INTRODUCTION
Focal nodular hyperplasia (FNH) is the second most common benign lesion of the liver after hemangioma. It usually occurs in women at the reproductive age, but has been reported in both sexes and at all ages[1]. FNH is composed of benign hepatocytes and prominent atypical branching artery present within a central fibrous scar. The exact pathogenesis of FNH is unknown but it is a local reactive hyperplastic response of hepatocytes to a congenital vascular anomaly[2,3]. On the basis of this theory, FNH is considered to be associated with cavernous hemangioma and vascular malformation of various organs and neoplasms of the brain[4]. Usually, FNH is a solitary lesion, but 20%-30% of the patients may have 2-5 FNH lesions and rarely present with more than five lesions[5]. FNH is usually a stable lesion that does not enlarge when studied for long periods of time, though very rare cases of FNH that progresses in size and number are available[6].
Gadolinium-EOB-DTPA (Eovist) is a new generation of tissue-specific (hepatobiliary) contrast agent that has been developed to enhance the performance of hepatic MR imaging[7,8] and to cause a preferential increase in liver intensity, leaving hepatic tumors as negative defects. Hence, an increased liver-tumor contrast can be obtained.
Herein, we report the findings of multiple progressive FNH lesions in a patient with hemosiderosis on computed tomography (CT) and Eovist magnetic resonance (MR) images. To our knowledge, this is the first case of FNH, which was enlarged due to hemosiderosis and regressed after treatment with phlebotomy.
CASE REPORT
Our institution does not require an institutional review board approval for this case report.
A 23-year-old female with a previous history of osteogenic sarcoma developed acute myeloblastic leukemia (AML) which was treated with a bone marrow transplant and chemoradiation. Radiologic examination then did not find liver and lung lesions. The patient had a history of hemosiderosis caused by blood transfusion during the treatment of AML. The HFE gene for hereditary hemochromatosis was negative. A previous CT examination 3 years after AML diagnosis revealed multiple enhanced FNH lesions (more than 10) in both liver lobes, with the largest located in the lateral segment of the left liver lobe. Liver biopsy demonstrated moderate hemosiderosis with hepatosteatosis, and biopsy of dominant lesions showed septal fibrosis surrounding regenerative nodules which were consistent with benign regenerative lesions. A repeat follow-up CT one year later demonstrated that the FNH lesions were increased in size with no change in their number (Figure 1). AML and sarcoma were in remission during her follow-up. Four years after the second CT examination, her liver function was elevated and she was referred to MR imaging. The MR imaging was performed with a superconducting imager (GE Healthcare, Milwaukee, WI) operating at 1.5 T using axial and coronal T2-weighted SSFSE (TR = 1520, TE = 89), axial T2-weighted FRFSE (TR = 10000, TE = 90) and T1-weighted gradient-echo (GRE) sequences (TR = 3.6/1.7, flip angle = 10°) before and after contrast injection. T1-weighted GRE images were acquired at 25-30 s (arterial phase), 70-90 s (portal venous phase), 3-5 min (equilibrium phase), and in a delayed phase following the administration of 6 mL Eovist (Bayer-Schering Pharma, Germany), acquired at 10 and 20 min after the injection. The section thickness was 5-7 mm. The MR imaging showed a diffusely decreased signal intensity of the liver and spleen as well as pancreas, most likely due to long-standing hemosiderosis, which was consistent with excessive iron deposition. The FNH lesions were hyperintense on pre-contrast T1-weighted images (Figure 2). Multiple hypointense FNH lesions were found on T2-weighted images (Figure 3A and B), which were enlarged and increased in number when compared to prior CT studies. The FNH lesions were enhanced at the arterial phase (Figure 3C) and remained hyperintense to the liver in all phases including the delayed hepatocyte specific phase (Figure 3D) with no parenchymal vasculature disruption and evidence of cirrhosis noted. Radiologic examination showed no accompanying liver hemangioma, vascular malformation and/or intracranial tumor. Due to the progressive course of FNH lesions, a further biopsy of dominant lesions was performed, which showed benign hepatocytes and malformed vessels embedded in fibrous stroma with proliferating bile ductules at the periphery of these vessels. These findings were consistent with FNH. Based on laboratory (elevated serum ferritin and iron saturation) and radiologic findings, monthly phlebotomy was started to remove the extra iron. Three months after the treatment, liver function tests and iron studies of the patient were remarkably improved. A repeat follow-up MR imaging 6 mo later showed that the size of FNH lesions was significantly reduced (Figure 4).
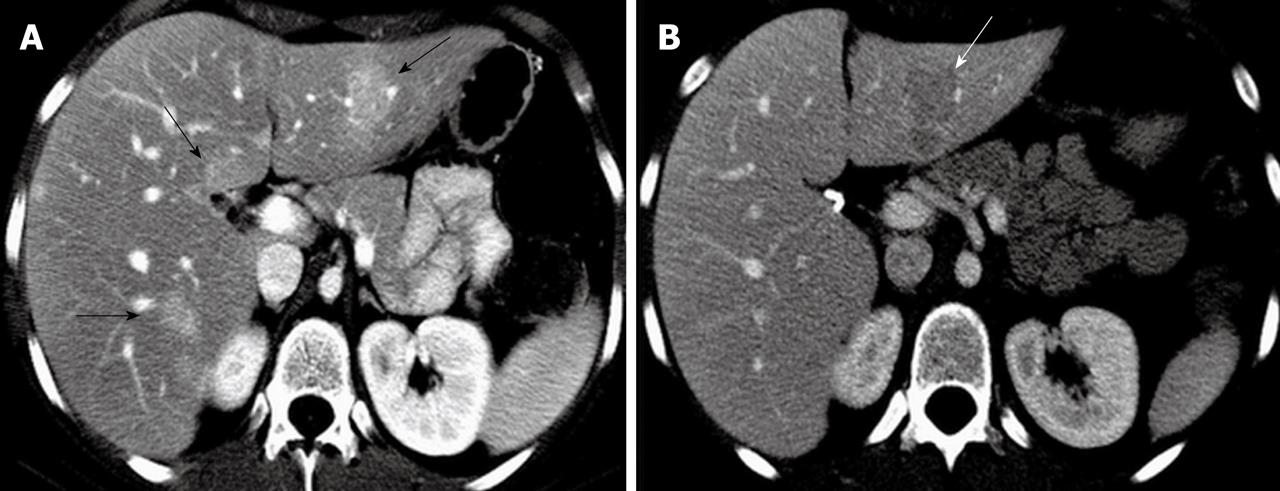
Figure 1 Arterial phase axial computed tomography showing enhanced focal nodular hyperplasia lesions in both liver lobes with the largest focal nodular hyperplasia lesion located in the lateral segment of the left liver lobe (arrows) (A) and portal venous phase axial computed tomography at 1 year follow-up demonstrating a hypodense focal nodular hyperplasia lesion in the left liver lobe with its size increased (arrow) (B).
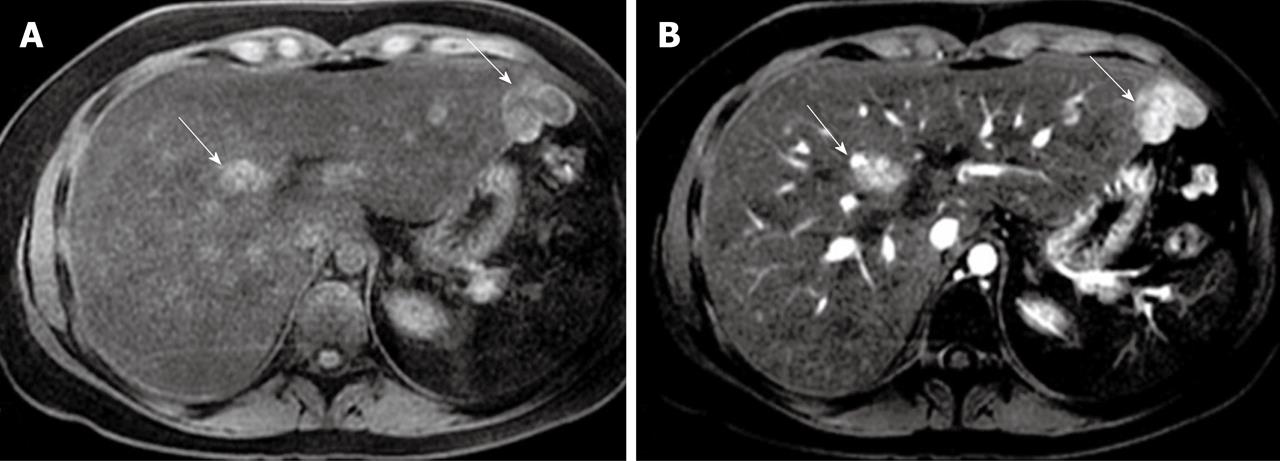
Figure 2 Axial pre-contrast T1-weighted magnetic resonance imaging demonstrating focal nodular hyperplasia lesions with an increased intensity (arrows) (A) and enhanced focal nodular hyperplasia lesions at the arterial phase (arrows) (B).
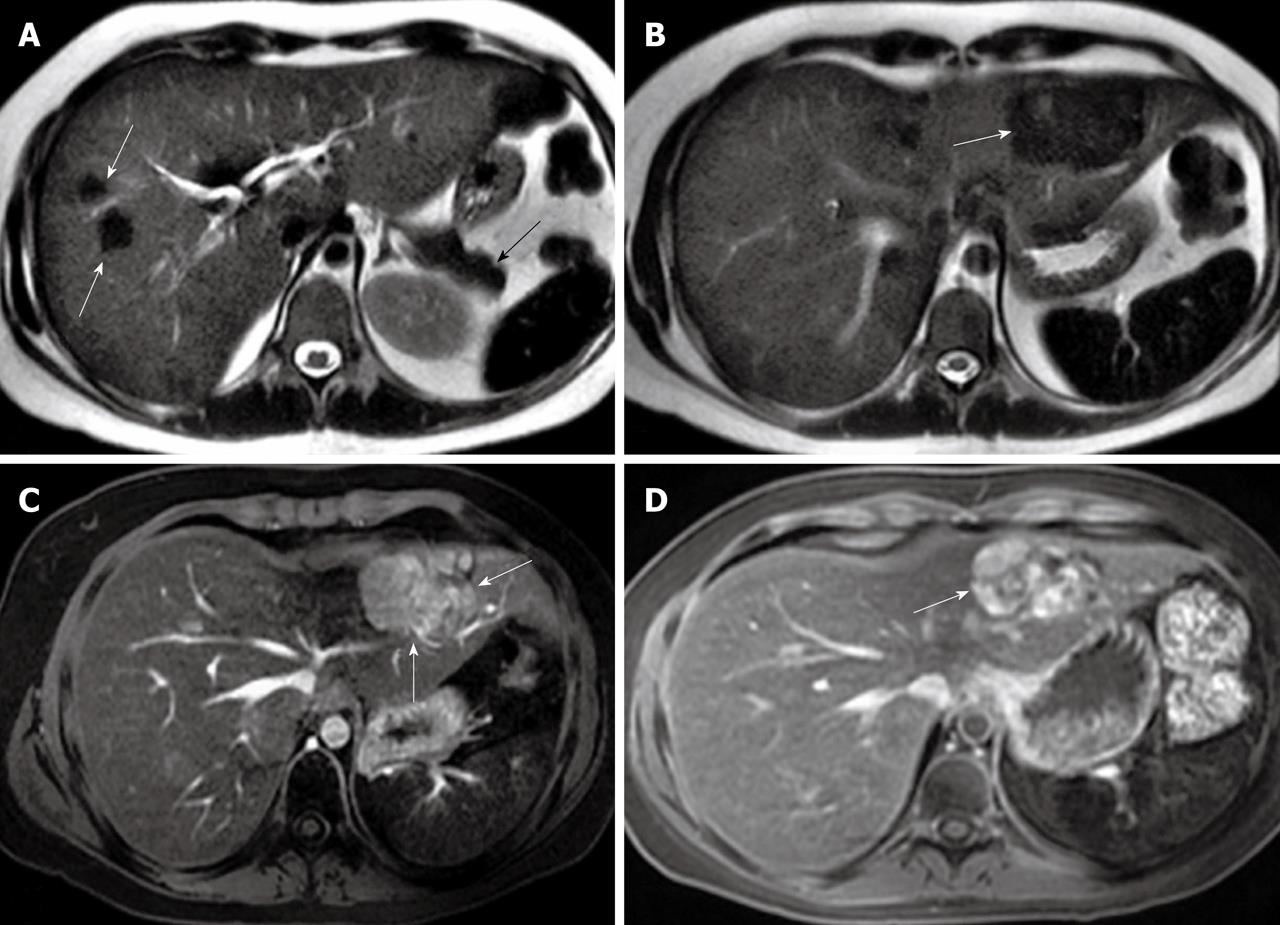
Figure 3 Axial T2-weighted magnetic resonance imaging showing hypointense focal nodular hyperplasia lesions in the right liver lobe (white arrows) with a diffusely decreased signal intensity of the liver and spleen as well as pancreas (black arrow) (A) and central hyperintense focal nodular hyperplasia lesions in the left liver lobe (arrow) (B), while Eovist-enhanced axial magnetic resonance imaging demonstrating enlarged focal nodular hyperplasia lesions in the left liver lobe in the arterial phase (arrows) (C) and hyperintense focal nodular hyperplasia lesions in the left liver lobe in the delayed phase (arrow) (D).
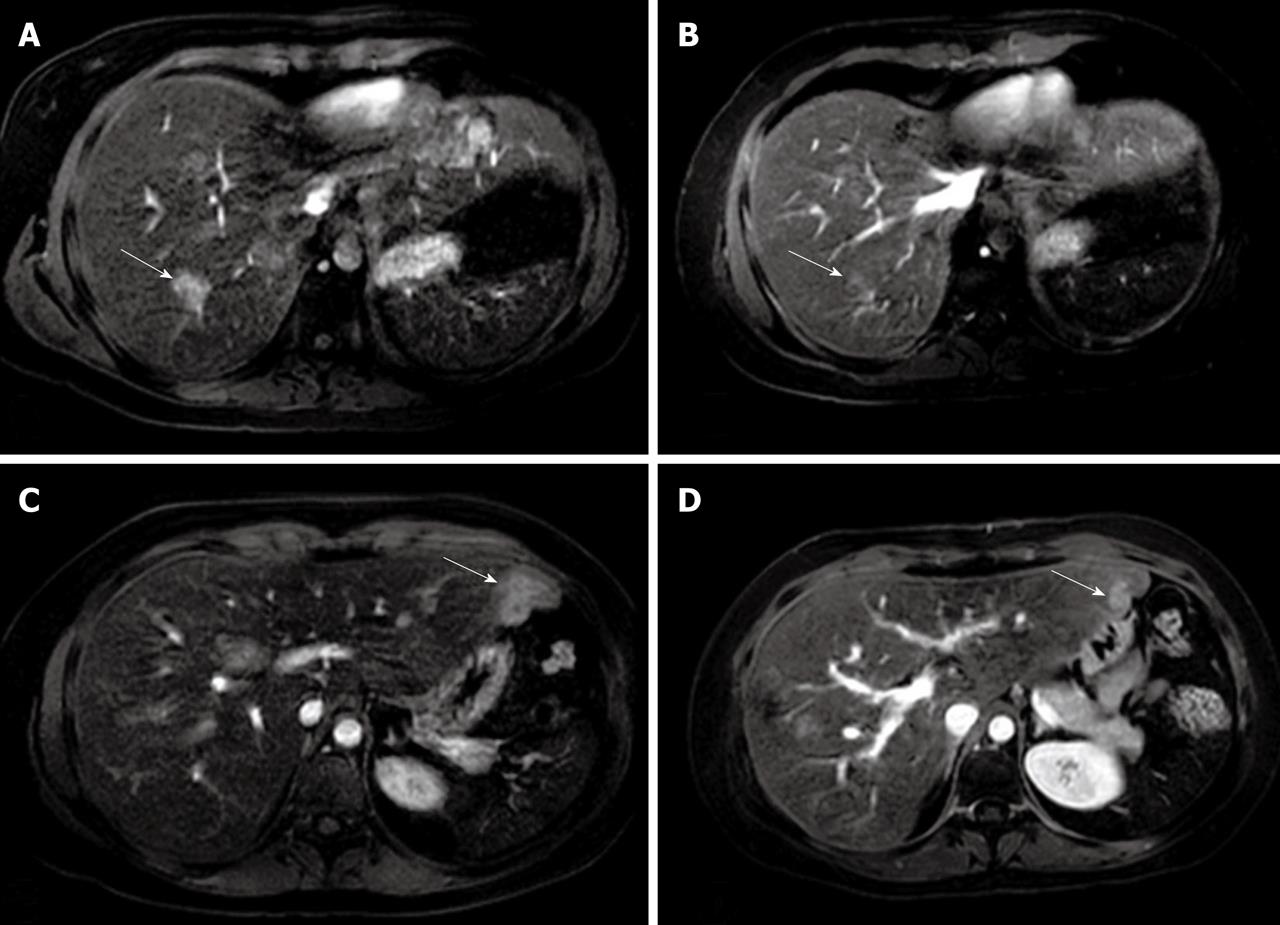
Figure 4 Eovist-enhanced axial magnetic resonance imaging demonstrating enhanced focal nodular hyperplasia lesions in the right liver lobe in arterial phase before treatment (arrow) (A), control magnetic resonance imaging 6 months later showing significantly decreased size of focal nodular hyperplasia lesions (arrow) (B), enhanced focal nodular hyperplasia lesions in the left liver lobe in the arterial phase before treatment (arrow) (C), and significantly decreased size of lesions in the left liver lobe at 6 month follow-up (arrow) (D).
DISCUSSION
FNH is a benign liver lesion characterized by a well circumscribed hyperplastic liver tissue with stellate fibrosis. Its pathogenesis still remains obscure but it is claimed to be a response to a preexisting vascular abnormality. FNH has a natural history of stability and lacks of complications. FNH, increasing in size and number during the clinical course, is extremely rare. To the best of our knowledge, there are selective cases reporting progressive FNH[9-13]. Höhler et al[9] have reported an enlarging FNH of the liver in a patient with genetic hemochromatosis, and claimed that FNH occurs rather by chance and is not induced by iron-overloading but appears to significantly influence its course. Predominant iron deposition in liver and other organs causes widespread tissue injury mainly due to the increased concentration of highly reactive free oxygen radicals. FNH is a primarily proliferative lesion involving differentiated hepatocytes which are more susceptible to stimulatory effects of free oxygen radicals. FNH lesions in our patient with hemosiderosis also increased in size and number during her follow-up in between five years but decreased in size shortly after phlebotomy. To our knowledge, FNH occurring in setting of hemosiderosis has not been reported in literature. We also believe that, because the lesion decreased in size after phlebotomy, the cause of its increased size in our patient was iron overload due to coexisting hemosiderosis.
The pathogenesis of multiple FNH lesions is unknown. FNH lesions following chemotherapy, radiation therapy and hematopoietic stem cell transplantation (HSCT) have been reported in adults and children[14,15]. The incidence of FNH following chemotherapy and radiotherapy has not been well defined. However, it has been shown that in the setting of HSCT, the incidence of FNH is 0.45% in pediatric patients[14]. In another series, of the 138 patients that underwent HSCT, 17 were shown to develop FNH with a median delay of 6.4 years[16]. Our patient had a history of HSCT due to AML and the liver lesions developed 3 years after treatment. We also believe that FNH lesions in our case occurred in the setting of HSCT.
Distinguishing FNH from regenerative cirrhotic nodules, hepatic adenoma, or hepatic carcinoma is difficult. MR imaging has a higher sensitivity (70%) and specificity (98%) for FNH than US and CT due to the fact that state-of-the-art MRI provides information about the soft-tissue characteristics and also vascularity of the lesions. Typically, FNH is iso- or hypointense on T1-weighted images (94%-100%), slightly hyper- or isointense on T2-weighted images (94%-100%), and has a hyperintense central scar on T2-weighted images (84%)[17]. The FNH lesions in our patient were hyperintense on T1-weighted images and hypointense on T2-weighted images, which we think may be due to the accompanying hepatosteatosis and significant iron overload.
After the introduction of tissue-specific contrast agents, the diagnostic accuracy of MR imaging for benign liver tumors has improved. There has been promising results in the characterization of adenoma and FNH with the superparamagnetic iron oxide (SPIO) ferucarbotran and hepatobiliary agents such as gadobenate dimeglumine (Gd-BOPTA) and mangafodipir trisodium (Mn-DPDP)[4,7,8,18,19]. The follow-up imaging of our patient was performed with Eovist (gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid, GD-EOB-DTPA), a recently approved contrast agent for hepatobiliary imaging[20]. It combines good extracellular properties for an early dynamic examination, is selectively taken up by hepatocytes, persists in the liver for more than 30 min, specifically shortens the T1 relaxation time of liver tissue and causes prolonged opacification of the normal liver parenchyma[21]. Thus, tumor cells in the liver without normal hepatocytes will not take up contrast and can thus be observed as hypointense. It has been demonstrated that benign hepatocyte-containing solid liver tumors such as liver adenoma or FNH exhibit prolonged tumor enhancement because of specific intracellular uptake of Eovist[22]. Similarly, in our case, the liver lesions enhanced at the arterial phase with a greater signal intensity than those at all phases including the delayed hepatocyte specific phase.
In conclusion, we present a case of multiple progressive FNH lesions in setting of hemosiderosis demonstrated by Eovist MR imaging. We believe that FNH lesions occur due to HSCT while the progressive course is due to the underlying iron overload.