Published online Feb 28, 2025. doi: 10.4329/wjr.v17.i2.102373
Revised: December 23, 2024
Accepted: January 18, 2025
Published online: February 28, 2025
Processing time: 133 Days and 18.5 Hours
In response to an ageing global population, the primary hip and knee arthroplasty rate continues to increase. Although an effective treatment, up to 25% patients may require revision arthroplasty during their lifetime, commonly due to periprosthetic loosening. Revision procedures are associated with significantly increased healthcare costs; therefore, timely and accurate diagnostics are critical for clinicians and patients. Loosening, which may be septic or aseptic, remains a challenge and requires thorough clinical examination and multimodal imaging evaluation. Plain radiographs remain an essential diagnostic tool but advanced imaging modalities such as computed tomography, magnetic resonance imaging and nuclear medicine are playing an increasingly important role. This comprehensive review, through outlining the available radiological modalities, their respective strengths and weaknesses and the pertinent imaging findings, may help radiologists and orthopaedic surgeons make more informed decisions in the management of periprosthetic loosening.
Core Tip: As the rate of joint arthroplasties continue to rise, so too does the rate of revision arthroplasty. One of the primary indications for revision surgery is periprosthetic loosening; however, diagnosis of this condition is challenging. In this review, we discuss some of the major breakthroughs and advances in imaging techniques that aim to address these challenges. Improvements in metal artefact reduction techniques in computed tomography and magnetic resonance imaging have markedly improved image quality and diagnosis. In addition, nuclear medicine imaging techniques such as single-photon emission computed tomography/computed tomography are proving to be an excellent aid in diagnosis.
- Citation: Shet SS, Kakish E, Murphy SC, Roopnarinesingh R, Power SP, Maher MM, Ryan DJ. Imaging evaluation of periprosthetic loosening: A primer for the general radiologist. World J Radiol 2025; 17(2): 102373
- URL: https://www.wjgnet.com/1949-8470/full/v17/i2/102373.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i2.102373
Hip and knee arthroplasties are among the fastest growing surgeries worldwide with > 480000 primary total hip arthroplasties (pTHAs) and > 260000 primary total knee arthroplasties (pTKAs) performed in the USA in 2019 alone. With an ageing population, this number is expected to rise to > 700000, and 1.2 million pTHAs and pTKAs, respectively being performed annually in the USA by 2040[1]. Although the success rates and overall satisfaction rates for both pTHA and pTKA are excellent, revision surgery remains a common occurrence. Currently, the overall 10-year survival rate is 93.6% and 95.6% for pTHA and pTKA, respectively, although lifetime survival is age dependent. Indeed, younger patients (age 46–50 years) have a lifetime risk of 22.6% for revision total hip arthroplasty (rTHA) and 25.2% for revision total knee arthroplasty (rTKA)[2,3].
Not surprisingly, with the rising numbers of pTHAs and pTKAs there is a proportionate increase in the rate of rTHA and rTKA, with which comes an increased cost of care, difficulty in resource allocation and an overall stress on healthcare systems worldwide[4]. Although there are various indications for rTHA and rTKA, the most common are aseptic loosening and infection. For pTHA failures, aseptic loosening accounts for 52%, and for pTKA 21.6%[5,6]. Infection is a more common cause for failed pTKA, being estimated to be responsible for 36.3% revisions[5,6].
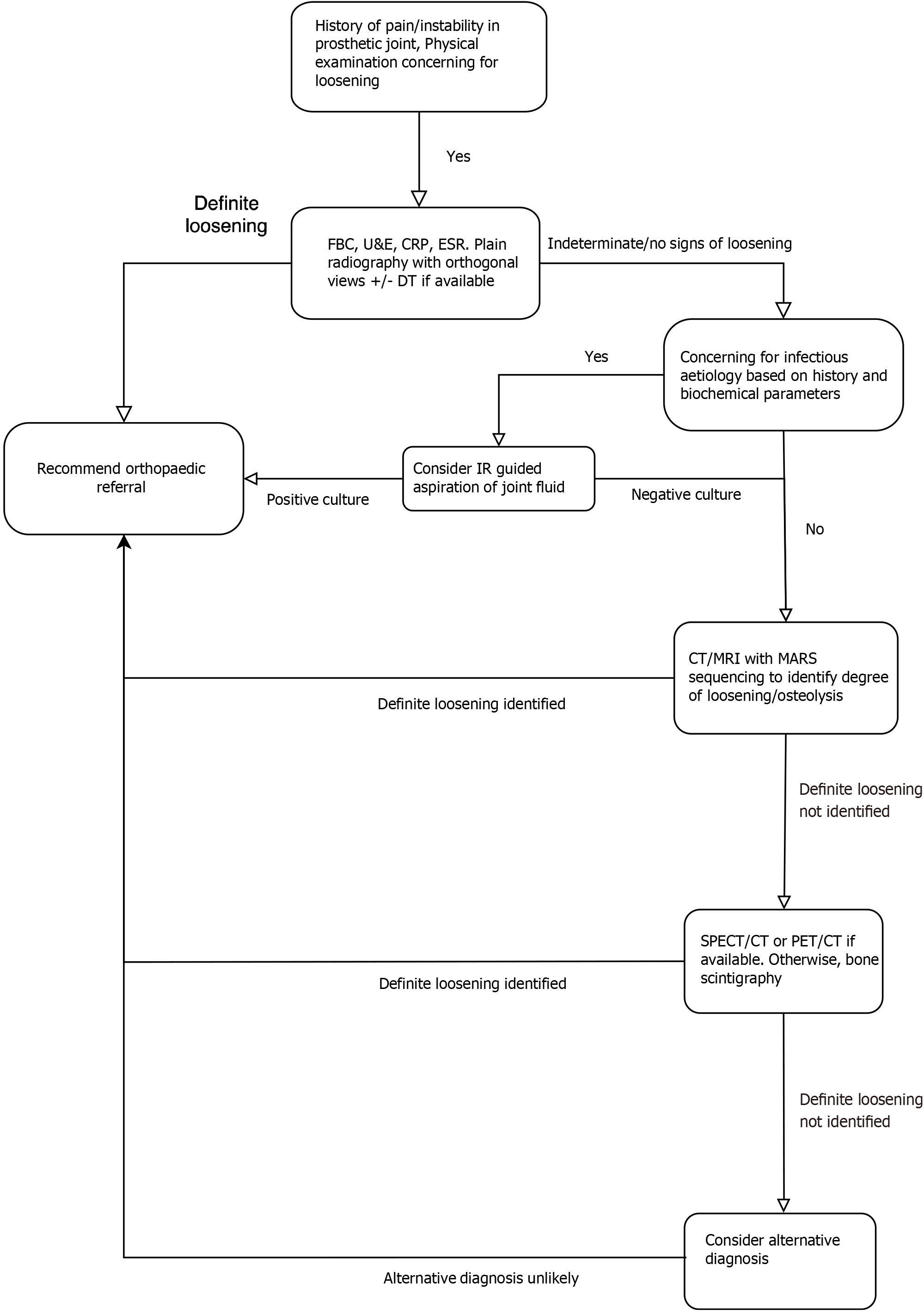
Serial radiographs play an important role in the diagnosis of periprosthetic loosening and various radiographic signs have been identified in the literature that indicate mechanical loosening[7]. However, limitations exist in the diagnostic capability of conventional radiographs, and they must be interpreted in conjunction with careful clinical evaluation[8]. One of the major limitations is that early loosening may not be detectable on plain radiography because evidence of bony integration of cementless implants is not visible. Therefore, an implant that appears to be firmly in place may in fact be loose[9].
As a result, recently, advanced imaging modalities such as computed tomography (CT), single-photon emission CT (SPECT), bone scintigraphy and magnetic resonance imaging (MRI) have been utilised more frequently in the diagnosis of periprosthetic loosening[10]. This review aims to provide general radiologists with a comprehensive overview of the current status of imaging in periprosthetic loosening and thus guide them in the diagnostic evaluation of these patients. Figures included within the article are accompanied by brief case descriptions with an aim of providing readers with real world examples of the utility of various imaging modalities.
During September 2024, PubMed and MEDLINE OVID were searched for relevant articles. MeSH terms included prosthesis failure, hip prosthesis, knee prosthesis and radiography. Studies published in English were included. Literature published within the last decade was focused on to consider evolving practice and technologies, however, older articles were not excluded from the literature review. Included figures were acquired from the institutional database of teaching cases and were anonymised prior to inclusion. As no identifiable patient information was collected, the clinical research ethics committee advised that formal ethical approval is not required.
Conventional radiography is the most commonly used modality in the evaluation of patients with suspected periprosthetic loosening. Typically, patients will present to the orthopaedic clinic or emergency department with a painful limb and difficulty in weightbearing. The first line imaging investigation is usually plain radiography with anteroposterior and lateral views of the affected limb. In addition to loosening, radiographs can be used to determine component alignment, polythene line wear, osteolysis, reactive bone formation as well as any evidence of periprosthetic fractures[11,12].
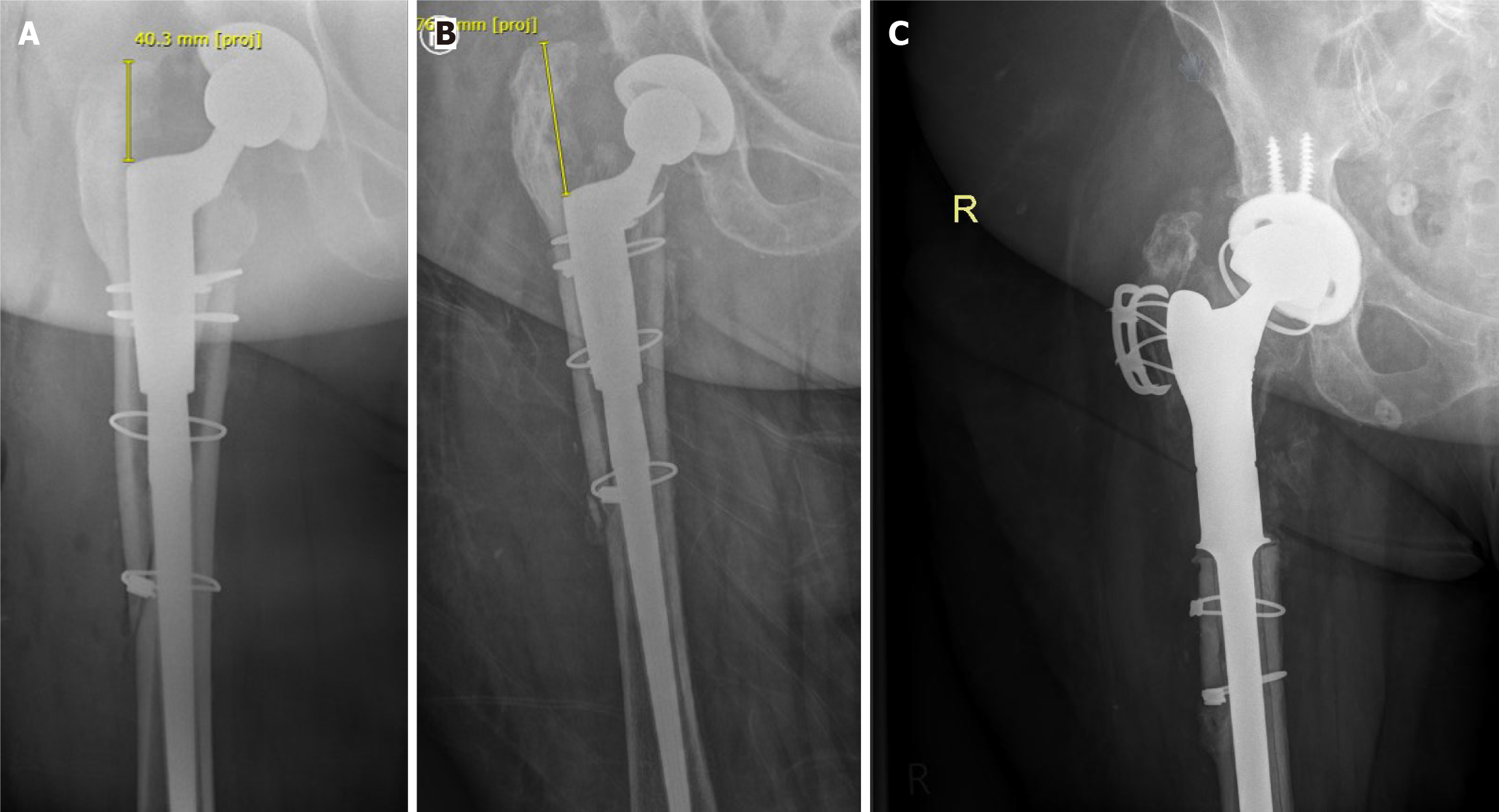
Numerous radiographic signs have been identified over the years that could indicate periprosthetic loosening. The most widely published being the presence of radiolucent lines > 2 mm around the prosthesis. Lucencies between 1 and 2 mm are generally considered to have indeterminate significance while lucencies < 1 mm are unlikely to be of clinical significance[13]. In addition to lucencies, loosening may be indicated by subsidence or movement/migration of the components. For example, in a THA prosthesis, vertical subsidence > 5 mm of the femoral component in indicative of loosening (Figure 1). Movement, as indicated by increased varus/valgus angulation of the femoral stem, is also indicative of loosening. Loosening may also be noted in the acetabular component and is commonly seen as superior or medial migration of the prosthetic cup.
In the case of a TKA prosthesis, subsidence may be noted when the tibial component is seen sinking into the tibial plateau. This is commonly associated with varus malalignment because of high medial load when compared with previous X-rays[14,15]. However, many of these hallmark signs require the presence of previous radiographs for compa
Although digital tomosynthesis (DT) has been used in breast imaging for many years, it is a relatively new technology in the field of musculoskeletal imaging. DT uses standard X-ray equipment with digital flat panel detectors to detailed images from low-dose projections obtained at different angles. Simply put, instead of a plain radiograph that captures an image at one angle, multiple images at different angles are captured and computer algorithms used to reconstruct the images in 3D depending on the pathology of interest. In evaluating periprosthetic loosening, DT with advanced imaging processing techniques such as iterative reconstruction with metal artefact reduction (MAR) can provide improved image quality and permits more accurate evaluation of the bone/implant interface compared to conventional radiography[17]. In addition, the radiation dose is lower compared to that with CT with similar specificity to CT and plain radiography at detecting loosening. Although sensitivity is similar to radiography, it is significantly lower than for CT. However, there is evidence to suggest interobserver agreement is better with DT than with plain radiographs[18].
DT has shown significant advantages over plain radiographs at detecting new bone formation around prostheses and ruling out loosening. In 2021, Totsuka et al[19] found that cancellous condensation around the stem, which appears as a radiolucent line on plain radiography, resulted in loosening being overcalled. DT, however, was able to provide more detailed information and exclude loosening for many of these patients and thus significantly alter their clinical course by avoiding unnecessary revision surgery[19]. Overall, although DT is unlikely to replace either plain radiographs or CT in evaluating loosening, it allows for more detailed evaluation of the bone/implant interface compared to plain radiography without an excessive radiation dose penalty.
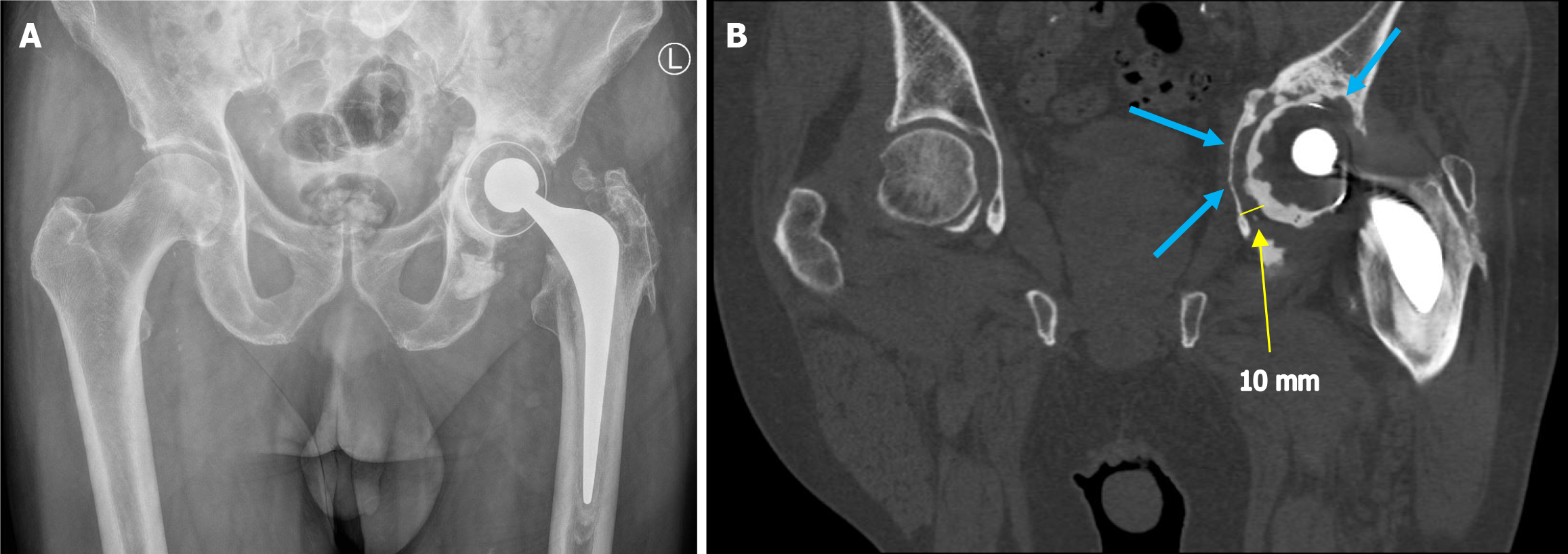
As a result of the limitations of plain radiographs and DT, CT with its ability to capture images in multiple planes, is often utilised to aid diagnosis. While plain radiographs may show equivocal findings, CT can characterise the extent and width of the periprosthetic lucency. In conjunction with careful clinical examination, this can help clinicians reach a diagnosis. Unfortunately, despite offering excellent visualisation of bony anatomy, CT images can be significantly affected by the presence of metal artefacts[20]. The artefacts produced can vary depending on the size, shape and material of the implant and in many cases, can cause difficulty in reaching a diagnosis[21]. These artefacts include image blurring as well as dark/bright streaks as a result of the metal implant blocking or scattering the X-rays. The composition of the metal component can affect the severity of artefacts quite significantly with metals such as titanium generating more artefacts in comparison to iron and platinum, for example[22].
Fortunately, MAR techniques have improved drastically in recent times and the diagnostic capability of CT has improved for these patients (Figure 2). Modern MAR techniques focus on manipulations in data acquisition, image reconstruction and post-processing to produce an image that allows the visualisation of the interface between the metal component and osseous tissue[23]. Similar to conventional radiographs, in CT, lucency > 2 mm around a prosthesis is indicative of loosening. However, in a symptomatic patient, lucency < 2 mm cannot satisfactorily exclude loosening. When radiographs are equivocal, CT scanning provides a fast and efficient means of clarifying the diagnosis[24].
An advanced type of CT technique, termed dual energy CT (DECT) was developed in the late 1970s and has allowed further improvements in image quality for cases with metal implants. In simple terms, DECT incorporates the use of two different X-ray energy spectra, which may be achieved by using two X-ray tubes operating at different energy levels, by rapidly switching the energy levels of a single X-ray tube or through the use of a dual layer detector. Comparing the attenuation characteristics at different energy levels allows for more accurate differentiation between materials thus improving image quality around metal implants.
In 2023, Foti et al[12] demonstrated the superiority of DECT over conventional CT and radiography in both diagnostic capability as well as in inter-reader agreement of the scan results in patients with TKA loosening. However, despite the improvements in CT, certain limitations exist. Firstly, DECT is a new development and the availability of these new scanners, particularly in smaller peripheral hospitals may be limited. Secondly, although advances have been made in MAR techniques, artefacts remain problematic and can cause diagnostic uncertainty in both conventional CT and DECT. Consequently, other additional imaging modalities have been utilised to aid diagnosis.
Ultrasound (US) as an imaging modality for musculoskeletal disorders is excellent and provides visualisation of muscle, bone, tendons and ligaments. US has the advantage of offering dynamic imaging with the benefit of no radiation exposure to the patient[25]. Unfortunately, US does not allow for the direct visualisation of periprosthetic loosening. Rather, it plays a role in the identification of adverse local tissue reactions (ALTR); for example, as seen with pseudotu
MRI has proved vital in the diagnosis of various metal artefact reduction pathology but its role in detecting periprosthetic loosening is less well established. Recognition of a loose component requires visualisation of the bone implant interface which is made difficult due to the presence of significant metal artefacts[27]. Recent times have seen improvements in MAR techniques applied to standard MRI sequences as well as the development of 3D multispectral imaging[27]. Two recent MRI techniques developed by Siemens and General Electric are slice encoding for metal artefact correction (SEMAC) and multi-acquisition variable resonance image combination (MAVRIC), respectively.
Conventional MRI uses a single resonant frequency to create images whereas in contrast, MAVRIC involves collecting data at various offset frequencies from the main resonance frequency. As a result, different parts of the frequency spectrum affected by the metal implant are sampled. Various sophisticated algorithms are then used to combine the multiple images obtained. By integrating the data from different frequencies, the final image aims to reduce artefacts caused by the implant and thus allow for better visualisation of the periprosthetic area region. SEMAC, rather than targeting the entire volume and relying on frequency offsets, focuses on correcting artefacts slice-by-slice. SEMAC attempts to correct distortions within each slice and subsequently combines the corrected slices to form a final image.
In cases where metal artefacts are severe, a hybrid approach combining the two techniques can be utilised for maximal artefact reduction. This technique is termed MAVRIC selective. There is evidence in the literature to show that MAVRIC selective can significantly reduce metal artefacts as compared to traditional MRI sequences and thus detailed evaluation of the periprosthetic bone–implant interface can be made possible[27,28].
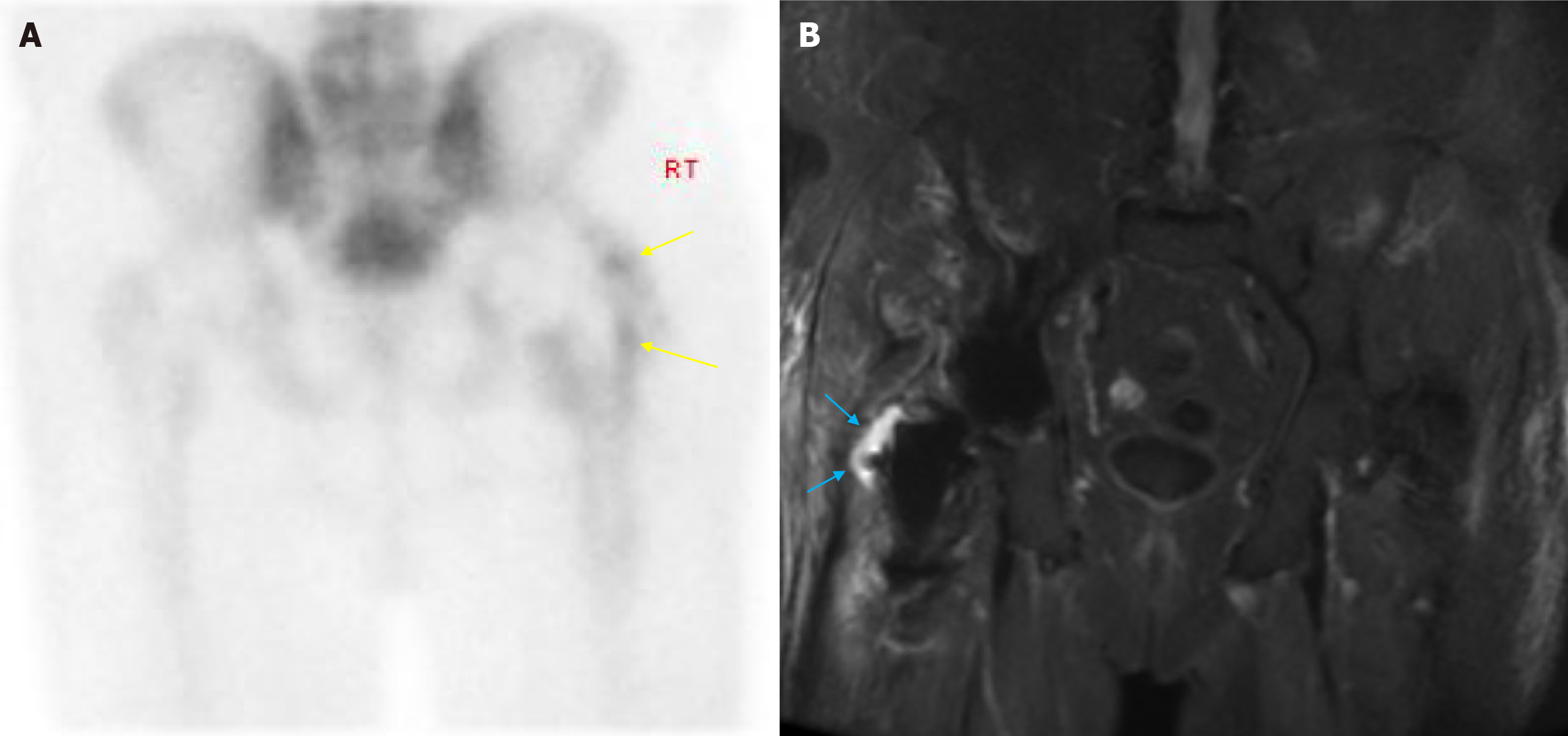
It is important for clinicians to be aware of these recent developments in MAR techniques to be better able to offer the patient the greatest chance of successful diagnosis. While metal artefacts have not been completely eradicated, newer techniques have improved the quality of MRI near implants and allowed for diagnosis of more pathology and post-operative complications[29]. Signs of loosening on MRI include formation of a fibrous membrane between the implant and bone, a fluid interface, or osteolysis around the prosthesis[30]. MRI is not routinely used for investigation of periprosthetic loosening in clinical practice because of the presence of artefacts and the availability of CT, which has faster acquisition times and lower costs.
Costs and slower image acquisition times compared to other modalities can significantly limit the use of MRI. However, due to the superior performance of MRI in characterising soft tissue lesions and collections, it still has a role to play in infection-related periprosthetic loosening (Figure 3). Unfortunately, despite advances in MAR sequences, metal artefacts have not been eliminated and careful clinical evaluation as well as thorough scrutiny of other available imaging is still required.
Various imaging modalities exist in the realm of nuclear medicine that have proven useful in the investigation of patients with suspected periprosthetic loosening. They include bone scintigraphy, positron emission tomography (PET) and SPECT. Each have their advantages and disadvantages and are discussed below. Bone scintigraphy has been extensively studied in joint arthroplasty and has been in use for several decades. Conventionally, technetium-99m labelled diphosphonates are used for this study. The incorporation of the radiotracer into bone is dependent on the blood supply to the bone and rate of metabolic activity. Areas of high metabolic activity result in increased uptake of the radiotracer thus aiding clinicians in the diagnosis of loosening and infection in joint arthroplasty. Unfortunately, although sensitive for loosening, studies have found bone scintigraphy to be nonspecific and differentiating between infection and aseptic loosening may be difficult (Figure 3). Therefore, correlation must be made with the patient’s clinical history, examination findings, and blood markers.
Some materials, such as hydroxyapatite-coated hip replacements, may result in high rates of false positives making a positive result in these studies hard to interpret. As with other imaging modalities discussed previously, careful clinical evaluation is essential for accurate diagnosis. Despite its low specificity, bone scintigraphy remains relatively cheap and accessible and can provide additional information to conventional plain radiographs.
SPECT is a type of cross-sectional nuclear imaging technique that uses single photon emitting radiopharmaceuticals to provide functional and physiological data on organs or pathological tissue. In the case of THA and TKA, SPECT combined with planar bone scintigraphy, can provide high sensitivity and specificity for loosening. However, this technique displays poor accuracy in the localisation of the area of increased uptake. Therefore, this limits its role clinically as the decision of which prosthetic component is to be revised, if any, can be difficult to make.
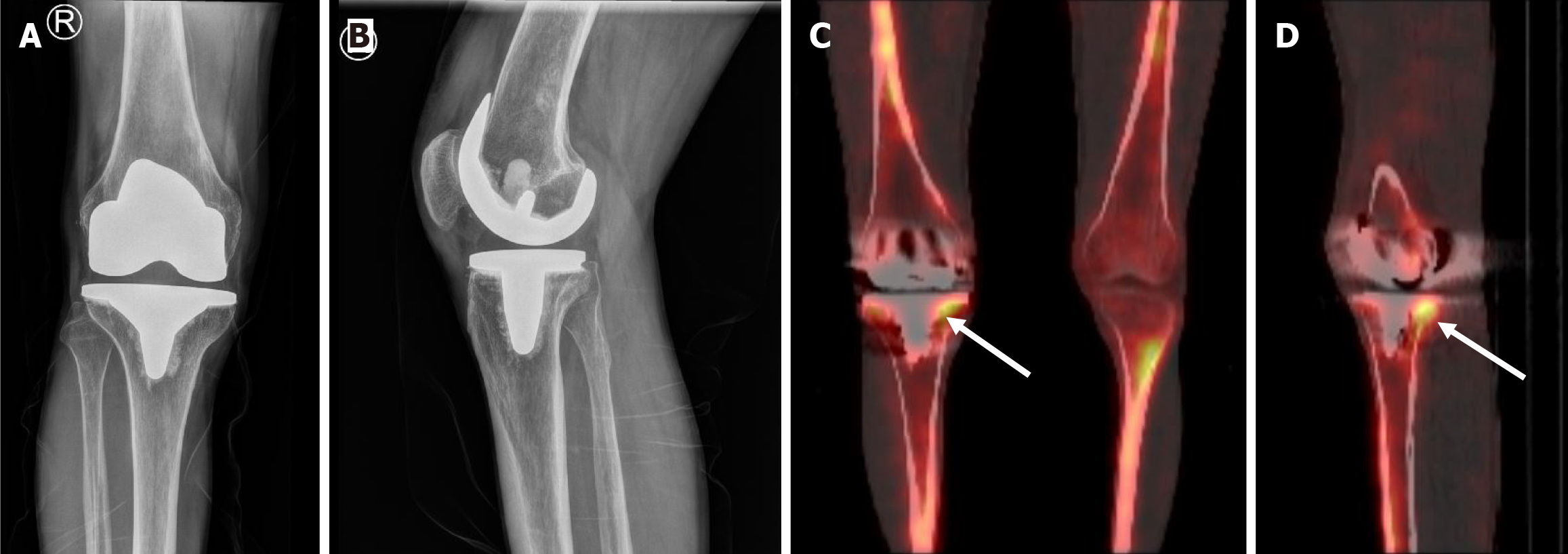
Fortunately, over the last decade, a hybrid technique combining the functional capability of SPECT with the anatomical detail of CT has been developed to address this shortcoming. This technique is proving to be increasing useful in diagnosis of loosening with high sensitivity and specificity reported[31-33] (Figure 4). In 2011, Hirschmann et al[34] described using SPECT/CT in evaluation of painful TKAs and found that SPECT/CT significantly changed the surgeons’ decision of revision with many patients who were initially planned for revision, being managed nonsurgically. In addition, malposition and patellofemoral osteoarthritis, both of which could be the cause of symptoms, were identified in TKA patients with the help of SPECT/CT[34].
Further evidence of the capability of SPECT/CT in diagnosing loosening was seen in a systematic review published by Peng et al[35] in 2021. The authors found that SPECT/CT had an impressive sensitivity and specificity of 0.94 and 0.89 in diagnosing aseptic loosening in TKA and THA, respectively. In fact, they recommended using SPECT/CT as the primary imaging modality in diagnosing periprosthetic loosening. Unfortunately, despite its excellent performance, high costs and high radiation doses limit its use[35].
PET is another nuclear imaging technique that involves the use of radiotracers to analyse changes in metabolism. A commonly used radiotracer is F-18 fluorodeoxyglucose, which can be used to illustrate changes in glucose metabolism at the cellular level. This is particularly useful in oncology and has been used for many years for monitoring cancer activity. In assessment of periprosthetic loosening, however, sodium fluoride (NaF) conjugated to F-18 is a more useful radiotracer. Previous studies have illustrated its effectiveness in characterising bone metabolism, and its use has been validated by comparison with bone biopsies. Used in isolation, 18(F)-NaF PET can provide information on areas of increased bony metabolic activity such as infection, inflammation and tumour, however, anatomical localisation is limited.
As a result, a combination of 18(F)-NaF PET and CT is often utilised to alleviate some of these shortcomings. This hybrid imaging technique provides precise information on anatomic localisation and aids in the identification of extraosseous disease not previously seen on PET in isolation[36,37]. The effectiveness of 18(F)-NaF PET/CT was demonstrated by Ullmark[38] in 2020, who identified patients with loosening that previously had normal or equivocal plain radiograph findings. These patients identified using 18(F)-NaF PET/CT all underwent revision surgery and were confirmed to have loose components intraoperatively[38]. Although effective, PET/CT scanning is an expensive process, even more so than SPECT/CT, and availability may be even more limited as a result. Regardless, due to cost of revision arthroplasty and the potential consequences of revision for the patient, when available, PET/CT may be considered for diagnosis of suspected periprosthetic loosening.
As the population in the western world continues to age, the number of primary hip and knee arthroplasties continues to rise. Consequently, the number of revision hip and knee arthroplasties being performed yearly are also rising due to complications such as aseptic loosening and infection. Accurate diagnosis of periprosthetic loosening is essential for early and effective patient management. However, diagnosis is not easy and requires careful clinical evaluation individualised to each patient.
While conventional radiography remains an excellent first-line diagnostic tool, it lacks the spatial resolution offered by CT and MRI and the functional information provided by nuclear medicine imaging. DT offers increased spatial resolution compared to plain radiography with a lower radiation dose than CT but lacks the high sensitivity of CT. Metal artefacts remain problematic and evaluation of the bone/implant interface in CT and MRI can often be obscured by artefacts. However, advances in MAR reconstruction and DECT have improved image quality, as demonstrated by the images in this review. US may be used as an adjunct to evaluate loosening, particularly in a metal-on-metal prosthesis where pseudotumour may be demonstrated; however, it cannot be used alone for diagnosis. Nuclear imaging techniques, particularly hybrid modalities such a SPECT/CT offer both functional information as well as increased spatial resolution compared to plain radiography. However, cost and accessibility remain an issue.
An integrated approach that combines careful clinical evaluation with the appropriate imaging modality is essential to accurately diagnose periprosthetic loosening and thus guide management. Through outlining the available radiological modalities, and their respective strengths and weaknesses, this article may help clinicians make informed decisions in the selection of imaging modalities for the diagnosis of periprosthetic loosening. Thus, there is the potential to minimise the number of unnecessary revision operations.
While orthopaedic surgeons should have strong involvement in the diagnostic pathway as early as possible, radiolo
Although careful clinical history and examination remain vital in diagnosing periprosthetic loosening, appropriate use of radiological investigations can provide vital information. Clinicians should be aware of the various image modalities available to aid in the diagnosis of the challenging condition as well as the advantages and disadvantages of each.
| 1. | Shichman I, Roof M, Askew N, Nherera L, Rozell JC, Seyler TM, Schwarzkopf R. Projections and Epidemiology of Primary Hip and Knee Arthroplasty in Medicare Patients to 2040-2060. JB JS Open Access. 2023;8:e22.00112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 218] [Reference Citation Analysis (0)] |
| 2. | Nugent M, Young SW, Frampton CM, Hooper GJ. The lifetime risk of revision following total hip arthroplasty. Bone Joint J. 2021;103-B:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Stone B, Nugent M, Young SW, Frampton C, Hooper GJ. The lifetime risk of revision following total knee arthroplasty : a New Zealand Joint Registry study. Bone Joint J. 2022;104-B:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Shichman I, Askew N, Habibi A, Nherera L, Macaulay W, Seyler T, Schwarzkopf R. Projections and Epidemiology of Revision Hip and Knee Arthroplasty in the United States to 2040-2060. Arthroplast Today. 2023;21:101152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 68] [Reference Citation Analysis (0)] |
| 5. | Postler A, Lützner C, Beyer F, Tille E, Lützner J. Analysis of Total Knee Arthroplasty revision causes. BMC Musculoskelet Disord. 2018;19:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 236] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 6. | Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, Kuskowski M, Cheng EY, Sharkey PF, Parvizi J, Stiehl JB, Mont MA. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Tigges S, Stiles RG, Roberson JR. Complications of hip arthroplasty causing periprosthetic radiolucency on plain radiographs. AJR Am J Roentgenol. 1994;162:1387-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Keogh CF, Munk PL, Gee R, Chan LP, Marchinkow LO. Imaging of the painful hip arthroplasty. AJR Am J Roentgenol. 2003;180:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Katzer A, Lœhr JF. Early loosening of hip replacements: causes, course and diagnosis. J Orthopaed Traumatol. 2003;4:105-116. [DOI] [Full Text] |
| 10. | Lohmann CH, Rampal S, Lohrengel M, Singh G. Imaging in peri-prosthetic assessment: an orthopaedic perspective. EFORT Open Rev. 2017;2:117-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Anil U, Singh V, Schwarzkopf R. Diagnosis and Detection of Subtle Aseptic Loosening in Total Hip Arthroplasty. J Arthroplasty. 2022;37:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 41] [Reference Citation Analysis (0)] |
| 12. | Foti G, Longo C, D'Onofrio M, Natali S, Piovan G, Oliboni E, Iacono V, Guerriero M, Zorzi C. Dual-Energy CT for Detecting Painful Knee Prosthesis Loosening. Radiology. 2023;306:e211818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Kleeblad LJ, van der List JP, Zuiderbaan HA, Pearle AD. Regional Femoral and Tibial Radiolucency in Cemented Unicompartmental Knee Arthroplasty and the Relationship to Functional Outcomes. J Arthroplasty. 2017;32:3345-3351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Mulcahy H, Chew FS. Current concepts in knee replacement: complications. AJR Am J Roentgenol. 2014;202:W76-W86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Srivastava A, Lee GY, Steklov N, Colwell CW Jr, Ezzet KA, D'Lima DD. Effect of tibial component varus on wear in total knee arthroplasty. Knee. 2012;19:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Rouzrokh P, Wyles CC, Kurian SJ, Ramazanian T, Cai JC, Huang Q, Zhang K, Taunton MJ, Maradit Kremers H, Erickson BJ. Deep Learning for Radiographic Measurement of Femoral Component Subsidence Following Total Hip Arthroplasty. Radiol Artif Intell. 2022;4:e210206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Blum A, Noël A, Regent D, Villani N, Gillet R, Gondim Teixeira P. Tomosynthesis in musculoskeletal pathology. Diagn Interv Imaging. 2018;99:423-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Gillet R, Teixeira P, Bonarelli C, Coudane H, Sirveaux F, Louis M, Blum A. Comparison of radiographs, tomosynthesis and CT with metal artifact reduction for the detection of hip prosthetic loosening. Eur Radiol. 2019;29:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Totsuka S, Nishino T, Watanabe R, Yamazaki M, Mishima H. New Evaluation Method for Bone Formation around a Fully Hydroxyapatite-Coated Stem Using Digital Tomosynthesis: A Retrospective Cross-Sectional Study. Diagnostics (Basel). 2021;11:2094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Math KR, Zaidi SF, Petchprapa C, Harwin SF. Imaging of total knee arthroplasty. Semin Musculoskelet Radiol. 2006;10:47-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Gotman I. Characteristics of metals used in implants. J Endourol. 1997;11:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 155] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Boas FE, Fleischmann D. CT artifacts: causes and reduction techniques. Imaging Med. 2012;4:229-240. |
| 23. | Wellenberg RHH, Hakvoort ET, Slump CH, Boomsma MF, Maas M, Streekstra GJ. Metal artifact reduction techniques in musculoskeletal CT-imaging. Eur J Radiol. 2018;107:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Mushtaq N, To K, Gooding C, Khan W. Radiological Imaging Evaluation of the Failing Total Hip Replacement. Front Surg. 2019;6:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Page P, Manske RC, Voight M, Wolfe C. MSK Ultrasound - An IJSPT Perspective. Int J Sports Phys Ther. 2023;18:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 26. | Robinson DJ, Lee S, Marks P, Schneider ME. Ultrasound Screening for Adverse Local Tissue Reaction after Hip Arthroplasty. Ultrasound Med Biol. 2017;43:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Burge AJ, Konin GP, Berkowitz JL, Lin B, Koff MF, Potter HG. What is the Diagnostic Accuracy of MRI for Component Loosening in THA? Clin Orthop Relat Res. 2019;477:2085-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Choi SJ, Koch KM, Hargreaves BA, Stevens KJ, Gold GE. Metal artifact reduction with MAVRIC SL at 3-T MRI in patients with hip arthroplasty. AJR Am J Roentgenol. 2015;204:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Yoon D, Doyle Z, Lee P, Hargreaves B, Stevens K. Clinical evaluation of isotropic MAVRIC-SL for symptomatic hip arthroplasties at 3 T MRI. Magn Reson Imaging. 2024;111:256-264. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Endo Y, Burge AJ, Koff MF, Lin B, Westrich GH, Boettner F, Chiu YF, Potter HG. Diagnostic Performance of MRI for Component Loosening in Total Knee Arthroplasty Compared with Radiography. Radiology. 2022;304:128-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Schillaci O, Danieli R, Manni C, Simonetti G. Is SPECT/CT with a hybrid camera useful to improve scintigraphic imaging interpretation? Nucl Med Commun. 2004;25:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Arıcan P, Okudan Tekin B, Şefizade R, Naldöken S, Baştuğ A, Özkurt B. The role of bone SPECT/CT in the evaluation of painful joint prostheses. Nucl Med Commun. 2015;36:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Jacene HA, Goetze S, Patel H, Wahl RL, Ziessman HA. Advantages of Hybrid SPECT/CT vs SPECT Alone. Open Med Imaging J. 2008;2:67-79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Hirschmann MT, Konala P, Iranpour F, Kerner A, Rasch H, Friederich NF. Clinical value of SPECT/CT for evaluation of patients with painful knees after total knee arthroplasty--a new dimension of diagnostics? BMC Musculoskelet Disord. 2011;12:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Peng Z, Jia Y, Li J, Wang G. Diagnostic Performance of Single-Photon Emission Computed Tomography/Computed Tomography in Aseptic Loosening: A Systematic Review and Meta-Analysis. J Arthroplasty. 2021;36:4003-4012.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 36. | Delank KS, Schmidt M, Michael JW, Dietlein M, Schicha H, Eysel P. The implications of 18F-FDG PET for the diagnosis of endoprosthetic loosening and infection in hip and knee arthroplasty: results from a prospective, blinded study. BMC Musculoskelet Disord. 2006;7:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Ullmark G, Nilsson O, Maripuu E, Sörensen J. Analysis of bone mineralization on uncemented femoral stems by [18F]-fluoride-PET: a randomized clinical study of 16 hips in 8 patients. Acta Orthop. 2013;84:138-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Ullmark G. Occult Hip Prosthetic Loosening Diagnosed by [18F] Fluoride-PET/CT. Arthroplast Today. 2020;6:548-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |