterogeneous, showing hyper and hypoechoic areas. Small lesions tend to be hypoechoic. Contrast-ultrasonography shows that HCC are enhanced in arterial phase, often with feeding vessels around and inside the tumor. It has a characteristic rapid wash-out in portal phase and frequently remains hypoenhanced in late phase (Figure 5).
For the diagnosis of HCC in cirrhotic patients, lesions over 2 cm in diameter need just one imaging technique showing typical findings or one imaging technique showing an AFP level over 400 μg. In nodules between 1 and 2 cm in diameter, two techniques showing typical imaging criteria are needed for the diagnosis. Follow-up every 3 mo is recommended for masses less than 1 cm in diameter[1].
Intrahepatic cholangiocarcinoma: It is generally a unique mass originated in small intrahepatic bile ducts. This tumor should be considered in patients with chronic primary sclerosing cholangitis, longstanding choledochocele, intrahepatic lithiasis, parasitic disease of the bile ducts, Caroli’s disease, and in patients exposed to thorotrast for radiographic procedures[1]. Jaundice is the most common clinical presentation[18] and usually associated with a high serum bilirubin level. Up to 80% of the cholangiocarcinomas present elevated values of serum CA 19-9 and 50% present elevated CEA[15].
Cholangiocarcinomas show different enhancement patterns depending on size of the lesion and different pathological components of the tumor[19]. In arterial phase, this tumor may show irregular peripheral rimlike hyperenhancement, heterogeneous hyperenhancement, homogeneous hyperenhancement or heterogeneous hypoenhancement. In portal phase, it presents complete wash out staying hypoenhanced. Even though there are many ultrasound patterns, the use of CEUS helps to differentiate between cholangiocarcinoma and HCC[20].
The liver is the most common site of metastasis from the gastrointestinal tract, pancreas, breast and lung. Colorectal cancer most commonly metastasizes to liver. Metastasis occurs in the most common malignant hepatic tumor. Generally, both hepatic lobes are involved[1].
Hypervascular metastases are associated to carcinoid tumors, melanomas, sarcomas, thyroid tumors and hypernephromas. They are completely enhanced in arterial phase with fast wash out and hypoenhanced in portal and late phases (Figure 6). Hepatic metastases can be classified into hypo and hypervascular.
Hypovascular metastases remain unenhanced during the three phases (Figure 7).
Intraoperative ultrasonography is the most sensitive imaging technique for diagnosing liver metastases and may be helpful in delineating the extent of disease and vascular landmarks during hepatic resection[21].
The vascular pattern characteristics of hepatic focal malignant on CEUS scan are summarized in Table 3.
| Arterial phase | Portal phase | Late phase | |
| Hepatocellular carcinoma | Hyperenhanced due to its arterial vascularization | Fast wash-out leaving an iso or hypoenhanced lesion | Hypoenhanced |
| Cholangiocarcinoma | Hyperenhanced in the border | Iso or hypoenhanced | Hypoenhanced |
| Hypervascular metastasis | Hyperenhanced, usually starting in the margin: ring sign | Iso or hypoenhanced | Hypoenhanced |
| Hypovascular metastasis | Hypoenhanced | Hypoenhanced | Hypoenhanced |
Recently, CE three-dimensional (3D) US has been incorporated to the diagnostic US technologies. This technique provides dynamic information for spatial volume. As compared with CEUS 2D, CEUS-3D acquires the data in a volume of interest (VOI) by scanning with a desired angle and allows reconstruction of images in three orthogonal planes and yields images similar to angiograms, with accurate visualization of the vascular characteristics of focal liver tumors[22,23]. A recent retrospective-prospective large study by Luo et al[5], found that dominant enhancement patterns are as diffuse enhancement or peripheral ring-like enhancement, followed by washout change in HCC or peripheral ring-like enhancement with venous washout for metastases. In the case of hemangiomas, the pattern is usually a nodular enhancement, whereas in FNH, the dominant pattern is observed in spoke-wheel arteries. Although CE 3D US shows a high sensitivity and specificity for differentiation of lesions and a good-excellent inter-observer agreement, no significant difference has been observed in prospective diagnosis accuracy of CE 3D US and CE 2D US.
However, some artifacts from the cardio-pulmonary motion, shadows of costal bones, and interference from abdominal gas might lead to reader’s misinterpretation. The role of this technique in both evaluation of focal liver lesions and assessment of the effect of percutaneous or TACE therapies on HCC should be further evaluated before its routine use is recommended.
CEUS is an emerging imaging technique that offers important advantages over CT and MRI. It is becoming the procedure of choice, using a low mechanical index, for the study and detection of focal liver lesions and a valuable complementary tool with CT and MRI in the diagnosis and follow-up of these lesions.
Peer reviewers: Kazushi Numata, MD, PhD, Associate Professor, Gastroenterological Center, Yokohama City University Medical Center, 4-57 Urafune-cho, Minami-ku, Yokohama, Kanagawa 232-0024, Japan; Hui-Xiong Xu, MD, PhD, Professor, Department of Medical Ultrasonics, Institute of Diagnostic and Interventional Ultrasound, Sun Yat-Sen University, 58 Zhongshan Road 2, Guangzhou 510080, Guangdong Province, China
S- Editor Cheng JX L- Editor Wang XL E- Editor Zheng XM
| 1. | Assy N, Nasser G, Djibre A, Beniashvili Z, Elias S, Zidan J. Characteristics of common solid liver lesions and recommendations for diagnostic workup. World J Gastroenterol. 2009;15:3217-3227. |
| 2. | von Herbay A, Vogt C, Willers R, Häussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med. 2004;23:1557-1568. |
| 3. | Wang WP, Wu Y, Luo Y, Li R, Zhou XD, Zhang J, Qian CW, Tan XY, Xu QH, Wang Y. Clinical value of contrast-enhanced ultrasonography in the characterization of focal liver lesions: a prospective multicenter trial. Hepatobiliary Pancreat Dis Int. 2009;8:370-376. |
| 4. | Jang HJ, Yu H, Kim TK. Imaging of focal liver lesions. Semin Roentgenol. 2009;44:266-282. |
| 5. | Luo W, Numata K, Morimoto M, Nozaki A, Ueda M, Kondo M, Morita S, Tanaka K. Differentiation of focal liver lesions using three-dimensional ultrasonography: retrospective and prospective studies. World J Gastroenterol. 2010;16:2109-2119. |
| 6. | Wilson SR, Burns PN. An algorithm for the diagnosis of focal liver masses using microbubble contrast-enhanced pulse-inversion sonography. AJR Am J Roentgenol. 2006;186:1401-1412. |
| 7. | Tranquart F, Claudon M, Correas JM. [Guidelines for the use of contrast agents in ultrasound]. J Radiol. 2005;86:1047-1054. |
| 8. | Wong GL, Xu HX, Xie XY. Detection of focal liver lesions in cirrhotic liver using contrast-enhanced ultrasound. World J Radiol. 2009;1:25-36. |
| 9. | Ladam-Marcus V, Mac G, Job L, Piot-Veron S, Marcus C, Hoeffel C. [Contrast-enhanced ultrasound and liver imaging: review of the literature]. J Radiol. 2009;90:93-106; quiz 107-108. |
| 10. | Tranquart F, Correas JM, Ladam Marcus V, Manzoni P, Vilgrain V, Aube C, Elmaleh A, Chami L, Claudon M, Cuilleron M. [Real-time contrast-enhanced ultrasound in the evaluation of focal liver lesions: diagnostic efficacy and economical issues from a French multicentric study]. J Radiol. 2009;90:109-122. |
| 11. | Nicolau Molina C, Fontanilla Echeveste T, Del Cura Rodríguez JL, Cruz Villalón F, Ripollés González T, Baudet Naveros B, Velasco Marcos MA, Garre Sánchez C, Huertas Arroyo R, Hernández García L. [Usefulness of contrast-enhanced ultrasonography in daily clinical practice: a multicenter study in Spain]. Radiologia. 2010;52:144-152. |
| 12. | Trillaud H, Bruel JM, Valette PJ, Vilgrain V, Schmutz G, Oyen R, Jakubowski W, Danes J, Valek V, Greis C. Characterization of focal liver lesions with SonoVue-enhanced sonography: international multicenter-study in comparison to CT and MRI. World J Gastroenterol. 2009;15:3748-3756. |
| 13. | von Herbay A, Westendorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound. 2010;38:1-9. |
| 14. | Rubin RA, Mitchell DG. Evaluation of the solid hepatic mass. Med Clin North Am. 1996;80:907-928. |
| 15. | Pons F, Llovet JM. Approaching focal liver lesions. Rev Esp Enferm Dig. 2004;96:567-573; 573-577. |
| 16. | Di Bisceglie A, Befeler AS. Tumors and cysts of the liver. Sleisenger and Fortran's gastrointestinal and liver disease. 9th ed. Philadelphia: Saunders-Elsevier 2010; 1569-1589. |
| 17. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. |
| 18. | Suzuki M, Takahashi T, Ouchi K, Matsuno S. The development and extension of hepatohilar bile duct carcinoma. A three-dimensional tumor mapping in the intrahepatic biliary tree visualized with the aid of a graphics computer system. Cancer. 1989;64:658-666. |
| 19. | Chen LD, Xu HX, Xie XY, Lu MD, Xu ZF, Liu GJ, Liang JY, Lin MX. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol. 2008;81:881-889. |
| 20. | Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010;20:743-753. |
| 21. | Kruskal JB, Kane RA. Intraoperative ultrasonography of the liver. Crit Rev Diagn Imaging. 1995;36:175-226. |
| 22. | Ohto M, Kato H, Tsujii H, Maruyama H, Matsutani S, Yamagata H. Vascular flow patterns of hepatic tumors in contrast-enhanced 3-dimensional fusion ultrasonography using plane shift and opacity control modes. J Ultrasound Med. 2005;24:49-57. |
| 23. | Yukisawa S, Ohto M, Masuya Y, Okabe S, Fukuda H, Yoshikawa M, Ebara M, Saisho H, Ohtsuka M, Miyazaki M. Contrast-enhanced three-dimensional fusion sonography of small liver metastases with pathologic correlation. J Clin Ultrasound. 2007;35:1-8. |
| 24. | Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15-24. |