Published online Aug 28, 2024. doi: 10.4329/wjr.v16.i8.348
Revised: August 13, 2024
Accepted: August 20, 2024
Published online: August 28, 2024
Processing time: 160 Days and 0.9 Hours
The rare co-occurrence of oligodendroglioma and arteriovenous malformation (AVM) in the same intracranial location.
In a 61-year-old man presenting with progressive headaches, is described in this case study. Preoperative multimodal imaging techniques (computed tomography, magnetic resonance imaging, magnetic resonance spectroscopy, digital subtrac
The preoperative utilization of multimodal imaging examination can help clini
Core Tip: This case report details the rare co-occurrence of oligodendroglioma and arteriovenous malformation in a 61-year-old male. Preoperative multimodal imaging, including computed tomography, magnetic resonance imaging, magnetic resonance spectroscopy, digital subtraction angiography, and computed tomography angiography, was crucial in accurately diagnosing the combined pathology, guiding the successful surgical removal. This study underscores the importance of comprehensive imaging to prevent misdiagnosis and highlights the feasibility of craniotomy for treating this rare condition.
- Citation: Guo P, Sun W, Song LX, Cao WY, Li JP. Multimodal imaging for the diagnosis of oligodendroglioma associated with arteriovenous malformation: A case report. World J Radiol 2024; 16(8): 348-355
- URL: https://www.wjgnet.com/1949-8470/full/v16/i8/348.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i8.348
The coexistence of oligodendroglioma and arteriovenous malformation (AVM) in the brain is a rare phenomenon, with a limited number of cases documented in the literature, primarily in case reports. Although oligodendroglioma and AVM are typically considered to be distinct diseases, the presence of both within a single lesion raises questions regarding the nature of this combined pathology[1,2]. Various hypotheses have been proposed to elucidate the potential mechanisms underlying the association of glioma with AVM in the same patient, including fortuitous coexistence, genetic predisposition, and a unique entity. Further investigation is required to better understand the pathophysiology of this rare occurrence[3,4]. Here, we describe a patient with an oligodendroglioma associated with major arteriovenous shunting and a vascular nidus mimicking an AVM, and highlight the use of multimodal imaging for the diagnosis of this type of disease.
A 61-year-old man presented with a progressive headache for 4 days.
The patient presented to our neurology outpatient clinic four days ago with the acute onset of headache, without any apparent precipitating factors. The patient denied experiencing dizziness, nausea, vomiting, unilateral limb numbness, unilateral limb weakness, or altered mental status.
The patient had no history of trauma, hypertension, or other medical conditions.
His history, personal history, and family history were unremarkable.
A neurological examination was performed on admission and yielded normal findings.
Laboratory examinations were normal.
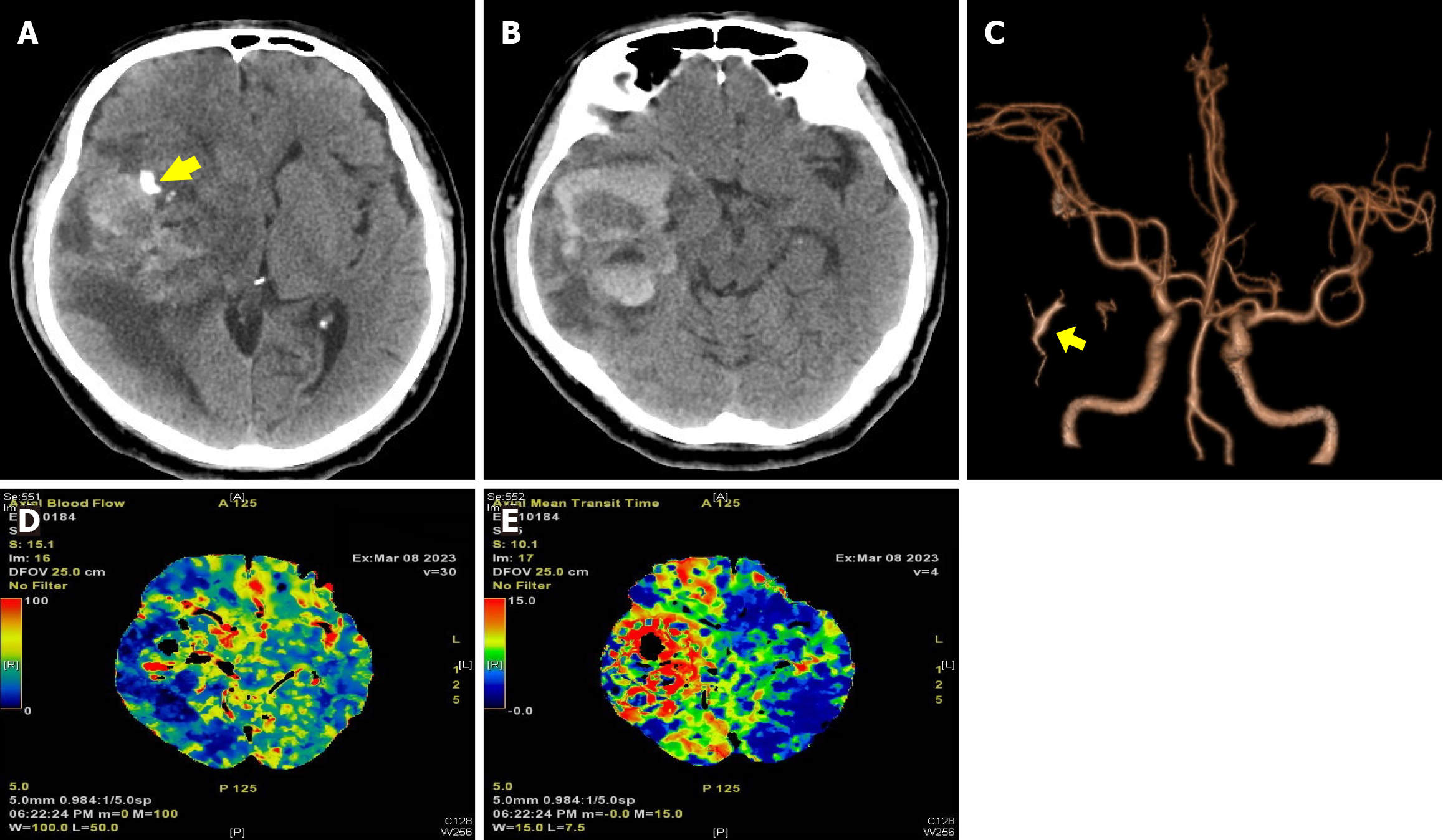
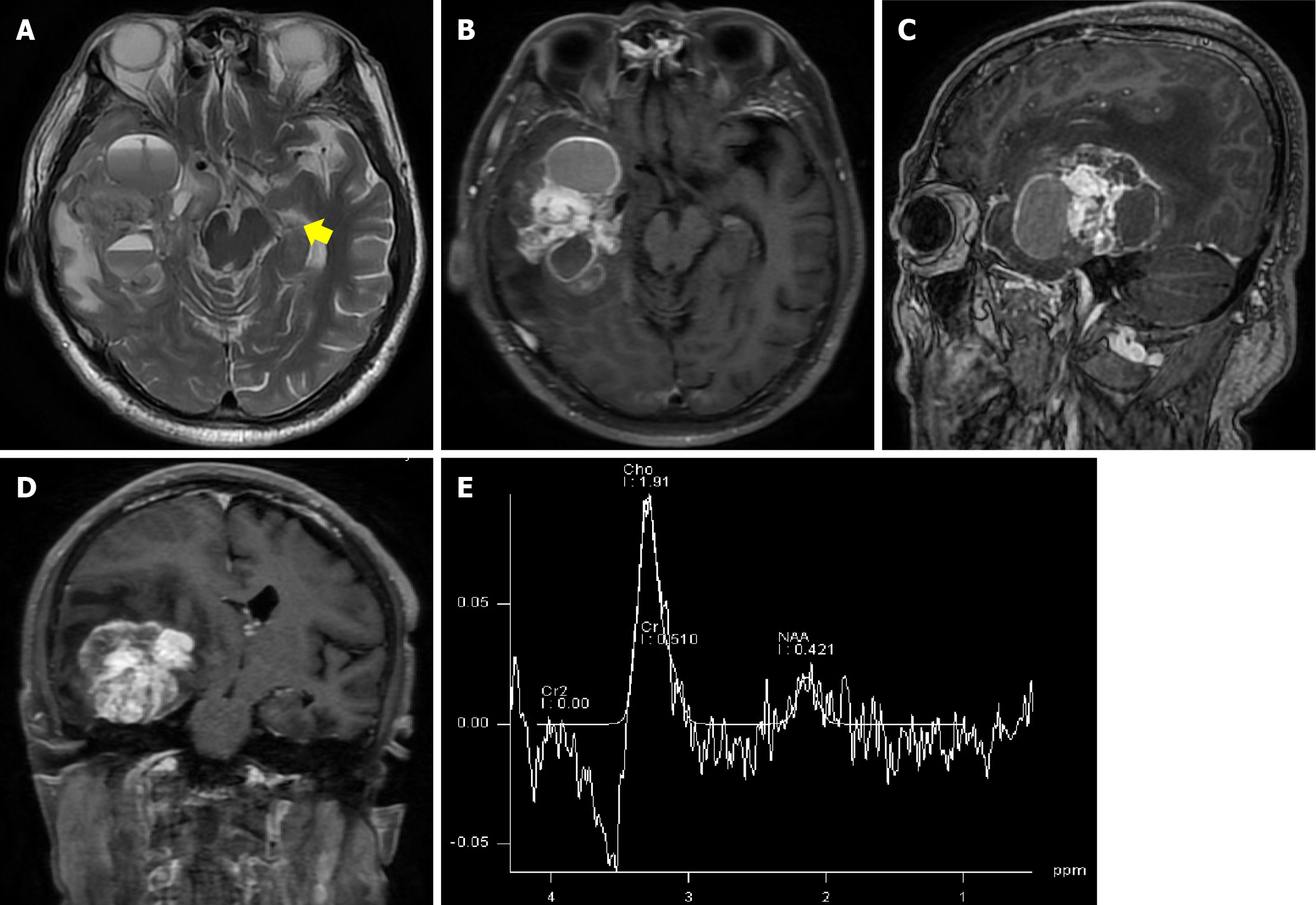
Computed tomography (CT) examination revealed a significant area of hemorrhage, edema, and calcification in the right temporal lobe, resulting in a midline shift to the left (Figure 1A and B). Multimodal imaging was conducted and yielded findings consistent with right middle cerebral artery elevation and an early lesion drainage vein [detected by CT angiography (CTA)] (Figure 1C). CT perfusion (CTP) imaging demonstrated increases in the regional cerebral blood flow (rCBF) and mean transit time in the lesion area (Figure 1D and E). Contrast-enhanced magnetic resonance imaging (MRI) revealed a 4.5 cm × 6.3 cm × 4.6 cm necrotic cystic lesion in the right hemisphere involving the basal ganglia, with a midline shift and uncal herniation. The lesion exhibited dark signaling and edema belts on T2-weighted images, and heterogeneous enhancement on contrast-enhanced T1-weighted images (Figure 2A–D). Magnetic resonance spectroscopy (MRS) demonstrated aberrant metabolic function (Figure 2E). Specifically, an elevated choline/creatine (Cr) peak ratio of 1.88 ppm and a severely decreased N-acetyl aspartate/Cr ratio of 1.49 ppm were observed within the lesion, consistent with the metabolic profile of glioma. Although the majority of the imaging findings supported the diagnosis of glioma, the presence of an early drainage vein on CTA images and dark vessel-like signals on T2-weighted images raised the possibility that a vascular malformation was present within the lesion, necessitating further angiographic evaluation.
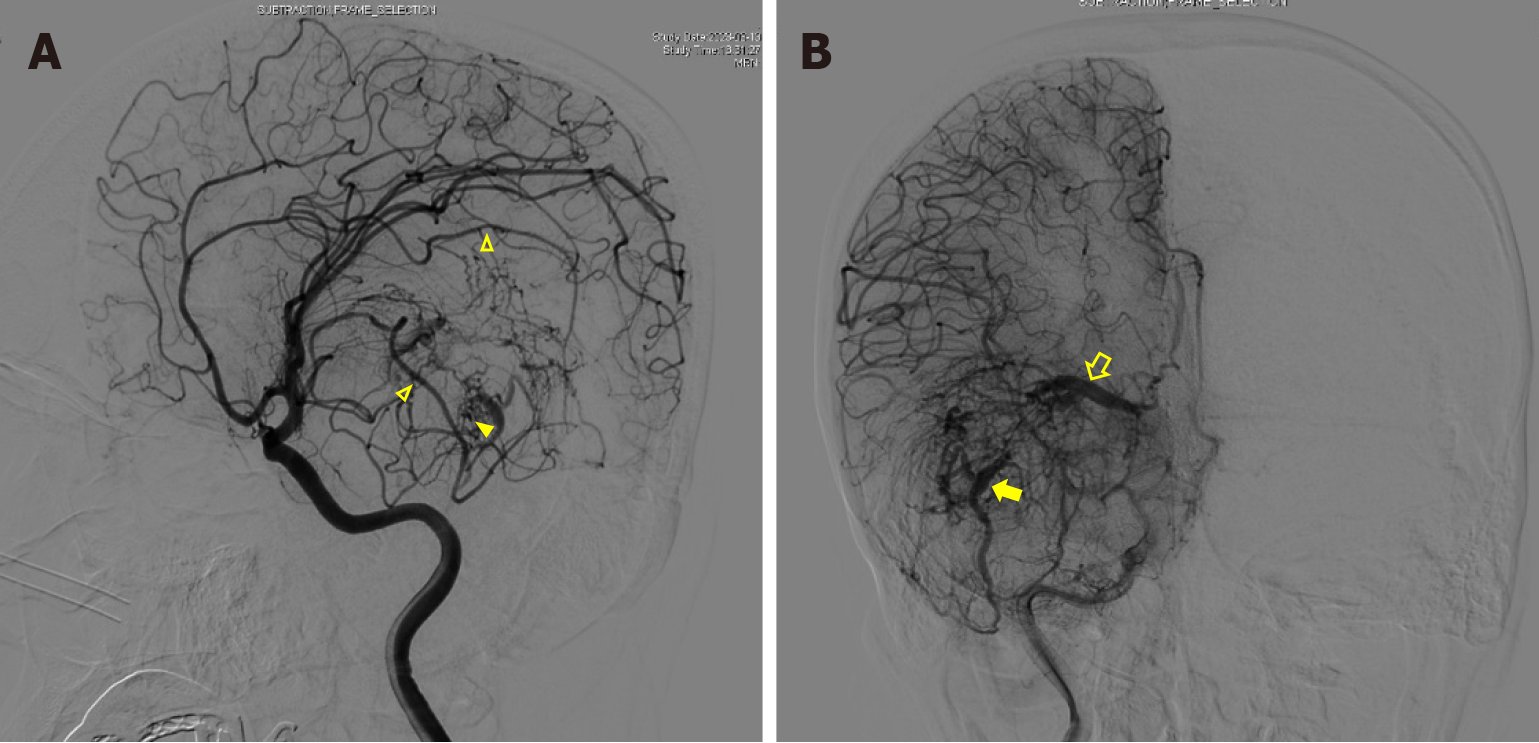
Cerebral digital subtraction angiography (DSA) was utilized to confirm the presence of an AVM. It revealed the presence of two primary feeding vessels originating from branches of the right middle artery. Additionally, two large drainage veins with a caput medusae distribution characteristic of a developmental venous anomaly were also identified. One vein drained into the great cerebral vein leading to the sinuses rectus, and the other drained through the superior petrosal sinus into the ipsilateral transverse sinus. Numerous small, tortuous blood vessels were observed to form a nidus between the feeding artery and the draining vein (Figure 3).
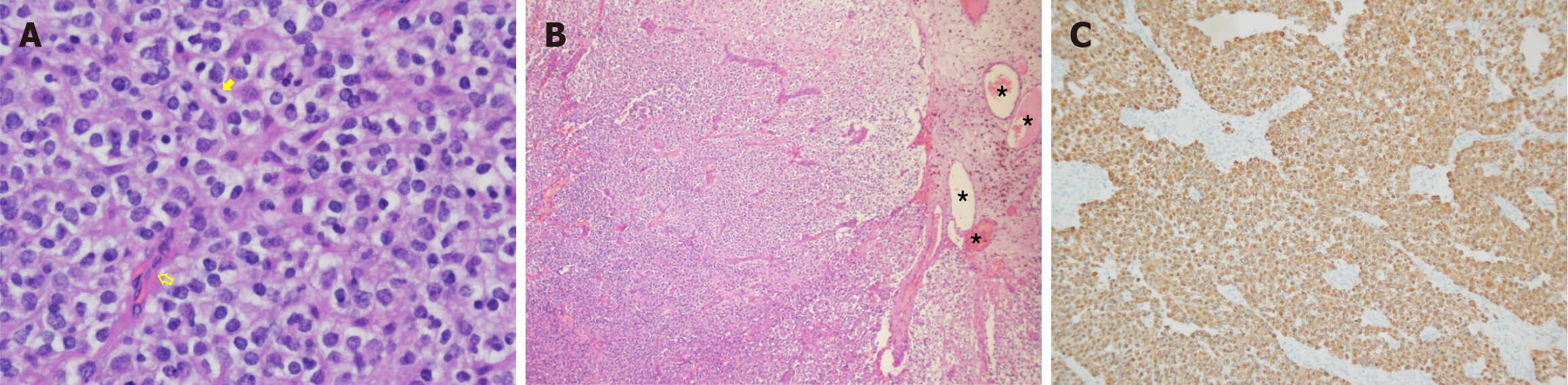
Pathological examination of the tumor revealed the diffuse and invasive growth of neoplastic glial cells with a single-cell morphology, round nuclei, clear borders, moderate atypia, perinuclear haloes, rare mitotic figures, branched proliferation of interstitial blood vessels, focal calcification, and patchy hemorrhage (Figure 4A and B). Immunohistochemical analyses demonstrated positive staining for glial fibrillary acidic protein (Figure 4C), S-100, and SRY-related HMG-box 10; negative staining for cytokeratins; scattered positive staining for p53; and a Ki-67 index of 30%. The final pathological diagnosis was oligodendroglioma (World Health Organization Grade II) associated with an AVM.
The patient underwent right temporal craniotomy, during which the tumor was observed to be grayish red with soft and tough consistencies, an uneven texture, and indistinct boundaries. It contained numerous slender blood vessels. The blood supply artery originating from the middle cerebral artery was occluded, and the tumor was removed in a piecemeal manner. Intraoperative fresh-frozen analysis confirmed the presence of low-grade gliomas, and microscopic examination indicated that the lesion had been nearly completely excised. As no preoperative embolization was conducted due to the lack of a facility for interventional embolization at our institution, the intraoperative bleeding volume was significant, at about 2000 mL. To mitigate the risks of intracranial hemorrhage and cerebral edema, the post-craniotomy removal of the tracheal intubation was delayed and the patient was transferred back to the surgical intensive care unit for further management.
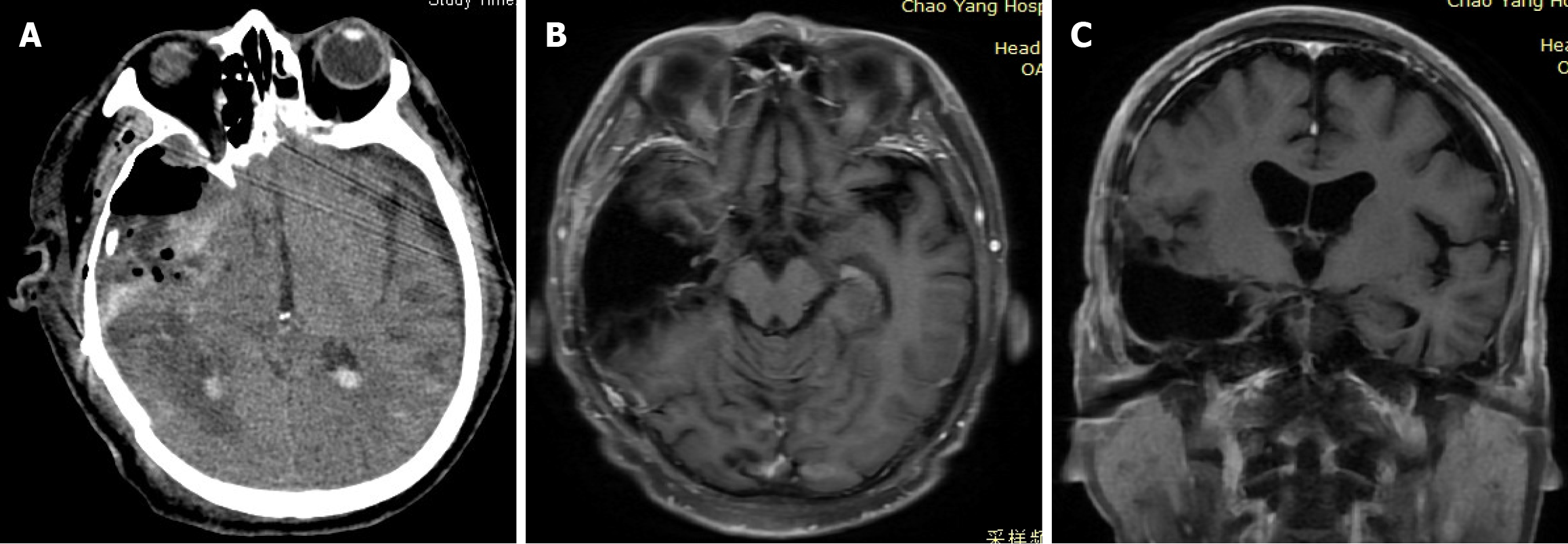
Postoperative head CT examination revealed minor hemorrhaging in the surgical cavity and resolution of the midline shift (Figure 5A). At the time of discharge, the patient exhibited no apparent neurological deficit; he had a modified Rankin Scale score of 5, a Glasgow Coma Scale score of 15, and a National Institutes of Health Stroke Scale score of 0. Following discharge, the patient underwent radiotherapy and chemotherapy as planned. A follow-up enhanced MRI examination performed 6 months postoperatively showed no definitive sign of recurrence (Figure 5B and C).
The coexistence of glioma and AVM has been rarely reported in the literature, primarily in case reports[5-21]. These lesions may present simultaneously in the same part or distinct regions of the brain, or occur sequentially in the same area[5,9,15]. Additionally, the literature contains isolated descriptions of the occurrence of de novo gliomas coexisting with AVMs in the same part of the brain[7,12]. In most cases, however, these mixed lesions are discovered simultaneously in the same cerebral region[4,6,8,10,11,13,14,16-21]. Vascular malformations can be associated with various glial components, including oligodendroglioma[4,7], pilocytic astrocytoma[10], pleomorphic xanthoastrocytoma[9,14], and the predominant type, glioblastoma[6,8,11-13,16,18-21]. Although the precise relationship between these lesions remains unclear, numerous studies suggest that vascular formation in gliomas and the development of AVMs are related to vascular endothelial growth factor (VEGF)[5]. Angiogenesis is stimulated by the upregulation of VEGF and other pro-angiogenic factors. VEGF overexpression in tumors, attributed to the upregulation of hypoxia-associated factors, induces aberrant vascular proliferation that is unable to adequately oxygenate the rapidly expanding tumor tissue, creating a cycle of hypoxia and dysfunctional angiogenesis[11]. Tumor-secreted VEGF binds to endothelial cells expressing VEGF receptors, promoting neoangiogenesis[11]. Contemporary research suggests that the rapid growth of gliomas necessitates a substantial increase in blood supply. Angiogenic factor levels are high in and around these tumors, facilitating the proliferation of new blood vessels. However, the convoluted nature of these vessels hinders their ability to adequately supply the growing neoplasms, resulting in a detrimental cycle that culminates in the development of vascular malforma
The incidence of intracranial AVMs coexisting with brain tumors (most commonly gliomas) is 0.1%[6]. The radiographic features differentiating AVM from glioma are frequently obscured, especially in the presence of acute or subacute hemorrhage[21], which often leads to misdiagnosis or missed diagnosis, posing significant challenges and risks for subsequent treatment. Reports document four cases of the preoperative misdiagnosis of AVMs[6,13,14,17]; in three of these cases, no intracranial bleeding was present and no preoperative CTA or DSA examination was performed[6,13,14]. In the fourth case, DSA findings were negative and postoperative pathological examination led to the diagnosis of pleomorphic xanthoastrocytoma with AVM[17]. In addition, three cases of the preoperative misdiagnosis of glioma in the absence of intracranial bleeding have been reported[8,10,12]; no preoperative MRI examination was performed in two of these cases[8,12]. In the third case, preoperative MRI examination was performed, but the presence of bleeding signals obscured the tumor signal, resulting in misdiagnosis[10]. In all of these cases, postoperative pathological examination or autopsy confirmed the diagnosis of glioma combined with vascular malformation[8,10,12]. The remaining 10 cases described in the literature were accurately diagnosed as gliomas combined with AVMs through preoperative MRI, CTA, or DSA examination[4,5,7,9,11,16,18-21]. Cases with intracranial bleeding are more prone to missed diagnosis gliomas, whereas those without bleeding tend to be missed diagnosis AVMs. Thus, the utilization of both MRI and DSA is essential for the accurate diagnosis of these lesions. Contrast-enhanced MRI plays a critical role in diagnosis because it enables the detection of intracranial tumors, elucidation of their connections with vascular malformations, and identification of the need for CTA or DSA examination to exclude the possibility of such malformations upon the identification of vascular lacunae in or around the tumor on T2-weighted images[11,12,17]. The diagnosis of occult AVM that are DSA-negative is advantageous compared to other examinations[17]. The primary factor contributing to most misdiagnoses and missed diagnoses is the lack of comprehensive multimodal imaging assessment. Clinicians must maintain a vigilant attitude toward examination and treatment. For cases without intracranial bleeding, the presence of abundant blood flow lacunae on T2-weighted MRI images necessitates further evaluation with DSA to rule out the presence of concurrent intracranial vascular malformations. In cases of intracranial bleeding in atypical locations, comprehensive multimodal
In the present case, the patient was admitted after a single-phase stroke CT examination revealed intracerebral hemorrhage in the right temporal lobe and basal ganglia. CTP demonstrated increased perfusion with elevated rCBF in the right temporal lobe. Subsequent contrast-enhanced MRI displayed heterogeneous enhancement within the hemorrhagic lesion, characterized by cystic necrotic regions and the limited presence of vascular lacunae. To mitigate ambiguity, additional MRS and DSA examinations were performed. MRS indicated the presence of a glioma, and DSA confirmed the existence of arteriovenous drainage within the lesion. Consequently, the preoperative utilization of multimodal imaging techniques can help healthcare providers to make the diagnosis of glioma coexisting with AVM, thereby facilitating subsequent therapeutic interventions.
Most existing reports on gliomas combined with AVMs are case studies. No standardized treatment protocol or guideline for this condition has been established. However, a review of the existing literature suggests that treatment planning in such cases should prioritize the addressing of the lesion responsible for the patient's presenting symptoms, particularly in cases of non-adjacent diseases. When the tumor is malignant, resection of the malformation may not be recommended due to the short median survival time and the risk of hemorrhage is approximately 2% per year[6,9]. The optimal treatment for mixed lesions in the same location is surgical resection, but this procedure is associated with significant challenges, especially regarding the intraoperative maintenance of hemostasis[4,7,8,11,13,14,16,17,19,20]. Consequently, preoperative embolization using methods similar to those applied for AVMs has been recommended to mitigate the risk of intraoperative bleeding[7,13,16,19,21]. Imai et al[16] first reported the use of a low concentration (20%) of n-butyl cyanoacrylate and a detachable coil to embolize the tortuous and dilated vessels inside the tumor. After embolization, the lesion was resected completely, with an intraoperative bleeding volume of 60 mL[16]. The authors advocated for the performance of preoperative embolization as a precautionary measure for tumors situated in eloquent regions and noted the need for preoperative provocation testing[16]. Although embolization is a technically routine procedure, the high vascular density, tumor infiltration of vessel walls, and absence of the glial plane typically found in AVM make vessel coagulation more challenging than with AVM in these cases[7]. Despite preoperative embolization, McKinney et al[7] experienced significant intraoperative bleeding and brain tissue protrusion, leading to the patient's death on the sixth postoperative day despite complete tumor resection. In the present case, preoperative embolization was not performed. During surgery, the tumor's rich vascularization and ample blood supply complicated hemostasis, resulting in approximately 2000 mL of blood loss during total excision. Hence, although craniotomy enables the concurrent resection of neoplastic and AVM components, the procedure is associated with significant intraoperative bleeding and hemostasis challenges. Interventional embolization before craniotomy for tumor removal is thus recommended[22].
The combination of glioma and AVM is an exceedingly rare condition, and the two lesions may mask each other when they are located at the same site in the brain. Multimodal preoperative examination can help clinicians minimize the risk of misdiagnosis or missed diagnosis, and provides valuable insights for subsequent treatment. Craniotomy for the removal of glioma and AVM is feasible, and preoperative interventional embolization is recommended to reduce intrao
| 1. | Zuccarello M, Giordano R, Scanarini M, Mingrino S. Malignant astrocytoma associated with arteriovenous malformation. Case report. Acta Neurochir (Wien). 1979;50:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Tews DS, Bohl JE, Van Lindert E, Ringel K. Association of oligodendroglioma-like cell proliferation and angiomatous vasculature-coincidence or pathogenetically related lesions? Clin Neuropathol. 1998;17:69-72. [PubMed] |
| 3. | Foy PM, Lozada L, Shaw MD. Vascular malformation simulating a glioma on computerized tomography. Case report. J Neurosurg. 1981;54:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 4. | Lombardi D, Scheithauer BW, Piepgras D, Meyer FB, Forbes GS. "Angioglioma" and the arteriovenous malformation-glioma association. J Neurosurg. 1991;75:589-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Harris OA, Chang SD, Harris BT, Adler JR. Acquired cerebral arteriovenous malformation induced by an anaplastic astrocytoma: an interesting case. Neurol Res. 2000;22:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ziyal IM, Ece K, Bilginer B, Tezel GG, Ozcan OE. A glioma with an arteriovenous malformation: an association or a different entity? Acta Neurochir (Wien). 2004;146:83-6; discussion 86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | McKinney JS, Steineke T, Nochlin D, Brisman JL. De novo formation of large arteriovenous shunting and a vascular nidus mimicking an arteriovenous malformation within an anaplastic oligodendroglioma: treatment with embolization and resection. J Neurosurg. 2008;109:1098-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Cemil B, Tun K, Polat O, Ozen O, Kaptanoglu E. Glioblastoma multiforme mimicking arteriovenous malformation. Turk Neurosurg. 2009;19:433-436. [PubMed] |
| 9. | Reynolds CF 3rd, Hoch CC, Buysse DJ, George CJ, Houck PR, Mazumdar S, Miller M, Pollock BG, Rifai H, Frank E. Sleep in late-life recurrent depression. Changes during early continuation therapy with nortriptyline. Neuropsychopharmacology. 1991;5:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Soltanolkotabi M, Schoeneman SE, Dipatri AJ, Hurley MC, Ansari SA, Rajaram V, Tomita T, Shaibani A. Juvenile pilocytic astrocytoma in association with arteriovenous malformation. Interv Neuroradiol. 2012;18:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Aucourt J, Jissendi P, Kerdraon O, Baroncini M. Neuroimaging features and pathology of mixed glioblastoma--AVM complex: a case report. J Neuroradiol. 2012;39:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Khanna A, Venteicher AS, Walcott BP, Kahle KT, Mordes DA, William CM, Ghogawala Z, Ogilvy CS. Glioblastoma mimicking an arteriovenous malformation. Front Neurol. 2013;4:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Gmeiner M, Sonnberger M, Wurm G, Weis S. Glioblastoma with the appearance of arteriovenous malformation: pitfalls in diagnosis. Clin Neurol Neurosurg. 2013;115:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Nagańska E, Matyja E, Pucko E, Ząbek M. The coexistence of pleomorphic xanthoastrocytoma and arteriovenous malformation. A case report. Folia Neuropathol. 2013;51:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Xhumari A, Rroji A, Enesi E, Bushati T, Sallabanda Diaz K, Petrela M. Glioblastoma after AVM radiosurgery. Case report and review of the literature. Acta Neurochir (Wien). 2015;157:889-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Imai T, Ohshima T, Nishizawa T, Shimato S, Kato K. Successful Preoperative Endovascular Embolization of an Extreme Hypervascular Glioblastoma Mimicking an Arteriovenous Malformation. World Neurosurg. 2016;86:512.e1-512.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Chen J, Chen L, Zhang C, He J, Li P, Zhou J, Zhu J, Wang Y. Glioma coexisting with angiographically occult cerebrovascular malformation: A case report. Oncol Lett. 2016;12:2545-2549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Lohkamp LN, Strong C, Rojas R, Anderson M, Laviv Y, Kasper EM. Hypervascular glioblastoma multiforme or arteriovenous malformation associated Glioma? A diagnostic and therapeutic challenge: A case report. Surg Neurol Int. 2016;7:S883-S888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Tunthanathip T, Kanjanapradit K. Glioblastoma Multiforme Associated with Arteriovenous Malformation: A Case Report and Literature Review. Ann Indian Acad Neurol. 2020;23:103-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Li CR, Chen SY, Yang MY, Cheng WY, Shen CC. A Rare Triploid Involving the Coexistence of Glioblastoma Multiforme, Arteriovenous Malformation and Intracranial Aneurysm: Illustrative Case and Literature Review. Medicina (Kaunas). 2023;59. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Faham B, Nsengiyumva EO, Jamal O, Makhchoune M, Naja A, Lakhdar A. Glioblastoma simulating an arteriovenous malformation: a case report. Ann Med Surg (Lond). 2023;85:2977-2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Thomas JM, Sasankan D, Surendran S, Abraham M, Rajavelu A, Kartha CC. Aberrant regulation of retinoic acid signaling genes in cerebral arterio venous malformation nidus and neighboring astrocytes. J Neuroinflammation. 2021;18:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |