Published online Jan 28, 2025. doi: 10.4329/wjr.v17.i1.100794
Revised: December 2, 2024
Accepted: January 9, 2025
Published online: January 28, 2025
Processing time: 147 Days and 21.2 Hours
Cardiovascular diseases and cancer are leading causes of morbidity and mortality. Patients with malignancies are at increased risk for cardiovascular complications including acute coronary syndromes, chemotherapy or radiation therapy related complications and cardiac metastasis.
We present a case of a 47-year-old female with metastatic cancer on immunotherapy presented with anterior ST elevation myocardial infarction followed by emergent percutaneous coronary intervention in the left anterior descending artery. Echocardiography after 72 hours showed thickening of inferior wall and cardiac magnetic resonance depicted inflammation and necrosis attributable to either cardiac metastasis or immunotherapy induced myocarditis. Biopsy was not performed because of treatment with antiplatelet drugs and a definite diagnosis was achieved after probationary administration of high-dose intravenous methylprednisolone that led to recovery.
In patients with malignancy, chemotherapy-induced cardiovascular complications and cardiac metastasis are common concerns and may coexist with common acute cardiovascular diseases including acute coronary syndromes. In such cases clinical suspicion aided by multimodality imaging is crucial for the diagnosis. A multidisciplinary team approach is required for prompt initiation of the appro
Core Tip: This case report presents a cardio oncology patient who presented with an acute coronary syndrome. However, the clinical presentation as well as the echocardiography findings were suspicious for an alternative diagnosis including immune checkpoint inhibitor-induced myocarditis or cardiac metastasis. Cardiac magnetic resonance aided the final diagnosis and the initiation of the appropriate treatment by the multidisciplinary team. The case highlights the significance of multimodality imaging for the management of cardio-oncology patients.
- Citation: Latsios G, Dimitroglou Y, Lazaros G, Alexopoulos N, Tolis I, Aggeli C, Tsioufis C. Differentiating between immune checkpoint inhibitor-induced myocarditis and cardiac metastasis in a cardio-oncology patient presenting with myocardial infarction: A case report. World J Radiol 2025; 17(1): 100794
- URL: https://www.wjgnet.com/1949-8470/full/v17/i1/100794.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i1.100794
Patients with malignancies are at increased risk for cardiovascular complications including acute coronary syndromes (ACS) and many antineoplasmatic drugs have been associated with cardiovascular side effects. In addition, malignant disease can lead to cardiac metastases which can mimic other cardiovascular diseases, albeit they usually remain silent[1]. We present a case of an oncology patient with history of cardiovascular disease who presented with ACS. A multidisciplinary diagnostic and therapeutic approach utilizing multimodality imaging led to the selection of the appropriate treatment.
A 47-year-old Caucasian woman was brought to the emergency department with acute onset of retrosternal chest pain radiating to her left arm, associated with diaphoresis and nausea.
Anginal chest pain in rest, typical for cardiac origin.
Her past medical history included ST-segment elevation myocardial infarction (STEMI) three months prior to presen
There is none personal and family history of the patient.
Cardiovascular examination was unremarkable.
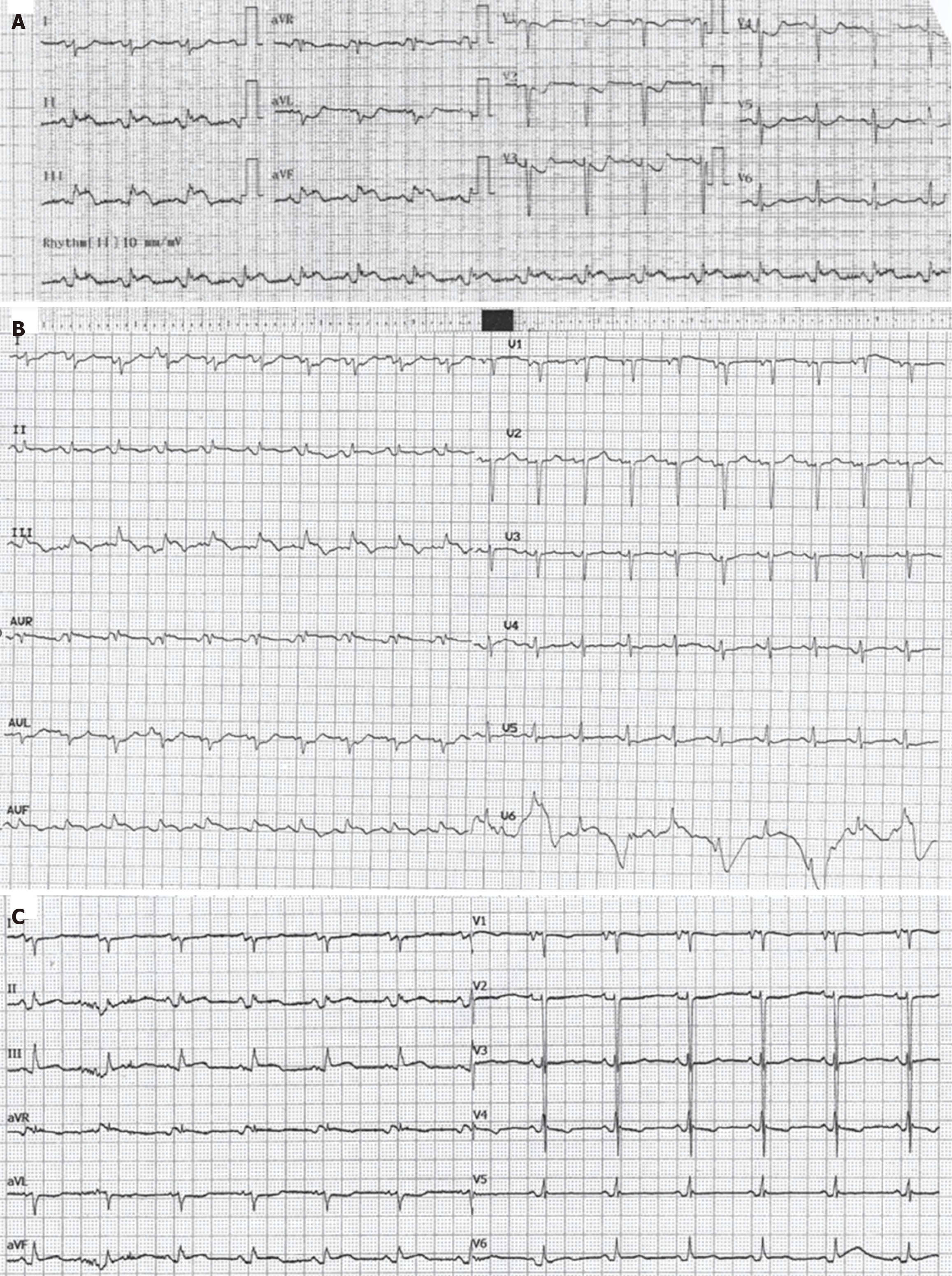
Echocardiography showed sinus rhythm with ST segment elevation in the inferior leads and ST segment depression in anterior and lateral leads (Figure 1A).
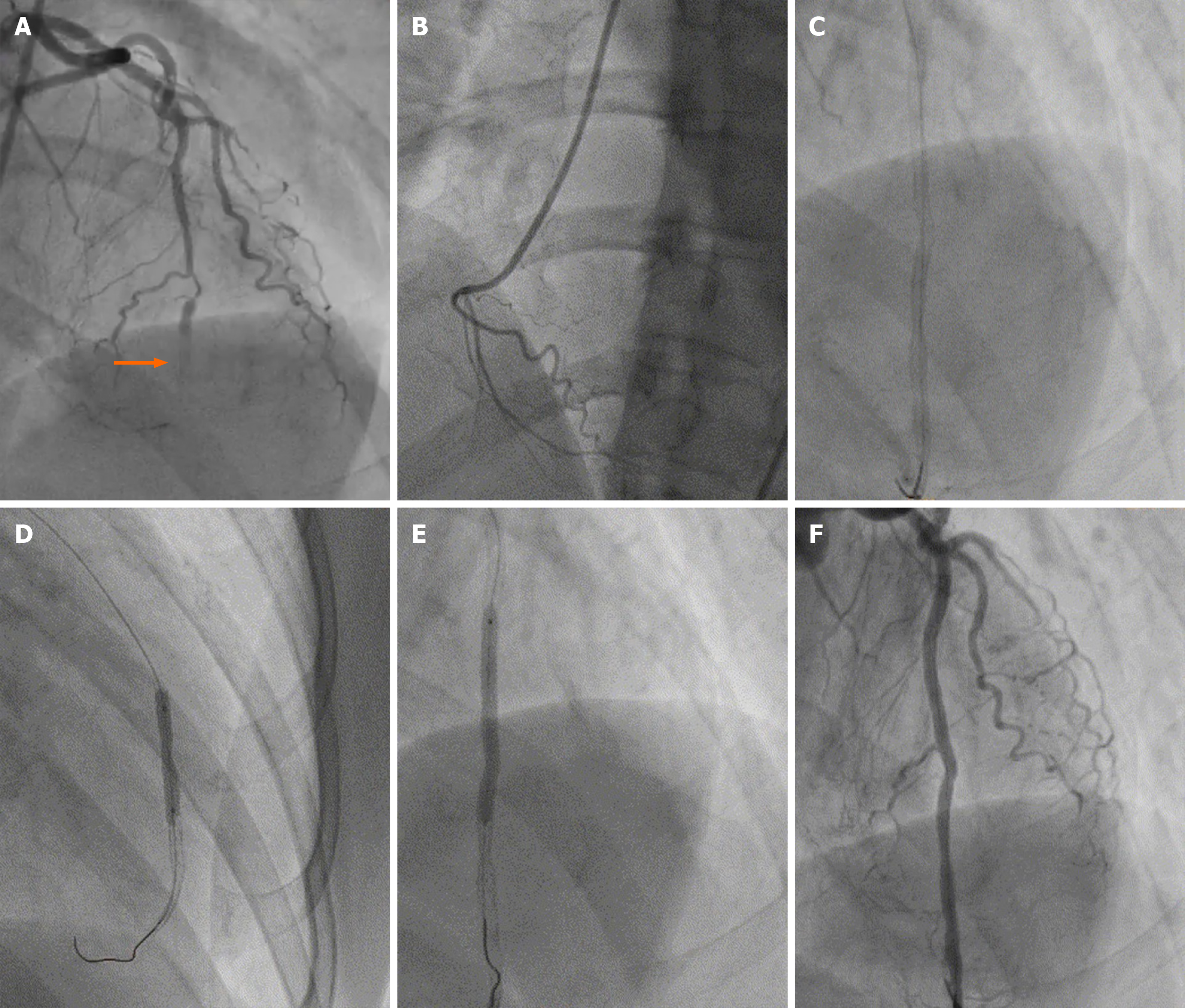
Based on a working diagnosis of STEMI the patient was immediately transferred to the catheterization laboratory. Coronary angiography revealed stent occlusion at the site of previous angioplasty, which was treated with PCI and further implantation of 2 drug-eluting stents (Figure 2).
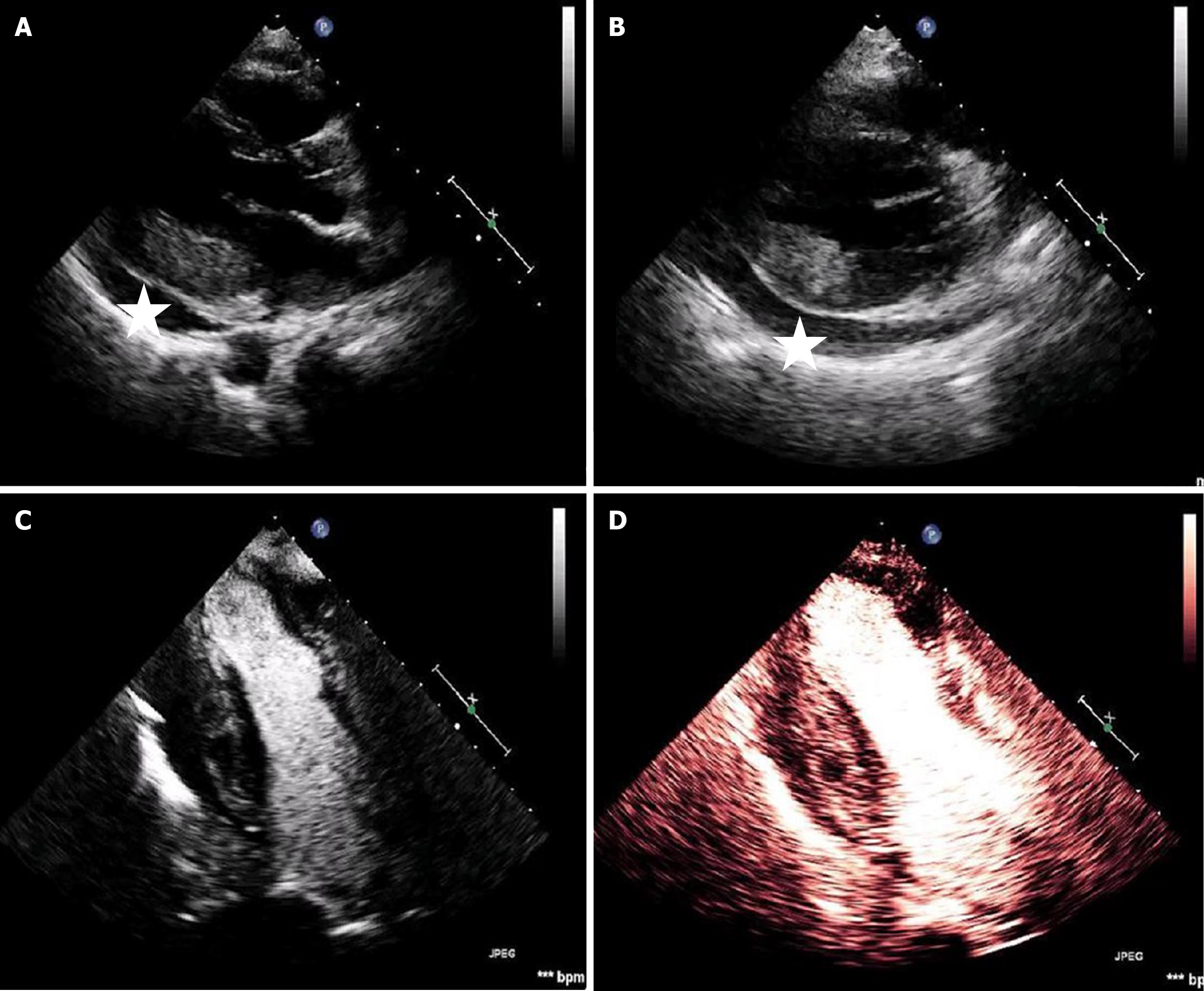
The patient was subsequently transferred to the coronary care unit and a transthoracic echocardiogram revealed moderate left ventricle systolic dysfunction with severe anterolateral wall hypokinesis and moderate posterolateral wall hypokinesis. Of particular interest were the increased thickness and echogenicity of the posterior and posterolateral wall. Reassessment 72 hours post-PCI showed marked and worsened thickening of the same segments, suspicious for myocardial edema. Moreover, assessment of myocardial perfusion with contrast echocardiography revealed perfusion heterogeneity in the inferior wall. A moderate pericardial effusion, with no signs of impending tamponade, was also present (Figure 3).
During her stay, troponin kinetics displayed a variable rather than typical biphasic pattern. Specifically, hs-troponin I levels peaked at 2300 pg/mL on the second day and varied from 800 to 2200 pg/mL till thirteen days after the peak value. Inflammation markers including C-reactive protein were markedly increased as well with a peak value of hs-C-reactive protein of 382 mg/L. The echocardiography changes of the inferior leads, persisted for several days after the initial treatment (Figure 1B and C). Differential diagnosis included intramyocardial hematoma, edema, thrombus, tumor infiltration, or preexisting hypertrophy - the latter excluded based on previous echo findings provided by the attending oncologist.
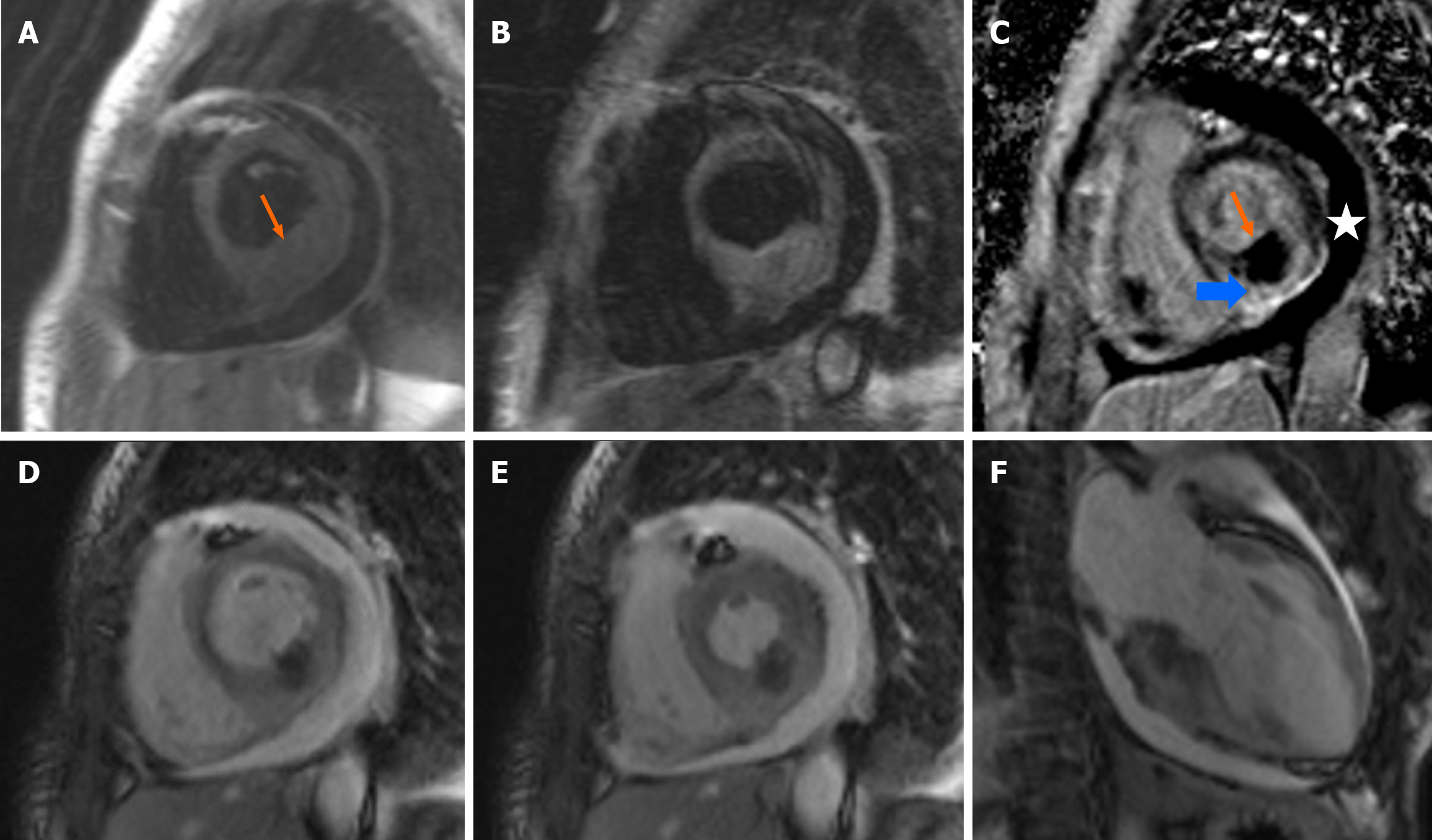
A cardiac magnetic resonance (CMR) imaging scan was carried out. The major findings were noted in the basal inferior - basal inferolateral wall, where there was severely increased wall thickness. In this area there was increased signal intensity in T2-weighted, in early- and in late-gadolinium enhancement images in the subepicardial and mesocardial regions, indicative of myocardial hyperemia or necrosis, with concomitant severely decreased signal intensity in the subendocardial region (Figure 4). Taken together, these findings were most probably indicative of tumor metastasis or acute myocarditis. Sudendocardial late gadolinium enhancement of the apex and anterolateral wall was compatible with the ischemic damage owing to myocardial infarction in the left anterior descending territory. Moderate circumferential pericardial effusion was also displayed, consistent with the transthoracic echocardiogram findings.
Based on the clinical, echocardiographic and CMR findings ICI-induced myocarditis was considered the most possible diagnosis. The diagnostic option of cardiac biopsy was thoroughly discussed and decided against, partly because the patient was on dual antiplatelet therapy.
A multidisciplinary medical team meeting was held and was agreed to administer ultra-high dose of intravenous methylprednisolone (500 mg per day for three days).
Thereafter, the patient remained asymptomatic and had an uncomplicated clinical recovery and was discharged after 18 days.
Cancer and heart disease share common risk factors and pathogenetic pathways[1]. Fluoropyrimidines and platinum compounds, vascular endothelial growth factor -inhibitors and radiotherapy increase the risk for ACS. Pathophysiologic mechanisms include endothelial injury, vasospasm and increased procoagulant activity[2]. Moreover, malignancy is a known predictor of stent thrombosis[3]. The patient presented was on oxaliplatin and suffered an initial ACS and subsequently had a recurrent myocardial infarction owing to thrombosis at the site of previous PCI. However, STEMI could not explain the clinical course and the echocardiographic assessment and further work-up was initiated. Imaging findings supported the presence of myocardial inflammation attributable to either cardiac metastasis or myocarditis.
Metastatic involvement of the myocardium could induce inflammation of the surrounding tissue. The incidence of cardiac metastasis in patients with malignancies is about 10%[4]. Most patients are asymptomatic, and symptoms depend on the size and the location of the tumor and include chest pain, dyspnea, palpitations and lower extremity edema mimicking other cardiovascular diseases. Multimodality imaging, including echocardiography, CMR, computed tomography and positron emission tomography, is essential for proper diagnosis and management.
Furthermore, recent evidence has shown that contemporary cancer treatment with ICI has been associated with myocarditis and pericarditis[5,6]. The CMR imaging pattern is similar to the one observed in other cases of drug-induced myocarditis and may include myocardial dysfunction, edema, and, in some cases, fibrosis. T1 mapping may also aid the diagnosis with CMR and according to a systematic review has among imaging biomarkers the higher concordance with endomyocardial biopsy and is also associated with patient prognosis[7]. The cause is considered immune-mediated, and the myocardium may be affected focally as in the case presented. A study reported that the prevalence of myocarditis in patients treated with ICI is 1.14% occurring at a median time of 34 days after initiation of immunotherapy[8]. Treatment with ultra-high doses of steroids was associated with better outcomes.
In favor of the diagnosis of myocarditis is the echocardiographic evaluation, since diffuse myocardial thickness was not noted at the initial echocardiography study and the patient had clinical and echocardiographic improvement. Moreover, myopericarditis which results from immunotherapy may present with a pericardial effusion and myopericardial enhancement similar to that of a malignant cause, thus differentiation might be challenging. On the other hand, CMR findings were also indicative of cardiac metastasis (Figure 3). New-onset pericardial effusion in a patient with a known malignancy might also indicate cardiac metastases. Biopsy was not performed because the patient was on dual antiplatelet therapy following stent thrombosis and the CMR findings were very helpful. Even though a definite diag
Acute cardiovascular care requires early recognition and prompt treatment, nevertheless complex molecular and pathophysiological pathways are yet to be fully understood[9]. This case highlights the importance of maintaining a high level of suspicion in patients with malignancy since ACS, chemotherapy-induced cardiovascular complications and cardiac metastasis may coexist. Multimodality imaging is crucial for the diagnosis and multidisciplinary approach by oncologists, cardiologists and imaging specialists is essential for optimal treatment.
| 1. | Koene RJ, Prizment AE, Blaes A, Konety SH. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation. 2016;133:1104-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 1040] [Article Influence: 115.6] [Reference Citation Analysis (0)] |
| 2. | Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, Lopez Fernandez T, Mohty D, Piepoli MF, Tamargo J, Torbicki A, Suter TM; ESC Scientific Document Group. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768-2801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1409] [Cited by in RCA: 1498] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 3. | van Werkum JW, Heestermans AA, Zomer AC, Kelder JC, Suttorp MJ, Rensing BJ, Koolen JJ, Brueren BR, Dambrink JH, Hautvast RW, Verheugt FW, ten Berg JM. Predictors of coronary stent thrombosis: the Dutch Stent Thrombosis Registry. J Am Coll Cardiol. 2009;53:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 540] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Butany J, Leong SW, Carmichael K, Komeda M. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol. 2005;21:675-680. [PubMed] |
| 5. | Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. 2018;71:1755-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 648] [Cited by in RCA: 1074] [Article Influence: 153.4] [Reference Citation Analysis (0)] |
| 6. | Oliveira C, Mainoli B, Duarte GS, Machado T, Tinoco RG, Esperança-Martins M, Ferreira JJ, Costa J. Correction to: Immunerelated serious adverse events with immune checkpoint inhibitors: systematic review and network metaanalysis. Eur J Clin Pharmacol. 2024;80:1597-1598. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Arcari L, Tini G, Camastra G, Ciolina F, De Santis D, Russo D, Caruso D, Danti M, Cacciotti L. Cardiac Magnetic Resonance Imaging in Immune Check-Point Inhibitor Myocarditis: A Systematic Review. J Imaging. 2022;8:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 8. | Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 3141] [Article Influence: 448.7] [Reference Citation Analysis (0)] |
| 9. | Latsios G, Toutouzas K, Tousoulis D. Care Pathways in ST Elevation Myocardial Infarction. Maybe a Modern Way to Hippocrates' Oath: "Harm Less and Help More". Cardiology. 2018;140:175-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |