Published online Sep 28, 2024. doi: 10.4329/wjr.v16.i9.380
Revised: August 8, 2024
Accepted: August 22, 2024
Published online: September 28, 2024
Processing time: 163 Days and 14.5 Hours
Prostatic artery embolization (PAE) is a promising but also technically demanding interventional radiologic treatment for symptomatic benign prostatic hyperplasia. Many technical challenges in PAE are associated with the complex anatomy of prostatic arteries (PAs) and with the systematic attempts to catheterize the PAs of both pelvic sides. Long procedure times and high radiation doses are often the result of these attempts and are considered significant disadvantages of PAE. The authors hypothesized that, in selected patients, these disadvantages could be mitigated by intentionally embolizing PAs of only one pelvic side.
To describe the authors’ approach for intentionally unilateral PAE (IU-PAE) and its potential benefits.
This was a single-center retrospective study of patients treated with IU-PAE during a period of 2 years. IU-PAE was applied in patients with opacification of more than half of the contralateral prostatic lobe after angiography of the ipsi
IU-PAE was performed in a total 13 patients (subgroup A, n = 7; subgroup B, n = 6). Dose-area product, fluoroscopy time and operation time in the IU-PAE group (9767.8 μGy∙m2, 30.3 minutes, 64.0 minutes, respectively) were significantly shorter (45.4%, 35.9%, 45.8% respectively, P < 0.01) compared to the control group. Clinical and imaging outcomes did not differ significantly between the IU-PAE group and the control group. In the 2 clinical failures of IU-PAE (both in subgroup A), the extent of prostatic infarction (demonstrated by iCEUS) was significantly smaller compared to the rest of the IU-PAE group.
In selected patients, IU-PAE is associated with comparable outcomes, but with lower radiation exposure and a shorter procedure compared to bilateral PAE. iCEUS could facilitate patient selection for IU-PAE.
Core Tip: In this retrospective study, intentionally unilateral prostatic artery embolization (IU-PAE) was performed in 13 patients with opacification of more than half of the contralateral prostatic lobe after angiography of the ipsilateral prostatic artery or with markedly asymmetric prostatic enlargement. Compared to bilateral PAE, IU-PAE was associated with significantly lower radiation exposure and a shorter procedure, but with no significant difference in clinical efficacy. Contrast-enhanced ultrasonography was applied during IU-PAE and revealed only limited prostatic infarction in the 2 clinical failures of IU-PAE, in contrast to the rest of the patients.
- Citation: Moschouris H, Stamatiou K. Intentionally unilateral prostatic artery embolization: Patient selection, technique and potential benefits. World J Radiol 2024; 16(9): 380-388
- URL: https://www.wjgnet.com/1949-8470/full/v16/i9/380.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i9.380
Unilateral prostatic artery embolization (PAE) for symptomatic benign hyperplasia is usually the result of failed catheterization of the contralateral prostatic artery (PA) due to pelvic arterial atherosclerotic stenoses, tortuosities, or due to unfavorable anatomy of the contralateral PA itself[1]. Unilateral PAE is also associated with reduced clinical efficacy (with a clinical success rate of 50% or lower)[1] compared to bilateral PAE, most likely as a result of the limited ischemic effect[2] and of early reperfusion from the contralateral branches. Exceptionally, an earlier small case series[3] de
The application of unilateral PAE when the largest part of the prostate has a unilateral arterial supply appears to be a reasonable way to maximize the efficacy of the procedure. A modality for on-site evaluation of the ischemic effect could also be helpful in this context. This report describes the patient selection, and technical aspects of intentionally unilateral PAE (IU-PAE) in a single tertiary center. The potential benefits of this approach are evaluated. Utilization of contrast-enhanced ultrasonography (CEUS) for intraprocedural monitoring of IU-PAE is also described.
This is a retrospective review of benign hyperplasia patients who were treated with IU-PAE at a single institution during a 2-year period (September 2021 to August 2023). The initial selection of patients for PAE was based on standard, widely accepted criteria[5]. Written informed consent was obtained from all patients prior to treatment. The study was approved by the institutional review board.
Preprocedural computed tomographic angiography (CTA) was conducted for vascular planning in all patients. IU-PAE was applied, if superselective angiography of the ipsilateral PA resulted in opacification of more than half of the contralateral prostatic lobe, as assessed visually by the operator during the intervention (subgroup A), or if markedly asymmetric prostatic enlargement was observed on CTA, with the dominant prostatic lobe occupying at least two thirds of the entire gland (subgroup B). Volumetry of the dominant lobe and of the entire prostate was performed with the ellipsoid formula using measurements (length, width and height) performed on the appropriate CTA images. Patients selected for IU-PAE should also fulfill at least one of the following criteria: Severe tortuosity or severe atheromatosis of the pelvic arteries, non-visualization, or visualization of a tiny (with a diameter of less than 1 mm) contralateral PA on preprocedural CTA. The tortuosity of the pelvic arteries was evaluated with a system used in a previous PAE study[6], as follows: Grade 1 (mild: Kinking < 30o in both pelvic sides); Grade 2 (moderate: Maximum kinking 30o-60o in at least 1 pelvic side); Grade 3 (severe: Multiple kinking 30o-60o in both sides and kinking of > 60o in at least 1 pelvic side). Angles were measured in coronal maximum intensity projection images of the preprocedural CTA. The severity of pelvic arterial atheromatosis was visually evaluated on CTA and was given a Grade (0, 1, 2 and 3, for no, mild, moderate and severe stenosis, respectively), also in line with a classification applied in a previous study[7].
The IU-PAE procedure differed from the standard PAE in that no attempt for identification and catheterization of contralateral PA(s) was performed during the intervention. Vascular access was obtained via the right or left common femoral artery with the Seldinger technique. The internal iliac artery was catheterized with a 5 French (Fr) angiographic catheter, usually with a reverse curve. The PA was catheterized with a 1.98 Fr microcatheter (Parkway Soft-Asahi Intecc Co.) and with a double-angled 0.016 microguidewire (Meister-Asahi). Distal advancement of the microcatheter in intraprostatic branches (“PErFecTED technique”)[8,9] was attempted in all cases. Embolization was performed with microspheres (Embosphere 100-300 or 300-500, Merit Medical) until complete flow stasis in the treated PA. At this point, contrast-enhanced ultrasonography [intraprocedural CEUS (iCEUS)] was performed transabdominally and infarction (represented by newly appearing, non-enhancing intraprostatic areas) was visually evaluated on 3 different prostate levels (base, middle part and apex) and compared to the whole prostate area. This visual estimate of prostatic infarction was semiquantitatively expressed in classes of ten percent, similar to a previous report[10]. All PAE procedures were performed with the same angiographic unit (Axiom Artis Zee, Siemens Healthineers, Erlangen, Germany) and by the same first operator with 5 years of previous experience in PAE. For iCEUS, a second-generation echo enhancer (SonoVue, Bracco, Milan, Italy) was administered in an antecubital vein and a low mechanical index, contrast-specific algorithm was utilized. iCEUS was performed with a portable ultrasonographic unit (M8 Mindray, Nanshan, Shenzhen, China).
Evaluation of clinical success was based on reduction of the International Prostate Symptom Score or on the relief from indwelling bladder catheter according to standard, widely accepted criteria[5]. Clinical assessment of all treated patients was scheduled within the first week post PAE, one and three months post PAE and then every three months. Complications were recorded and graded using the modified (for PAE) Clavien-Dindo system[11]. Prostate shrinkage and prostate infarction were evaluated postoperatively with transabdominal ultrasonography: Unenhanced ultrasonography (US) was performed at least twice: (1) During the patient’s first visit, within one week post PAE; and (2) Three months post PAE. Prostatic volume (PV) was calculated with the ellipsoid formula using the measurements of transabdominal US. Post-PAE CEUS was performed at least once (during the patient’s first visit, within one week post PAE). The extent (volume) of each prostatic infarction was calculated using the ellipsoid formula; the volumes of all prostatic infarcts were summed and divided by PV, thus calculating the percentage of prostatic infarction, in line with previous work[2].
Radiation parameters [DAP, fluoroscopy time (FT)] and operation time (OT) (calculated from the moment of arterial introducer placement until its removal) were also recorded. IU-PAE patients were compared to a control group of patients who had undergone bilateral PAE during the same period and with the same equipment, angiographic protocol and with the same catheterization and embolization materials.
Descriptive statistics were calculated for quantitative and qualitative data. Several variables of the IU-PAE group were compared with the corresponding variables of the control group. Depending on the type and distribution of each variable, the appropriate test was utilized (Welch’s t test, Mann-Whitney U test, Z test). Pearson correlation coefficient was used to assess the correlation between the visual estimate of prostatic infarction (measured by iCEUS during the procedure) and the percentage of prostatic infarction (measured by CEUS within the first week post PAE). The Kaplan-Meier method was used to calculate the clinical success rates of PAE over time; the log-rank test was used to evaluate differences in clinical success rates between the IU-PAE group and the control group, and between subgroups A and B. Statistical significance was defined as P < 0.05.
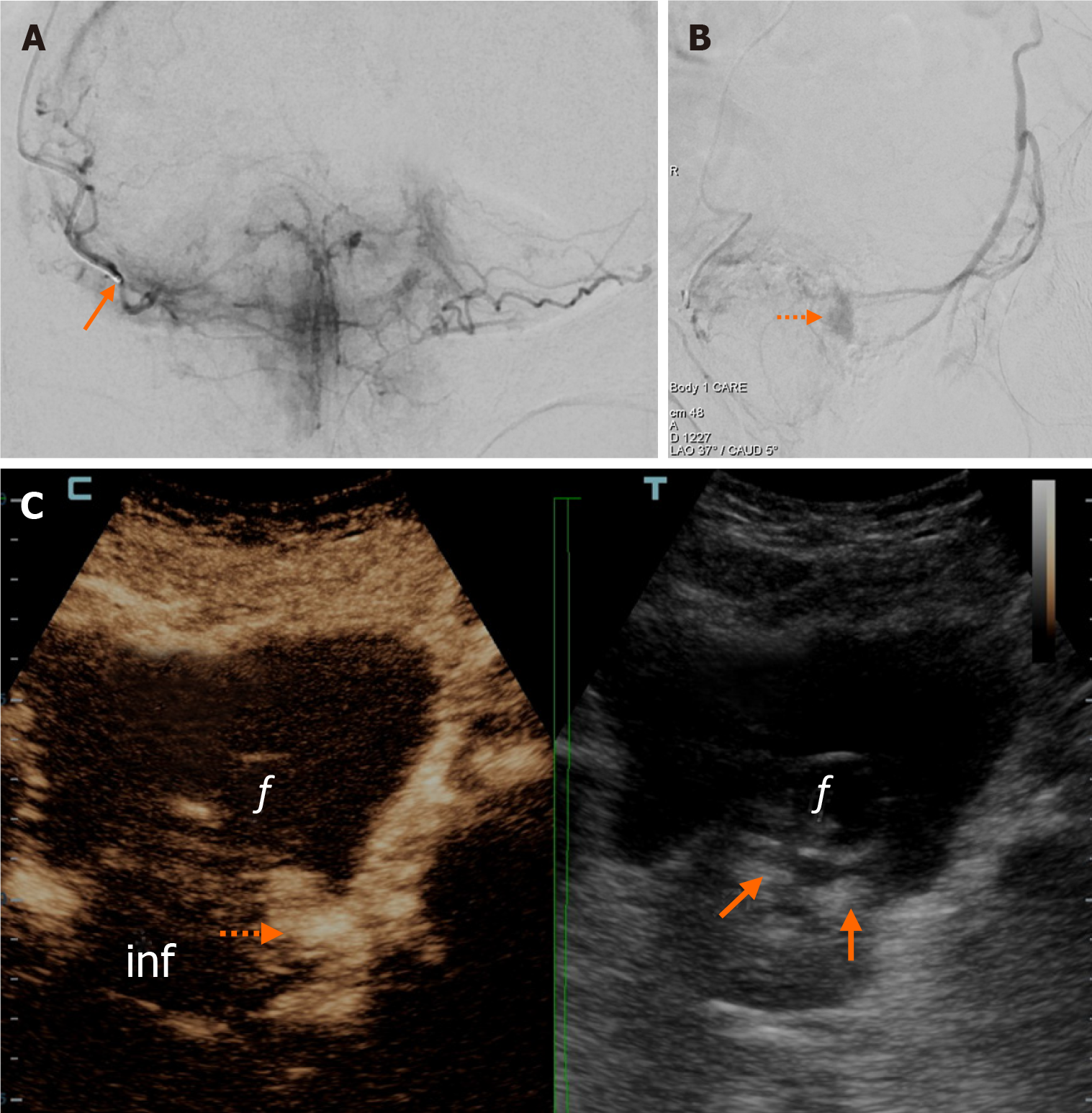
IU-PAE was performed in a total 13 patients during the study period (Table 1). Subgroup A was represented by 7/13 patients. After catheterization and superselective angiography of the ipsilateral PA, complete opacification of the contralateral lobe was observed in 2/7 patients; incomplete opacification exceeding half of the contralateral lobe was observed in 5/7 patients. In 2/7 patients (one with complete contralateral opacification and one with opacification of more than half of the contralateral lobe) the microcatheter could be advanced in contralateral prostatic branches. Regarding iCEUS, in 5/7 patients of subgroup A, infarcts were demonstrated in both prostatic lobes, occupying at least 20% of the entire prostate on visual evaluation (Figure 1). In 2/7 patients, iCEUS demonstrated small infarcts limited to the ipsilateral lobe and occupying less than 10% of the entire prostate. Of note, both of these patients had undergone embolization with the relatively larger microspheres (300-500 microns).
| IU-PAE (n = 13) | Control (n = 30) | P value | |
| Age (mean ± SD, years) | 76.5 ± 8.2 | 71.8 ± 10.6 | 0.166 |
| BMI (mean ± SD, kg/m2) | 28.7 ± 4.6 | 27.0 ± 2.8 | 0.300 |
| PV (mean ± SD, mL) | 106.7 ± 43.8 | 92.8 ± 27.1 | 0.330 |
| Grade of tortuosity (mean ± SD) | 2.2 ± 0.9 | 2.1 ± 0.8 | 0.736 |
| Grade of atheroma (mean ± SD) | 1.5 ± 0.7 | 1.2 ± 0.6 | 0.360 |
| LUTS, proportion of pts | 9/13 | 22/30 | 0.779 |
| IPSS (mean ± SD) | 26.0 ± 4.0 | 26.5 ± 3.9 | 0.708 |
| IBC proportion of pts | 4/13 | 8/30 | 0.779 |
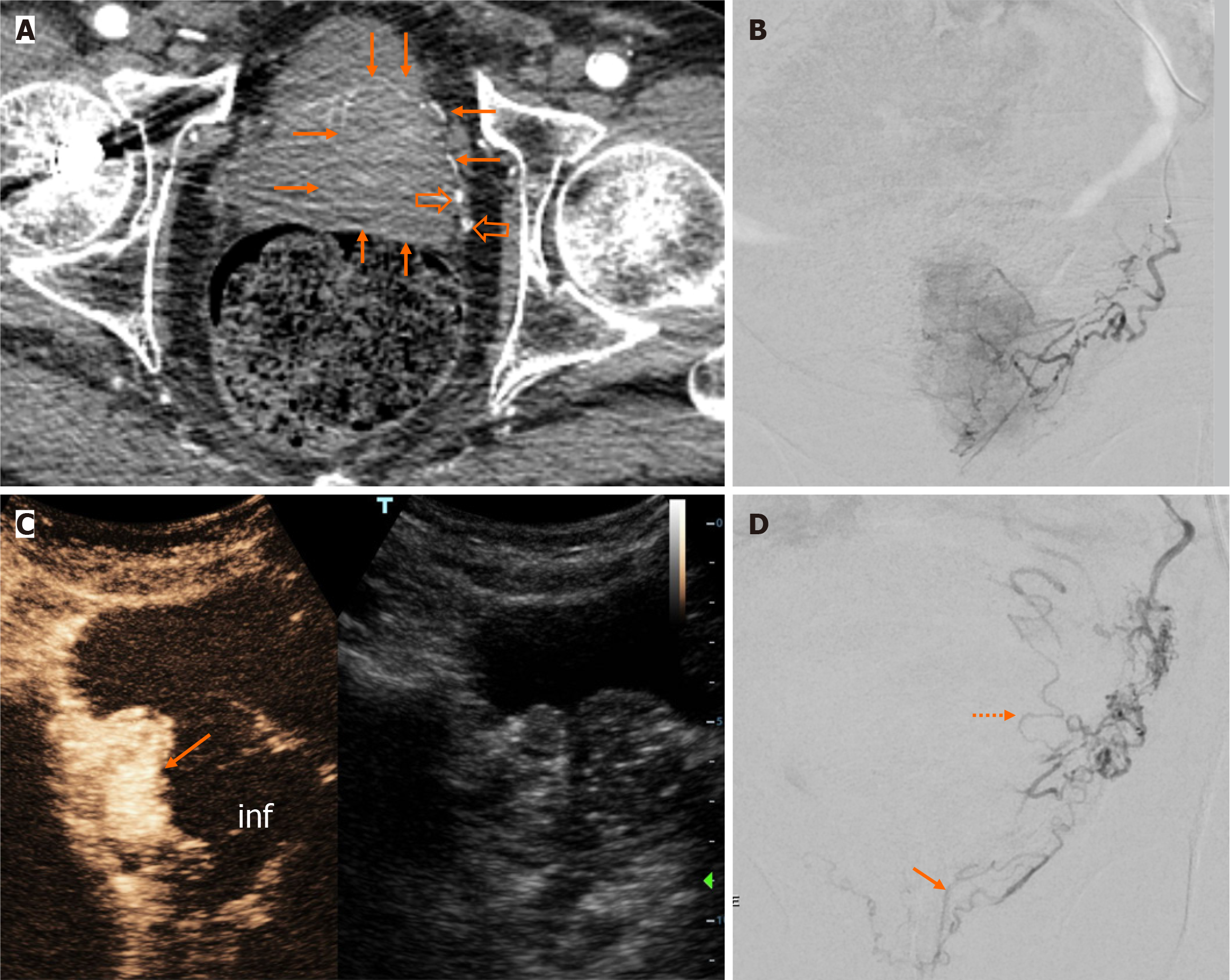
Subgroup B was represented by 6/13 patients, with a volume of the dominant prostatic lobe representing 67%-90% (mean: 74.7%) of the entire PV. In all patients of subgroup B, catheterization and injection of the embolic agent were limited to the PA(s) of the dominant lobe. The latter was fed by a single PA in 5 cases and by a duplicate PA in one case. Immediately post-embolization, iCEUS showed ipsilateral infarction occupying at least 30% of the prostate in all 6 patients (Figure 2). Additionally, in 2 of them, limited infarction of the contralateral lobe was also observed.
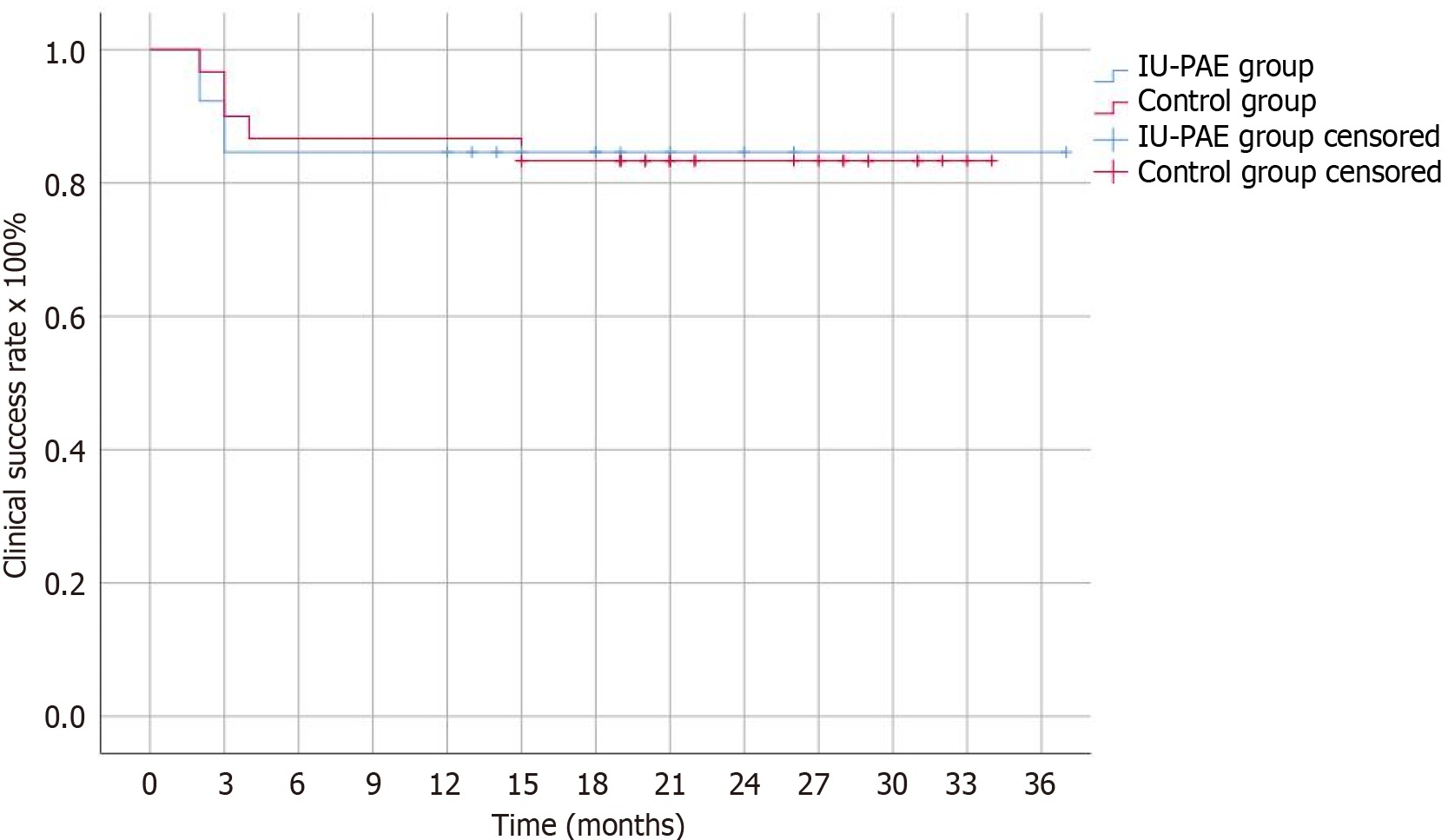
Radiation parameters (DAP, FT) and OT in the IU-PAE group (9767.8 μGy∙m2, 30.3 minutes, 64.0 minutes, respectively) were significantly lower (45.4%, 35.9%, 45.8% respectively, P < 0.01) compared to the control group of 30 patients who underwent bilateral PAE (Table 2). Regarding efficacy, the clinical success rates of IU-PAE were 92.3%, 84.6%, 84.6%, 84.6% and 84.6% at 2, 3, 6, 12 and 24 months post-PAE, respectively. These did not significantly differ from the clinical success rates of the control group (Figure 3). Follow-up time ranged from 2-37 (mean: 17) months.
| IU-PAE (n = 13) | Control (n = 30) | P value | |
| Operation time (mean ± SD, minutes) | 64.0 ± 20.2 | 118.2 ± 22.6 | < 0.001a |
| Fluoroscopy time (mean ± SD, minutes) | 30.3 ± 10.6 | 47.3 ± 14.8 | 0.002a |
| DAP (mean ± SD, μGy∙m2) | 9767.8 ± 5873.5 | 17891.5 ± 9087.1 | 0.004a |
| “PErFecTED” technique (proportion of pts) | 7/13 | 14/30 | 0.667 |
| Embo with 100-300 vs 300-500 (proportion of pts) | 10/3 | 23/7 | 0.984 |
| MC advancement in contralateral side (proportion of pts) | 2/13 | 1/30 | 0.155 |
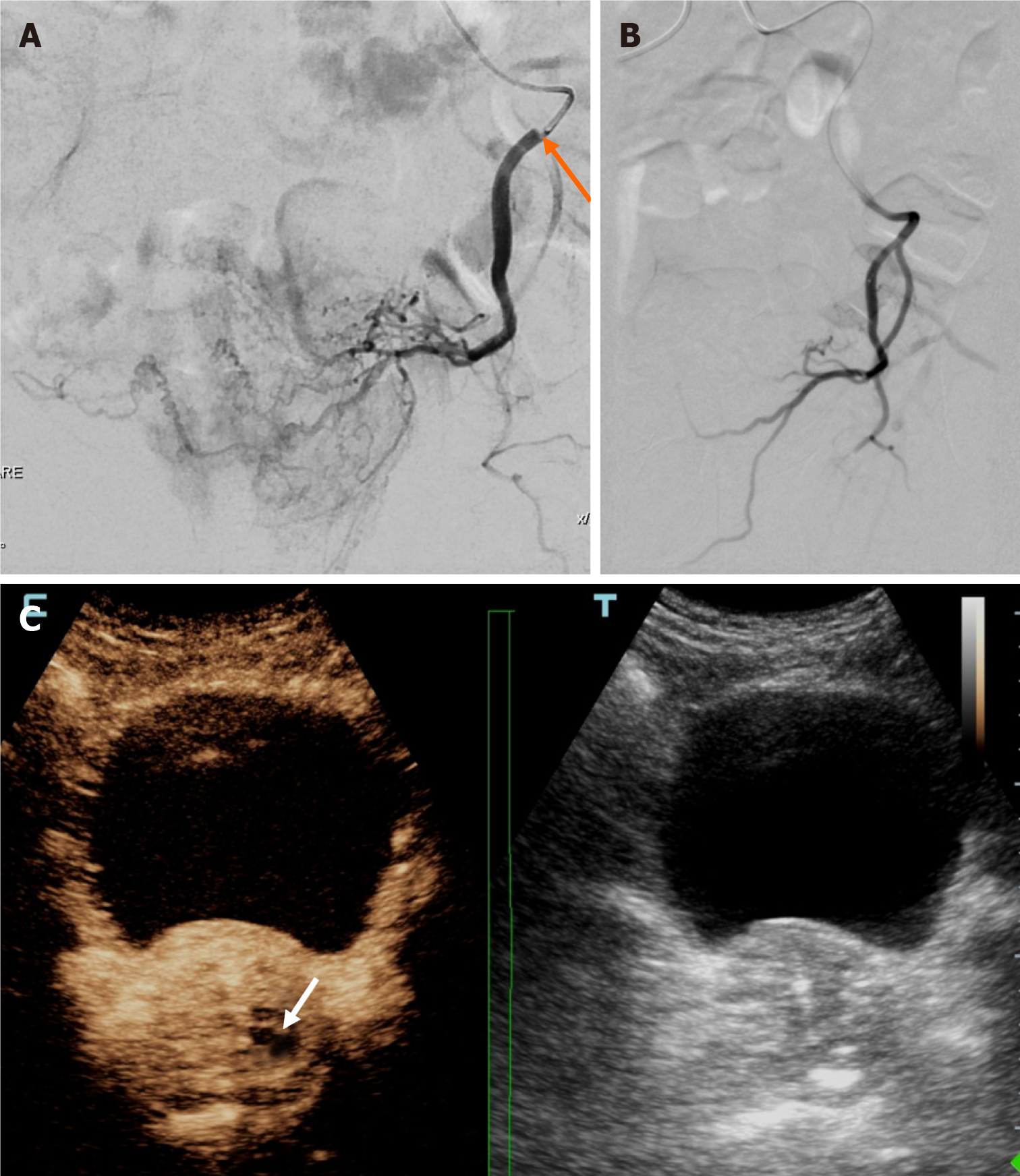
Early clinical failures of IU-PAE were observed in the 2 aforementioned patients with limited unilateral prostatic infarction (Figure 4). Although both clinical failures occurred in subgroup A, clinical success rates did not significantly differ between subgroup A and subgroup B (P = 0.173). The percentage of prostatic infarction and the degree of prostate shrinkage were also lower in subgroup A; however, these differences were not statistically significant either (31.1% ± 17.6% vs 41.8% ± 13.1%, P = 0.389 and 26.6% ± 9.5% vs 29.7% ± 16.2%, P = 1.000).
Only 2 minor complications (acute urinary retention, n = 1; inguinal hematoma, n = 1) occurred in the IU-PAE group, vs 7 minor complications in the control group (acute urinary retention, n = 3; hemospermia, n = 1, mild rectal bleeding, n = 2; urinary infection, n = 1). Prostate shrinkage and the extent of prostatic infarction were also comparable between the IU-PAE group and the control group. A more detailed comparison between the IU-CEUS group and control group in terms of imaging and clinical outcomes is provided in Table 3. Finally, there was excellent correlation between iCEUS and post-PAE CEUS regarding the evaluation of prostatic infarction (r = 0.95, P < 0.001), although prostatic infarction tended to be overestimated with iCEUS (mean visual estimate of prostatic infarction with iCEUS: 49.2% ± 22.2%; mean percentage of prostatic infarction with post-PAE CEUS: 36.1% ± 16.0%).
| IU-PAE (n = 13) | Control (n = 30) | P value | |
| Percentage of prostatic infarction1 (mean ± SD, %) | 36.1 ± 16.0 | 33.1 ± 14.5 | 0.478 |
| PV reduction2 (mean ± SD, %) | 28.0 ± 12.5 | 32.9 ± 9.6 | 0.065 |
| IPSS reduction2 (mean ± SD, %) | 58.0 ± 16.3 | 56.6 ± 22.3 | 0.819 |
| Clinical success rate2 (%) | 84.6 | 90.0 | 0.995 |
| Complications3 - proportion of pts | 2/13 | 7/30 | 0.555 |
In this report, IU-PAE was performed in 2 subgroups of patients. In subgroup A, opacification of more than half of the contralateral prostatic lobe was observed after catheterization of the ipsilateral PA, with or without additional advancement of the microcatheter in contralateral prostatic branches. Subgroup A is somewhat similar to the case series of Amouyal et al[3], although in the present work we also included patients with more than half, but less than total opacification of the contralateral lobe. Two patients showed early clinical failures in subgroup A. As these patients underwent embolization with the relatively larger microspheres (300-500 microns), we speculate that these microspheres failed to cross the tiny intraprostatic anastomoses with contralateral prostatic branches and never reached the contralateral lobe. This is supported by the respective iCEUS findings (i.e., limited, unilateral infarction in these 2 cases, as opposed to more extensive and bilateral infarction in the rest of the patients of subgroup A).
Subgroup B consisted of patients with markedly asymmetric prostatic enlargement. The “dominant” prostatic lobe (which was exclusively embolized) was arbitrarily defined as the lobe that occupied at least two thirds of the entire gland. In an earlier study[12], it was also arbitrarily defined that the prostate was dominantly vascularized by a unilateral PA, when the proportion of prostatic arterial supply via one side PA was over 70%. Of note, such a dominant unilateral arterial supply of the prostate was noted in 67.3% of the patients in this study; however, it was not reported whether this feature was exploited in order to perform IU-PAE. In the present series, unilateral embolization of the dominant lobe resulted in a significant extent of prostatic infarction and in satisfactory prostate shrinkage and clinical improvement. It is likely that the relatively larger size of the arteries in the dominant lobe facilitated the antegrade flow of the embolic agent and the accumulation of microspheres into the more distal prostatic branches and thus increased the efficacy of embolization.
Compared to bilateral PAE in the control group, IU-PAE was associated with a significant reduction in radiation exposure, with a 45.4% reduction in DAP and 35.9% reduction in FT. The herein reported mean DAP of IU-PAE (9767.8 μGy∙m2), is lower than the corresponding value reported in the aforementioned small series of bilateral PAE through a single PA[3]; it is also lower than the mean DAP reported in a previous work (13981.5 μG∙m2), where the same equipment as in the present study was used and several techniques (but not IU-PAE) were combined to minimize radiation exposure during PAE[13]. The IU-PAE procedure was also significantly faster compared to bilateral PAE; this could be a crucial advantage when anxious and less cooperative patients are treated. Remarkably, these benefits of IU-PAE were not associated with any compromise in short- to mid-term clinical efficacy. The satisfactory clinical success rate of IU-PAE (84.6% up to 30 months post PAE) is clearly higher than that of “traditional” unilateral PAE[1] and comparable to that of bilateral PAE. However, it should be acknowledged that the long-term efficacy of IU-PAE has not been investigated and there is (at least theoretically) a risk of early reperfusion from the untreated contralateral branches.
In this work, iCEUS was applied to monitor prostatic infarction in the angio-suite, immediately post embolization. iCEUS proved feasible and practical, with no significant delay in the procedure and no additional radiation. Although iCEUS did not alter treatment strategy in the present work, it could be used in this way in the future: If iCEUS demon
Several limitations of this study should be acknowledged: The small sample size (13 patients) limits the generalizability of the findings. No long-term follow-up data were available. Selection criteria for IU-PAE were arbitrarily set by the authors. Imaging evaluation was performed with US and computed tomography; magnetic resonance imaging (which is probably the most accurate and comprehensive modality for peri-interventional imaging of the prostate)[21] was not utilized.
This work provides additional evidence regarding IU-PAE. In selected patients, this approach appears to be comparable to bilateral PAE in terms of safety and short- to mid-term clinical efficacy, but with significantly lower radiation exposure and significantly shorter OT. Clearly, larger studies are required to validate the herein presented findings, and to explore the long-term outcomes of IU-PAE. iCEUS appears to be a practical modality for on-site monitoring of the ischemic effect of embolization, with a potential role in patient selection for IU-PAE. iCEUS also appears to be a feasible and versatile variant of the standard CEUS technique and its place in the context of other interventional radiology procedures warrants further research.
| 1. | Bilhim T, Pisco J, Rio Tinto H, Fernandes L, Campos Pinheiro L, Duarte M, Pereira JA, Oliveira AG, O'Neill J. Unilateral versus bilateral prostatic arterial embolization for lower urinary tract symptoms in patients with prostate enlargement. Cardiovasc Intervent Radiol. 2013;36:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Moschouris H, Stamatiou K, Malagari K, Marmaridou K, Kladis-Kalentzis K, Kiltenis M, Papadogeorgopoulos N, Tsavari A, Manoloudaki K. The value of contrast-enhanced ultrasonography in detection of prostatic infarction after prostatic artery embolization for the treatment of symptomatic benign prostatic hyperplasia. Diagn Interv Radiol. 2019;25:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Amouyal G, Pellerin O, Del Giudice C, Déan C, Thiounn N, Sapoval M. Bilateral Arterial Embolization of the Prostate Through a Single Prostatic Artery: A Case Series. Cardiovasc Intervent Radiol. 2017;40:780-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 4. | Svarc P, Hagen T, Waltenburg H, Andersson C, Bläckberg M, Baco E, Taudorf M, Røder MA, Lindgren H, Kløw NE, Lönn LB. Center experience and other determinants of patient radiation exposure during prostatic artery embolization: a retrospective study in three Scandinavian centers. Eur Radiol. 2022;32:2404-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cornelis FH, Bilhim T, Hacking N, Sapoval M, Tapping CR, Carnevale FC. CIRSE Standards of Practice on Prostatic Artery Embolisation. Cardiovasc Intervent Radiol. 2020;43:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Enderlein GF, Lehmann T, von Rundstedt FC, Aschenbach R, Grimm MO, Teichgräber U, Franiel T. Prostatic Artery Embolization-Anatomic Predictors of Technical Outcomes. J Vasc Interv Radiol. 2020;31:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Hacking N, Vigneswaran G, Maclean D, Modi S, Dyer J, Harris M, Bryant T. Technical and Imaging Outcomes from the UK Registry of Prostate Artery Embolization (UK-ROPE) Study: Focusing on Predictors of Clinical Success. Cardiovasc Intervent Radiol. 2019;42:666-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Dias US Jr, de Moura MRL, Viana PCC, de Assis AM, Marcelino ASZ, Moreira AM, Leite CC, Cerri GG, Carnevale FC, Horvat N. Prostatic Artery Embolization: Indications, Preparation, Techniques, Imaging Evaluation, Reporting, and Complications. Radiographics. 2021;41:1509-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Carnevale FC, Moreira AM, Antunes AA. The "PErFecTED technique": proximal embolization first, then embolize distal for benign prostatic hyperplasia. Cardiovasc Intervent Radiol. 2014;37:1602-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Barat M, Boeken T, Moussa N, Di Gaeta A, Déan C, Thioun N, Del Giudice C, Pellerin O, Sapoval M. Contrast-Enhanced Ultrasonography for the Early Evaluation of Prostate Artery Embolization. Cardiovasc Intervent Radiol. 2020;43:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 11. | Moreira AM, de Assis AM, Carnevale FC, Antunes AA, Srougi M, Cerri GG. A Review of Adverse Events Related to Prostatic Artery Embolization for Treatment of Bladder Outlet Obstruction Due to BPH. Cardiovasc Intervent Radiol. 2017;40:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Zhang G, Wang M, Duan F, Yuan K, Li K, Yan J, Chang Z, Wang Y. Radiological Findings of Prostatic Arterial Anatomy for Prostatic Arterial Embolization: Preliminary Study in 55 Chinese Patients with Benign Prostatic Hyperplasia. PLoS One. 2015;10:e0132678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Moschouris H, Stamatiou K, Spanomanolis N, Vasilopoulos A, Tzamarias S, Malagari K. A Retrospective, Single-Center Study of Technical-Procedural Factors Affecting Radiation Dose During Prostatic Artery Embolization. Cureus. 2022;14:e27728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Maclean D, Francis Bryant CT, Vigneswaran G, Bryant TJ, Harris M, Somani B, Modi S. Comprehensive Review on Current Controversies and Debate in Prostate Artery Embolization. Turk J Urol. 2022;48:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Lucas-Cava V, Sánchez-Margallo FM, Moreno-Lobato B, Dávila-Gómez L, Lima-Rodríguez JR, García-Martínez V, López-Sánchez C, Sun F. Prostatic artery occlusion: initial findings on pathophysiological response in a canine prostate model. Transl Androl Urol. 2022;11:1655-1666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Wang MQ, Zhang JL, Xin HN, Yuan K, Yan J, Wang Y, Zhang GD, Fu JX. Comparison of Clinical Outcomes of Prostatic Artery Embolization with 50-μm Plus 100-μm Polyvinyl Alcohol (PVA) Particles versus 100-μm PVA Particles Alone: A Prospective Randomized Trial. J Vasc Interv Radiol. 2018;29:1694-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Sun F, Crisóstomo V, Báez-Díaz C, Sánchez FM. Prostatic Artery Embolization (PAE) for Symptomatic Benign Prostatic Hyperplasia (BPH): Part 2, Insights into the Technical Rationale. Cardiovasc Intervent Radiol. 2016;39:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Kisilevzky N, Faintuch S. MRI assessment of prostatic ischaemia: best predictor of clinical success after prostatic artery embolisation for benign prostatic hyperplasia. Clin Radiol. 2016;71:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Schmidt VF, Schirren M, Heimer MM, Kazmierczak PM, Cyran CC, Wildgruber M, Seidensticker M, Ricke J, Solyanik O. Semi-Automatic MRI Feature Assessment in Small- and Medium-Volume Benign Prostatic Hyperplasia after Prostatic Artery Embolization. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 20. | Wang RL, Lin FF, Ruan DD, Li SJ, Zhou YF, Luo JW, Fang ZT, Tang Y. A correlation study between prostate necrosis rate calculated by 3D Slicer software and clinical efficacy of prostatic artery embolization, along with an analysis of predictors of clinical success after prostatic artery embolization. Abdom Radiol (NY). 2024;49:927-938. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Walker SM, Turkbey B. Role of mpMRI in Benign Prostatic Hyperplasia Assessment and Treatment. Curr Urol Rep. 2020;21:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |