Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.439
Revised: August 14, 2024
Accepted: August 27, 2024
Published online: September 28, 2024
Processing time: 195 Days and 14.6 Hours
Factor XIII (FXIII) deficiency is a rare yet profound coagulopathy. FXIII plays a pivotal role in hemostasis, and deficiencies in this factor can precipitate un
In this case study, a 53-year-old male devoid of significant medical history presented with recurrent intracranial hemorrhages and a hematoma in the right hip. Subsequent genetic analysis revealed a homozygous mutation in the ACE gene, confirming the diagnosis of acquired FXIII deficiency.
This case underscores the significance of considering acquired deficiencies in clotting factors when evaluating patients with unexplained bleeding episodes.
Core Tip: Factor XIII (FXIII) deficiency is a rare yet profound coagulopathy. FXIII plays a pivotal role in hemostasis, and deficiencies in this factor can precipitate unchecked or spontaneous hemorrhaging. Immunological assays for detecting FXIII inhibitors are indispensable for diagnosing acquired FXIII deficiency; however, the availability of suitable testing facilities is limited, resulting in prolonged turnaround times for these assays. In this case study, a 53-year-old male devoid of significant medical history presented with recurrent intracranial hemorrhages and a hematoma in the right hip. Subsequent genetic analysis revealed a homozygous mutation in the ACE gene, confirming the diagnosis of acquired FXIII deficiency. This case underscores the imperative of contemplating acquired coagulopathies in individuals experiencing unexplained and recurrent hemorrhagic episodes. Furthermore, it underscores the value of thorough genetic analysis in uncovering uncommon coagulation anomalies, which can substantially influence patient care and prognosis. Subsequent investigations could delve into elucidating the pathophysiological mechanisms underpinning mutations in the ACE gene and their interplay with coagulation pathways. Acquired FXIII deficiency, albeit uncommon, warrants consideration in individuals presenting with inexplicable hemorrhagic episodes. Genetic testing emerges as pivotal in the diagnosis and therapeutic approach to such instances.
- Citation: Wang L, Zhang N, Liang DC, Zhang HL, Lin LQ. Acquired factor XIII deficiency presenting with multiple intracranial hemorrhages and right hip hematoma: A case report. World J Radiol 2024; 16(9): 439-445
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/439.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.439
Factor XIII (FXIII) deficiency represents an uncommon and frequently overlooked congenital hemorrhagic disorder typified by compromised fibrin stabilization, leading to an elevated predisposition to spontaneous and recurrent hemorrhagic episodes[1]. Although FXIII deficiency is acknowledged for its correlation with intracranial hemorrhages, the simultaneous occurrence of bleeding in diverse anatomical locales, such as the cranium and hip, remains a clinical rarity[2].
Presented is a case involving a 53-year-old male exhibiting recurrent intracranial hemorrhages and a right hip hematoma, devoid of notable medical antecedents, ultimately diagnosed with acquired FXIII deficiency. The deficit of FXIII, a pivotal clotting factor in the terminal phases of the coagulation cascade, presents distinctive hurdles in both diagnosis and management owing to its rarity and the array of clinical manifestations it may entail.
In FXIII deficiency, intracranial hemorrhages are extensively documented, frequently resulting in profound neurological ramifications[3]. Nevertheless, the manifestation of bleeding in extracranial locales, such as the hip, adds another layer of intricacy to this case[4]. A comprehensive comprehension of the interrelationships among clotting factors and the ramifications of FXIII deficiency is imperative for elucidating the underlying mechanisms contributing to the multifocal hemorrhagic incidents observed in this instance.
Delving into this case, our aim is to illuminate the clinical intricacies of FXIII deficiency, underscoring the imperative for heightened awareness among healthcare providers to promptly diagnose and appropriately manage this rare bleeding disorder. Furthermore, this report adds to the evolving understanding of the diverse and potentially life-threatening manifestations of FXIII deficiency, ultimately fostering improved patient outcomes through enhanced diagnostic accuracy and personalized therapeutic strategies[5].
In February 2023, a 53-year-old male without a medical history of hypertension, diabetes, heart disease, stroke, or renal disease underwent surgery for a right hip hematoma at a local hospital. The operation, which lasted three hours, resulted in a blood loss of 100 mL, fluid administration of 2000 mL, and a urine output of 200 mL. The procedure was conducted under general anesthesia, utilizing fentanyl and propofol for sedation and analgesia. Notably, anticoagulant and antiplatelet medications were not administered.
In June 2023, he was hospitalized with a fever persisting for 20 days following cerebral hemorrhage surgery. The admission diagnosis encompassed intracranial infection, postoperative left temporal lobe cerebral hemorrhage, pulmonary infection, stage 2 high blood pressure (very high risk), postoperative right hip hemorrhage, and right hip skin infection.
The patient denied having a history of high blood pressure, diabetes, heart disease, stroke or kidney disease. Immunization history: Vaccinations have been administered in full compliance with national guidelines. The patient denies any history of hypersensitivity to food or medications. Anticoagulant and antiplatelet agents were not utilized. Underwent inguinal hernia repair over a decade ago. The patient denies any history of trauma, blood transfusion, poisoning, or chronic medication use. There is no history of addictive substance use. The patient also reports no other medical conditions, including anemia, epistaxis, gingival or dental bleeding, or ecchymosis.
Despite the absence of a family history of genetic disorders and no reported similar cases among other family members, the patient's routine blood tests, morphology, coagulation profile, liver and kidney functions, platelet aggregation, antiphospholipid antibodies, and thromboelastography revealed no significant abnormalities (Table 1).
| FXIII antigen (%) | INR | APTT ratio | Fibrinogen (mg/dL) | |
| Reference ranges | 75.2-154.8 | 0.80-1.20 | 24.5-31.7 | 1.80-4.00 |
| At diagnosis | 49.2 | 1.06 | 29.6 | 5.3 |
| Last blood sample | 37.7 | 0.98 | 27.3 | 5.14 |
The patient is alert and fully responsive. Nutritional status is robust, with normal developmental progress. There are no indications of anemia, jaundice, cyanosis, or edema. Vital signs are as follows: Body temperature 36.8 °C, pulse rate 72 beats per minute, respiratory rate 18 breaths per minute, and blood pressure 120/75 mmHg. No evidence of dyspnea or palpitations is observed.
Notably, FXIII antigen testing indicated levels below the normal range (49.2%, reference range 75.2%-154.8%). Following informed consent from the spouse, peripheral venous blood samples from the patient, his brother, and son were submitted to Di’an Medical Laboratory for whole-exome sequencing. This analysis revealed a homozygous mutation in the ACE gene c.140A>G (p.Gln47Arg), confirmed through sanger sequencing. While the patient exhibited heterozygosity for this mutation, his brother and son tested negative, indicating an acquired condition (Table 2).
| Patient | Reference ranges | |
| Coagulation-active factor VII (%) | 90.8 | 50-150 |
| Coagulation-active factor VIII (%) | 170.7 | 50-150 |
| Antithrombin III activity (%) | 100.5 | 70-125 |
| Fibrinolytic-antifibrinolytic complex (ng/mL) | 1.14 | < 0.85 |
| Thrombin regulatory protein antigen (IU/mL) | 12.08 | 3.82-13.35 |
| Platelet count (x109/L) | 352 | 125-350 |
| Protein C activity (%) | 104.7 | 70-140 |
| Protein S Activity (%) | 113.5 | 55-130 |
| IgG (mg/dL) | 9.63 | 8.60-17.40 |
| IgA (mg/dL) | 1.94 | 1.00-4.20 |
| IgM (mg/dL) | 0.63 | 0.30-2.20 |
| C3 (mg/dL) | 0.70-1.40 | 1.1 |
| C4 (mg/dL) | 0.10-0.40 | 0.32 |
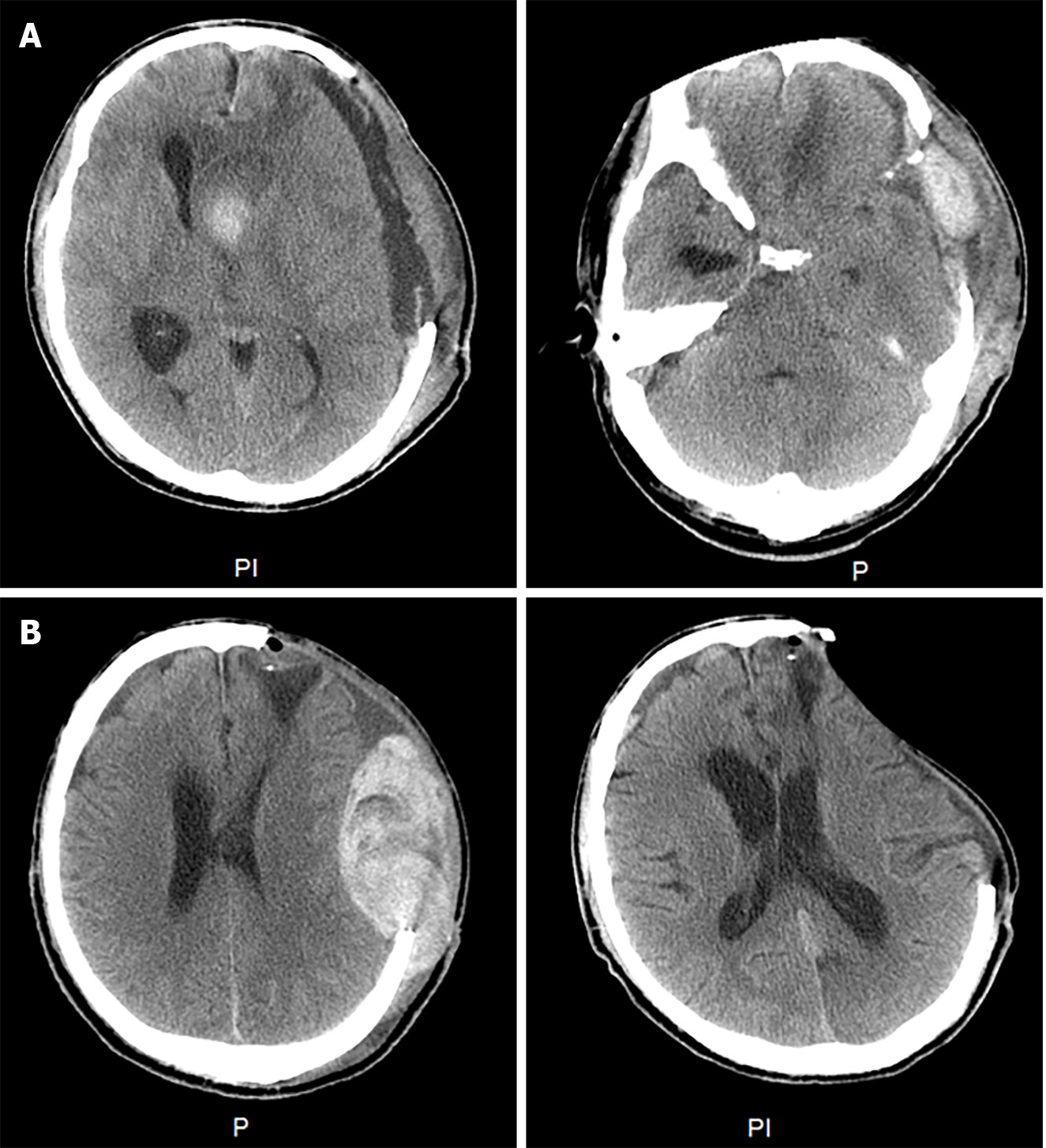
On May 24, 2023, a neuro endoscopic evacuation of a left temporal lobe hematoma was performed on the patient. However, on June 5, 2023, bilateral mydriasis developed, and a head computed tomography (CT) scan revealed significant brain swelling and midline shift, necessitating an emergency craniotomy for hematoma evacuation and left-sided decompressive craniectomy. Subsequently, the patient experienced postoperative fever, and lumbar puncture indicated intracranial infection, which was managed with meropenem and vancomycin. On June 6, a subcutaneous hematoma emerged, leading to a left temporal extradural hematoma evacuation. Despite the procedure’s repetition on June 10 due to recurrence, the patient’s consciousness deteriorated on June 16 (Glasgow coma scale score E1V1M3), exhibiting unequal pupils and sluggish light reflex. A CT scan revealed increased subcutaneous effusion and pronounced rightward midline shift, indicative of local brain herniation. Another hematoma evacuation was conducted, followed by ongoing intensive care unit management (Figure 1A).
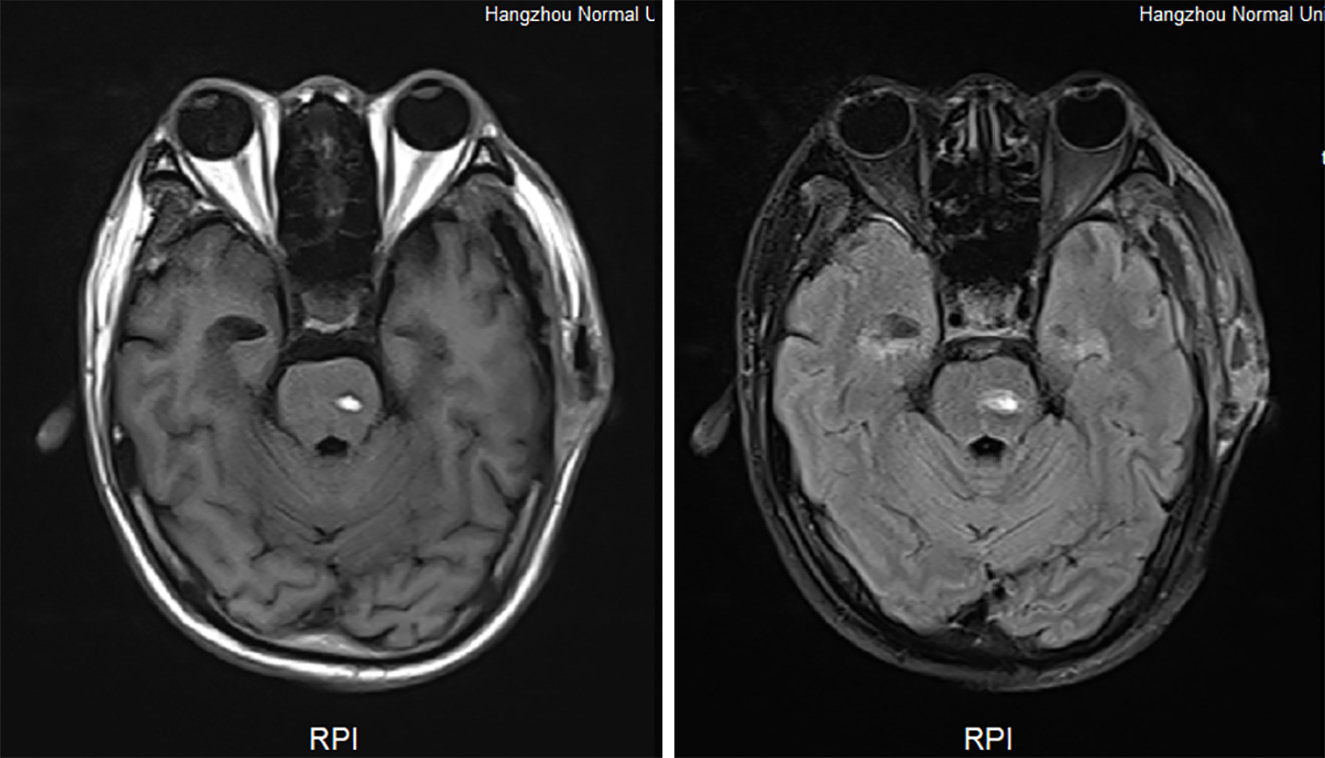
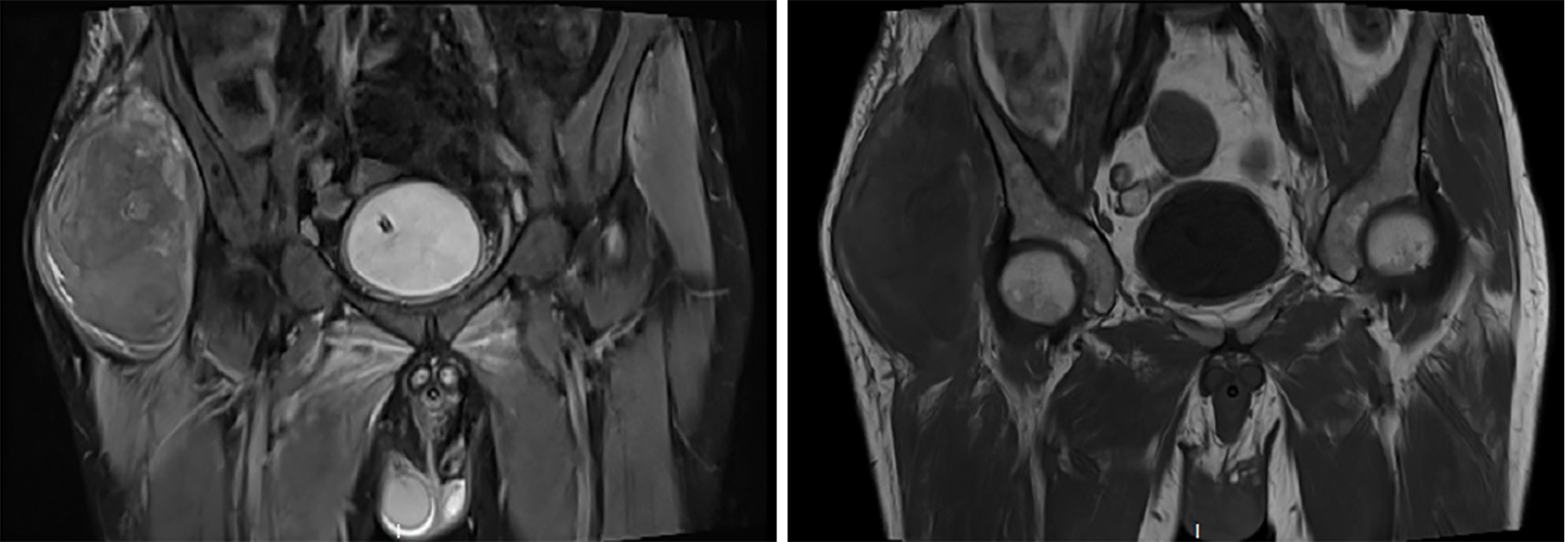
During hospitalization, a decline in the patient’s hemoglobin levels was observed, and on June 30, swelling at the right hip incision site was noted. Subsequent CT imaging of the hip revealed a hematoma, while a head magnetic resonance imaging unveiled new brainstem hemorrhage, subdural/epidural hematoma at the left temporoparietal region, bleeding in the anterior horn of the left lateral ventricle, and ventricular hemorrhage. Hemostatic and plasma transfusion therapies were administered to address these conditions (Figure 2 and Figure 3). Following up on July 16, a repeat head CT scan revealed increased subcutaneous bleeding in the right surgical area and a slight rise in midline shift to the right. Intermittent transfusions of cryoprecipitate were provided to enhance coagulation (Figure 1B).
Given the clinical presentation and genetic analysis results, the diagnosis of acquired FXIII deficiency was established for the patient.
As part of the treatment regimen, weekly infusions of 10 units of cryoprecipitate were administered as prophylaxis against future bleeding episodes. From July 19, 2023, through December 29, 2023.
Subsequently, the patient exhibited improved consciousness, characterized by clear mentation and restored mobility.
Acquired FXIII deficiency originates from the production of FXIII inhibitors by autoantibodies targeting FXIII[6]. This autoimmune phenomenon typically emerges in individuals of middle to advanced age, with a tendency towards onset in older individuals and no apparent gender predilection. Patients afflicted with acquired FXIII deficiency, characterized by the presence of FXIII inhibitors, are predisposed to severe episodes of bleeding. These inhibitors can hinder the activity, function, or alter the reactivity of fibrin substrates by obstructing the activation of FXI[7]. Consequently, this interference results in abnormal cross-linking between fibrin monomers, leading to the formation of unstable plasma fibrin clots. Clinically, this manifests as severe and recurrent spontaneous bleeding, spanning various anatomical sites[8]. Common presentations encompass bruising of the skin and mucosa, hematoma formation in soft tissues and muscles, visceral bleeding, postoperative bleeding delays, and, in severe instances, intracranial hemorrhage[9]. The wide array of bleeding manifestations underscores the intricate nature of acquired FXIII deficiency, underscoring the necessity for clinicians to exercise vigilance in identifying and managing this condition[10,11]. The profound impact on hemostasis, demonstrated by the instability of fibrin clots, mandates a multidisciplinary approach for accurate diagnosis, treatment, and ongoing patient care[12].
This case highlights a distinct occurrence of acquired FXIII deficiency, an uncommon hematological anomaly often eclipsed by more prevalent coagulation disorders. FXIII serves as a pivotal enzyme in the clotting cascade, crucial for fibrin cross-linkage and clot stabilization. Deficiency in FXIII can precipitate severe and recurring episodes of bleeding, mirroring the observations in our patient[13]. The patient’s recurrent intracranial hemorrhages, notably following surgical procedures, alongside the emergence of a right hip hematoma in the absence of significant medical or familial bleeding history, presented a diagnostic conundrum. The lack of conventional risk factors such as hypertension, diabetes, or prior cerebrovascular ailments further added complexity to the clinical scenario. The persistent recurrence of hemorrhagic events despite surgical interventions and routine postoperative management suggested an underlying coagulopathic condition[14].
In the context of FXIII deficiency, intracranial hemorrhage poses a particularly grave risk due to its high mortality and morbidity rates[15]. The patient’s deteriorating neurological status, marked by declining Glasgow coma scale scores and pupil asymmetry, warranted multiple neurosurgical interventions. The persistence of hemorrhagic events despite these interventions underscored the necessity for reevaluation of diagnosis and treatment strategies. The definitive diagnosis of acquired FXIII deficiency was established through genetic analysis, revealing a homozygous mutation in the ACE gene. Notably, this mutation is not conventionally associated with coagulation disorders, suggesting a unique phenotype or a secondary mutation influencing FXIII levels. The patient’s heterozygous genotype and the absence of the mutation in his immediate family members imply an acquired rather than an inherited etiology[16].
The management of acquired FXIII deficiency predominantly revolves around FXIII replacement therapy. Our patient exhibited a favorable response to prophylactic cryoprecipitate transfusions, which provide a concentrated source of FXIII[17]. The cessation of hemorrhagic episodes following the initiation of this treatment underscores the critical role of timely diagnosis and appropriate therapeutic interventions in such clinical scenarios. Additionally, this case prompts critical inquiry into the necessity of screening for coagulation disorders in individuals presenting with unexplained intracranial hemorrhage[18]. Existing guidelines typically do not advocate for routine coagulation factor assessments in the absence of suggestive clinical history[19]. Nonetheless, this case serves as a poignant reminder that rare coagulopathies may manifest even in patients lacking significant bleeding antecedents or identifiable risk factors.
Moreover, the treatment of individuals afflicted with acquired FXIII deficiency necessitates a multidisciplinary strategy, engaging hematologists, neurosurgeons, and critical care experts. Balancing the risk of recurrent hemorrhage, the prospect of immune-mediated inhibitor formation, and the requirement for sustained prophylactic measures demands meticulous consideration of the patient's holistic well-being and quality of life[20].
In conclusion, this case underscores the imperative of contemplating acquired coagulopathies in individuals experiencing unexplained and recurrent hemorrhagic episodes. Furthermore, it underscores the value of thorough genetic analysis in uncovering uncommon coagulation anomalies, which can substantially influence patient care and prognosis. Subsequent investigations could delve into elucidating the pathophysiological mechanisms underpinning mutations in the ACE gene and their interplay with coagulation pathways[21].
Acquired FXIII deficiency, albeit uncommon, warrants consideration in individuals presenting with inexplicable hemorrhagic episodes. Genetic testing emerges as pivotal in the diagnosis and therapeutic approach to such instances.
| 1. | Inbal A, Lubetsky A, Krapp T, Castel D, Shaish A, Dickneitte G, Modis L, Muszbek L, Inbal A. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94:432-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 48] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Dorgalaleh A. The History of Factor XIII Deficiency. Semin Thromb Hemost. 2024;50:34-42. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Yan MTS, Rydz N, Goodyear D, Sholzberg M. Acquired factor XIII deficiency: A review. Transfus Apher Sci. 2018;57:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Sandouno TM, Bachir H, Alaoui HB, Hamaz S, Eloumri AA, Berrimi M, Serraj K. Acute spontaneous subdural hematoma as an inaugural presentation of systemic lupus erythematosus with acquired factor XIII deficiency: a case report. Pan Afr Med J. 2021;39:207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Ichinose A; Japanese Collaborative Research Group on AH13. Autoimmune acquired factor XIII deficiency due to anti-factor XIII/13 antibodies: A summary of 93 patients. Blood Rev. 2017;31:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Aljabry M. Factor XIII deficiency in the Saudi population, an underestimated bleeding risk. Review article and an illustrative case report with dental complications. Saudi Dent J. 2023;35:305-309. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Di Micco P, Gussoni G, Pieralli F, Campanini M, Dentali F, Fontanella A. Acquired Factor XIII Deficiency Inducing Recurrent and Fatal Bleeding, Description of a Case. J Blood Med. 2020;11:43-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Benedetto C, Marozio L, Tavella AM, Salton L, Grivon S, Di Giampaolo F. Coagulation disorders in pregnancy: acquired and inherited thrombophilias. Ann N Y Acad Sci. 2010;1205:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Schroeder V, Kohler HP. Factor XIII deficiency: an update. Semin Thromb Hemost. 2013;39:632-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É. Factor XIII: a coagulation factor with multiple plasmatic and cellular functions. Physiol Rev. 2011;91:931-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 11. | Fadoo Z, Merchant Q, Rehman KA. New developments in the management of congenital Factor XIII deficiency. J Blood Med. 2013;4:65-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Hethershaw EL, Cilia La Corte AL, Duval C, Ali M, Grant PJ, Ariëns RA, Philippou H. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J Thromb Haemost. 2014;12:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Schroeder V, Kohler HP. New developments in the area of factor XIII. J Thromb Haemost. 2013;11:234-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Ivaskevicius V, Windyga J, Baran B, Schroeder V, Junen J, Bykowska K, Seifried E, Kohler HP, Oldenburg J. Phenotype-genotype correlation in eight Polish patients with inherited Factor XIII deficiency: identification of three novel mutations. Haemophilia. 2007;13:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Marco A, Marco P. Autoimmune Acquired Factor XIII Deficiency: A Case Report. J Blood Med. 2021;12:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Muszbek L, Pénzes K, Katona É. Auto- and alloantibodies against factor XIII: laboratory diagnosis and clinical consequences. J Thromb Haemost. 2018;16:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Ogawa Y. [Acquired autoimmune coagulation factor XIII/13 deficiency]. Rinsho Ketsueki. 2020;61:799-808. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 18. | Ariëns RA, Lai TS, Weisel JW, Greenberg CS, Grant PJ. Role of factor XIII in fibrin clot formation and effects of genetic polymorphisms. Blood. 2002;100:743-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Ejaz M, Saleem A, Ali N, Tariq F. Factor XIII deficiency with intracranial haemorrhage. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Pelcovits A, Schiffman F, Niroula R. Factor XIII Deficiency: A Review of Clinical Presentation and Management. Hematol Oncol Clin North Am. 2021;35:1171-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Shahraki H, Dorgalaleh A, Fathi M, Tabibian S, Teimourian S, Mollanoori H, Khiabani A, Zaker F. How to Assess Founder Effect in Patients with Congenital Factor XIII Deficiency. Int J Hematol Oncol Stem Cell Res. 2020;14:265-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |