Published online Mar 28, 2025. doi: 10.4329/wjr.v17.i3.100168
Revised: January 14, 2025
Accepted: February 14, 2025
Published online: March 28, 2025
Processing time: 230 Days and 11.5 Hours
Acromegaly is caused by a pituitary neuroendocrine tumor (PitNET) with excessive production of growth hormone (GH), leading to multisystem complications. Previous studies have identified predictors of disease persistence following surgery and poor response to medical treatment, including tumor size, vertical and horizontal extensions of the adenoma, hyperintensity in T2-weighted magne
To evaluate PitNET volume as a complementary prognostic factor in patients with acromegaly.
This is a retrospective descriptive study with an analytical component evaluating the correlation between the volumetric analysis of GH-producing PitNETs, IGF-1 levels before and after surgery, disease control during follow-up, and the line of therapy required for disease control in a cohort of patients treated at two centers: Endocrinology Department of the Central Military Hospital and Centros Médicos Colsanitas, Bogotá, Colombia.
A total of 77 patients with acromegaly (42 men, 35 women) were included in this study. The mean age at diagnosis was 42 years (SD: 12), with a mean disease duration of 9.9 years (SD: 7.2). The mean pituitary tumor volume was 4358 mm³ (SD: 6291, interquartile range [IQR]: 13602). Patients with controlled acromegaly had a mean PitNET volume of 3202 mm³ (SD: 4845, 95%CI: 621-5784) compared to 5513 mm³ (SD: 7447, 95%CI: 1545-9482) in the uncontrolled group (P = 0.15). A PitNET volume exceeding 3697 mm³ was associated with a higher likelihood of requiring third or fourth-line therapy (50% vs 36%; P = 0.03).
PitNET volume was associated with the need for higher-line therapy to manage acromegaly but did not correlate with long-term disease control or with pre- or postsurgical IGF-1 levels. Nevertheless, a trend towards an inverse relationship between tumor volume and future disease control was observed. While macroadenoma classification remains crucial, among patients with macroadenomas, those with a volume exceeding 3697 mm³ could have worse prognosis.
Core Tip: Acromegaly is caused by a pituitary neuroendocrine tumor (PitNET) with excessive growth hormone (GH) production, leading to multisystem complications. Previous studies have identified predictors of poor prognosis, including tumor size, extensions, T2-weighted magnetic resonance imaging hyperintensity, granulation density, and GH/insulin-like growth factor 1 levels. In this study, PitNET volume was associated with the need for higher-line therapy and showed a trend toward larger volumes in uncontrolled patients, suggesting that adenoma volume could be an additional prognostic factor in acromegaly.
- Citation: Alvarez M, Donato A, Rincon J, Rincon O, Lancheros N, Mancera P, Guzman I. Evaluation of pituitary tumor volume as a prognostic factor in acromegaly: A cross-sectional study in two centers. World J Radiol 2025; 17(3): 100168
- URL: https://www.wjgnet.com/1949-8470/full/v17/i3/100168.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i3.100168
Acromegaly is an infrequent disorder caused by excessive production of growth hormone (GH) and insulin-like growth factor 1 (IGF-1), which leads to multisystem complications[1-3] . It has an average annual incidence of 0.2 and 1.1 cases per 100000 people per year and a prevalence of between 2.8 and 13.7 cases per 100000[3,4]. Hormone-secreting pituitary neuroendocrine tumors (PitNETs) account for more than 95% of acromegaly cases[4,5].
Excessive GH and IGF-1 levels are associated in the long term with soft tissue overgrowth and alterations in bone formation dynamics. These changes contribute to alterations in physical appearance and multisystem dysfunctions, including diabetes mellitus, cardiovascular disease, and cancer, which collectively increase mortality rates by up to 18%[2,3,5,6]. Cardiovascular diseases and cancer are identified as the primary causes of death[7].
Acromegaly is diagnosed by measuring IGF-1 levels and clinical findings. Contrast-enhanced magnetic resonance imaging (MRI) of the sella turcica is the method of choice for identifying a pituitary adenoma[3,6], which corresponds to a macroadenoma (> 10 mm) in 70% of cases[3,7]. MRI assesses not only the tumor size but also the presence of other complications such as invasion or extension of the cavernous sinus, sphenoid, carotid, and the compression of the optic chiasm[2].
The primary treatment for acromegaly is transsphenoidal resection of the adenoma. Remission is achieved in 80% and 50% of cases with microadenomas and macroadenomas, respectively, following surgery[2,6]. Remission is defined as the normalization of IGF-1 levels adjusted for age and sex. Predictors of postoperative remission include surgical experience, low GH or IGF-1 levels, and smaller tumor size. Macroadenomas exhibit a higher recurrence rate, especially those with invasive characteristics[7]. Therefore, pre- and postoperative GH and IGF-1 levels serve as crucial predictors of long-term remission[2]. Higher preoperative GH levels and cavernous sinus invasion are predictive of postoperative outcomes[6]. In cases of disease persistence or recurrence after surgery, or for patients who are not surgical candidates, medical treatment options include first- or second-generation somatostatin analogues, GH receptor antagonist, and dopamine agonists[1].
In previous studies, predictors of persistent and recurrent acromegaly following surgery, as well as predictors of poor response to first-line medical therapies, have included factors such as tumor size, horizontal or vertical extensions of the adenoma, pre- and postoperative levels of IGF-1 and GH, T2-weighted MRI hyperintensity of adenomas, granulation density, expression of somatostatin receptors 2 and 5, and aryl hydrocarbon receptor-interacting protein (AIP) expression.
The objective of this study was to evaluate the correlation between pituitary adenoma volume as an additional prognostic factor in patients with acromegaly.
This retrospective descriptive study with an analytical component included patients diagnosed with acromegaly and treated at the Endocrinology Department of the Central Military Hospital and Centros Médicos Colsanitas in Bogotá, Colombia, from 2020 to 2023. A total of 124 patients were evaluated.
Demographic data and MRI findings performed prior to any treatment were collected. Acromegaly was diagnosed based on the biochemical demonstration of non-suppressed GH following an oral glucose tolerance test and IGF-1 levels above the normal range, adjusted for age and sex. IGF-1 levels were measured using an immunoassay from Immunodiagnostic Systems in most of the patients (IDS-iSYS).
Electronic medical records were reviewed, and imaging data, including adenoma diameters, intensities, and volumes, were obtained from MRI records. Eligibility criteria required that MRI scans were performed using high-resolution techniques, comprising at least one T2-weighted coronal slice, one T1- or T2-weighted sagittal sequence, and one T1-enhanced coronal sequence with gadolinium contrast enhancement. Patients without pituitary gland MRI or hormonal studies were excluded. Patients with previous pituitary surgery or medical therapy for acromegaly were also excluded.
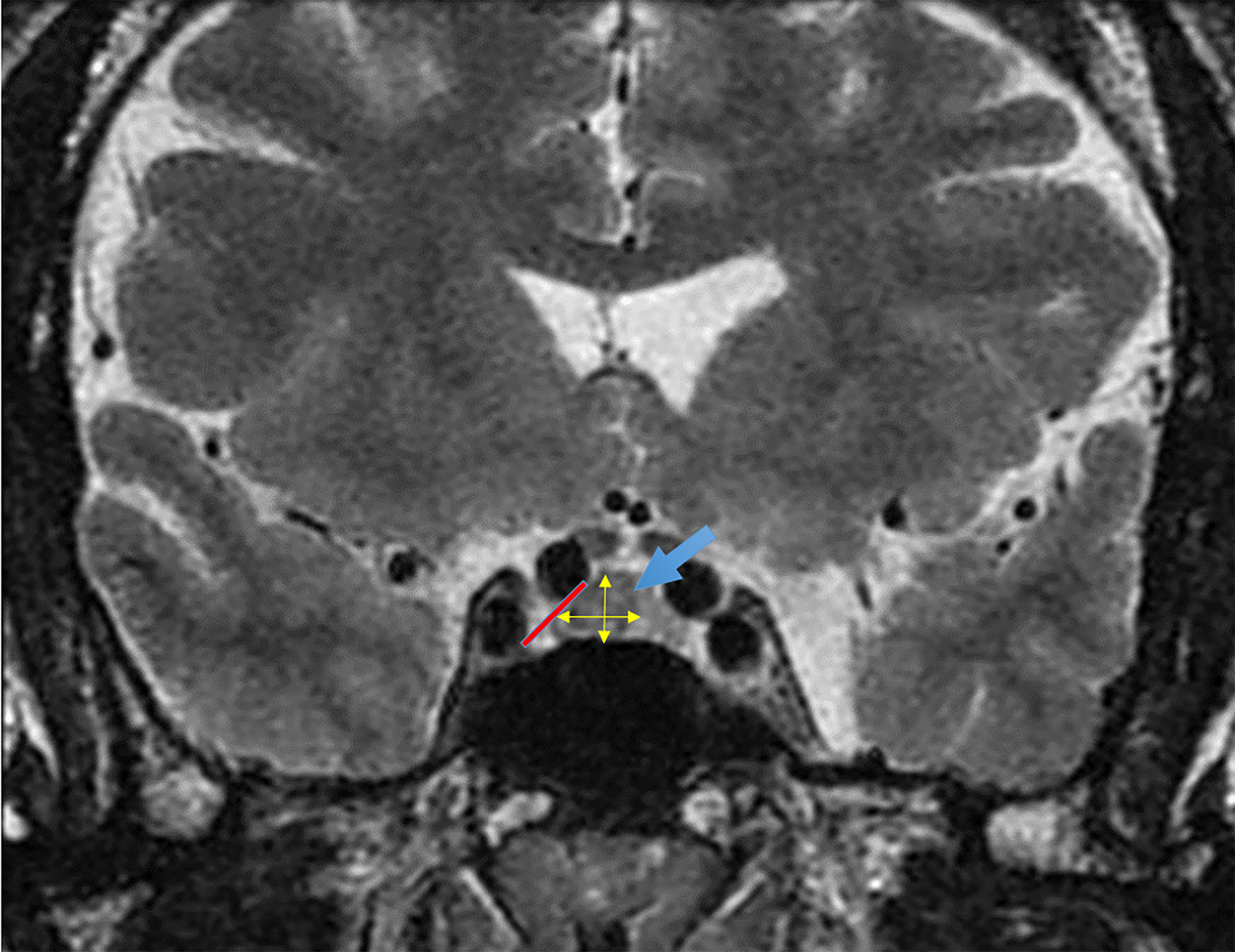
After applying the inclusion criteria, 77 of 124 patients were eligible for the study. Data collected for each patient included age at diagnosis, sex, IGF-1 levels at diagnosis and 3 to 6 months postsurgery, medical treatment received, and volume of the PitNET. The characteristics of the PitNET were recorded as length, height, width, volume, horizontal extension to the cavernous sinus, vertical extension (suprasellar and inferior), and T2-weighted hyperintensity. PitNET volume was calculated as in previous studies[8,9] using the simplified ellipsoid volume formula: Volume = (length × height × width)/2 (Figure 1). The T2 signal of the adenoma was classified as hyperintense or non-hyperintense.
The study assessed the correlation between MRI-measured pituitary tumor volume and IGF-1 levels (pre- and postsurgery), disease control during follow-up—defined as persistent normalization of IGF-1 by an endocrinologist—and the treatment line required. Treatment lines were categorized as follows: First-line (surgery alone), second-line (first-generation somatostatin analogs), third-line (pegvisomant, pasireotide, or cabergoline plus first-generation somatostatin analog), and fourth-line (radiotherapy).
Descriptive data are presented based on their nature: Quantitative data are presented as the mean ± standard deviation (SD) or median with interquartile ranges (IQRs), according to the Shapiro-Wilk test; qualitative variables are presented as absolute and relative values. The χ2 test was used to compare categorical variables, with Fisher's correction as required. The t-test was performed for the comparison of continuous variables between groups. Non-parametrically distributed data were assessed using the Mann-Whitney U test. Relationships between variables were examined using Spearman correlation analysis. P < 0.05 was considered statistically significant.
A total of 77 patients with acromegaly participated in the study, comprising 42 men (54.5%) and 35 women (45.5%), with a mean age at diagnosis of 42 years (SD: 12). The average follow-up duration from diagnosis was 9.9 years (SD: 7.2). Among the participants, 25 (32%) had microadenomas and 51 (66%) had macroadenomas. The mean pituitary tumor volume was 4358 mm³ (SD: 6291, IQR: 13602). Acromegaly was considered controlled in 44 patients (57%) and not controlled in 33 patients (42%; Table 1).
| Variable | |
| Female | 35 |
| Age at diagnosis, years (SD-IQR) | 42 (12-46) |
| Time from diagnosis, years (SD-IQR) | 9.9 (7.2-25) |
| Microadenoma, n (%) | 26 (32) |
| Macroadenoma, n (%) | 51 (66) |
| T2 weighted hyperintensity, n (%) | 16 (20) |
| Adenoma volume, mm3 (SD-IQR) | 4358 (6291-13602) |
| Vertical extension (supraselar or sphenoid sinus), n (%) | 22 (29) |
| Horizontal extension (cavernous sinus), n (%) | 20 (27) |
| IGF-1 at diagnosis, ng/mL (SD-IQR) | 564.6 (334-859) |
| IGF-1 after surgery, ng/mL, (SD-IQR) | 331 (198-478) |
| Treatment line 0 (No surgical therapy), n (%) | 12 (15) |
| Treatment line 1, n (%) | 16 (20) |
| Treatment line 2, n (%) | 20 (25) |
| Treatment line 3, n (%) | 16 (20) |
| Treatment line 4, n (%) | 13 (16) |
| Controlled disease, n (%) | 44 (57) |
| Uncontrolled disease, n (%) | 33 (42) |
MRI T2 hyperintensity was observed in 16 patients (20%). Vertical extension was present in 22 patients (29%), and horizontal extension into the cavernous sinus was noted in 20 patients (27%). The distribution of patients across each line of therapy was as follows: 15 for line 1, 20 for line 2, 16 for line 3, and 12 for line 4. Transsphenoidal resection surgery was performed in 67 patients (87%), with 15 patients (19%) receiving it as the sole therapy. Treatment with first-generation somatostatin analogs was administered to 20 patients (25%). Sixteen patients required third-line therapy with pasireotide, pegvisomant, or cabergoline (20%). Radiotherapy was performed in 12 patients (15%).
The mean PitNET volume was 3202 mm³ (SD: 4845, 95% confidence interval [CI]: 621-5784) for controlled patients and 5513 mm³ (SD: 7447, 95%CI: 1545-9482) for uncontrolled patients (P = 0.15; Table 2). A PitNET volume exceeding 3697 mm³ was associated with a higher likelihood of requiring third or fourth-line therapy (50% vs 36%; P = 0.03).
| N = 77 | Controlled | Not controlled | P value |
| T2-weighted hyperintensity (n: 16), n (%) | 8 (50) | 8 (50) | 0.57 |
| Macroadenoma (n: 51), n (%) | 28 (54) | 23 (45) | 0.88 |
| Microadenoma (n: 26), n (%) | 16 (60) | 10 (40) | |
| Adenoma volume | 3202 (SD: 4845) (95%CI: 621-5784) | 5513 (SD: 7447) (95%CI: 1545-9482) | 0.15 |
| Vertical extension (n: 22), n (%) | 9 (40) | 13 (59) | 0.07 |
| Horizontal extension (n: 20), n (%) | 10 (50) | 10 (50) | 0.60 |
Among patients with T2 hyperintensity, 8 of 16 (50%) achieved disease control, compared to 36 of 61 (59%) without T2 hyperintensity (P = 0.51). A higher proportion of patients with microadenomas achieved control compared to those with macroadenomas (61% vs 52%), while a greater percentage of patients with macroadenomas had uncontrolled disease (25% vs 10%; P = 0.62) (Table 2). Control of acromegaly was more frequent in patients without vertical extension (66% vs 34%; P = 0.07) and without horizontal extension (59% vs 45%; P = 0.60) (Table 2).
The percentage elevation of IGF-1 adjusted for sex and age at diagnosis was higher in macroadenomas (270 ng/mL; SD: 25, 95%CI: 217-324) compared to microadenomas (150 ng/mL; SD: 21, 95%CI: 190-290) (P = 0.003). There was no difference in the percentage elevation of postoperative IGF-1 levels between patients with microadenomas and macroadenomas (P = 0.13). Additionally, there was no statistically significant difference in the percentage elevation of IGF-1 levels preoperatively (P = 0.87) and postoperatively (P = 0.76) based on pituitary volume.
The time from diagnosis was longer in patients with controlled disease (11.9 years; SD: 1.0, 95%CI: 9.7-14.1) compared to those with uncontrolled disease (7.3 years, SD: 1.1, 95%CI: 4.9-9.6; P = 0.005).
Previous studies have reported multiple parameters as predictors of acromegaly outcomes, including tumor size, preoperative levels of GH and IGF-1, granulation density and intensity on T2 images, somatostatin 2 and 5 receptor expression, AIP expression, and E cadherin; however, which factor is more robustly predictive is still unclear[10-25]. In this study we evaluated PitNET volume as a predictor of control of acromegaly.
Several studies have previously investigated MRI predictors of prognosis in acromegaly, including adenoma size greater than 1 cm (macroadenoma), invasion or extension into the cavernous sinus, sphenoid sinus, carotid artery, and compression of the optic chiasm, as well as T2 hyperintensity[26-32].
One of the most consistent findings is the poorer prognosis associated with macroadenomas, which exhibit higher rates of postsurgical persistence and poorer disease control in the medium to long term[30-32]. In most series, approximately 70% of acromegaly cases are attributed to macroadenomas, and remission rates are 80% and 50% for microadenomas and macroadenomas, respectively. In our study, we observed a similar proportion of 66% for macroadenomas. There was a higher percentage of IGF-1 elevation at diagnosis in patients with macroadenomas and a trend towards less disease control in patients with macroadenomas (45%) compared to microadenomas (40%); however, this trend did not reach statistical significance, likely due to the small sample size.
We found a statistically significant higher need for third- and fourth-line therapies in patients with PitNET volumes categorized as greater than 3697 mm³, as 50% of patients with volumes exceeding this threshold required third- and fourth-line treatments. This finding indicates a worse disease prognosis, higher treatment costs, and longer time to achieve disease control, given that delays in escalating therapeutic lines are a well-documented issue in the management of acromegaly and is of utmost importance, as it suggests that among patients with macroadenomas, there may be two distinct groups: Those with a volume exceeding this threshold, who have a worse prognosis, and those with a volume below this threshold.
Additionally, we observed a trend of larger PitNET volumes in patients with uncontrolled disease. Although not statistically significant, patients who are currently controlled had smaller tumor volumes at diagnosis compared to those who remain uncontrolled, who exhibited larger adenoma volumes. It is likely that the sample size was insufficient to detect a statistically significant difference in this regard.
Assessing the volume of pituitary adenomas may provide a more comprehensive measure of the tumor, as it could better reflect the horizontal and vertical invasion of the adenoma compared to the single measurement of 1 cm used to classify adenomas. Some previous studies have described the relationship between tumor volume and hormone levels in acromegaly. In this study, the progression of GH and IGF-1 levels was evaluated in a group of 11 patients, revealing a decrease in hormonal levels proportional to the reduction in tumor volume[9]. However, this study did not assess long-term disease control or the future need for higher therapeutic lines. In our study, although no statistical difference in hormonal levels was observed, likely due to a non-normal data distribution or small sample size, we identified tumor volume as a predictor of the need for higher lines of treatment. Identifying predictors of poor long-term disease control and the need for therapeutic escalation is crucial, as there is an urgent need for predictors that can alert clinicians to make early therapy adjustments and achieve early disease control. This remains one of the main challenges in the current management of acromegaly.
Another study demonstrated that adenoma volume can serve as a predictor of surgical success and disease control in acromegaly[31]. In a cohort of 27 patients, adenoma volumes were measured using semi-automatic segmentation tools via MRI, and volumes < 2000 mm³ were associated with a higher likelihood of remission (94%) compared to those with larger volumes (50%). In our study, which included a larger sample size, we observed that adenomas with volumes exceeding 3697 mm³ were linked to an increased need for advanced therapeutic lines to achieve disease control. However, while the volume thresholds show some overlap, these findings are not directly comparable. As previously noted, the lack of a universal strategy for measuring adenoma volume complicates its standardized application in clinical practice[25].
T2-weighted MRI hyperintensity has been identified as a poor prognostic factor in multiple studies[29]. However, in our study, this characteristic was found in only 16 patients, half of whom did not have disease control. This limited occurrence explains why it was not a statistically significant factor in this cohort of patients.
Regarding pre- and postoperative IGF-1 levels adjusted for age and sex, we found that patients with macroadenomas had higher preoperative IGF-1 levels compared to microadenomas. However, when analyzing according to PitNET volume, there was no direct correlation between volume and pre- or postoperative IGF-1 levels. Previous studies have demonstrated the importance of IGF-1 levels as a prognostic factor in acromegaly[32]. This finding may be related to the fact that IGF-1 levels were evaluated as a percentage of elevation relative to the normal range for sex and age, rather than as a continuous variable. This approach was used to enhance reliability, considering that the normal values in this study vary depending on the laboratory technique, with reference ranges differing by sex and age in each population.
In our study, we found a significantly longer disease duration in controlled patients compared to uncontrolled patients. The longer disease duration observed in controlled patients compared to uncontrolled patients is likely related to delays in therapeutic escalation, a well-documented issue in patients with acromegaly. These delays occur not only between the onset of symptoms and diagnosis but also between diagnosis and the first surgery, as well as in the escalation of subsequent therapies until disease control is achieved—a process that often spans several years.
Other factors previously described as predictors of disease control, such as sex, adenoma size, and cavernous sinus invasion, were not statistically significant in our evaluation of disease control, likely due to the small sample size. However, we did find a statistically significant difference in disease duration, with longer disease duration in the controlled group compared to the uncontrolled group. We interpret this finding as indicative of the chronic nature of acromegaly, which is challenging to manage and often necessitates multiple therapeutic interventions over time, including the long-term efficacy of radiotherapy in disease management.
This study had limitations due to its retrospective nature. MRI readings were extracted from medical or imaging records. Conducted across two different centers, patient characteristics were heterogeneous. Nevertheless, patient medical records were meticulously documented in both institutions, and the diagnostic and follow-up equipment had similar characteristics.
The volume of GH-producing PitNET correlated with the need for escalated therapeutic regimens to achieve disease control in acromegaly patients but did not correlate with long-term disease control or with pre- or postsurgical IGF-1 levels. A trend towards an inverse relationship between tumor volume and future disease control was observed. While macroadenoma classification remains essential, considering the scientific evidence, the underlying pathophysiology, and the three-dimensional growth pattern of adenomas, volumetric assessment could serve as a valuable adjunct prognostic factor in acromegaly.
| 1. | Melmed S. Acromegaly. N Engl J Med. 1990;322:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 322] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Cardinal T, Collet C, Wedemeyer M, Singer PA, Weiss M, Zada G, Carmichael JD. Postoperative GH and Degree of Reduction in IGF-1 Predicts Postoperative Hormonal Remission in Acromegaly. Front Endocrinol (Lausanne). 2021;12:743052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Dal J, Feldt-Rasmussen U, Andersen M, Kristensen LØ, Laurberg P, Pedersen L, Dekkers OM, Sørensen HT, Jørgensen JO. Acromegaly incidence, prevalence, complications and long-term prognosis: a nationwide cohort study. Eur J Endocrinol. 2016;175:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Crisafulli S, Luxi N, Sultana J, Fontana A, Spagnolo F, Giuffrida G, Ferraù F, Gianfrilli D, Cozzolino A, Cristina De Martino M, Gatto F, Barone-Adesi F, Cannavò S, Trifirò G. Global epidemiology of acromegaly: a systematic review and meta-analysis. Eur J Endocrinol. 2021;185:251-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Horchani A, Bayar I, Ben Amor B, Hellara I, Abdelali M, Neffati F. L’acromégalie : une pathologie endocrinienne à lourdes conséquences. Ann Biol Clin (Paris). 2022;80: 268-273. |
| 6. | Bray DP, Mannam S, Rindler RS, Quillin JW, Oyesiku NM. Surgery for acromegaly: Indications and goals. Front Endocrinol (Lausanne). 2022;13:924589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Melmed S. Pituitary-Tumor Endocrinopathies. N Engl J Med. 2020;382:937-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 354] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 8. | Fink AM, Vidmar S, Kumbla S, Pedreira CC, Kanumakala S, Williams C, Carlin JB, Cameron FJ. Age-related pituitary volumes in prepubertal children with normal endocrine function: volumetric magnetic resonance data. J Clin Endocrinol Metab. 2005;90:3274-3278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Schwyzer L, Starke RM, Jane JA Jr, Oldfield EH. Percent reduction of growth hormone levels correlates closely with percent resected tumor volume in acromegaly. J Neurosurg. 2015;122:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kreutzer J, Vance ML, Lopes MB, Laws ER Jr. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J Clin Endocrinol Metab. 2001;86:4072-4077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 180] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Abosch A, Tyrrell JB, Lamborn KR, Hannegan LT, Applebury CB, Wilson CB. Transsphenoidal microsurgery for growth hormone-secreting pituitary adenomas: initial outcome and long-term results. J Clin Endocrinol Metab. 1998;83:3411-3418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Jane JA Jr, Starke RM, Elzoghby MA, Reames DL, Payne SC, Thorner MO, Marshall JC, Laws ER Jr, Vance ML. Endoscopic transsphenoidal surgery for acromegaly: remission using modern criteria, complications, and predictors of outcome. J Clin Endocrinol Metab. 2011;96:2732-2740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Galm BP, Buckless C, Swearingen B, Torriani M, Klibanski A, Bredella MA, Tritos NA. MRI texture analysis in acromegaly and its role in predicting response to somatostatin receptor ligands. Pituitary. 2020;23:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Potorac I, Petrossians P, Daly AF, Schillo F, Ben Slama C, Nagi S, Sahnoun M, Brue T, Girard N, Chanson P, Nasser G, Caron P, Bonneville F, Raverot G, Lapras V, Cotton F, Delemer B, Higel B, Boulin A, Gaillard S, Luca F, Goichot B, Dietemann JL, Beckers A, Bonneville JF. Pituitary MRI characteristics in 297 acromegaly patients based on T2-weighted sequences. Endocr Relat Cancer. 2015;22:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Colao A, Ferone D, Marzullo P, Lombardi G. Systemic complications of acromegaly: epidemiology, pathogenesis, and management. Endocr Rev. 2004;25:102-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 838] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 16. | Oldfield EH, Jane JA Jr, Thorner MO, Pledger CL, Sheehan JP, Vance ML. Correlation between GH and IGF-1 during treatment for acromegaly. J Neurosurg. 2017;126:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Stonesifer LD, Jordan RM, Kohler PO. Somatomedin C in treated acromegaly: poor correlation with growth hormone and clinical response. J Clin Endocrinol Metab. 1981;53:931-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Ribeiro-Oliveira A Jr, Barkan A. The changing face of acromegaly--advances in diagnosis and treatment. Nat Rev Endocrinol. 2012;8:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Fleseriu M, Biller BMK, Freda PU, Gadelha MR, Giustina A, Katznelson L, Molitch ME, Samson SL, Strasburger CJ, van der Lely AJ, Melmed S. A Pituitary Society update to acromegaly management guidelines. Pituitary. 2021;24:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 20. | Akkaya E, Akgun MY, Sebnem Durmaz E, Aydın S, Mefkure Ozkaya H, Comunoglu N, Kizilkilic O, Gazioglu N, Kadioglu P, Tanriover N. T2-weighted magnetic resonance imaging as a novel predictor of surgical remission in newly diagnosed pituitary macroadenomas presenting as acromegaly. J Clin Neurosci. 2021;90:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Puig-Domingo M, Resmini E, Gomez-Anson B, Nicolau J, Mora M, Palomera E, Martí C, Halperin I, Webb SM. Magnetic resonance imaging as a predictor of response to somatostatin analogs in acromegaly after surgical failure. J Clin Endocrinol Metab. 2010;95:4973-4978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, Pivonello R. Acromegaly. Nat Rev Dis Primers. 2019;5:20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 250] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 23. | Heck A, Ringstad G, Fougner SL, Casar-Borota O, Nome T, Ramm-Pettersen J, Bollerslev J. Intensity of pituitary adenoma on T2-weighted magnetic resonance imaging predicts the response to octreotide treatment in newly diagnosed acromegaly. Clin Endocrinol (Oxf). 2012;77:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Heck A, Emblem KE, Casar-Borota O, Bollerslev J, Ringstad G. Quantitative analyses of T2-weighted MRI as a potential marker for response to somatostatin analogs in newly diagnosed acromegaly. Endocrine. 2016;52:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Chuang CC, Lin SY, Pai PC, Yan JL, Toh CH, Lee ST, Wei KC, Liu ZH, Chen CM, Wang YC, Lee CC. Different Volumetric Measurement Methods for Pituitary Adenomas and Their Crucial Clinical Significance. Sci Rep. 2017;7:40792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Anik I, Cabuk B, Gokbel A, Selek A, Cetinarslan B, Anik Y, Ceylan S. Endoscopic Transsphenoidal Approach for Acromegaly with Remission Rates in 401 Patients: 2010 Consensus Criteria. World Neurosurg. 2017;108:278-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Erkan B, Barut O, Akbas A, Akpinar E, Akdeniz YS, Tanriverdi O, Gunaldi O. Results of Endoscopic Surgery in Patients with Pituitary Adenomas : Association of Tumor Classification Grades with Resection, Remission, and Complication Rates. J Korean Neurosurg Soc. 2021;64:608-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Ruiz S, Gil J, Biagetti B, Venegas E, Cámara R, Garcia-Centeno R, Gálvez MÁ, Picó A, Maraver S, González I, Abellán P, Trincado P, Herrera M, Olvera P, Xifra G, Bernabeu I, Serra-Soler G, Azriel S, García L, Carvalho D, Jordà M, Valassi E, Puig J, Puig-Domingo M. Magnetic resonance imaging as a predictor of therapeutic response to pasireotide in acromegaly. Clin Endocrinol (Oxf). 2023;99:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 29. | Nista F, Corica G, Castelletti L, Khorrami K, Campana C, Cocchiara F, Zoppoli G, Prior A, Rossi DC, Zona G, Ferone D, Gatto F. Clinical and Radiological Predictors of Biochemical Response to First-Line Treatment With Somatostatin Receptor Ligands in Acromegaly: A Real-Life Perspective. Front Endocrinol (Lausanne). 2021;12:677919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 30. | Almeida JP, Ruiz-Treviño AS, Liang B, Omay SB, Shetty SR, Chen YN, Anand VK, Grover K, Christos P, Schwartz TH. Reoperation for growth hormone-secreting pituitary adenomas: report on an endonasal endoscopic series with a systematic review and meta-analysis of the literature. J Neurosurg. 2018;129:404-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Tirosh A, Papadakis GZ, Chittiboina P, Lyssikatos C, Belyavskaya E, Keil M, Lodish MB, Stratakis CA. 3D Volumetric Measurements of GH Secreting Adenomas Correlate with Baseline Pituitary Function, Initial Surgery Success Rate, and Disease Control. Horm Metab Res. 2017;49:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Coopmans EC, Korevaar TIM, van Meyel SWF, Daly AF, Chanson P, Brue T, Delemer B, Hána V, Colao A, Carvalho D, Jaffrain-Rea ML, Stalla GK, Fajardo-Montañana C, Beckers A, van der Lely AJ, Petrossians P, Neggers SJCMM. Multivariable Prediction Model for Biochemical Response to First-Generation Somatostatin Receptor Ligands in Acromegaly. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |