Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.482
Revised: August 16, 2024
Accepted: September 3, 2024
Published online: September 28, 2024
Processing time: 77 Days and 16.7 Hours
Immune checkpoint inhibitors (ICIs) are therapeutic agents for advanced and metastatic non-small cell lung cancer (NSCLC) with high clinical antitumor efficacy. However, immune-related adverse events occur in 20% of these patients and often requiring treatment with immunosuppressive agents, such as corticosteroids. Consequently, this may increase the risk of patients to opportunistic infections. Pneumocystis jirovecii pneumonia (PJP), a rare but serious opportunistic infection typically observed in patients with human immunodeficiency virus, can also occur in cancer patients undergoing long-term glucocorticoid treatment.
We report a case of a 56-year-old male with squamous NSCLC treated with triplimab combined with paclitaxel, carboplatin, and radical thoracic radiation therapy. Following this regimen, he developed acute kidney injury (AKI) with elevated creatinine levels. After concurrent radical chemoradiotherapy ended, he developed a grade 3 immune-related AKI. High-dose corticosteroids were admi
PJP is rare but can occur in patients with ICI adverse events and should be differentiated from tumor progression or immune-related adverse events. Thoracic radiation may increase risk, necessitating careful monitoring and prevention.
Core Tip: A patient with squamous lung cancer was treated with triplimab combined with paclitaxel, carboplatin, and radical thoracic radiation therapy. Despite the good therapeutic effect, he developed a grade 3 immune-related acute kidney injury, prompting high-dose corticosteroids treatment. Eight weeks later, the patient developed severe pneumonia with spontaneous pneumothorax, and was diagnosed with Pneumocystis jirovecii pneumonia (PJP) co-infection with the herpes simplex virus 1 and cytomegalovirus. PJP is rare but might occur in patients with immune checkpoint inhibitor adverse events, highlighting the need to be differentiated from tumor progression or immune-related adverse events.
- Citation: Zheng YW, Pan JC, Wang JF, Zhang J. Pneumocystis pneumonia in stage IIIA lung adenocarcinoma with immune-related acute kidney injury and thoracic radiotherapy: A case report. World J Radiol 2024; 16(9): 482-488
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/482.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.482
Immunotherapy-related adverse reactions have increased owing to the widespread use of immunotherapies. Glucocorticoid therapy is an effective method for inhibiting overactivated immune responses[1]. Long-term glucocorticoid therapy renders the patient immunocompromised, and more patients with cancer are at risk of opportunistic infections[2]. Pneumocystis jirovecii (P. jirovecii) pneumonia (PJP) is an opportunistic infection caused by P. jirovecii. PJP is prevalent among patients with human immunodeficiency virus (HIV) infection but rarely in patients with cancer. Patients presenting with PJP may exhibit fever, cough, dyspnea, and respiratory failure in severe cases.
Here, we report a rare case of PJP with spontaneous pneumothorax in a 56-year-old male patient with advanced non-small cell lung cancer (NSCLC) who received eight weeks of glucocorticoid therapy to treat an immune-related acute kidney injury (AKI). Owing to prompt diagnosis and use of sulfamethoxazole, trimethoprim, and other antipathogenic drugs, the patient recovered fully.
The patient diagnosed with squamous lung cancer 4 months ago presented with sudden dyspnea for the past 3 days.
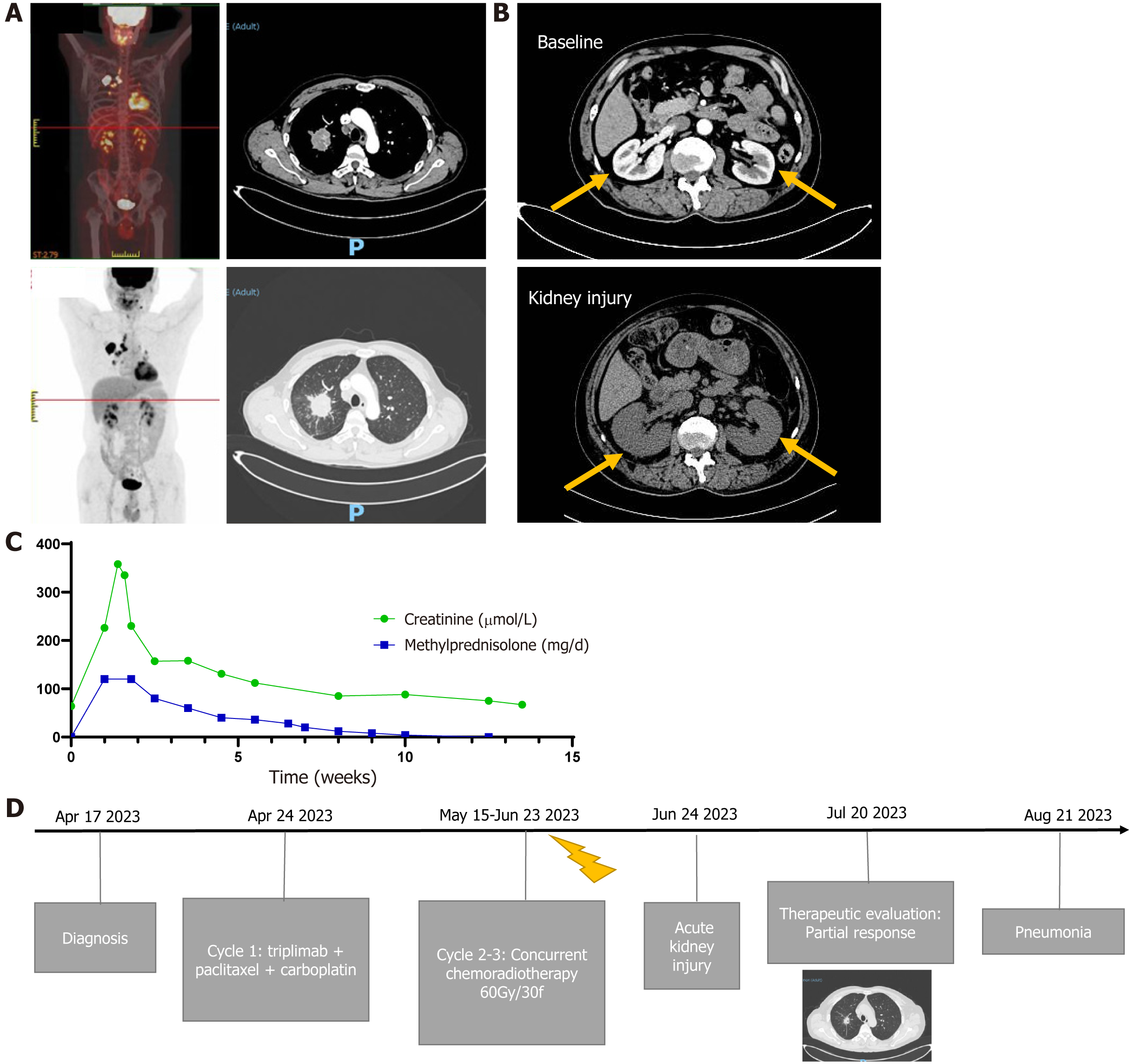
A 56-year-old man who was a former smoker with an Eastern Cooperative Oncology Group performance status of 0 was diagnosed with stage IIIA NSCLC (cTabN2M0, squamous lung cancer, PD-L1 10%; Figure 1A). After multidisciplinary discussion, including consideration of the patient's will, triplimab (a PD-1 inhibitor) combined with radical chemoradiotherapy was chosen as the treatment regimen. After one cycle of induction treatment, the patient received triplimab and concurrent chemoradiotherapy from May 15, 2023, to June 23, 2023. After completing thoracic radiation therapy, AKI was observed on June 24, 2023. The level of creatinine suddenly increased to 226 μmol/L, increasing further to 358 µmol/L two days later. Computed tomography (CT) revealed that the volume of the bilateral kidney increased by approximately 20% without hydronephrosis (Figure 1B), and the patient refused a renal biopsy. The patient subsequently received methylprednisolone (60 mg) twice per day and antibiotics for bacterial infection prevention. The creatinine level decreased gradually, and methylprednisolone was slowly tapered in tandem (Figure 1C). On July 20, 2023, chest imaging revealed a partial response without radiation pneumonia. The timeline of treatment is showed in Figure 1D.
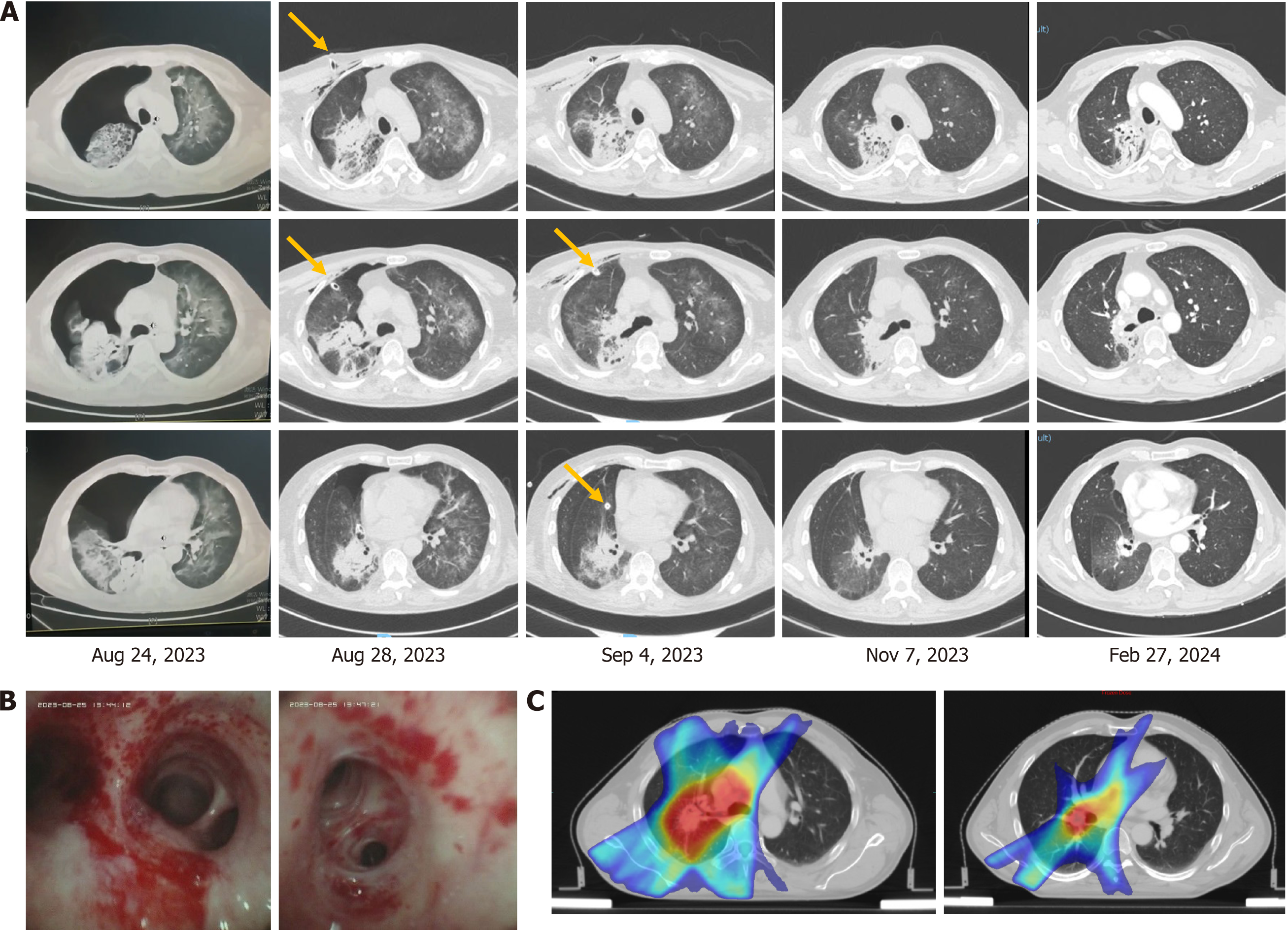
In August 2023, the patient was administered prednisone (10 mg) once daily (equivalent to 8 mg of methylprednisolone). On August 24, 2023, he suddenly developed fever and dyspnea, which worsened over three days, and the patient was urgently admitted to the hospital.
The patient had no significant medical history.
No family history of any malignant disease was reported.
Upon admission, his vital signs were unstable: Blood pressure, 138/89 mmHg; body temperature, 38.2 °C; heart rate, 115 beats/minute; and breathing rate, 24 breaths/minute. The oxygen saturation during oxygen inhalation was 95%. Breath sounds over the right lung were diminished, and moist rales were heard on the left lung.
Blood test results were as follows: White blood cell count: 5.06 × 109/L; lymphocyte count: 0.65 × 109/L; C-reactive protein: 52.58 mg/L; and procalcitonin level: 0.077 ng/mL. The tests for pathogens (traditional laboratory test results) were negative. Serological test results for Aspergillus were normal.
CT showed diffuse lesions in both lungs and right pneumothorax (Figure 2A). After closed drainage of the right thoracic cavity, diffuse lesions in the right lung became more severe. Bronchoscopy with bronchoalveolar lavage was performed since no neoplasm was observed, and transbronchial biopsy deemed unnecessary. Bronchoscopy revealed numerous yellowish-white secretions in the bronchus, bronchial mucosal hyperemia, erosion, and bleeding (Figure 2B).
PJP infection was suspected, and thus the patient received piperacillin-tazobactam (4.5 g) three times per day combined with sulfamethoxazole and trimethoprim (TMP-SMX) (1.2 g/0.24 g) three times per day as empiric antibiotic therapy. The lavage fluid was sent to KingMed Diagnostics for targeted sequencing of multiple respiratory pathogens. Three days later, the next-generation sequencing of the lavage fluid confirmed PJP, herpes simplex virus 1, and cytomegalovirus.
Acyclovir (0.25 g) was administered twice a day. One week later, the inflammation and symptoms were alleviated. Piperacillin-tazobactam and acyclovir were used for two weeks, and TMP-SMX was used continuously. Two months later, the inflamed bilateral lung was absorbed; however, the area with radiation developed chronic inflammation, with slight displacement of the mediastinum (Figure 2A and C).
On February 27, 2024, chronic inflammation was absorbed further and TMP-SMX was discontinued. The patient’s daily activities eventually returned to the previous level. No further immunotherapies were administered. The patient remains alive without recurrence as of April 01, 2024.
PJP is a fungal infection that commonly affects immunocompromised patients and can be life-threatening in severe cases. The World Health Organization has listed it as one of 19 priority invasive fungal diseases, calling for increased research and public health action[3]. Typically, at-risk patients are those with underlying disease states that alter host immunity, such as HIV infection, transplant recipients, or those taking immunosuppressive therapies and medications[4]. The incidence of PJP in patients with solid tumors was documented at 0.013% (20/151718)[5].
Over the past 20 years, immune checkpoint inhibitors (ICIs) have been widely used[6]. However, these therapies can result in a variety of immune-related adverse events that can occur in any organ, including the kidneys[7]. AKI is the most common form of nephrotoxicity and is classically related to acute interstitial nephritis[8]. A noninvasive modality for the definite diagnosis of ICI-AKI remains unavailable[9,10]; however, CT imaging showed that the volume of the bilateral kidney increased in our patient. The estimated incidence of AKI directly related to ICI is approximately 3–5%[11]. Most patients had stage 1 or 2 while 10% had stage 3 AKI[12]. In our case, the patient had stage 3 AKI; fortunately, efficient and timely glucocorticoid therapy resulted in the recovery of kidney function. However, glucocorticoids can significantly impact both the innate and adaptive immune responses, and long-term steroid use increases the risk of opportunistic infections.
Our patient developed PJP and viral infection after receiving glucocorticoids for more than two months. PJP prophylaxis is recommended for patients expected to receive ≥ 20 mg daily prednisone equivalent for ≥ 4 weeks in the National Comprehensive Cancer Network guidelines of Management of Immunotherapy-Related Toxicities (Version 1.2024). Additionally, a study by Shah et al[13] highlighted the degree of immunosuppression and the relative risk of opportunistic infections. In 112 patients who received 20 mg daily of a prednisone equivalent for four weeks to manage immune-related adverse events, only eight had opportunistic infections; among them, one patient developed PJP[13]. Similarly, Sadek et al[14] revealed that only two PJP cases were found in patients treated with an ICI (480 patients received ICIs during that period). The incidence of PJP after steroid use due to immune-related adverse reactions is considerably low, and only six cases have been reported in the literature (Table 1). Considering the relatively common adverse effects of TMP-SMX at prophylactic doses[4], we wonder whether PJP prophylaxis is efficacious or necessary in all patients with cancer receiving steroids for immune-related adverse events. Conversely, steroids were frequently used in patients with cancer for a variety of other reasons. PJP has also been observed in patients with cancer receiving corticosteroids for malignant spinal cord compression[15] and weight loss[16]. Miyake et al[17] reported that the incidence of PJP in immunosuppressed non-HIV patients was 0.18% (32/17733), a monthly average dose of ≥ 13.7 mg daily prednisolone was a significant independent risk factors for PJP, and prophylaxis with ≥ 34.3 mg/day of TMP-SMX is to be recommended[17].Therefore, further studies are required to determine whether patients with cancer require precise PJP prophylaxis.
| References | Country | Age and Sex | Cancer type | Patient condition | Treatment | AE and immunosuppression | Pathogens | Prognosis |
| Schwarz et al[23], 2019 | Austria | 79, Male | NSCLC | ECOG performance status 2, history of smoking, COPD | Chemotherapy-thoracic radiation-nivolumab | Immune-related pneumonitis, steroids for 6 weeks | Pneumocystis jirovecii | Died |
| 53, Male | NSCLC | ECOG performance status 0, history of smoking | Chemotherapy-thoracic radiation + nivolumab | Immune-related pneumonitis, steroids, and mycophenolate mofetil for 5 weeks | Pneumocystis jirovecii, cytomegalovirus | Died | ||
| Duarte et al[15], 2020 | Belgium | 68, Male | Melanoma | NA | Nivolumab-ipilimumab | Immune-related hepatitis and colitis, steroids for 10 weeks and infliximab | Pneumocystis jirovecii | Recovered |
| 24, Female | Hodgkin’s lymphoma | NA | Multi cycle chemotherapy-pembrolizumab | Macrophage-activating syndrome, steroids for 6 months | Pneumocystis jirovecii | Recovered | ||
| Arriola et al[24], 2015 | England | 69, Female | Melanoma | Chronic lymphocytic leukemia | Chemotherapy-ipilimumab | Immune-related colitis, steroids for 12 weeks, and infliximab | Pneumocystis jirovecii | Recovered |
| 63, Female | Melanoma | NA | Ipilimumab | A capillary leak syndrome, steroids for 4 weeks | Pneumocystis jirovecii | Recovered |
In addition to steroids, multiple other factors, such as lymphocytopenia and radiation to the chest, may contribute to PJP in patients with solid tumors in a composite manner[3]. In patients with lymphocytopenia, especially those with low CD4+ T cell counts, P. jirovecii can proliferate, causing a mononuclear cell response with inflammation. McAleese et al[18] advocated prophylaxis in patients with a lymphocyte count < 0.6 × 109/L. Fu et al[19] reported seven patients with thoracic neoplasms experiencing radiation pneumonitis complicated by PJP. Similar to radiation pneumonia, PJP presents with various atypical radiographic characteristics, including the relationship between photographic findings and the planning target volume. Similarly, the right side of the lung that received radiation had a more severe infection in our case, which resulted in pneumothorax. Pneumothorax is a rare complication of PJP, occurring in only 3% of the HIV-positive patients with PJP[20]. This finding indicates that thoracic radiation may worsen the risk of PJP.
With the emergence of targeted therapies and immunotherapies, as well as the continuous development of novel radiotherapies, we have entered an era of novel treatment paradigms for locally advanced NSCLC[21]. The feasibility of induction with ICIs and chemotherapy before definitive chemoradiotherapy for locally advanced-NSCLC has been explored[22]. Notably, multiple factors interacted with each other in our case; although radiation pneumonia did not occur, handling immune-related adverse events leading to opportunistic infections still worsened the lung injury. In addition, we also differentiated PJP from immune and radiation pneumonia during treatment. Because no sign of inflammation was evident one month before the symptoms, immune and radiation pneumonia were not initially considered. A short-term reexamination after anti-inflammatory treatments confirmed the validity of our judgment.
A special feature of our case was that the patient developed double-lung PJP complicated by viral pneumonia accompanied by spontaneous pneumothorax during immune-related adverse event treatment. The patient’s prognosis was good after timely anti-inflammatory treatments. Appropriate chemoprophylaxis to reduce the risk of PJP is necessary with comprehensive consideration of steroid use, lymphocytopenia, other chemotherapies, immunotherapies, and radiation therapy.
| 1. | Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, Miller WH Jr, Calabrese L. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17:504-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 2. | Chastain DB, Spradlin M, Ahmad H, Henao-Martínez AF. Unintended Consequences: Risk of Opportunistic Infections Associated With Long-term Glucocorticoid Therapies in Adults. Clin Infect Dis. 2024;78:e37-e56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Xue T, Kong X, Ma L. Trends in the Epidemiology of Pneumocystis Pneumonia in Immunocompromised Patients without HIV Infection. J Fungi (Basel). 2023;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Weyant RB, Kabbani D, Doucette K, Lau C, Cervera C. Pneumocystis jirovecii: a review with a focus on prevention and treatment. Expert Opin Pharmacother. 2021;22:1579-1592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Takeda K, Harada S, Hayama B, Hoashi K, Enokida T, Sasaki T, Okamoto K, Nakano K, Ohkushi D. Clinical characteristics and risk factors associated with Pneumocystis jirovecii infection in patients with solid tumors: study of thirteen-year medical records of a large cancer center. BMC Cancer. 2021;21:987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Liu SM, Zheng MM, Pan Y, Liu SY, Li Y, Wu YL. Emerging evidence and treatment paradigm of non-small cell lung cancer. J Hematol Oncol. 2023;16:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 7. | O'Leary CL, Pierce N, Patel SP, Naidoo J. Immune-Related Toxicity in NSCLC: Current State-of-the-Art and Emerging Clinical Challenges. J Thorac Oncol. 2024;19:395-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 8. | Miao J, Sise ME, Herrmann SM. Immune checkpoint inhibitor related nephrotoxicity: Advances in clinicopathologic features, noninvasive approaches, and therapeutic strategy and rechallenge. Front Nephrol. 2022;2:1017921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Capaccione KM, Valiplackal JP, Huang A, Roa T, Fruauff A, Liou C, Kim E, Khurana S, Maher M, Ma H, Ngyuen P, Mak S, Dumeer S, Lala S, D'souza B, Laifer-Narin S, Desperito E, Ruzal-Shapiro C, Salvatore MM. Checkpoint Inhibitor Immune-Related Adverse Events: A Multimodality Pictorial Review. Acad Radiol. 2022;29:1869-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Longhitano E, Muscolino P, Lo Re C, Ferrara SA, Cernaro V, Gembillo G, Tessitore D, Speranza D, Figura F, Santarpia M, Silvestris N, Santoro D, Franchina T. Immune Checkpoint Inhibitors and the Kidney: A Focus on Diagnosis and Management for Personalised Medicine. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Rao Ullur A, Côté G, Pelletier K, Kitchlu A. Immunotherapy in oncology and the kidneys: a clinical review of the evaluation and management of kidney immune-related adverse events. Clin Kidney J. 2023;16:939-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Knox A, Cloney T, Janssen H, Solomon BJ, Alexander M, Ruderman I, John T. Immune-related acute kidney injury in Australian non-small cell lung cancer patients: Real-world results. Lung Cancer. 2023;184:107325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 13. | Shah NJ, Cook MR, Wu T, Lev-Ari S, Blackburn MJ, Serzan MT, Alaoui A, Ahn J, Atkins MB. The Risk of Opportunistic Infections and the Role of Antibiotic Prophylaxis in Patients on Checkpoint Inhibitors Requiring Steroids. J Natl Compr Canc Netw. 2022;20:800-807.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 14. | Sadek M, Loizidou A, Drowart A, Van den Wijngaert S, Gomez-Galdon M, Aspeslagh S. Pneumocystis Infection in Two Patients Treated with Both Immune Checkpoint Inhibitor and Corticoids. J Immunother Precis Oncol. 2020;3:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Duarte C, Gilbert D, Sheridan AD, PharmaD SDW, Lam ET. Pneumocystis jirovecii Pneumonia in Patients With Metastatic Prostate Cancer on Corticosteroids for Malignant Spinal Cord Compression: Two Case Reports and a Guideline Review. Oncology (Williston Park). 2020;34. [PubMed] |
| 16. | Sanka P, Hsu A. A Case of Pneumocystis jirovecci in a Patient with Non-Small Cell Lung Cancer Treated with Immunotherapy. R I Med J (2013). 2023;106:11-13. [PubMed] |
| 17. | Miyake K, Kawamura T, Nakahara Y, Sasaki S. A single-center, person-month-based analysis of the risk of developing Pneumocystis pneumonia (PCP) in immunosuppressed non-HIV patients: Preventive effects of trimethoprim-sulfamethoxazole. J Infect Chemother. 2023;29:1097-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | McAleese J, Mooney L, Walls GM. Reducing the Risk of Death From Pneumocystis jirovecii Pneumonia After Radical Radiation Therapy to the Lung. Clin Oncol (R Coll Radiol). 2021;33:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Fu Z, Yang X, Bi N, Zhai Y, Chen D, Wang W, Deng L, Zhang T, Zhou Z, Liang J. Radiation pneumonitis complicated by Pneumocystis carinii in patients with thoracic neoplasia: a clinical analysis of 7 cases. Cancer Commun (Lond). 2019;39:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Christe A, Walti L, Charimo J, Rauch A, Furrer H, Meyer A, Huynh-Do U, Heverhagen JT, Mueller NJ, Cavassini M, Mombelli M, van Delden C, Frauenfelder T, Montet X, Beigelman-Aubry C, Arampatzis S, Ebner L. Imaging patterns of Pneumocystis jirovecii pneumonia in HIV-positive and renal transplant patients - a multicentre study. Swiss Med Wkly. 2019;149:w20130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Miao D, Zhao J, Han Y, Zhou J, Li X, Zhang T, Li W, Xia Y. Management of locally advanced non-small cell lung cancer: State of the art and future directions. Cancer Commun (Lond). 2024;44:23-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 44] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Zhang T, Wang J, Zhou Z, Liu W, Xiao Z, Deng L, Feng Q, Wang X, Lv J, Ma X, Xue Q, Wang J, Wang Z, Bi N. Induction Immune Checkpoint Inhibitors and Chemotherapy Before Definitive Chemoradiation Therapy for Patients With Bulky Unresectable Stage III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2023;116:590-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 23. | Schwarz M, Kocher F, Niedersuess-Beke D, Rudzki J, Hochmair M, Widmann G, Hilbe W, Pircher A. Immunosuppression for Immune Checkpoint-related Toxicity Can Cause Pneumocystis Jirovecii Pneumonia (PJP) in Non-small-cell Lung Cancer (NSCLC): A Report of 2 Cases. Clin Lung Cancer. 2019;20:e247-e250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Arriola E, Wheater M, Krishnan R, Smart J, Foria V, Ottensmeier C. Immunosuppression for ipilimumab-related toxicity can cause pneumocystis pneumonia but spare antitumor immune control. Oncoimmunology. 2015;4:e1040218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |