Published online Aug 28, 2024. doi: 10.4329/wjr.v16.i8.337
Revised: August 6, 2024
Accepted: August 15, 2024
Published online: August 28, 2024
Processing time: 96 Days and 23.6 Hours
Postoperative aortobronchial fistula (ABF) is a rare complication that can occur in 0.3%-5.0% of patients over an extended period of time after thoracic aortic surgery. Direct visualization of the fistula via imaging is rare.
To investigate the relationship between computed tomography (CT) findings and the clinical signs/symptoms of ABF after thoracic aortic surgery.
Six patients (mean age 71 years, including 4 men and 2 women) with suspected ABF on CT (air around the graft) at our hospital were included in this retro
ABF detection after surgery was found to have a mean and median of 14 and 13 years, respectively. Initial signs and symptoms were asymptomatic in 4 patients, bloody sputum was found in 1 patient, and fever was present in 1 patient. The complications of ABF included graft infection in 2 patients and graft infection with hemoptysis in 2 patients. Of the 6 patients, 3 survived, 2 died, and 1 was lost to follow-up. The locations of the ABFs were as follows: 1 in the ascending aorta; 1 in the aortic arch; 2 in the aortic arch leading to the descending aorta; and 2 in the descending aorta. ABFs were directly confirmed by CT in 4/6 (67%) patients. Peri-graft dirty fat (4/6, 67%) and peri-graft ring enhancement (3/6, 50%) were associated with graft infection, endoleaks and pseudoaneurysms were associated with hemoptysis (2/6, 33%).
Asymptomatic ABF after thoracic aortic surgery can be confirmed on chest CT. CT is useful for the diagnosis of ABF and its complications.
Core Tip: This retrospective study included six patients with aortobronchial fistula based on computed tomography (CT) findings of peri-graft air, with aortobronchial fistula being detected a mean of 14 years after thoracic aortic surgery. Asymptomatic aortobronchial fistula can be confirmed on chest CT as direct communication between dilated peripheral bronchi and peri-graft air. CT is useful for the diagnosis of aortobronchial fistula and its complications.
- Citation: Tsuchiya N, Inafuku H, Yogi S, Iraha Y, Iida G, Ando M, Nagano T, Higa S, Maeda T, Kise Y, Furukawa K, Yonemoto K, Nishie A. Direct visualization of postoperative aortobronchial fistula on computed tomography. World J Radiol 2024; 16(8): 337-347
- URL: https://www.wjgnet.com/1949-8470/full/v16/i8/337.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i8.337
Postoperative aortobronchial fistula (ABF) is a rare complication that occurs in 0.4%-5% of patients after thoracic aortic surgery[1-4]. Hemoptysis of varying degrees is the typical manifestation of ABF[5]. Other symptoms include chest or back pain and dyspnea. Imaging findings of ABF include indirect signs such as periaortic hematoma, pulmonary hemorrhage around the aorta (focal ground-glass opacities), signs of graft infection (periaortic enhancement and effusion, air in the vessel wall, increased density of fat tissue) and/or stent deviation[5,6]. Direct visualization of the fistula by imaging, including computed tomography (CT) and bronchoscopy, is rare[7]. The results of conservative treatment for ABF have been poor (50% in-hospital mortality rate), and treatments have included surgical or thoracic endovascular aortic repair (TEVAR) repair or lung resection[5]. ABF with an infectious cause always has a poor prognosis[5].
The existence of air in the vessel wall after a thoracic aortic procedure (peri-graft air) suggests ABF with or without graft infection. While there are severe ABF cases with hemoptysis and infection, we treated asymptomatic cases where we identified peri-graft air on CT. The asymptomatic ABFs that are found incidentally as peri-graft air on CT have not been investigated, and how to manage these ABFs remains unclear. Because high-resolution CT has recently been used to detect small fistulas such as bronchopleural fistulas, we hypothesize that ABFs can also be visualized directly on CT.
The purpose of this study was to determine the relationship between CT findings and clinical symptoms of ABF in patients after thoracic aortic surgery, with a particular focus on peri-graft air and direct visualization of ABFs on CT.
The institutional review board approved this single-center retrospective observational study and waived informed consent.
The study participants were retrospectively selected from patients seen at our institution between January 2004 and September 2022. The radiology picture archiving and communication system (PACS) was initially used to select patients, and selection was further refined by review of patients’ electronic medical records. The study inclusion criterion included peri-graft air on chest CT after thoracic aorta surgery. The exclusion criterion included peri-graft air caused by a condition other than ABF (e.g., postoperative changes immediately after surgery, aorto-cutaneous fistula, aorto-intestinal fistula, or pseudo-ABF due to positive-pressure ventilation). Of 166 reports by radiologists were reviewed to identify the presence of peri-graft air on chest CT. Clinical data were extracted from the participants’ medical records and included the following: Age, sex, primary disease for which thoracic aortic surgery was indicated, operative procedure, severe complications (graft infection/hemoptysis), treatments for severe complications, outcomes, durations between surgery and CT, durations between severe complications and CT, and symptoms when CT was performed. The diagnosis of graft infection was determined by cardiovascular surgeons and radiologists and was based on symptoms of infection, bacteremia, and findings on gallium scintigraphy or positron emission tomography.
Chest CT with or without contrast medium was performed as part of a routine clinical evaluation. Three different scanners were used: LightSpeed VCT (64-row scanner, GE Medical Systems, Milwaukee, WI, United States), Aquilion ONE (320-row scanner, Canon Medical Systems, Otawara, Japan) and Aquilion precision (160-row scanner, Canon Medical Systems, Otawara, Japan). CT scans were performed while the patient held their breath at full inspiration while the patient was in the supine position. For CT angiography (CTA), iodine-based contrast medium (100 mL, iopamiron injection 370 syringe, Bayer Holding Ltd. Japan) was administered at a rate of 5.0 mL/s via a peripheral venous line. Automatic bolus tracking was performed in the descending aorta at a level of Th7 with a trigger of 250 Hounsfield units. Following CTA, the late phase was obtained 70 seconds later. The scanning parameters were as follows: Voltage, 120 kVp; Current, automatic exposure control; Collimation, 0.5 (Canon) or 0.625 mm (GE); Rotation time, 0.5 second; Matrix, 512 × 512; and Slice thickness, 1 (Canon) or 1.25 mm (GE).
All CT images known to have peri-graft air as a finding suggestive of ABF were selected from each patient and were imaged during the following times over the course of the fistula: (1) Initial CT with peri-graft air; (2) At the onset of severe complications; and (3) The latest CT during follow-up. The CT images were evaluated independently by two board-certified diagnostic radiologists who were blinded to the clinical information. Discrepancies were resolved by consensus. The readers used the reconstruction function of the interpretation system to evaluate the images. The following findings of peri-graft air and the surrounding region were tallied by each reader: (1) Location of peri-graft air; (2) Fluid collection; (3) Ring enhancement; (4) Direct confirmation of ABF; (5) Endoleak; (6) Pseudoaneurysm; (7) Bronchodilation; (8) Atelectasis; (9) Dirty fat sign; and (10) Pulmonary hemorrhage. The following findings were evaluated only on enhanced CT images: (1) Ring enhancement; (2) Endoleak; and (3) Pseudoaneurysm.
Descriptive statistics were employed to characterize the occurrence of each imaging finding. JMP-pro15 software (SAS Institute Japan, Tokyo, Japan) was used for statistical analysis.
During the study period, we identified 203 patients from the PACS database with peri-graft air on CT after thoracic aorta surgery. A total of 195 patients with peri-graft air as a postoperative change were excluded. We excluded an additional 2 patients with an aortocutaneous fistula and pseudo-ABF due to positive pressure ventilation (Figure 1). The size of the study population was fixed, because of the retrospective nature of the study, and a total of 6 patients (4 males and 2 females; mean age, 71 years; age range, 57-83) were ultimately included in this study.
Patient characteristics are summarized in Table 1. Peri-graft air was detected at a mean of 14 and median of 13 years after surgery. The initial symptoms were asymptomatic in 4 patients (67%), hemoptysis in 1 patient, and fever in 1 patient. Severe complications included graft infections in 4 patients (66.6%) and hemoptysis in 3 patients (50%). Two patients developed graft infection and hemoptysis 2 years and 7 years after asymptomatic ABF was detected, respectively. There were 2 deaths (33.3%), 3 survivors (50%), and 1 patient lost to follow-up (16.7%), with a mean observation period of 7 years from the initial detection of peri-graft air on chest CT. Patient details are summarized in Table 2.
| Characteristics | n | Precent | |
| Age | Mean age (years) | 71 | |
| Gender | Male | 4/6 | 66.7 |
| Female | 2/6 | 33.3 | |
| Surgery | Aortic replacement | 2/6 | 33.3 |
| Aortic replacement + TEVAR | 3/6 | 50 | |
| TEVAR | 1/6 | 16.7 | |
| Mean period surgery to ABF detection | 14 years | ||
| Complication | Hemoptysis | 3/6 | 50 |
| Graft infection | 4/6 | 66.6 | |
| Mean period surgery to complication | 15.5 years | ||
| Mean period ABF to complication | 4 years | ||
| Treatment | Conservative treatment | 4/6 | 66.7 |
| Abscess drainage | 1/6 | 16.7 | |
| Aortic replacement | 1/6 | 16.7 | |
| Outcome | Deaths | 2/6 | 33.3 |
| Alive | 3/6 | 50 | |
| Lost to follow-up | 1/6 | 16.7 | |
| Mean observation period | 7 years | ||
| Case | Primary disease | Surgery | Period: Surgery to ABF | Complication | Period ABF to complication | Treatment | Outcome | Observation period |
| F: 73 years | Dissection Marfan syndrome | Replacement + TEVAR | 5 years | No | No | No | Alive | 5 months |
| F: 74 years | Aneurysm | Replacement TEVAR (10 years after replacement) | 10 years (1 year) | Graft infection hemoptysis | 2 years | Drainage | Died hemoptysis | 6 years |
| M: 67 years | Aortic coarctation Aneurysm | Replacement | 26 years | Graft infection hemoptysis | Simultaneous | Conservative | Alive | 2 years |
| M: 73 years | Infected aneurysm | TEVAR | 1 year | Graft infection | Simultaneous | Conservative | Died sepsis | 1 year |
| M: 57 years | Aneurysm | Replacement TEVAR (14 years later from replacement) | 16 years (3 years) | Graft infection hemoptysis | 7 years | Replacement TEVAR | Alive | 18 years |
| M: 83 years | Aneurysm | Replacement | 23 years | No | No | No | Lost to follow-up | 7 years |
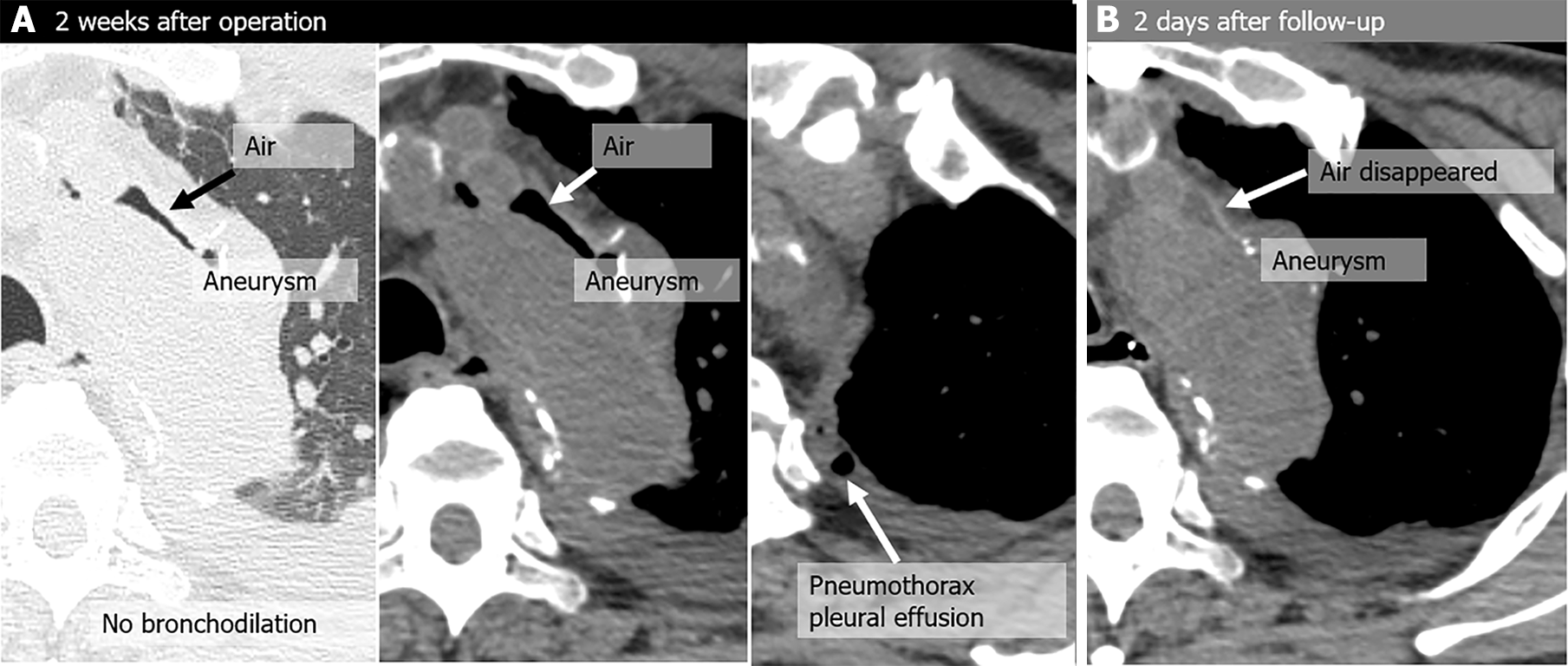
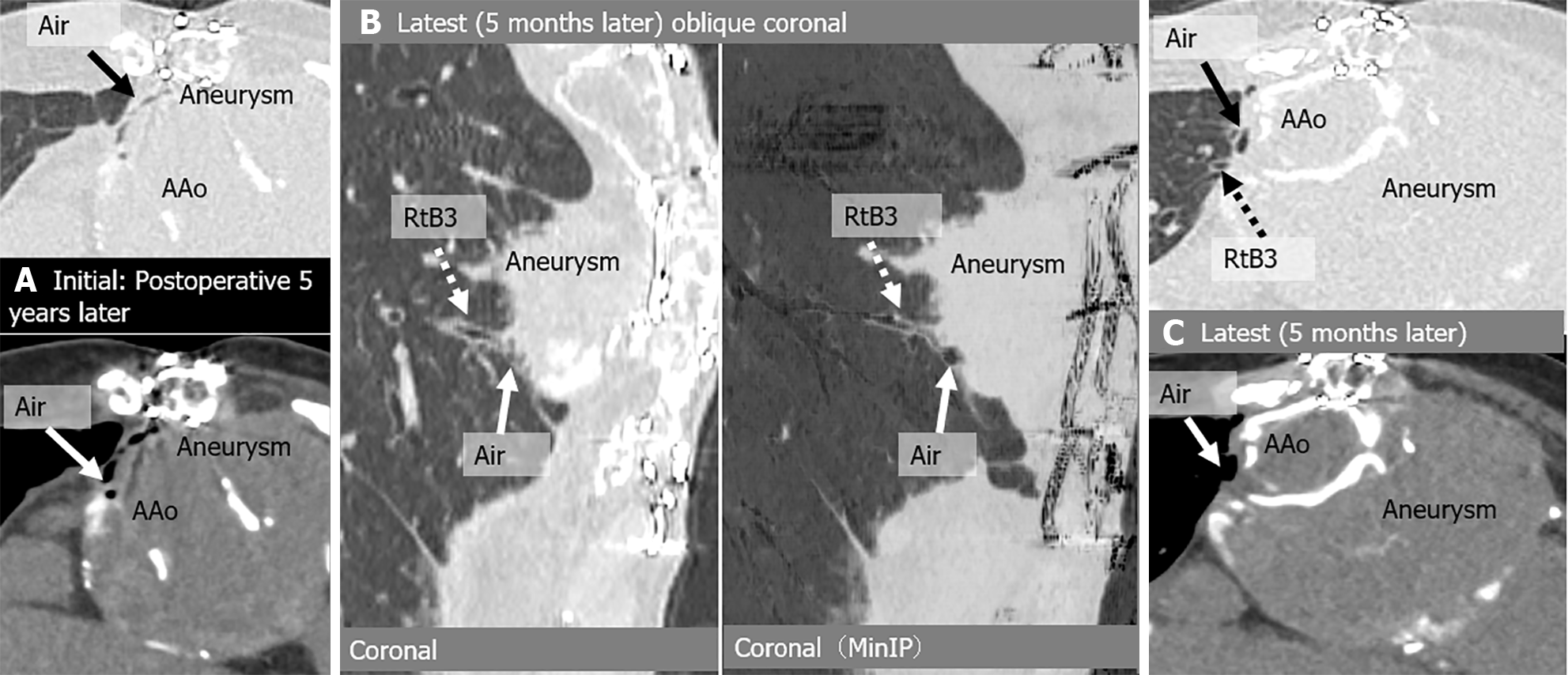
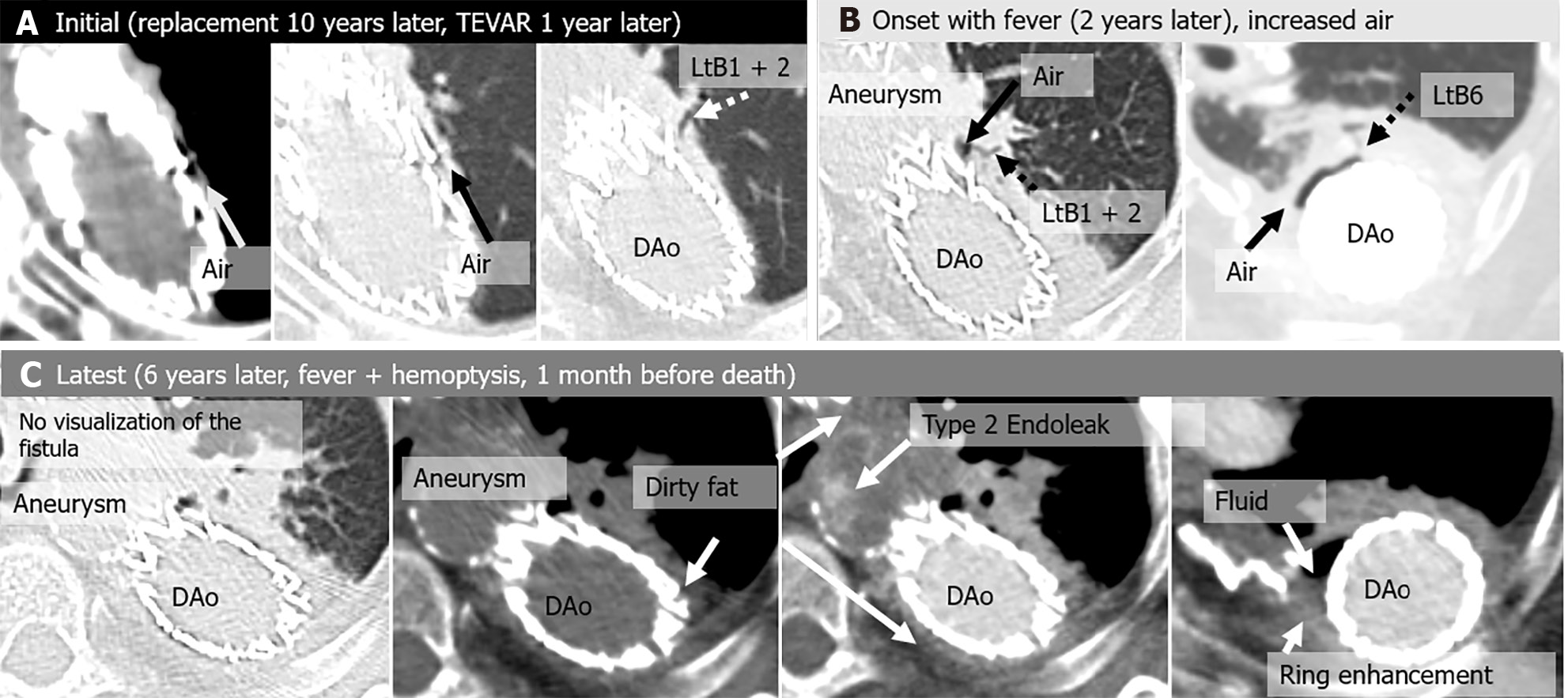
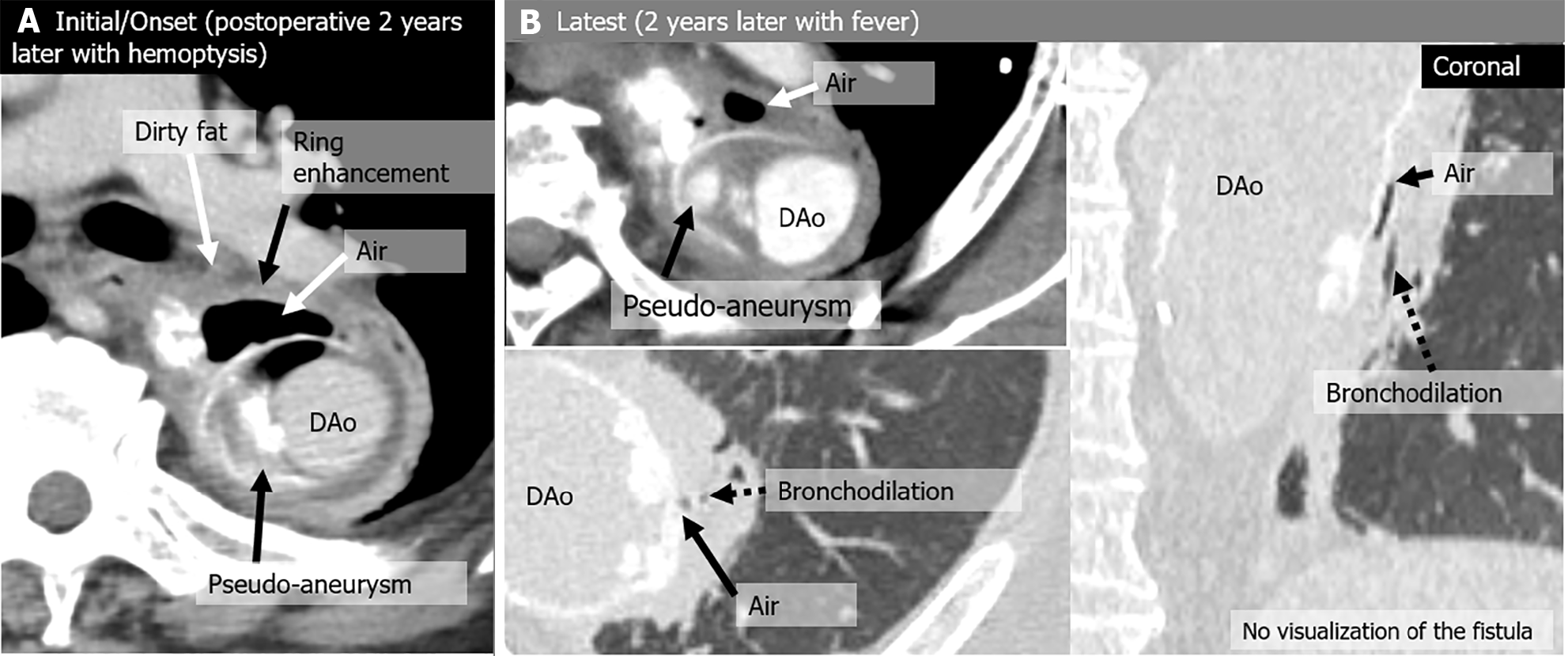
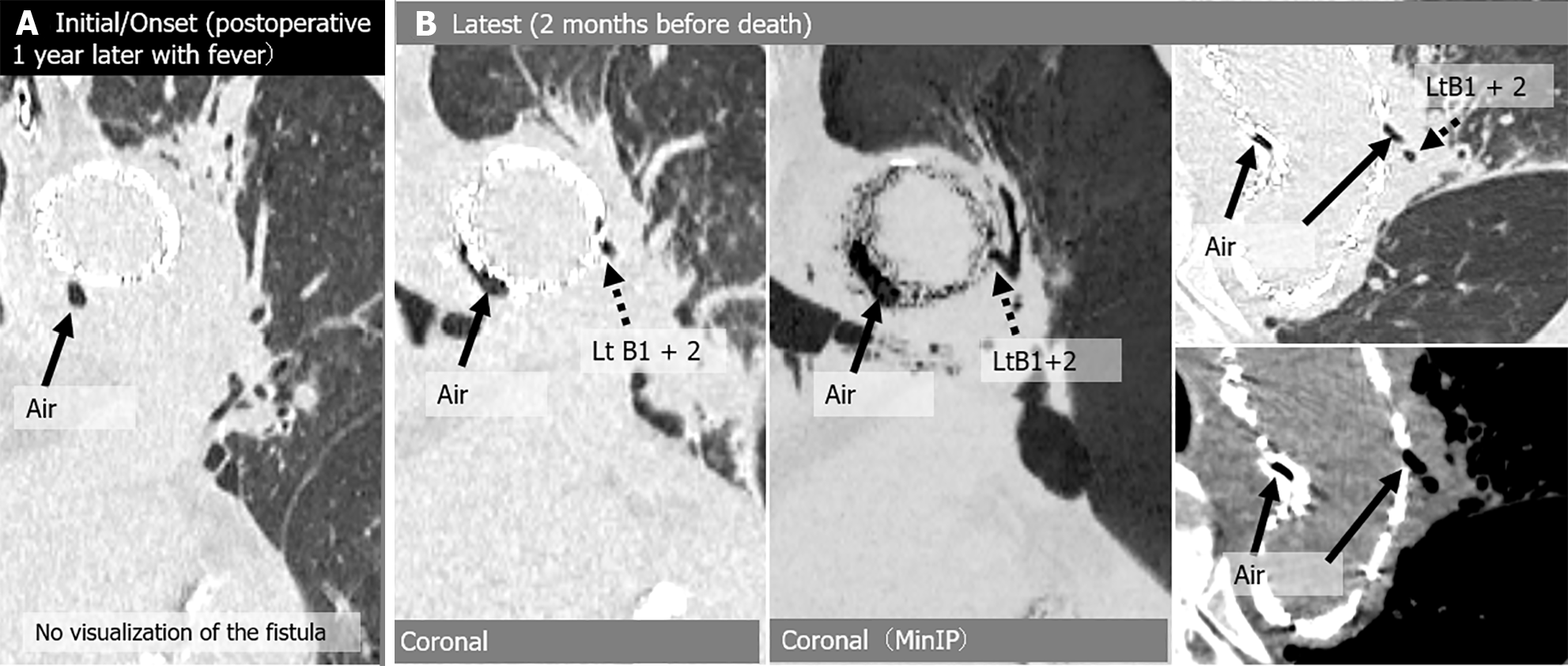
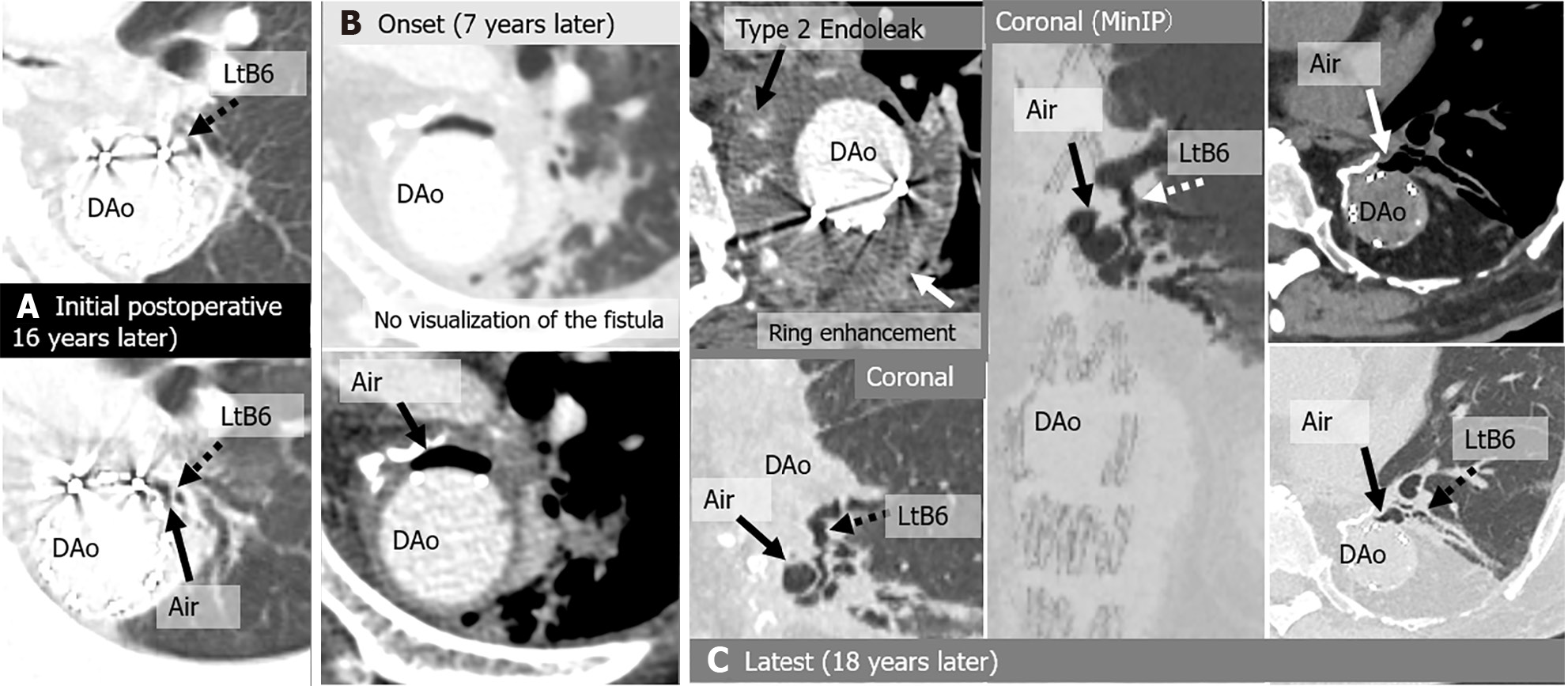
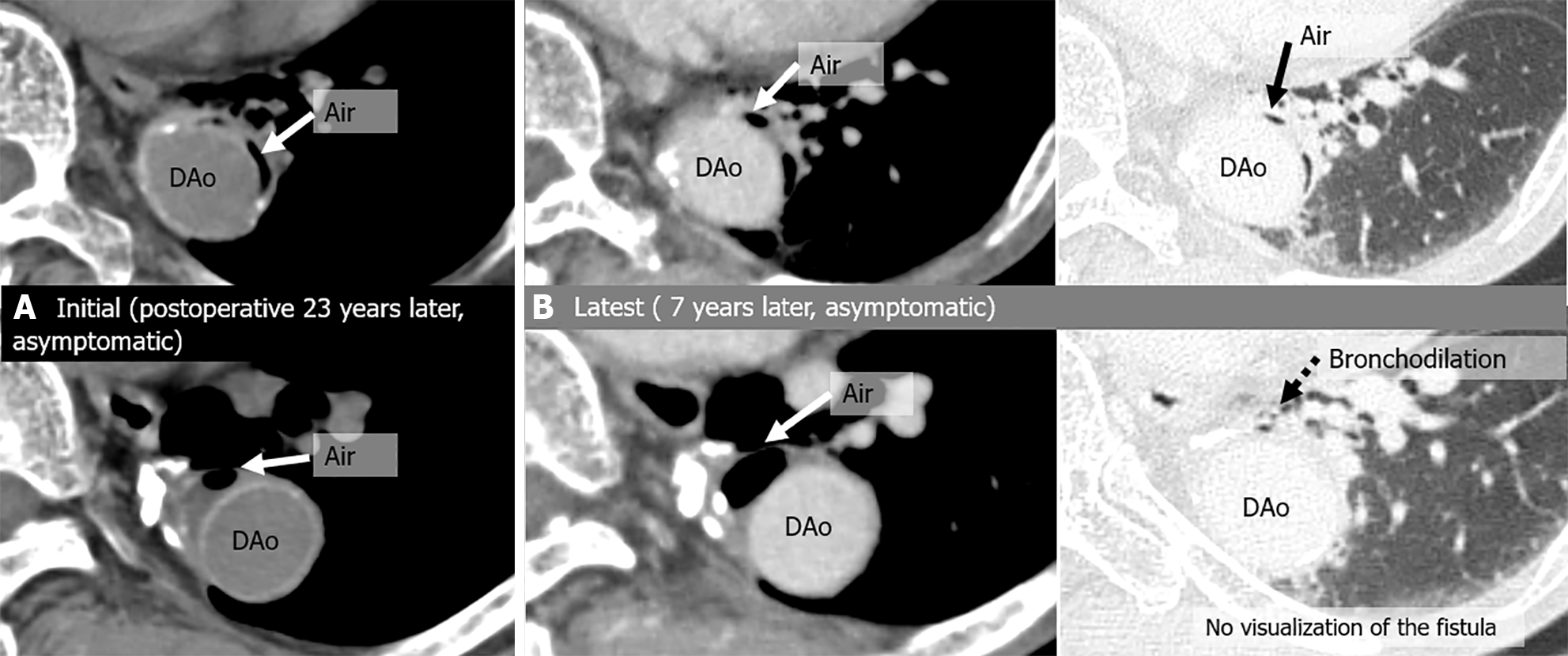
A total of 112 chest CT exams were performed on the 6 patients after they were initially diagnosed with peri-graft air on chest CT. During follow-up, the amount of peri-graft air increased or decreased in all the study patients. The locations of the ABFs were as follows: 1 in the ascending aorta 1 in the aortic arch; 2 in the aortic arch leading to the descending aorta; and 2 in the descending aorta. ABFs were directly confirmed on CT scans in 4 of the 6 (67%) patients, with a predominant appearance on the left side (3/4, 75%). Among the 112 CT exams, the following 16 were analyzed in detail: 6 initial scans (2 of which were also the onset of severe complications), 4 scans at the onset of severe complications, and 6 scans at the latest follow-up (Table 3 and Table 4). ABF was directly confirmed on CT scans in 7 of 14 exams (50%). Direct confirmation of ABF was not possible for 5 of the 7 scans of symptomatic patients (71.4%). Peri-graft dirty fat (4/4, 100%) and peri-graft ring enhancement (3/3, 100%) were associated with graft infections. Endoleak and pseudoaneurysm were associated with hemoptysis (2/2, 100%). The CT images of the 6 patients are shown in Figures 2, 3, 4, 5, 6 and 7.
| Initial CT (n = 6) | Onset CT (n = 4) | Follow-up CT (n = 6) | |||||
| n | % | n | % | n | % | ||
| Symptoms | Asymptomatic | 4/6 | 66.7 | - | 3/6 | 50 | |
| Fever | 1/6 | 16.7 | 3/4 | 75 | 3/6 | 50 | |
| Hemoptysis | 1/6 | 16.7 | 1/4 | 25 | 1/6 | 16.7 | |
| Locations of ABF | Ascending aorta | 1/6 | 16.7 | 0/4 | 0 | 1/6 | 16.7 |
| Aortic arch | 2/6 | 33.3 | 1/4 | 25 | 1/6 | 16.7 | |
| Aortic arch descending aorta | 1/6 | 16.7 | 2/4 | 50 | 2/6 | 33.3 | |
| Descending aorta | 2/6 | 33.3 | 1/4 | 25 | 2/6 | 33.3 | |
| Image findings | Direct confirmation of ABF | 3/6 | 50 | 1/4 | 25 | 3/6 | 50 |
| Fluid collection | 4/6 | 66.7 | 4/4 | 100 | 4/6 | 66.7 | |
| Ring enhancement | 1/2 | 50 | 3/3 | 100 | 1/2 | 50 | |
| Endoleak/Pseudoaneurysm | 1/2 | 50 | 3/3 | 100 | 1/2 | 50 | |
| Bronchodilation | 6/6 | 100 | 4/4 | 100 | 6/6 | 100 | |
| Atelectasis | 5/6 | 83.3 | 4/4 | 100 | 5/6 | 83.3 | |
| Dirty fat sign | 2/6 | 33.3 | 4/4 | 100 | 3/6 | 50 | |
| Pulmonary hemorrhage | 0/6 | 0 | 0/4 | 0 | 0/6 | 0 | |
| Case | CT | Fever | Hemoptysis | Peri-graft air location | Around graft with air (peri-graft air) | |||||||
| Fluid | Ring enhancement | Endoleak/Pseudo-aneurysm | Direct confirmation of ABF | Bronchodilation | Atelectasis | Dirty fat sign | Pulmonary hemorrhage | |||||
| 1 | Initial | No | No | As | No | - | - | Rt B3 | Rt B3 | No | No | No |
| Latest | No | No | As | No | - | - | Rt B3 | Rt B3 | No | No | No | |
| 2 | Initial | No | No | Arc | (+) | - | - | Lt B1 + 2 | Lt B1 + 2 | (+) | No | No |
| Onset | (+) | No | Arc-Des | (+) | (+) | Type 2 endoleak | Lt B1 + 2, B6 | Lt B1 + 2, B6 | (+) | (+) | No | |
| Latest | (+) | (+) | Arc-Des | (+) | (+) | Type 2 endoleak | No | (+) | (+) | (+) | No | |
| 3 | Initial/Onset | No | (+) | Arc-Des | (+) | (+) | Pseudoaneurysm | No | (+) | (+) | (+) | No |
| Latest | (+) | No | Arc-Des | (+) | - | - | No | (+) | (+) | (+) | No | |
| 4 | Initial/Onset | (+) | No | Arc | (+) | - | - | No | (+) | (+) | (+) | No |
| Latest | (+) | No | Arc | (+) | - | - | Lt B1 + 2 | Lt B1 + 2 | (+) | (+) | No | |
| 5 | Initial | No | No | Des | No | No | No | Lt B6 | Lt B6 | (+) | No | No |
| Onset | (+) | (+) | Des | (+) | (+) | Type 2 endoleak | No | Lt B6 | (+) | (+) | No | |
| Latest | No | No | Des | No | - | - | Lt B6 | Lt B6 | (+) | No | No | |
| 6 | Initial | No | No | Des | (+) | - | - | No | (+) | (+) | No | No |
| Latest | No | No | Des | (+) | No | No | No | (+) | (+) | No | No | |
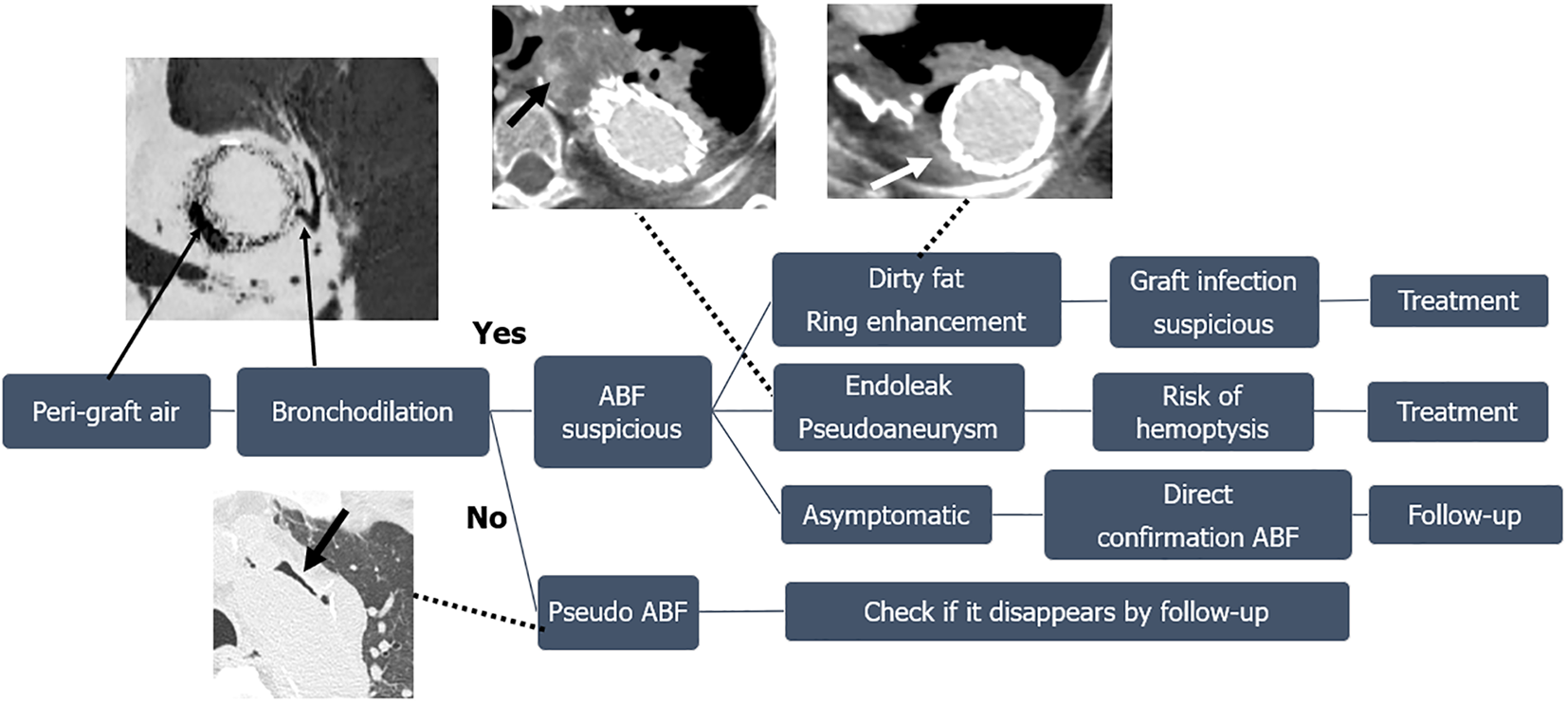
This study revealed that asymptomatic ABFs occurring after thoracic aortic surgery can be directly confirmed by chest CT. Patients with ABFs accompanied by complications had CT scans with specific imaging findings in addition to clinical symptoms; important imaging findings included the presence of endoleaks and pseudoaneurysms in patients with hemoptysis and peri-graft dirty fat and peri-graft ring enhancement in patients with graft infections. Figure 8 shows a flow chart based on our results of the steps of a diagnostic assessment identifying peri-graft air after surgery on the thoracic aorta.
Von Segesser et al[1] reported that ABFs occurred in 8 (5.6%) of 145 patients who underwent surgery for descending thoracic aorta/thoracoabdominal aortic aneurysms. The prevalence of ABFs has increased in parallel with the spread of TEVAR. The rates of occurrence of ABFs after TEVAR were reported by Luehr et al[2] Chiesa et al[3] and Czerny et al[4] to be 0.5%, 0.4%, and 0.6%, respectively. These studies focused on patients with symptomatic ABFs and did not include patients with asymptomatic ABFs. In our study, more than half of the patients diagnosed with ABF because of peri-graft air on CT were asymptomatic, indicating that the frequencies of ABFs reported in the past may be underestimated. ABF has been reported to occur as early as 3 weeks and as late as 25 years after interventions[7]. Our study revealed that the intervals between the time of operation and the onset of ABFs ranged from 1 to 26 (mean 15.5) years after aortic procedures.
In most cases, ABF was recognized as a late effect several years after surgery, but only one case occurred within 1 year, and in that case, the underlying disease was an infected aortic aneurysm. In infected aortic aneurysms, an ABF may occur preoperatively, and there is also a risk of ABF recurrence postoperatively. Half of the patients who had asymptomatic ABFs remained asymptomatic, while the other half developed complications several years after surgery. In other words, the finding of peri-graft air does not necessarily mean the need for immediate treatment, but it is a sign that careful follow-up is required.
Direct visualization of the fistula on CT is rare[7]; however, we found that an ABF was directly confirmed on CT scans in 50% of our patients, and CT contributed to the definitive diagnosis of ABF. When limited to patients with symptomatic ABF and complications, the rate of direct visualization of ABF on CT scans was as low as 29%. We speculate that pus and blood accumulate in the ABF when symptoms are present. A possible reason why it has been reported that it is difficult to visualize ABF directly on CT images is that only patients with symptomatic ABFs were examined by CT.
Dilation of the peripheral bronchi near peri-graft air was observed in all our patients and is an important diagnostic finding for ABFs. We excluded one patient with pseudo-ABF due to positive-pressure ventilation from this study because the patient’s scan did not show signs of bronchodilation (Figure 1). A case of pseudo-ABF triggered by increased airway pressure has been reported[8]. It is necessary to distinguish a pseudo-ABF from a true ABF that can cause complications, and the absence of bronchodilation may be the key imaging finding.
Peri-graft atelectasis was also observed in almost all our patients. Continuous stimulation and compression of the lung by adjacent lesions such as an aortic aneurysm or pseudoaneurysm, surgical sutures, and aortic stent grafts lead to inflammation, scarring, and ultimately ABFs[7]. Atelectasis around a graft is one of the CT findings that can lead to the formation of an ABF, and the finding of dilated peripheral bronchi within the region of atelectasis may be a risk for ABF.
An important imaging finding of hemoptysis caused by an ABF is the presence of an endoleak or a pseudoaneurysm. Bleeding from an endoleak or a pseudoaneurysm reaches the airways via the ABF and presents as hemoptysis. CTA is essential for evaluating endoleaks and pseudoaneurysms and should be performed in patients with hemoptysis.
Pulmonary hemorrhage can be evaluated by plain CT, but in this study, none of the patients experienced pulmonary hemorrhage. Therefore, pulmonary hemorrhage may be less common as an indirect finding of ABF.
Among the signs suggestive of graft infection, peri-graft dirty fat sign and peri-graft ring enhancement are helpful. Fluid collection around the graft does not always indicate graft infection; contrast-enhanced CT is required to show that the fluid collection is due to an abscess.
This study has several limitations. First, this was a retrospective single-center study with a small number of patients. Second, CT imaging protocols, such as the use of contrast agents, are inconsistent, resulting in missing values. Third, it was difficult to establish a strict definition of ABF in this study. However, in terms of the findings used for diagnosis, our patients diagnosed with ABF were not inconsistent to previous ABF-reported cases. Finally, treatment strategies for our patients were affected by various factors, such as the patient's condition, wishes, and underlying disease; therefore, the imaging findings are just one of the references.
CT imaging can detect incidental asymptomatic ABFs occurring after surgery on the thoracic aorta, allowing for early diagnosis and intervention before the onset of severe symptoms. Early detection may improve patient outcomes and reduce the risk of complications.
| 1. | von Segesser LK, Tkebuchava T, Niederhäuser U, Künzli A, Lachat M, Genoni M, Vogt P, Jenni R, Turina MI. Aortobronchial and aortoesophageal fistulae as risk factors in surgery of descending thoracic aortic aneurysms. Eur J Cardiothorac Surg. 1997;12:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Luehr M, Etz CD, Nozdrzykowski M, Garbade J, Lehmkuhl L, Schmidt A, Misfeld M, Borger MA, Mohr FW. Emergency open surgery for aorto-oesophageal and aorto-bronchial fistulae after thoracic endovascular aortic repair: a single-centre experience†. Eur J Cardiothorac Surg. 2015;47:374-82; discussion 382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Chiesa R, Melissano G, Marone EM, Marrocco-Trischitta MM, Kahlberg A. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg. 2010;39:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Czerny M, Eggebrecht H, Sodeck G, Weigang E, Livi U, Verzini F, Schmidli J, Chiesa R, Melissano G, Kahlberg A, Amabile P, Harringer W, Horacek M, Erbel R, Park KH, Beyersdorf F, Rylski B, Blanke P, Canaud L, Khoynezhad A, Lonn L, Rousseau H, Trimarchi S, Brunkwall J, Gawenda M, Dong Z, Fu W, Schuster I, Grimm M. New insights regarding the incidence, presentation and treatment options of aorto-oesophageal fistulation after thoracic endovascular aortic repair: the European Registry of Endovascular Aortic Repair Complications. Eur J Cardiothorac Surg. 2014;45:452-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Yuan SM. Aortobronchial fistula. Gen Thorac Cardiovasc Surg. 2020;68:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Gulati A, Kapoor H, Donuru A, Gala K, Parekh M. Aortic Fistulas: Pathophysiologic Features, Imaging Findings, and Diagnostic Pitfalls. Radiographics. 2021;41:1335-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Picichè M, De Paulis R, Fabbri A, Chiariello L. Postoperative aortic fistulas into the airways: etiology, pathogenesis, presentation, diagnosis, and management. Ann Thorac Surg. 2003;75:1998-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Yie K. Cough, Aortobronchial Fistula, and Air Migration in the Remnant Aneurysm Sac: An Unforeseen Path? Ann Vasc Dis. 2020;13:93-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |