Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.389
Revised: August 20, 2024
Accepted: September 2, 2024
Published online: September 28, 2024
Processing time: 162 Days and 9.4 Hours
Osteoid osteoma is a benign bone tumor with characteristic clinical symptomatology. The selected method for its treatment is percutaneous radiofrequency ablation. However, percutaneous cryoablation is an alternative method with certain advantages.
To evaluate percutaneous computed tomography (CT)-guided cryoablation for the treatment of osteoid osteoma in young patients and adults.
A total of 25 patients were treated with percutaneous CT- guided cryoablation for osteoid osteomas between October 2020 and March 2023 at a single institution. All patients were above 14-years-old (mean age, 24-years-old), and all procedures were performed under local anesthesia. Of the 25 patients, 8 were female and 17 were male. Tumor sites included the femur (n = 9), medial malleolus (n = 4), sacral ala (n = 4), facets (n = 4), humerus (n = 3), and tibia (n = 1). One cryoprobe was used in each procedure and, when possible, the lesion was covered by the ice-ball using an extraosseous position without penetrating the nidus. All necessary ther
All patients treated had complete response (100% clinical success rate) starting on the day of the procedure. Technical success was achieved in all cases. Visual analog scale (VAS) scores at 1 year were 0, compared to a mean VAS score of 8.5 ± 1 (SD) before the procedure. No recurrences were reported at the 1-year follow-up and no complications were observed. In 11/25 cases, an extraosseous position of the cryoprobe was used with less procedural time achieving technical and clinical success and no complications with less patient discomfort. All patients were discharged from the hospital on the same day as the procedure.
Cryoablation of osteoid osteomas is an efficacious and safe procedure with durable clinical results. Its greatest advantage is that the procedure can be performed under local anesthesia using an extraosseous position of the cryoprobe when possible.
Core Tip: This study aims to evaluate percutaneous cryoablation for the treatment of osteoid osteomas. Our results prove that cryoablation is safe and effective as a treatment option for these tumors and has certain advantages over other ablative methods. Cryoablation has the advantage of extraosseous positioning of the cryoprobe, avoiding bone drilling, less procedural and post-procedural pain, making it possible for the procedure to be performed strictly under local anesthesia and the ability to treat larger lesions with a single probe placement.
- Citation: Michailidis A, Panos A, Samoladas E, Dimou G, Mingou G, Kosmoliaptsis P, Arvaniti M, Giankoulof C, Petsatodis E. Cryoablation of osteoid osteomas: Is it a valid treatment option? World J Radiol 2024; 16(9): 389-397
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/389.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.389
Osteoid osteoma is a benign bone tumor, ranking third in frequency among bone tumors and accounting for about 14% of all cases. The first case was described in 1930, and it was recognized as a distinct entity in 1935[1]. This tumor predominantly occurs during the second decade of life, affecting males four times more often than females. Patients typically present with dull, night-time pain that subsides after taking aspirin or non-steroidal anti-inflammatory drugs (NSAIDs)[2]. The tumor consists of a nidus surrounded by thick sclerotic bone, and the nidus is formed by prostaglandin-producing cells. Although the exact pain mechanism remains unclear[3], the composition of the nidus is believed to contribute to the pain.
Osteoid osteomas are most commonly found in the cortex of the femur, tibia, and vertebral pedicles. Diagnosis is typically based on the characteristic clinical presentation of night pain and is confirmed by imaging techniques such as X-rays, computed tomography (CT), magnetic resonance imaging (MRI), or even SPECT scans[4]. CT scans are preferred for diagnosing osteoid osteomas because they clearly depict the nidus and assist in differentiating the tumor from conditions like Brodie's abscess and osteomyelitis. MRI is highly sensitive in detecting surrounding bone marrow and soft tissue edema, which can resemble tumors and osteomyelitis. Double-density scintigraphy is also effective in imaging osteoid osteomas and is often followed by a CT scan[5].
Treatment options include conservative management with analgesics or NSAIDs while awaiting spontaneous regression, and surgical excision, which comes with disadvantages like large incisions, prolonged hospitalization, and the potential need for reoperation in cases of incomplete excision. Minimally invasive techniques, such as trephine excision of the nidus and radiofrequency (RF) ablation, considered the gold standard, have been established as effective solutions that improve outcomes and offer better post-procedure recovery for patients. Percutaneous cryoablation is a well-established method for treating bone tumors and other malignancies, offering optimal oncologic outcomes[6]. Its major advantages in bone ablation include the ability to penetrate dense bone, reduced procedural pain, and the visualization of the ice ball. Additionally, cryoablation can create larger ablation volumes, which is particularly beneficial for treating osteoid osteomas with eccentric shapes, potentially improving treatment outcomes.
Our study aims to highlight the efficacy and safety of performing cryoablation under local anesthesia in a series of 25 cases of osteoid osteoma, reinforcing the validity of this method as a treatment option.
Cryoablation therapy was performed on 25 patients, all diagnosed with osteoid osteoma. These patients were initially examined at our orthopedic outpatient department before being referred to the interventional radiology department for treatment. This retrospective study was approved by the scientific committee of our hospital, which oversees research ethics, and written consent was obtained from all patients for reviewing their clinical and imaging data. The study included patients over 14-years-old with typical clinical presentation and imaging findings of osteoid osteoma. Exclusion criteria included coagulation disorders, pain of different origin, acute infection, and previous interventions for treating osteoid osteoma. One patient who had undergone two unsuccessful RF ablations for a femoral osteoid osteoma, resulting in massive periostitis, was excluded from the study.
The clinical presentation in all cases was characteristic night pain relieved by aspirin or NSAIDs. Diagnosis was confirmed through imaging, including MRI, unenhanced CT scans, and in some cases, Technetium-99 bone scans. Typical CT findings included a lucent nidus surrounded by reactive sclerotic bone. MRI showed bone edema, and intravenous gadolinium administration revealed osteoid osteoma nidus. Bone scintigraphy was used when additional diagnostic information was needed and typically showed focal uptake and the characteristic double-density sign. Of the 25 patients, 8 were female and 17 were male, with a mean age of 28-years-old (range: 15 years to 53 years). The procedures were performed between October 2020 and March 2023. Tumor sites included the femur (n = 9), medial malleolus (n = 4), sacral ala (n = 4), facets (n = 4), humerus (n = 3), and tibia (n = 1) (Table 1).
| No. | Sex | Age | Location | VAS pre | VAS post (1-12 months) | Complication |
| 1 | Female | 53 | Sacrum | 7 | 1 | Nothing |
| 2 | Female | 18 | Medial malleolus | 8 | 0 | ‘’ |
| 3 | Male | 41 | Humerus | 9 | 0 | ‘’ |
| 4 | Male | Femur | 9 | 0 | ‘’ | |
| 5 | Male | 24 | O5 facet | 7 | 0 | ‘’ |
| 6 | Male | 16 | Femur | 8 | 0 | ‘’ |
| 7 | Female | 27 | O3 facet | 8 | 1 | ‘’ |
| 8 | Female | 16 | Medial malleolus | 10 | 0 | ‘’ |
| 9 | Male | 18 | Femur | 8 | 0 | ‘’ |
| 10 | Male | 45 | Sacrum | 7 | 0 | ‘’ |
| 11 | Male | 19 | Femur | 8 | 1 | ‘’ |
| 12 | Male | 20 | Humerus | 9 | 1 | ‘’ |
| 13 | Male | 18 | Tibia | 9 | 0 | ‘’ |
| 14 | Male | 23 | Femur | 8 | 0 | ‘’ |
| 15 | Male | 18 | Humerus | 8 | 0 | ‘’ |
| 16 | Female | 51 | Sacrum | 7 | 1 | ‘’ |
| 17 | Female | 19 | Medial malleolus | 8 | 0 | ‘’ |
| 18 | Male | 44 | Humerus | 9 | 0 | ‘’ |
| 19 | Male | Femur | 9 | 0 | ‘’ | |
| 20 | Male | 26 | O4 facet | 7 | 0 | ‘’ |
| 21 | Male | 17 | Femur | 8 | 0 | ‘’ |
| 22 | Female | 29 | O5 facet | 8 | 1 | ‘’ |
| 23 | Female | 15 | Medial malleolus | 10 | 0 | ‘’ |
| 24 | Male | 19 | Femur | 8 | 0 | ‘’ |
| 25 | Male | 40 | Sacrum | 7 | 0 | ‘’ |
All procedures were performed under CT guidance with local anesthesia using 1% lidocaine at the skin entry site and the periosteum. Paracetamol (1 g) and tramadol (100-200 mg) were administered intravenously before the procedure for pain control. Prophylactic IV antibiotics (1 g cefoxitin) were given to all patients. Even patients requiring direct nidus penetration were treated under local anesthesia. The entire procedure was conducted under strictly aseptic conditions. The cryoprobe was positioned either adjacent to the lesion without penetrating the cortex or directly into the nidus with bone penetration, depending on the location of the osteoid osteoma.
To ensure complete coverage of the lesion from an extraosseous position, the cryoprobe was placed parallel to the cortex, covering the entire lesion with the ice ball (11/25 cases). In other cases, the nidus was penetrated with a bone trocar, and the cryoprobe was then coaxially inserted. The access needle was withdrawn at least 3 cm to expose the cryoprobe and prevent contact between the ice ball and the needle.
For each procedure, an IceSeed Cryoprobe 17 g or IceSphere Cryoprobe 17 g (Boston Scientific, Marlborough, MA, United States) was used, capable of creating an ice ball measuring 15 mm × 20 mm and 23 mm × 29 mm, respectively, at -20°C. A freeze-thaw-freeze protocol was applied, consisting of two 10-minute freezing cycles with an intermediate thawing cycle, divided into a 9-minute passive thaw and a 1-minute active thaw. The ice ball formation was monitored by consecutive CT scans, appearing as a hypodense area in the soft tissues, but not visible in the bone, with the ability to penetrate sclerotic bone.
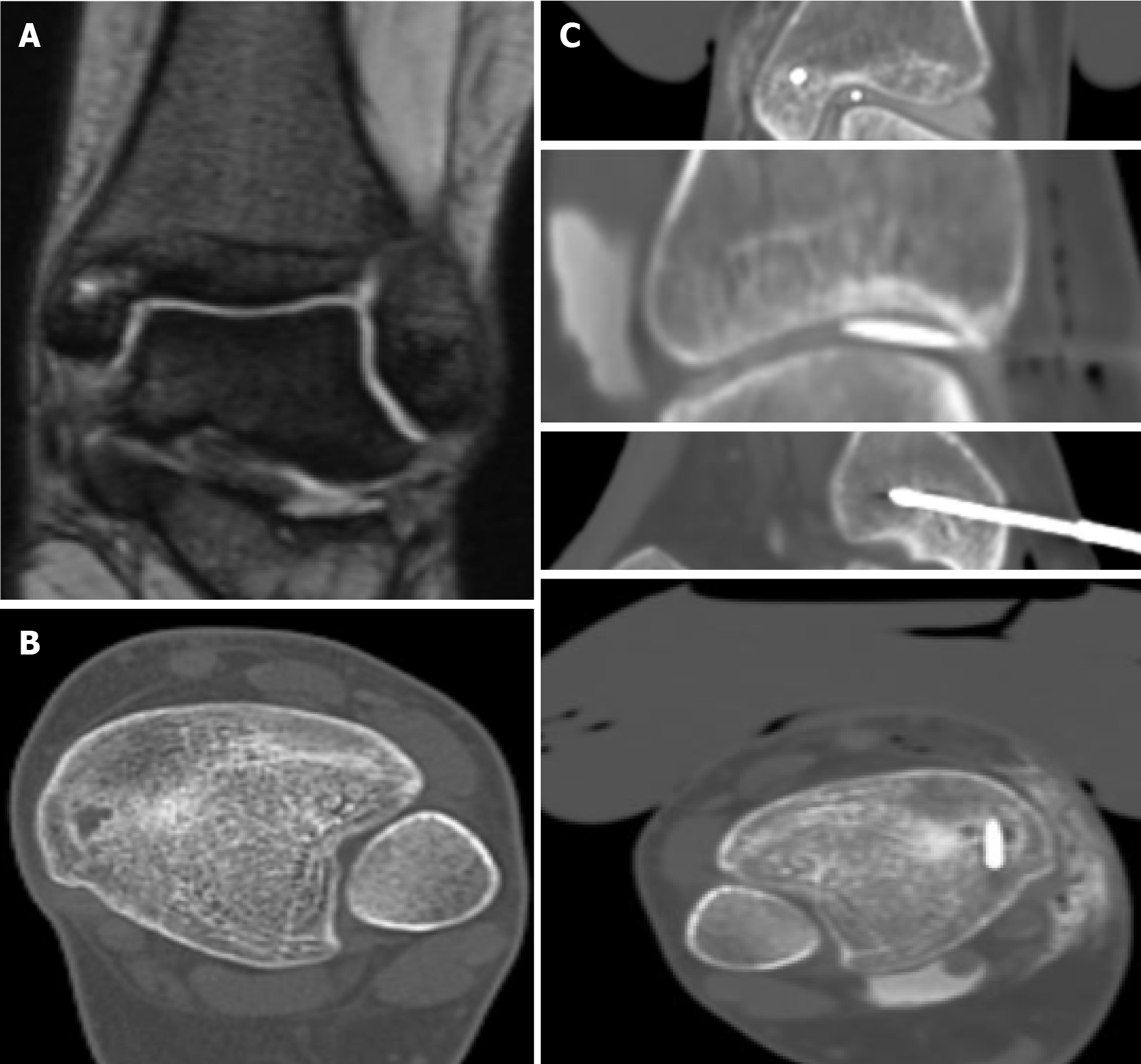
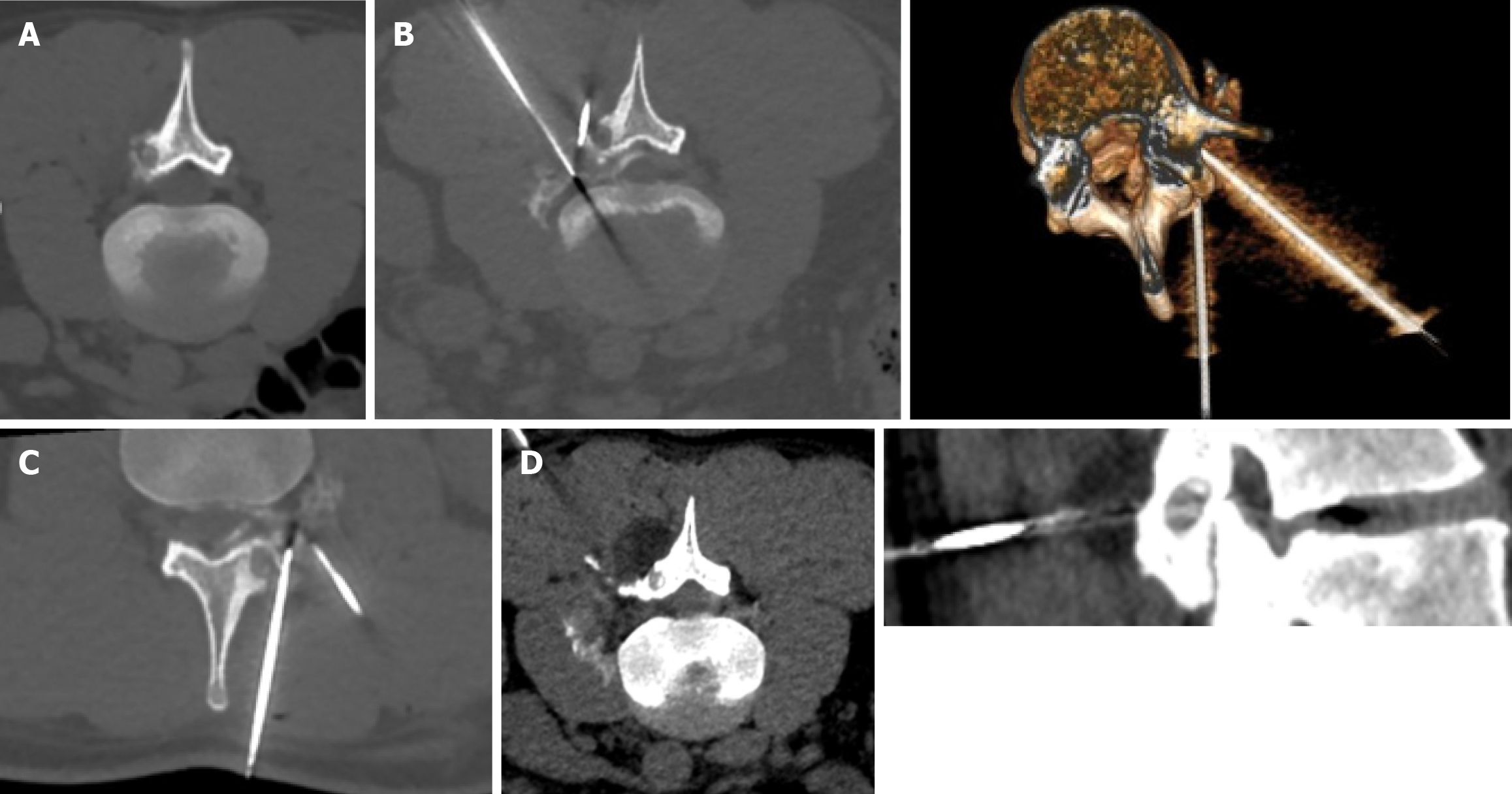
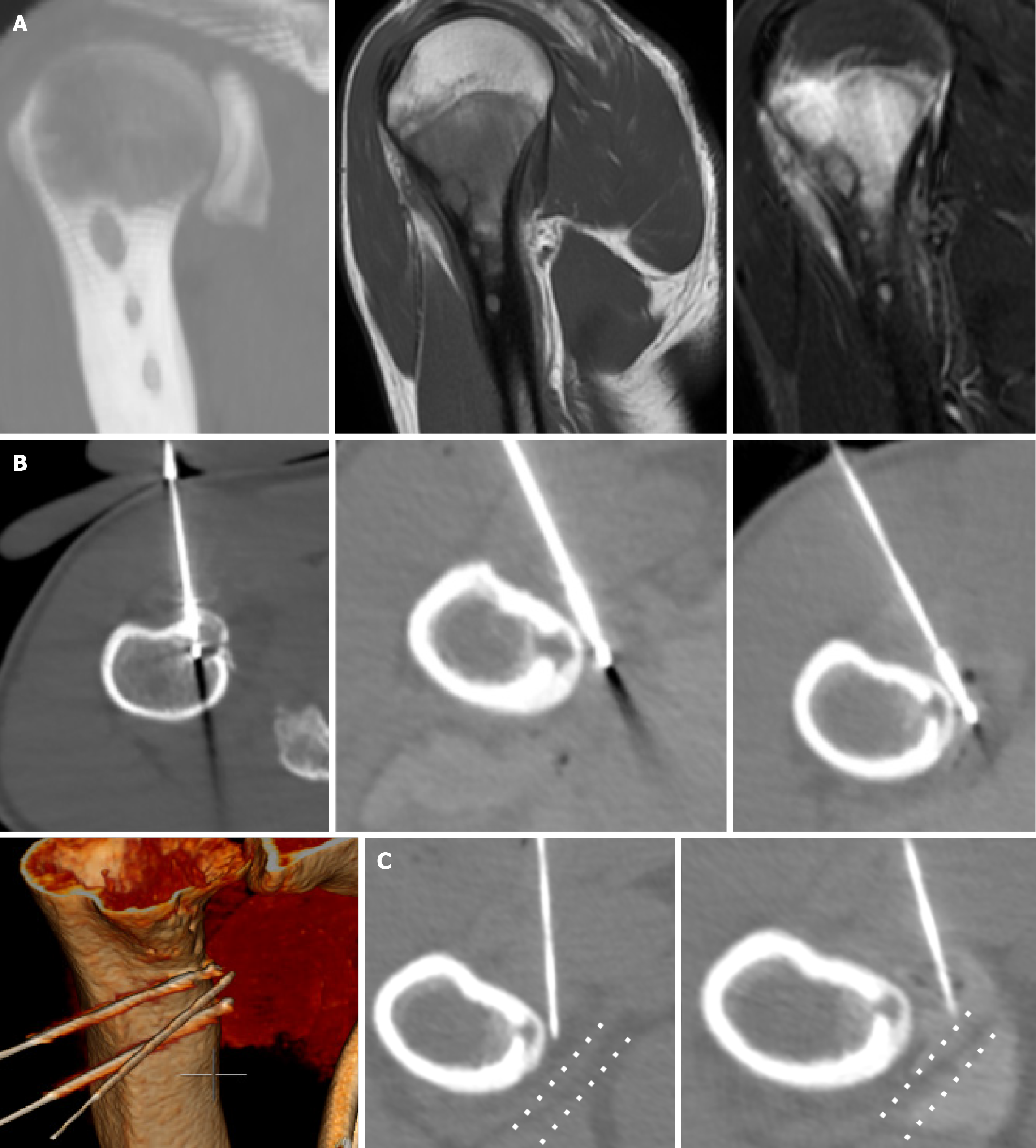
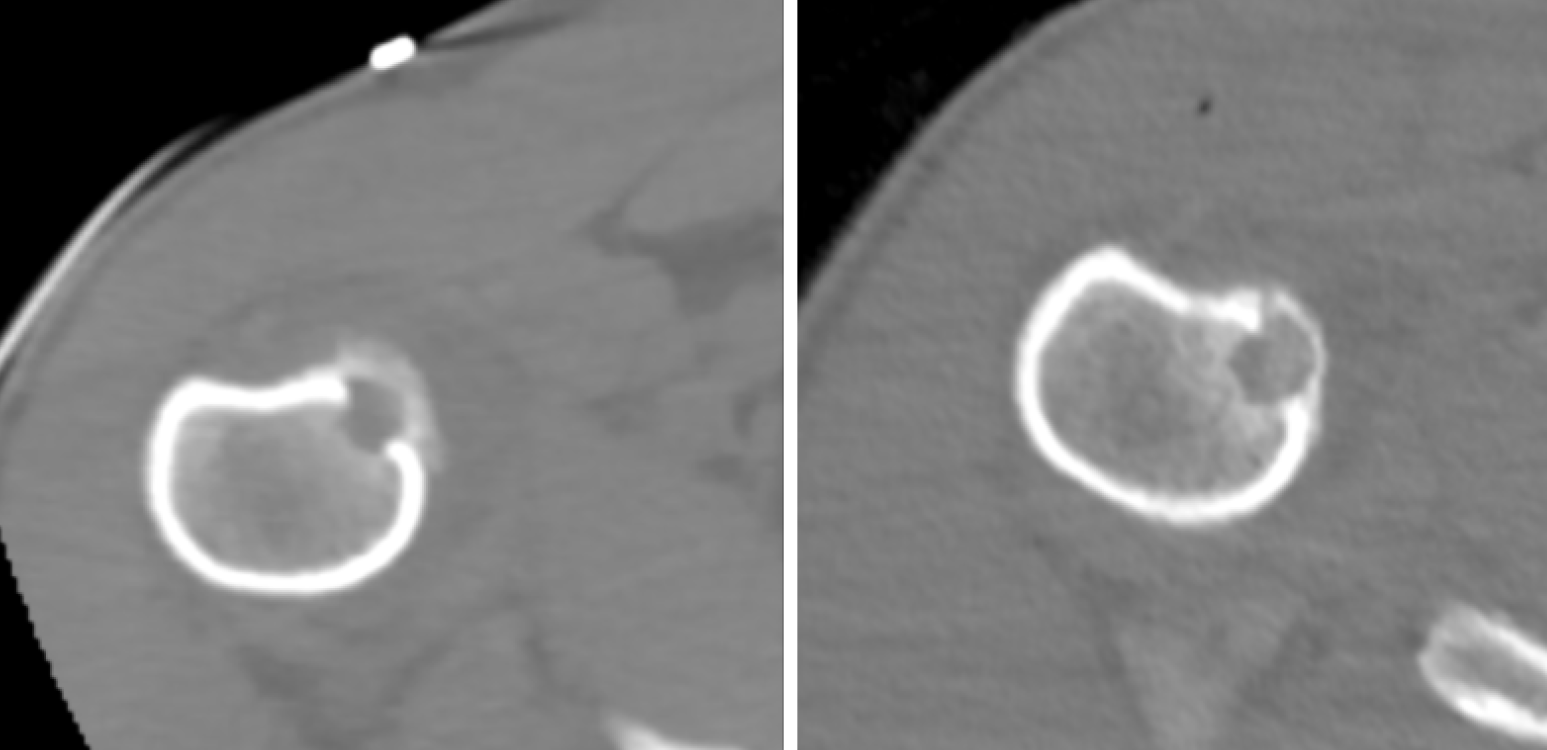
When the ice ball adequately covered the lesion and the cryosphere approached within 1 cm of a critical structure, the generator power was reduced to 60%. Depending on the case, passive and active thermoprotection techniques were applied to avoid thermal injury to adjacent sensitive structures, such as nerves, cartilage, and skin. Techniques like hydrodissection, neurostimulation, and thermocouple placement were used to monitor the temperature and function of adjacent nerve roots. A warm glove combined with hydrodissection was used for skin protection, while direct injection of warm saline was employed for joint protection (Figures 1, 2, and 3).
Performing the procedure under local anesthesia allowed communication with patients to assess any discomfort and enabled real-time nerve monitoring by asking patients to perform specific movements related to the nerve at risk. At the end of the procedure, the ice ball adequately enclosed the nidus of the osteoid osteoma. Peri- and post-procedural pain was significantly less compared to RF ablation, and all patients were able to ambulate and were discharged on the same day after the procedure.
Anti-inflammatory drugs were prescribed for 4 days, and patients were advised to refrain from heavy sports activities for 2 weeks.
All patients were clinically evaluated 1 week after the procedure to assess any complications and to evaluate their post-procedural visual analog scale (VAS) scores. Clinical data were also collected at 6 months and 1 year after the procedure. No imaging studies were conducted if patients experienced complete relief of their symptoms. Complete pain relief at 1 year was defined as clinical success.
For statistical analyses in this study, the Shapiro-Wilk test was employed to assess the normality of continuous variables. Depending on the results of this test, continuous variables were then described using either the mean (SD) or the median (IQR). Categorical variables, such as sex, were reported as frequencies and percentages.
To evaluate differences in outcomes over time, such as pain levels before and after treatment, the Wilcoxon signed-rank test was used for comparing related samples. Additionally, generalized estimating equation (referred to as GEE) models were applied to examine changes in pain and other variables at different time points. The GEE models are particularly advantageous for analyzing longitudinal non-normal data, as they utilize all available data for each individual.
Statistical significance was determined using a P-value threshold of less than 0.05. The analyses were performed using IBM SPSS 27.0 (IBM Corp., Armonk, NY, United States).
Technical success was achieved in all cases, with the ice ball adequately covering the lesion. All procedures were performed under local anesthesia. An extraosseous position of the cryoprobe was used in 11 out of 25 cases (Figure 4). Biopsies were performed in only 2 cases where the nidus of the osteoid osteoma was larger than 1 cm.
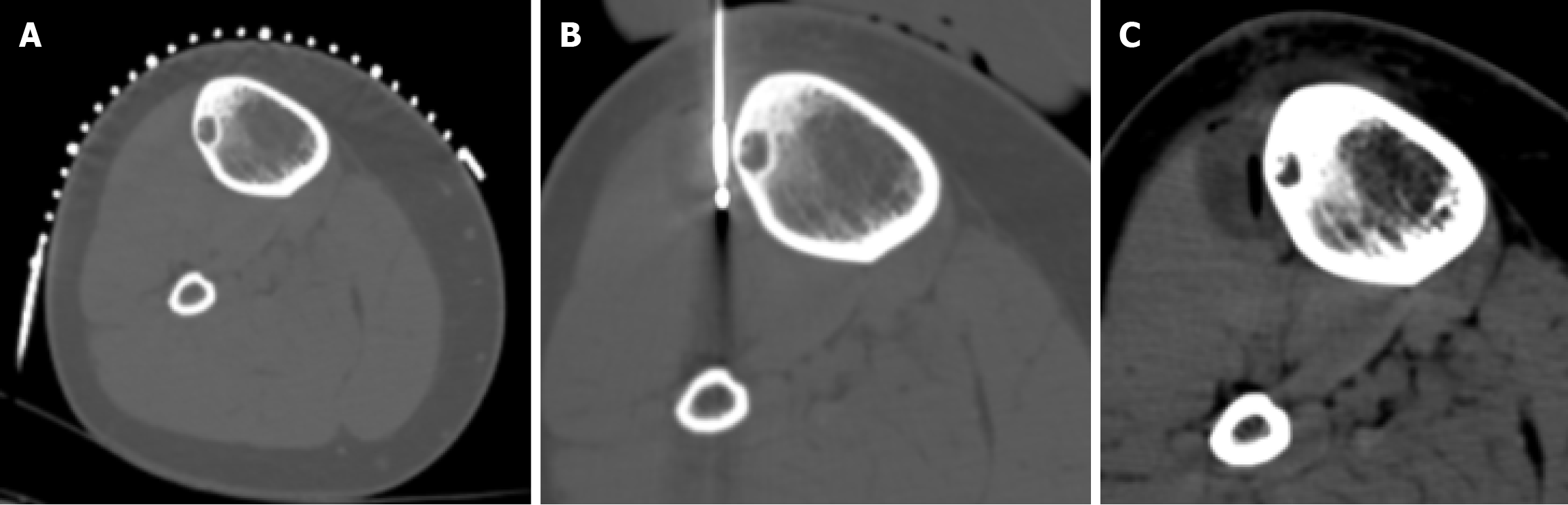
Patient data were collected at 1 month, 6 months, and 1 year after the procedure, with all cases having at least a 1-year follow-up. All patients experienced complete pain relief starting the day after the procedure and reported no discomfort during follow-up. VAS scores at 1 year were 0, compared to a mean VAS score of 8.5 ± 1 (SD) before the procedure (Figure 5). No recurrences were reported at the 1-year follow-up, and no complications were observed.
All patients reported that they tolerated the procedure very well, with no discomfort and no post-procedural issues.
We also compared the results of the 11 cases where an extraosseous position was used vs the 14 cases where an intraosseous position of the cryoprobe was positioned via bone penetration. Technical success was achieved in both groups. The procedural time was similar between the intraosseous group and the subset of extraosseous positioning cases where hydrodissection was used [60 ± 10 (SD) minutes]. However, in the 4/11 extraosseous positioning cases where hydrodissection was not required, the procedural time was significantly faster [30 ± 10 (SD) minutes]. Neither group reported complications with minimal periprocedural pain, but patients in the extraosseous group reported less proce
Osteoid osteoma, a benign bone tumor, presents a unique challenge in clinical management. The current study explores the efficacy and safety of percutaneous CT-guided cryoablation as a treatment option for osteoid osteomas in adults. The findings reveal compelling evidence supporting the validity of cryoablation in this context. The primary outcome of the study was a 100% clinical success rate, and it underscores the effectiveness of cryoablation in treating osteoid osteomas. This aligns with previous research that has demonstrated the ability of cryoablation to induce complete response and relief from the characteristic night pain associated with osteoid osteomas.
The excellent outcomes of cryoablation have been highlighted in other studies, as well. Le Corroller et al[7] presented a series of 50 cases that treated osteoid osteoma with cryoablation techniques. The patients had no post-procedural pain, VAS scores decreased from 8 before the procedure to 0 at 6-months follow-up, and had only 1 case that cryoablation failed due to bad positioning of the probe. Coupal et al[8] reported similar outcomes in a 10-case series. All patients had reduced pain after the procedure and in the follow-up. All cases had technical and clinical success, and no complications were reported. Finally, Whitmore et al[9] reported 29 cases that included adults and children. They achieved 100% technical success rate and had long-term clinical outcomes of cryoablation around 90%. The high technical success rate further solidifies cryoablation as a reliable therapeutic option for this particular benign bone tumor.
An intriguing aspect of the study is the utilization of local anesthesia and the preference for an extraosseous position of the cryoprobe. These choices contribute to the overall patient experience and also underscore the adaptability of cryoablation in diverse clinical settings. Performing the procedure under local anesthesia is a notable advancement[10], potentially reducing the procedural burden on patients and minimizing the need for general anesthesia, which may be associated with additional risks.
The deliberate selection of an extraosseous position for the cryoprobe, without penetrating the nidus when possible, adds a layer of sophistication to the technique. This approach enhances the safety profile of the procedure and highlights the precision achievable with CT guidance. The ability to tailor the procedure to the individual characteristics of the lesion, considering its location and morphology, speaks to the versatility of cryoablation.
The cryoprobe was inserted at an extraosseous position when the lesion was accessible from outside the bone and not deeply embedded within it. This approach offers several advantages, such as avoiding bone penetration, which reduces procedural pain and shortens the procedural time. By not penetrating the bone, the risk of complications associated with bone damage is minimized. Additionally, this technique can effectively treat the lesion if the ice ball adequately covers the tumor and if any critical structures outside the bone are properly protected with techniques like hydrodissection and temperature monitoring, particularly for nearby nerves.
In the study, 11 patients underwent cryoablation with the cryoprobe positioned at an extraosseous location, while the remaining patients had the probe inserted intraosseously. Both groups achieved complete pain relief, with no reported recurrences or complications at the 1-year follow-up. The patients treated with extraosseous positioning benefited from reduced procedural pain and shorter procedural times due to the non-invasive nature of this approach. The main challenge with the extraosseous technique is ensuring that the ice ball adequately covers the lesion while also protecting surrounding anatomical structures. However, in this study, proper protection of critical structures, such as nerves, was achieved using hydrodissection and temperature monitoring, leading to successful outcomes without clinical implications.
Overall, both techniques were effective, but the extraosseous approach offers advantages in terms of reduced invasiveness and patient comfort, provided that adequate precautions are taken to protect nearby structures. This point highlights the versatility of cryoablation techniques and their ability to be tailored to the specific characteristics of each lesion.
An important consideration in any interventional procedure is its safety profile. In this study, the absence of reported complications and the same-day discharge of patients following cryoablation speak to the safety and minimal inva
Drawing comparisons with established treatment modalities for osteoid osteomas enriches the discussion. While surgical excision[12], trephine excision, and RF ablation have been cornerstones in the management of osteoid osteomas, cryoablation and RF ablation are both effective techniques for treating osteoid osteomas with distinct advantages. Cryoablation is particularly noted for its lower periprocedural and postprocedural pain[13]. This is largely due to the anesthetic effect of the cold temperatures used during the procedure, which can significantly reduce discomfort. Another key advantage of cryoablation is the ability to visualize the ablation zone in real-time via imaging, as the "ice ball" formed around the cryoprobe is clearly visible. This visualization ensures precise targeting of the lesion and helps protect surrounding tissues. Moreover, cryoablation allows for the treatment of larger lesions by creating larger ablation zones. This is extremely useful in osteoid osteomas with excessive periosteal reaction and eccentric shape, which is an in
In contrast, RF ablation offers the advantage of faster ablation times and a more established track record, with extensive long-term data supporting its use[15]. However, it lacks the ability to visualize the ablation zone as clearly as cryoablation and generally requires direct bone penetration, which may contribute to higher periprocedural pain[16]. RF ablation also lacks the ability of treating larger lesions. In such cases, the RF probe must be repositioned after the first treatment or multiple RF probes should be used. This comparative analysis suggests that cryoablation could offer unique advantages in specific clinical scenarios.
The clinical implications of these findings extend beyond statistical significance. Cryoablation is efficacious and appears to positively impact the patient experience. Considering its application in young patients and adults, the discussion should explore how cryoablation may influence recovery, hospital stay, and overall quality of life. The study prompts further investigation into age-related considerations and variations in treatment response.
As with any study, limitations exist. Acknowledging these limitations, such as the relatively small sample size and the absence of long-term follow-up, enhances the transparency of the research. Identifying areas for further research, such as larger-scale trials, extended follow-up periods, or comparative studies against alternative treatments[17,18], provides a roadmap for future investigations.
The study's findings robustly support cryoablation as an efficacious and safe treatment option for osteoid osteomas in adults. The emphasis on local anesthesia, extraosseous positioning, and the meticulous attention to safety considerations contribute to the evolving landscape of interventional radiology. While further research is warranted to establish the long-term efficacy and comparative advantages of cryoablation, this study marks a significant step forward in expanding the therapeutic options available for managing osteoid osteomas in adult patients.
| 1. | Jaffe HL. "OSTEOID-OSTEOMA". Arch Surg. 1935;31:709. [RCA] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 394] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Tepelenis K, Skandalakis GP, Papathanakos G, Kefala MA, Kitsouli A, Barbouti A, Tepelenis N, Varvarousis D, Vlachos K, Kanavaros P, Kitsoulis P. Osteoid Osteoma: An Updated Review of Epidemiology, Pathogenesis, Clinical Presentation, Radiological Features, and Treatment Option. In Vivo. 2021;35:1929-1938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Onashvili N, Loria G, Gogichaishvili T, Mariamidze A, Sirbiladze M, Sainishvili N. Computed tomography guided radiofrequency ablation of multifocal osteoid osteoma. Radiol Case Rep. 2020;15:1275-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Çakar M, Esenyel CZ, Seyran M, Tekin AÇ, Adaş M, Bayraktar MK, Coşkun Ü. Osteoid osteoma treated with radiofrequency ablation. Adv Orthop. 2015;2015:807274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Lee EH, Shafi M, Hui JH. Osteoid osteoma: a current review. J Pediatr Orthop. 2006;26:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 6. | Parmeggiani A, Martella C, Ceccarelli L, Miceli M, Spinnato P, Facchini G. Osteoid osteoma: which is the best mininvasive treatment option? Eur J Orthop Surg Traumatol. 2021;31:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Le Corroller T, Vives T, Mattei JC, Pauly V, Guenoun D, Rochwerger A, Champsaur P. Osteoid Osteoma: Percutaneous CT-guided Cryoablation Is a Safe, Effective, and Durable Treatment Option in Adults. Radiology. 2022;302:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Coupal TM, Mallinson PI, Munk PL, Liu D, Clarkson P, Ouellette H. CT-guided percutaneous cryoablation for osteoid osteoma: initial experience in adults. AJR Am J Roentgenol. 2014;202:1136-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Whitmore MJ, Hawkins CM, Prologo JD, Marshall KW, Fabregas JA, Yim DB, Monson D, Oskouei SV, Fletcher ND, Williams RS. Cryoablation of Osteoid Osteoma in the Pediatric and Adolescent Population. J Vasc Interv Radiol. 2016;27:232-7; quiz 238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Pusceddu C, Vergantino E, Santucci D, Marsico S, Cappucci M, Vaccarino F, Beomonte Zobel B, Grasso RF, Faiella E. Percutaneous Cryoablation under Conscious Sedation: A Safe, Effective and Painless Option for the Treatment of Pediatric Osteoid Osteoma. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Wu B, Xiao YY, Zhang X, Zhao L, Carrino JA. CT-guided percutaneous cryoablation of osteoid osteoma in children: an initial study. Skeletal Radiol. 2011;40:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Shu M, Ke J. The surgical management of osteoid osteoma: A systematic review. Front Oncol. 2022;12:935640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Baal JD, Pai JS, Chen WC, Joseph GB, O'Donnell RJ, Link TM. Factors Associated with Osteoid Osteoma Recurrence after CT-Guided Radiofrequency Ablation. J Vasc Interv Radiol. 2019;30:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Santiago E, Pauly V, Brun G, Guenoun D, Champsaur P, Le Corroller T. Percutaneous cryoablation for the treatment of osteoid osteoma in the adult population. Eur Radiol. 2018;28:2336-2344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Vogl TJ, Bialek M, Eichler K, Hammerstingl R, Bielfeldt J, Zangos S, Scholtz JE, Adwan H. Short- and Long-Term Outcomes after Radiofrequency Ablation of Osteoid Osteomas. J Pers Med. 2024;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 16. | Motamedi D, Learch TJ, Ishimitsu DN, Motamedi K, Katz MD, Brien EW, Menendez L. Thermal ablation of osteoid osteoma: overview and step-by-step guide. Radiographics. 2009;29:2127-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Shanmugasundaram S, Nadkarni S, Kumar A, Shukla PA. Percutaneous Ablative Therapies for the Management of Osteoid Osteomas: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2021;44:739-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Tsoumakidou G, Thénint MA, Garnon J, Buy X, Steib JP, Gangi A. Percutaneous Image-guided Laser Photocoagulation of Spinal Osteoid Osteoma: A Single-Institution Series. Radiology. 2016;278:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |