Published online Feb 28, 2025. doi: 10.4329/wjr.v17.i2.104518
Revised: January 23, 2025
Accepted: February 19, 2025
Published online: February 28, 2025
Processing time: 64 Days and 18.5 Hours
Gastric adenocarcinoma with enteroblastic differentiation (GAED) is one of the common subtypes of alpha-foetoprotein (AFP)-producing gastric cancer. GAED frequently results in venous invasion and liver metastasis, the latter being particularly linked to a poor prognosis. So far, the evidence for liver metastases from AFP-producing gastric cancer is only focused on those from gastric hepatoid adenocarcinoma, owing to their imaging similarities with hepatocellular carci
A 65-year-old man who had undergone a pyloric gastrectomy for GAED two years ago was found to have a liver tumor in the hepatic segment 7, accompanied by elevated serum AFP levels. Dynamic contrast-enhanced computed tomogra
The imaging features of blood flow alternations resulting from vascular invasion may be crucial to diagnosing liver metastases from GAED.
Core Tip: Accurate diagnosis of liver metastases from gastric adenocarcinoma with enteroblastic differentiation (GAED) and distinguishing it from other potential differentials, particularly hepatocellular carcinoma, is crucial owing to its association with a poor prognosis. The presence of elevated serum alpha-foetoprotein levels, as well as histopathological changes in the perilesional tissues surrounding the tumor, are the key characteristic findings of liver metastases from GAED. This report is the first to describe the characteristic imaging features of liver metastases from GAED based on the radiopathological correlation.
- Citation: Irizato M, Minamiguchi K, Fujita Y, Yamaura H, Onaya H, Taiji R, Tanaka T, Inaba Y. Distinctive imaging features of liver metastasis from gastric adenocarcinoma with enteroblastic differentiation: A case report. World J Radiol 2025; 17(2): 104518
- URL: https://www.wjgnet.com/1949-8470/full/v17/i2/104518.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i2.104518
Gastric adenocarcinoma with enteroblastic differentiation (GAED) is a type of alpha-foetoprotein (AFP)-producing gastric cancer, similar to other tumors like gastric hepatoid adenocarcinoma (GHA) and gastric yolk sac tumor. Histologically, GAED is characterized by a glycogen-rich clear cytoplasm and fetal gut-like structures, along with the expression of one or more of the following enteroblastic markers: Glypican-3, spalt-like transcription factor 4, and AFP[1,2]. Notably, GAED occurs more frequently with venous invasion and liver metastasis compared to conventional gastric cancer and is associated with a poorer overall survival prognosis[1,3,4]. Liver metastasis from GAED is observed in 31%-41.5% of cases and is clinically important for both diagnosis and prognostication of GAED[1,3]. Although several studies have reported imaging findings for liver metastasis from AFP-producing gastric cancer, most of the existing literature focuses on liver metastases from GHA[5-8]. Previous studies also have emphasized the need for caution in diagnosing liver metastases from GHA, given the similarity of these imaging findings with those from hepatocellular carcinoma (HCC)[9,10]. Given the lack of comprehensive reporting on the imaging findings of hepatic metastasis from GAED, we present a rare case of liver metastasis from GAED and discuss the challenges met in differentiating it from HCC due to the presence of arterial enhancement and fat content.
An asymptomatic 65-year-old male patient of GAED was found to have a liver mass on computed tomography (CT).
The patient had undergone pyloric gastrectomy for GAED two years ago. The follow-up CT revealed a liver mass, for which he was referred to our hospital.
The postoperative pathology revealed GAED (pT3N0M0, stage 2A).
There was no relevant history of tumor or liver disease.
No abnormalities were noted in the physical examination.
The patient had significantly elevated serum AFP levels (283 ng/mL; normal value: < 10 ng/mL), while other tumor markers were within normal ranges.
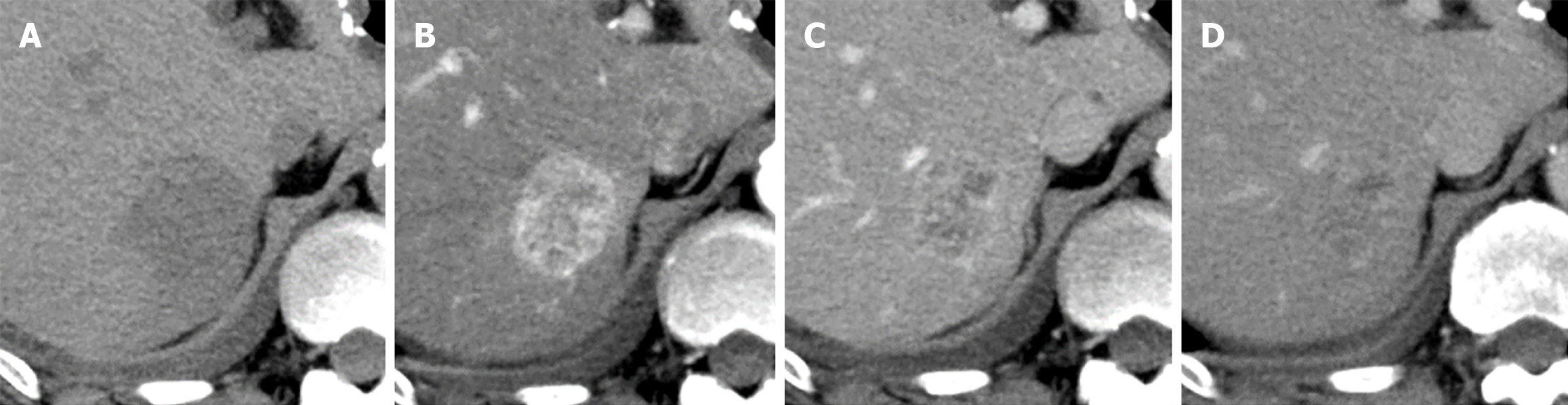
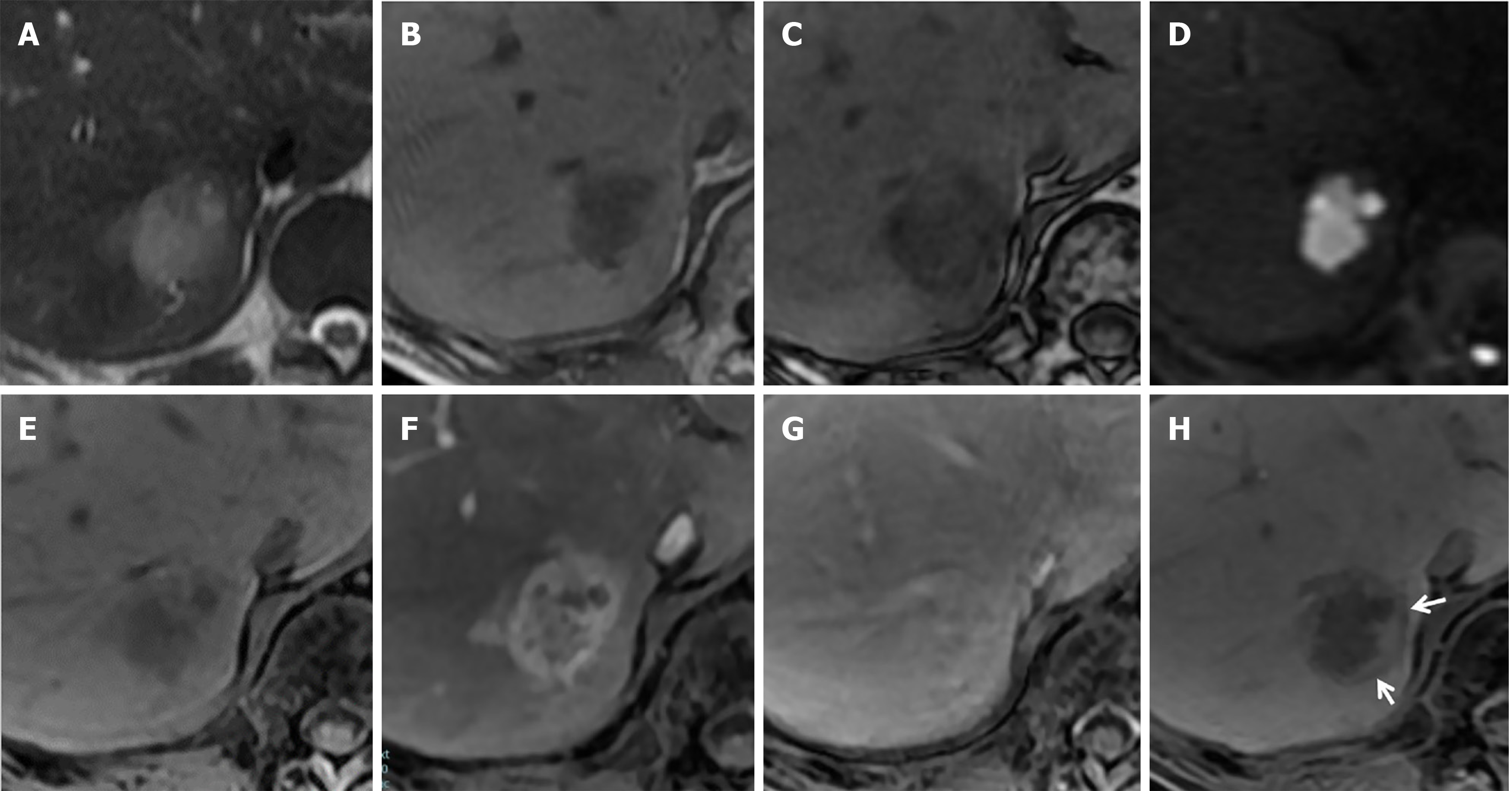
Contrast-enhanced CT revealed a well-defined oval mass (3.5 cm) in segment 7 of the liver (Figure 1). In the arterial phase, the tumor showed peripheral-dominant enhancement; in the delayed phase, the peripheral tumor area showed the same degree of enhancement as the surrounding liver parenchyma, and the central area was progressively enhanced. Next, gadolinium-ethoxybenzyl-diethylenetriamine penta-acetic acid-enhanced magnetic resonance imaging (MRI) was performed which showed the tumor as a mildly hyperintense structure with indistinct borders on T2-weighted images and hyperintense on diffusion-weighted images, demonstrating a contrast enhancement pattern similar to that of contrast-enhanced-CT (Figure 2). The periphery of the tumor showed a signal drop in chemical shift imaging and the same signal intensity as the liver parenchyma in the hepatobiliary phase (HBP), corresponding to the hyperenhancement area in the arterial phase. The HBP image also demonstrated a rim-like hypointensity surrounding the tumor.
The clinical and imaging findings were suggestive of HCC or liver metastasis as a differential diagnosis.
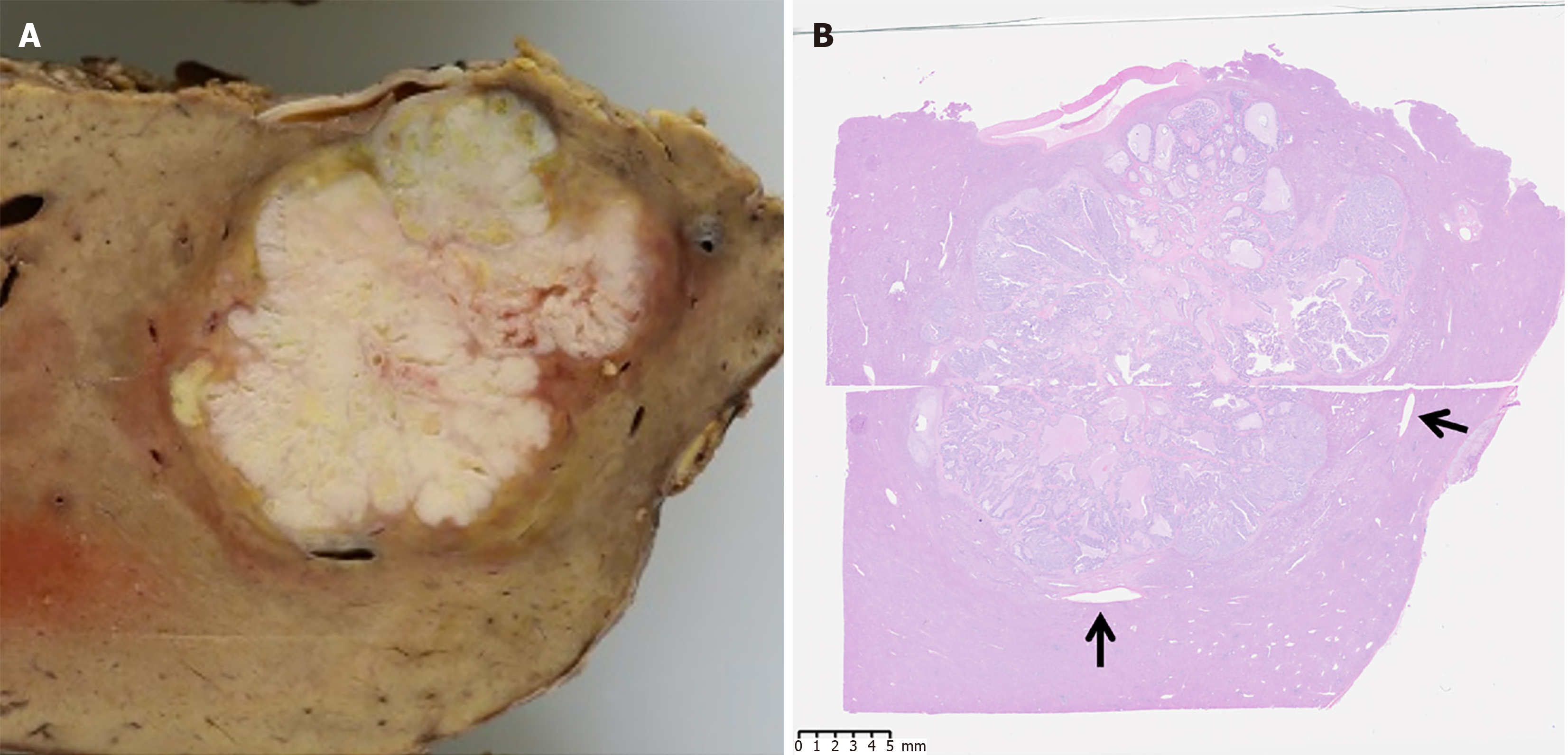
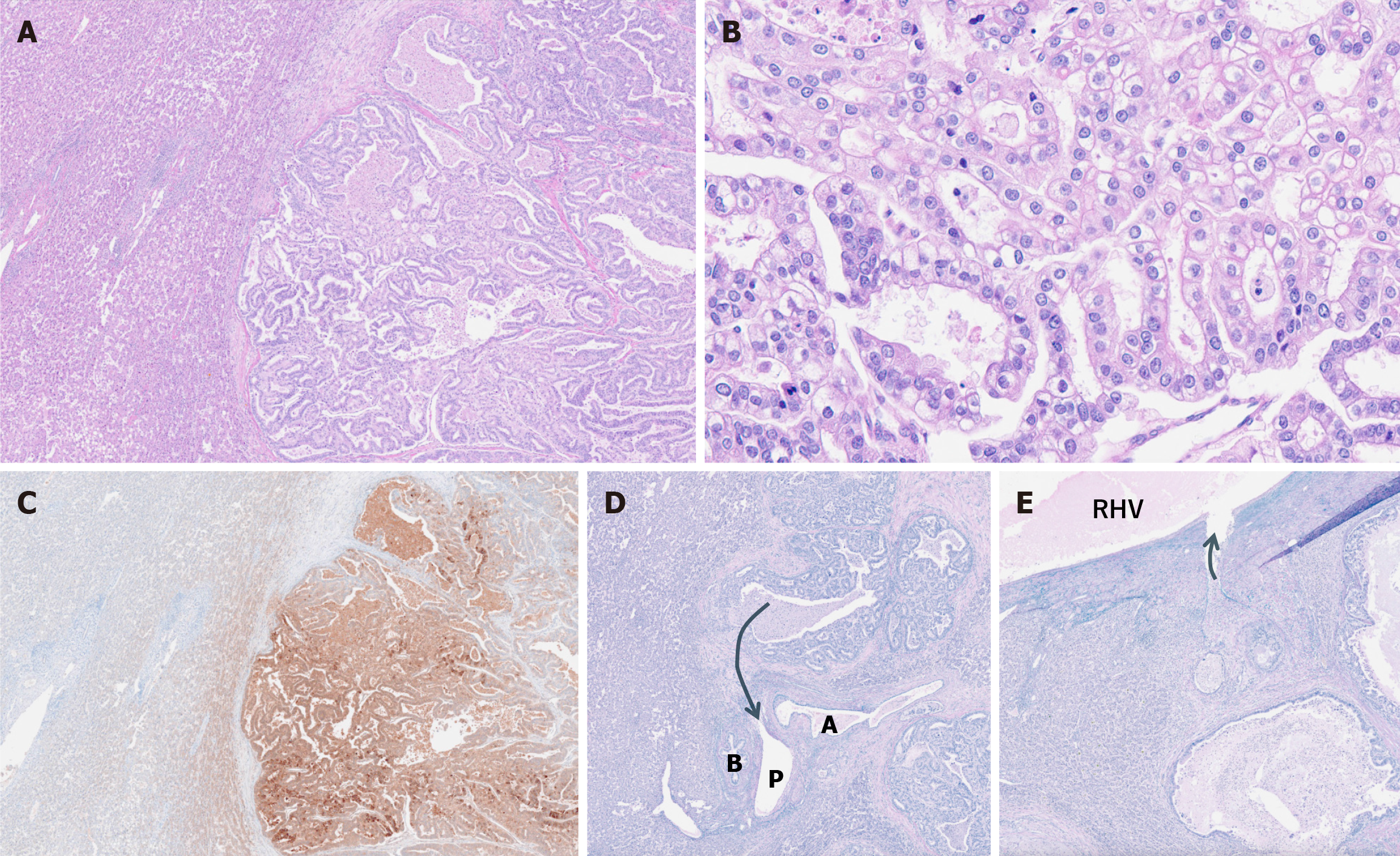
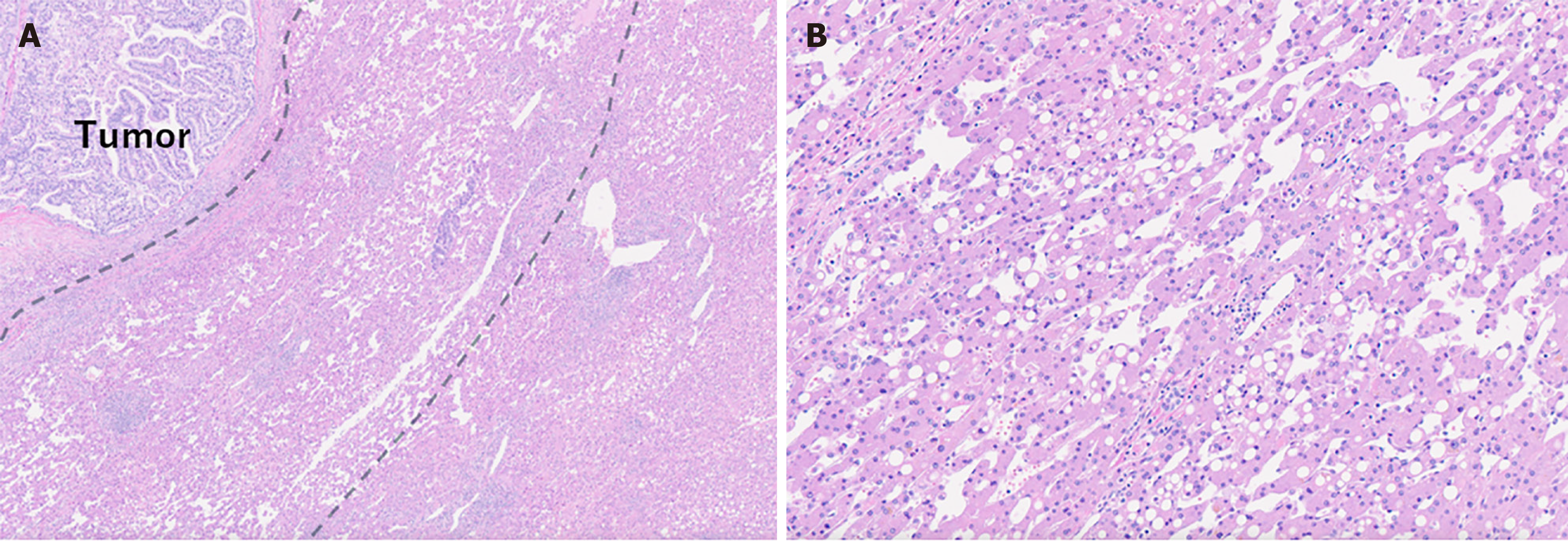
A partial hepatic resection was performed. Macroscopically, we observed a yellowish-white lobulated mass without a well-defined capsule, with dilation of the portal vein surrounding the tumor (Figure 3). Histopathological examination of the resected specimen revealed tumor cells with clear cytoplasm forming a glandular structure (Figure 4A and B), along with testing positive for AFP in the immunohistochemical testing (Figure 4C). These findings were similar to those of the previously resected gastric adenocarcinoma. Furthermore, Elastin van Gieson staining showed tumor invasion of the portal vein surrounding the tumor and the hepatic vein branch touching the tumor (Figure 4D and E). Additionally, hepatic steatosis and sinusoidal dilation were observed in the liver parenchyma surrounding the tumor (Figure 5). The dilated sinusoids were partially positive for CD34, suggestive of arterial blood supply to the tumor. The liver tumor was ultimately determined as metastasis from GAED.
Six months after the surgery, the patient continues to receive chemotherapy, with no evidence of new metastatic lesions.
In our patient, the radiopathological correlation demonstrated the liver metastases from GAED showed circumferential perilesional enhancement in the arterial phase on both contrast-enhanced CT and MRI. Presumably, an arterio-portal shunt caused by the narrowing, obliteration, or compression of sinusoids is the underlying functional mechanism of the perilesional enhancement in liver metastases[11]. Further histological examination demonstrated the presence of a tumor thrombus in the peritumoral portal vein, which may have contributed to the development of an arterio-portal shunt leading to perilesional enhancement. Vascular thrombosis has been described as an important imaging feature of liver metastases from GHA[5]. However, to date, only a few reports have addressed the association between liver metastases from GAED and tumor thrombus. Ge et al[12] recently described that 11 out of 16 patients with GAED (68.8%) exhibited vascular thrombosis. Additional studies with larger cohorts are necessary to determine whether vascular thrombosis is a characteristic feature of GAED and GHA.
Our case also showed a signal drop on chemical shift imaging, which reflects steatosis in the area consistent with perilesional enhancement. Peritumoral hepatic steatosis is often observed in liver metastases from insulinoma and has also recently been reported in metastatic somatostatin-producing neuroendocrine neoplasm[13,14]; however, there are no reports of fat deposition around tumors associated with blood flow changes. A previous study reported fatty degeneration of hepatocytes with sinusoidal dilation in the congested liver as a result of heart failure[15]. In our patient, fat deposition was observed in the peritumoral hepatocytes surrounding the dilated sinusoid, potentially indicating fat degeneration resulting from congestion caused by the tumor thrombus in the hepatic vein.
Additionally, we observed a rim-like hypointensity surrounding the tumor in HBP; pathological examination revealed no capsule, but a dilated portal vein in the corresponding area. HBP is an imaging sequence that histologically reflects the expression of organic anion-transporting polypeptide 1B3 on the cells. In HCC, microscopic portal vein tumor invasion induces hemodynamic changes, leading to a reduction of organic anion-transporting polypeptide B3 expression in the surrounding liver parenchyma; this alternation may explain the imaging finding of peritumoral hypointensity[16]. The underlying mechanism for the rim-like hypointensity observed in our patient remains unknown, but it is plausible that the hemodynamic changes in the portal vein, induced by the tumor thrombus in the portal and hepatic veins, may be responsible.
Tumor size is an essential factor in determining the treatment strategy. If the tumor size is overestimated, the patient could be deprived of appropriate treatment options. Several imaging sequences have been reported to assess the appropriate size of liver tumors[17,18]. The arterial phase is not ideal for evaluating tumor size, as it complicates the differentiation between the perilesional and tumor enhancement zones. As a potential pitfall, if the liver parenchyma surrounding the tumor also shows hyper-enhancement, as observed in our case, it may be misidentified as part of the tumor, leading to inaccurate measurement of the tumor size.
Both angiomyolipoma and HCC should be considered in the differential diagnosis of hepatic tumors with arterial enhancement and fat deposition. Angiomyolipoma typically does not show an increase in AFP, and early venous return is considered as the characteristic imaging feature[19]. However, steatohepatitic HCC (SH-HCC) is a histological variant of HCC characterized by fat deposition, with the distinctive radiological features of arterial enhancement, delayed washout, and the presence of diffuse or focal fatty deposits[20]. In the present case, the presence of elevated AFP levels and the HBP findings - the rim-like hypointensity (interpreted as a tumor capsule) and the hyperintensity lesions (indicating fat deposits and arterial enhancement) - were considered part of the tumor, leading to the inclusion of SH-HCC as a differential diagnosis. However, there was no washout in the delay phase, which was used to differentiate SH-HCC from liver metastases from GAED.
We reported a rare case of liver metastasis from GAED and provided detailed imaging features for both CT and MRI. The specific imaging characteristics observed in this case, including perilesional enhancement, fat deposits, and rim-like hypointensity, suggest histological invasiveness (vascular invasion) of the liver metastases from GAED. Further studies with more cases related to liver metastases from GAED are required to confirm these imaging findings.
We would like to extend our sincere appreciation to surgeon for their expert advice and critical insights throughout the preparation of this manuscript. They contributed significantly to refining our conclusions.
| 1. | Murakami T, Yao T, Mitomi H, Morimoto T, Ueyama H, Matsumoto K, Saito T, Osada T, Nagahara A, Watanabe S. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016;19:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Yamazawa S, Ushiku T, Shinozaki-Ushiku A, Hayashi A, Iwasaki A, Abe H, Tagashira A, Yamashita H, Seto Y, Aburatani H, Fukayama M. Gastric Cancer With Primitive Enterocyte Phenotype: An Aggressive Subgroup of Intestinal-type Adenocarcinoma. Am J Surg Pathol. 2017;41:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Abe D, Akazawa Y, Yatagai N, Hayashi T, Ueyama H, Mine S, Fukunaga T, Nagahara A, Yao T, Saito T. Clinicopathological characteristics of gastric adenocarcinoma with enteroblastic differentiation and gastric adenocarcinoma with enteroblastic marker expression. Virchows Arch. 2023;483:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Matsumoto K, Ueyama H, Matsumoto K, Akazawa Y, Komori H, Takeda T, Murakami T, Asaoka D, Hojo M, Tomita N, Nagahara A, Kajiyama Y, Yao T, Watanabe S. Clinicopathological features of alpha-fetoprotein producing early gastric cancer with enteroblastic differentiation. World J Gastroenterol. 2016;22:8203-8210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Lin YY, Chen CM, Huang YH, Lin CY, Chu SY, Hsu MY, Pan KT, Tseng JH. Liver metastasis from hepatoid adenocarcinoma of the stomach mimicking hepatocellular carcinoma: Dynamic computed tomography findings. World J Gastroenterol. 2015;21:13524-13531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Chang MY, Kim HJ, Park SH, Kim H, Choi DK, Lim JS, Park MS, Kim MJ, Kim H. CT features of hepatic metastases from hepatoid adenocarcinoma. Abdom Radiol (NY). 2017;42:2402-2409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Yuan J, Wang G, Liu M, Gong Z. MRI features of hepatic metastasis from hepatoid adenocarcinoma of the stomach: A case report. Radiol Case Rep. 2022;17:2295-2298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Zhu H, Li Q, Qian L. Liver metastasis from hepatoid adenocarcinoma of the stomach: a case report and literature review. Front Oncol. 2024;14:1297062. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Díaz-González Á, Monclús E, Darnell A, Solé M, Bruix J. Liver Metastases From Gastric Adenocarcinoma Mimicking Multinodular Hepatocellular Carcinoma. Hepatology. 2018;68:2042-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Jo JM, Kim JW, Heo SH, Shin SS, Jeong YY, Hur YH. Hepatic metastases from hepatoid adenocarcinoma of stomach mimicking hepatocellular carcinoma. Clin Mol Hepatol. 2012;18:420-423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Yu JS, Rofsky NM. Hepatic metastases: perilesional enhancement on dynamic MRI. AJR Am J Roentgenol. 2006;186:1051-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Ge X, Hua M, Zhan Y. Gastric adenocarcinoma with intestinal progenitor cell differentiation: a morphologically underdiagnosed and more invasive distinctive type of gastric adenocarcinoma. Am J Cancer Res. 2024;14:3885-3895. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Atwell TD, Lloyd RV, Nagorney DM, Fidler JL, Andrews JC, Reading CC. Peritumoral steatosis associated with insulinomas: appearance at imaging. Abdom Imaging. 2008;33:571-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shao Y, Gui Y, Cheng Y, Xu J, Chang X, Lv K. Case report: Peritumoral hepatic steatosis in a patient with a metastatic somatostatin-producing oligosymptomatic neuroendocrine neoplasm. Front Oncol. 2022;12:1013017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Wells ML, Venkatesh SK. Congestive hepatopathy. Abdom Radiol (NY). 2018;43:2037-2051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Kim KA, Kim MJ, Jeon HM, Kim KS, Choi JS, Ahn SH, Cha SJ, Chung YE. Prediction of microvascular invasion of hepatocellular carcinoma: usefulness of peritumoral hypointensity seen on gadoxetate disodium-enhanced hepatobiliary phase images. J Magn Reson Imaging. 2012;35:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 17. | Huh J, Park J, Kim KW, Kim HJ, Lee JS, Lee JH, Jeong YK, Shinagare AB, Ramaiya NH. Optimal Phase of Dynamic Computed Tomography for Reliable Size Measurement of Metastatic Neuroendocrine Tumors of the Liver: Comparison between Pre- and Post-Contrast Phases. Korean J Radiol. 2018;19:1066-1076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Dong SY, Yang YT, Wang WT, Zhu S, Sun W, Zeng MS, Rao SX. Hepatobiliary phase images of gadoxetic acid-enhanced MRI may improve accuracy of predicting the size of hepatocellular carcinoma at pathology. Acta Radiol. 2022;63:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Yoshimura H, Murakami T, Kim T, Nakamura H, Hirabuki N, Sakon M, Wakasa K, Inoue Y. Angiomyolipoma of the liver with least amount of fat component: imaging features of CT, MR, and angiography. Abdom Imaging. 2002;27:184-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Inui S, Kondo H, Tanahashi Y, Fukukura Y, Sano K, Morisaka H, Saito K, Kondo F, Fukusato T, Furui S, Oba H. Steatohepatitic hepatocellular carcinoma: imaging findings with clinicopathological correlation. Clin Radiol. 2021;76:160.e15-160.e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |