Published online Aug 28, 2024. doi: 10.4329/wjr.v16.i8.294
Revised: August 22, 2024
Accepted: August 27, 2024
Published online: August 28, 2024
Processing time: 22 Days and 10.3 Hours
Jaw and maxillofacial bone lesions encompass a wide variety of both neoplastic and non-neoplastic pathologies. These lesions can arise from various tissues, including bone, cartilage, and soft tissue, each presenting distinct challenges in diagnosis and treatment. While some pathologies exhibit characteristic imaging features that aid in diagnosis, many others are nonspecific. This overlap often necessitates a multimodal imaging approach, combining techniques such as radiographs, computed tomography, and magnetic resonance imaging to achieve a diagnosis or narrow the diagnostic considerations. This article provides a comprehensive review of the imaging approach to jaw and maxillofacial bone tumors, including updates on the 2022 World Health Organization classification of these tumors. The relevant anatomy of the jaw and dental structures that is important for accurate imaging interpretation is discussed.
Core Tip: The imaging approach to jaw and maxillofacial bone tumors is multifaceted and pivotal in accurately diagnosing these lesions. Achieving accurate diagnosis and effective management requires a comprehensive understanding of jaw and dental anatomy, coupled with a nuanced interpretation of imaging modalities. Computed tomography scans are the primary tool for evaluating these lesions, offering detailed information on lesion size, shape, location, margins, internal matrix, and involvement of adjacent teeth. Magnetic resonance imaging complements this by providing high-resolution soft tissue contrast. Key imaging features for interpreting jaw and maxillofacial bone lesions include radiodensity, marginal definition, loculation pattern, relationship to adjacent teeth, erosion of teeth or bone, internal matrix appearance, patterns of osseous expansion, and the presence of soft tissue components. Among these, radiodensity is particularly important as it helps determine the nature of the jaw lesions and guides the diagnostic process.
- Citation: Choi WJ, Lee P, Thomas PC, Rath TJ, Mogensen MA, Dalley RW, Wangaryattawanich P. Imaging approach for jaw and maxillofacial bone tumors with updates from the 2022 World Health Organization classification. World J Radiol 2024; 16(8): 294-316
- URL: https://www.wjgnet.com/1949-8470/full/v16/i8/294.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i8.294
Jaw and maxillofacial bone lesions encompass a wide variety of both neoplastic and non-neoplastic pathologies[1-5]. These lesions can arise from various tissues, including bone, cartilage, and soft tissue, each presenting distinct challenges in diagnosis and treatment. While some pathologies exhibit characteristic imaging features that aid in diagnosis, many others are nonspecific[1-4,6,7]. This overlap often necessitates a multimodal imaging approach, combining techniques such as radiographs, computed tomography (CT), and magnetic resonance imaging (MRI) to achieve a more accurate diagnosis.
Radiologists play a pivotal role in assessing these lesions and providing potential differential diagnoses to guide patient management. Their expertise not only helps in identifying the nature of the lesion but also in determining its extent and involvement with surrounding structures, which are crucial for planning surgical or therapeutic interventions. A thorough understanding of the anatomy and the spectrum of pathologies in this region is vital for interpreting imaging accurately.
This article aims to provide a comprehensive review of imaging approaches for jaw and maxillofacial bone tumors, serving as a valuable resource for radiologists, oral surgeons, and other healthcare professionals when encountering these lesions. It includes an overview of the anatomy of the jaw and dental structures, which is essential for a better understanding of these pathologies. Additionally, the article covers the latest updates from the 2022 World Health Organization (WHO) classification of these tumors. This classification system standardizes the diagnosis and categorization of lesions, improving communication among healthcare providers and supporting research and treatment strategies.
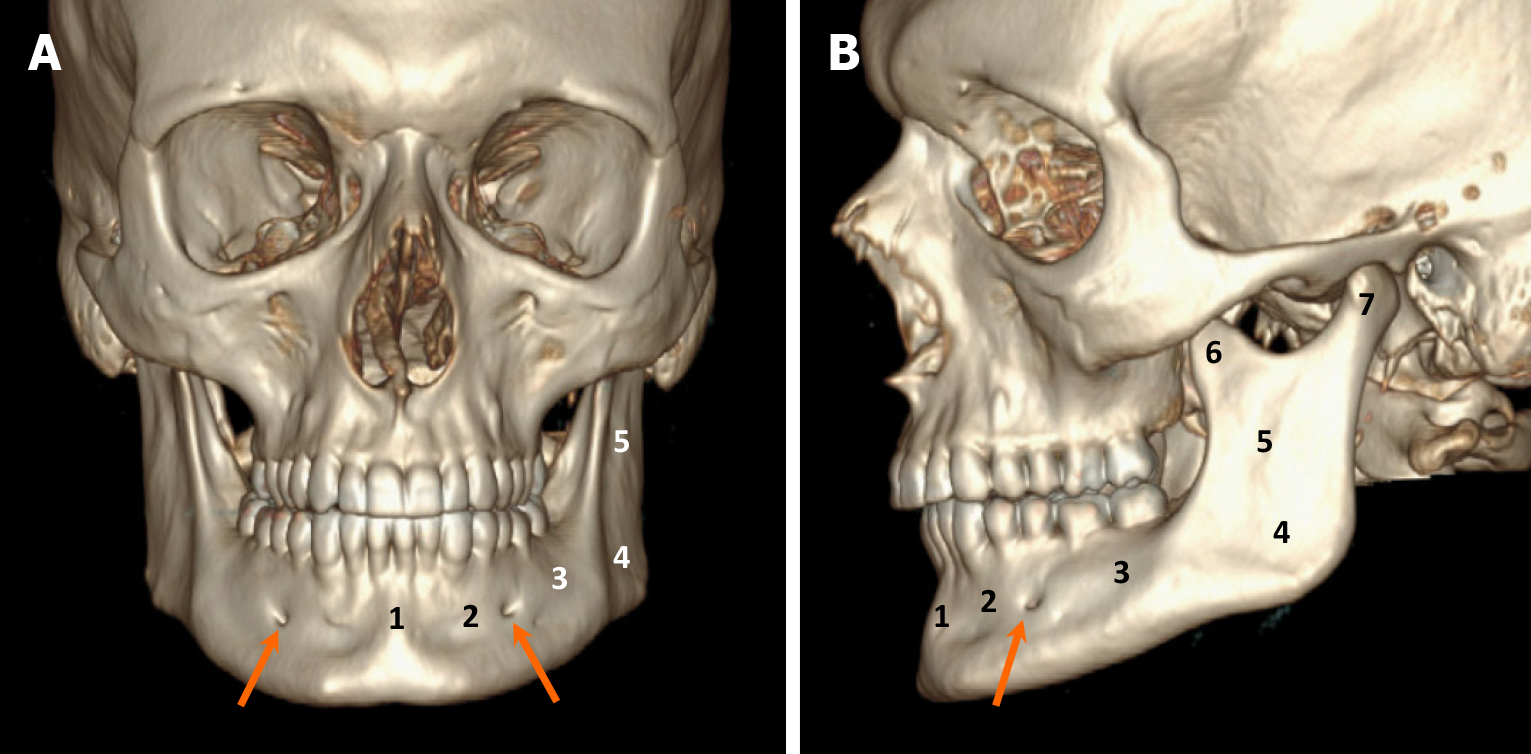
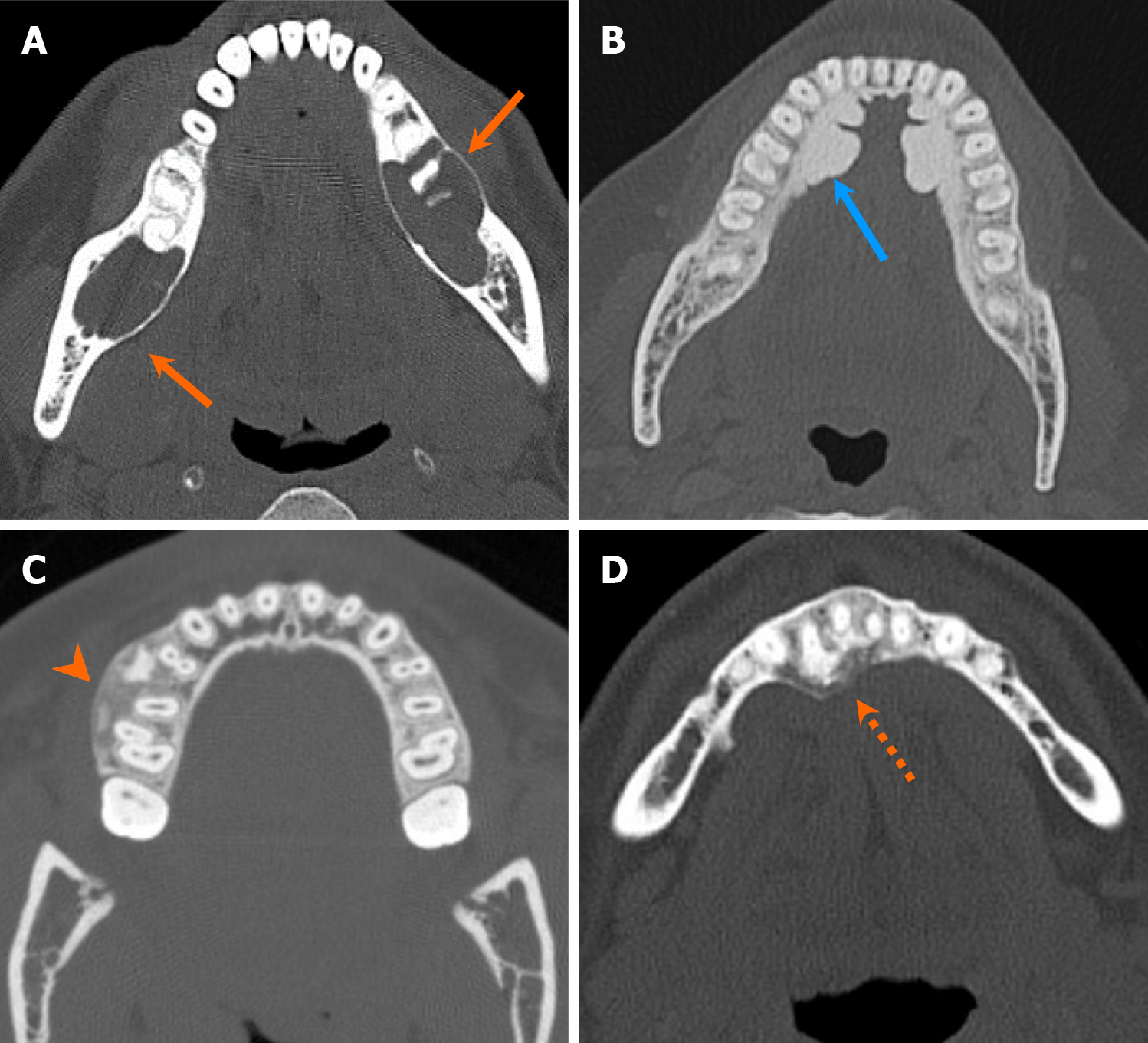
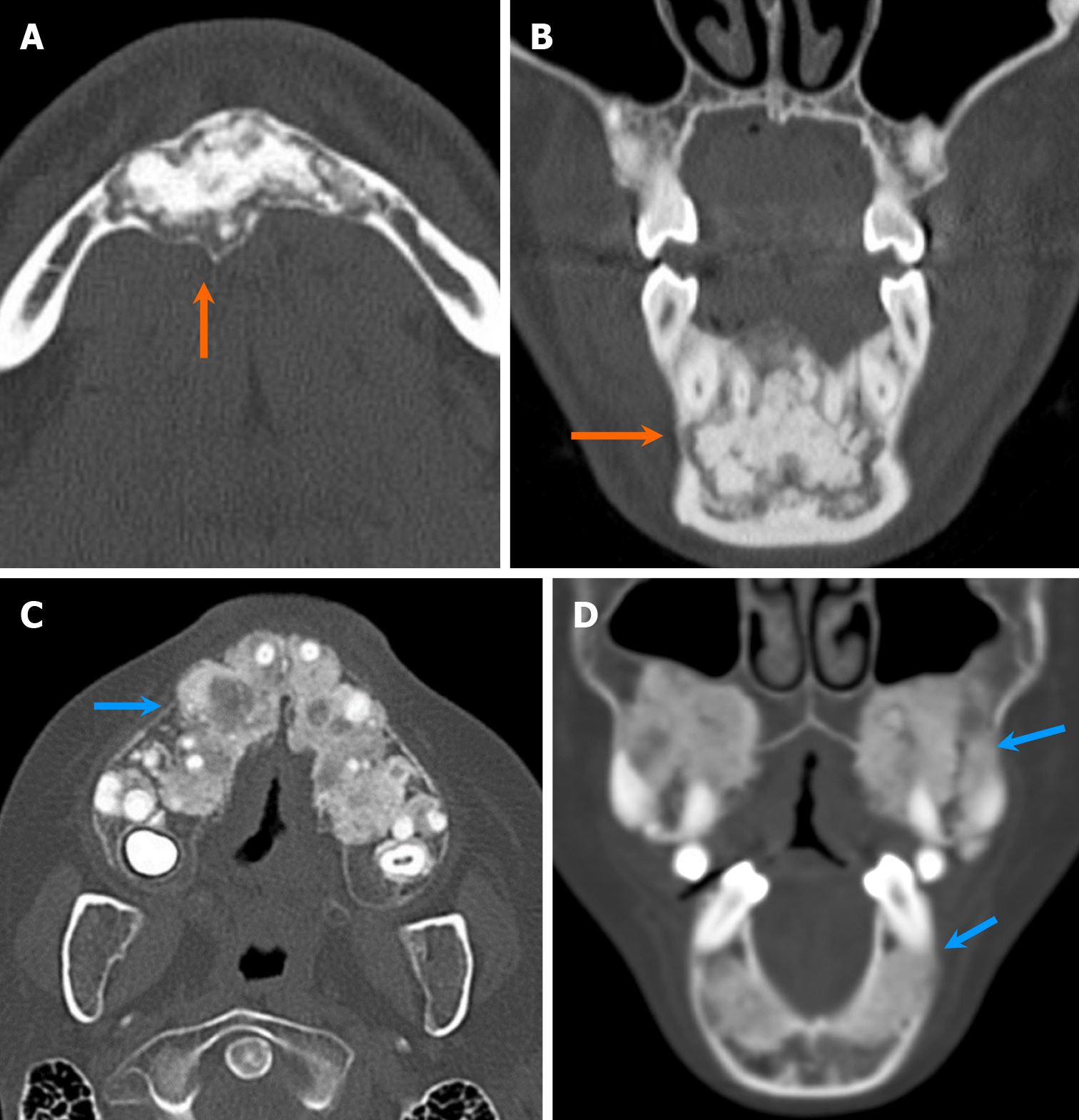
The jaw bones primarily consist of the mandible and maxilla. The mandible, forming the lower jaw, is a horseshoe-shaped bone, while the maxilla forms the upper jaw. The tooth-bearing portions of both the mandible and maxilla are referred to as the alveolar processes, covered by the gingiva. The mandible has various parts, including the symphysis, parasymphysis, body, angle, ramus, as well as coronoid and condylar processes. The mandibular ramus is a vertically oriented bone extending from the mandibular angle inferiorly to the coronoid and condylar processes superiorly. The space between these two processes is known as the “mandibular notch”. The condylar process forms the temporomandibular joint with the overlying temporal bone, while the coronoid process serves as an insertion site for the temporalis muscle. On the medial aspect of the mandibular ramus lies the mandibular foramen, which connects to the inferior alveolar canal (IAC) (so called mandibular canal), housing the inferior alveolar nerve (a branch of CN V3), along with an artery and vein. The inferior alveolar nerve exits the mandible through the mental foramen which is located near the first and second premolar teeth. Subsequently, the inferior alveolar nerve changes its name to the mental nerve. The second smaller terminal branch of the inferior alveolar nerve, called the incisive nerve, continues to course in the parasymphyseal region of the mandible within the mandibular incisive canal, innervating the canines and the incisors. The lower part of the mandible below the IAC is referred to as the basilar bone[8]. The anatomy of the mandible is illustrated in Figure 1.
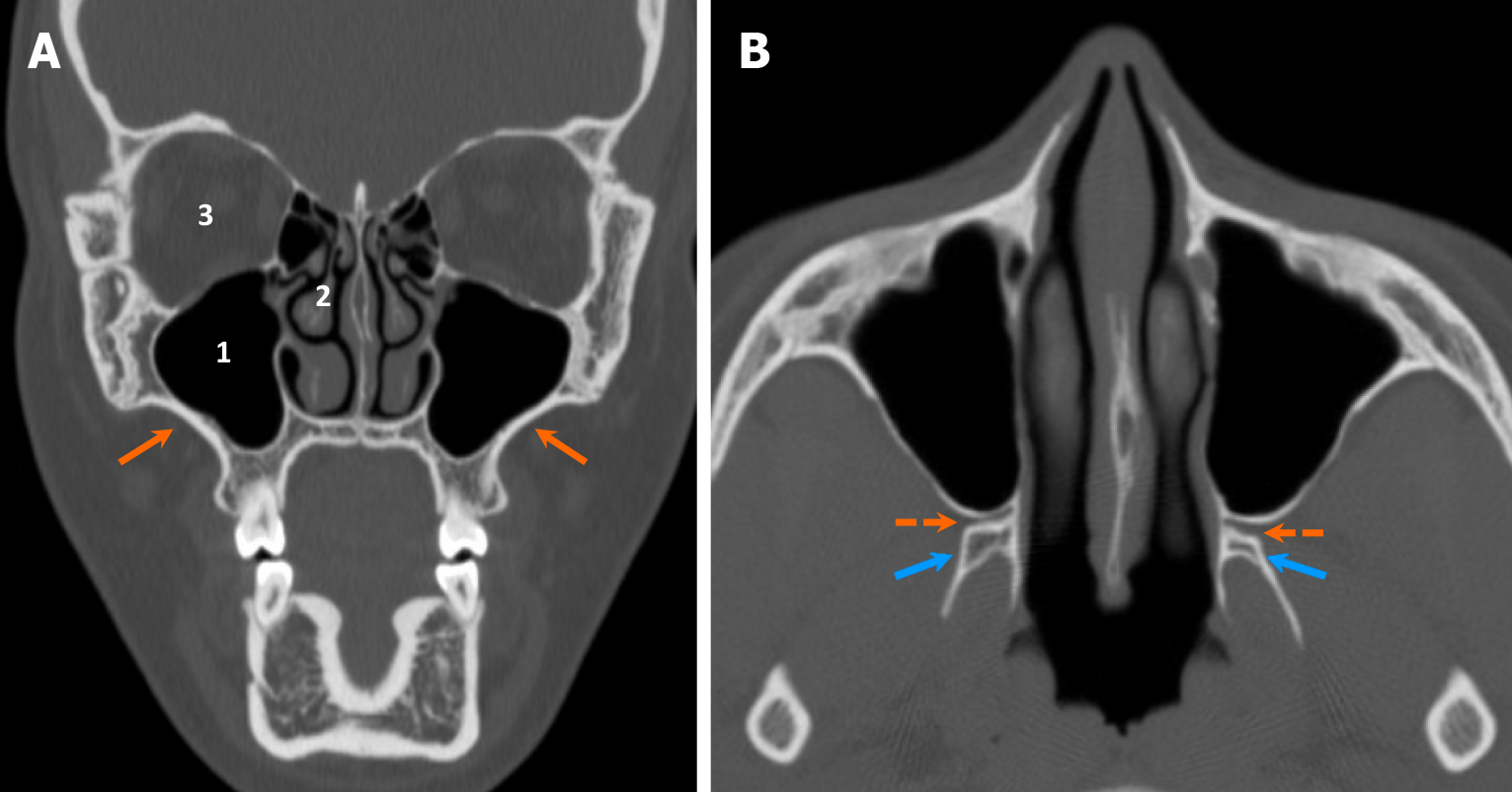
The maxilla consists of paired bones that constitute the upper jaw and the majority of the midface (Figure 2). The maxillary sinuses are situated in the mid portion of the maxilla. The superior aspect of the maxilla serves as the inferior orbital wall. Medial to the maxillary sinuses lie the nasal cavities and nasal septum. The hard palate originates from the palatine processes of the maxilla anteriorly and the horizontal plates of the palatine bones posteriorly. This structure divides the nasal cavities from the oral cavity. The pterygoid process of the sphenoid, including medial and lateral pterygoid plates, are located posterior to the maxillary alveolar process. The pterygopalatine fossa is a space located between the posterior wall of the maxillary sinus and the pterygoid process of the sphenoid bone. It serves as an important location for the pterygopalatine ganglion (CN V2). The fossa communicates with various structures: The inferior orbital fissure superiorly, the foramen rotundum superiorly and posteriorly, the lesser and greater palatine canals inferiorly, the posterior nasal cavity medially through the sphenopalatine foramen, and the infratemporal fossa laterally through the pterygomaxillary fissure[8].
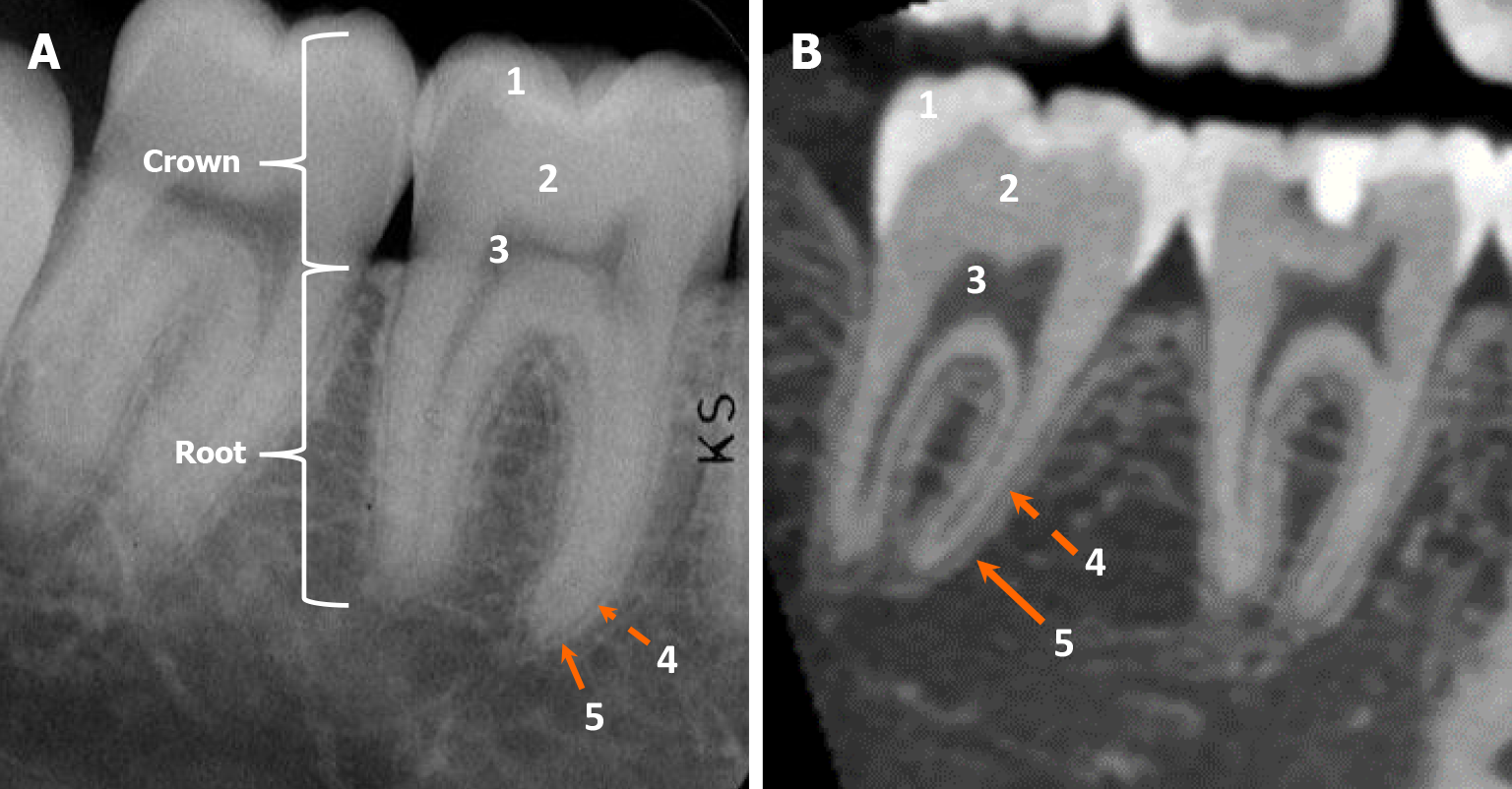
A tooth comprises enamel, dentin, cementum, and pulp. The part of the tooth protruding from the alveolar process is referred to as the “anatomic crown”, while the portion embedded in the bone is known as the “root”. The constriction where the crown and the root meet is called the “cementoenamel junction”[8,9]. Enamel is the densely mineralized part of the crown, produced by ameloblasts during embryonic development[8]. Cementum is a thin layer of mineralized connective tissue produced by cementoblasts and primarily composed of hydroxyapatite and collagen. It covers the surface of dental roots. Cementum serves various functions, including supporting the tooth, anchoring it to the bone through the periodontal ligaments (PDL), adapting the tooth’s position during eruption or movement, acting as an insertion site for the PDL to the tooth, and facilitating the repair of resorbed root surfaces[9,10].
Dentin is a calcified tissue located deep to the enamel and cementum, produced by odontoblasts during embryonic development[8]. Radiologically, dentin appears less radiodense relative to enamel but isodense to cementum. Consequently, dentin and cementum cannot be radiologically separated[8-10]. The PDL is depicted as a thin radiolucent area on the outer surface of the dental root, covered by a thin radiodense line of the lamina dura. The pulp cavity is situated in the central part of the tooth, containing odontoblasts, fibroblasts, as well as blood vessels and nerves. The neurovascular bundle of the tooth enters the root apex through the apical foramen and extends into the pulp cavity within the crown[8-10]. The dental anatomy is illustrated in Figure 3.
Various imaging modalities are available for assessing jaw and maxillofacial bone tumors, including radiography, CT, MRI, and occasionally nuclear imaging studies. An orthopantomogram, also known as a panoramic radiograph, is a diagnostic imaging modality commonly utilized for the initial evaluation of dental pathology and its relationship with adjacent structures such as the maxillary sinus and IAC, due to its wide availability and low radiation dose. This technique provides a broad view of both jaws and the temporomandibular joints. However, it has limitations, including low spatial resolution, overlapping structures, and potential distortion from artifacts[11]. While it effectively differentiates between radiolucent, radio-opaque, and mixed-type lesions and has the advantage of a low radiation dose, its ability to provide detailed lesion evaluation is restricted by its two-dimensional nature. It remains valuable for examining straightforward cases, such as radicular cysts, but less effective for detailed analysis[12].
CT stands as the primary tool for evaluating jaw lesions due to its ability to provide precise assessments of lesion size, shape, location, margin, internal matrix, and involvement with adjacent teeth. Additionally, it can offer insights into the soft-tissue extent of the lesion. Therefore, CT is typically used for a more detailed evaluation of jaw and maxillofacial lesions that are initially detected on an orthopantomogram. Two main types of CT, namely dental cone-beam CT scan (CBCT) and conventional CT scan, exist. Dental CBCT enables the creation of three-dimensional images of the oral and maxillofacial area, resembling images from conventional CT but with a reduced radiation dose. Images obtained with dental CBCT have higher spatial resolution and are less affected by beam-hardening artifacts compared to conventional CT scans. However, it is important to note that dental CBCT images can only be generated in a bone-window, making them inadequate for evaluating soft tissue[11,12].
MRI serves as a complementary tool to radiography and CT scans, with its primary advantage lying in its high soft tissue contrast resolution achieved without the use of ionizing radiation. MRI can evaluate internal soft tissue components, fluid-fluid levels, enhancement patterns, and other pertinent imaging features of the lesion. Moreover, it can offer insights into the biophysical properties of the lesion, such as diffusion restriction on diffusion-weighted imaging or tumor vascularity through MR perfusion[11,13]. Nuclear imaging studies, including positron emission tomography/CT, white blood cell scans, and Gallium scans, are occasionally used to assist in the evaluation of jaw and maxillofacial bone tumors. Positron emission tomography/CT is primarily employed for tumor staging, while white blood cell and Gallium scans are used as ancillary tools to investigate suspected infections[11].
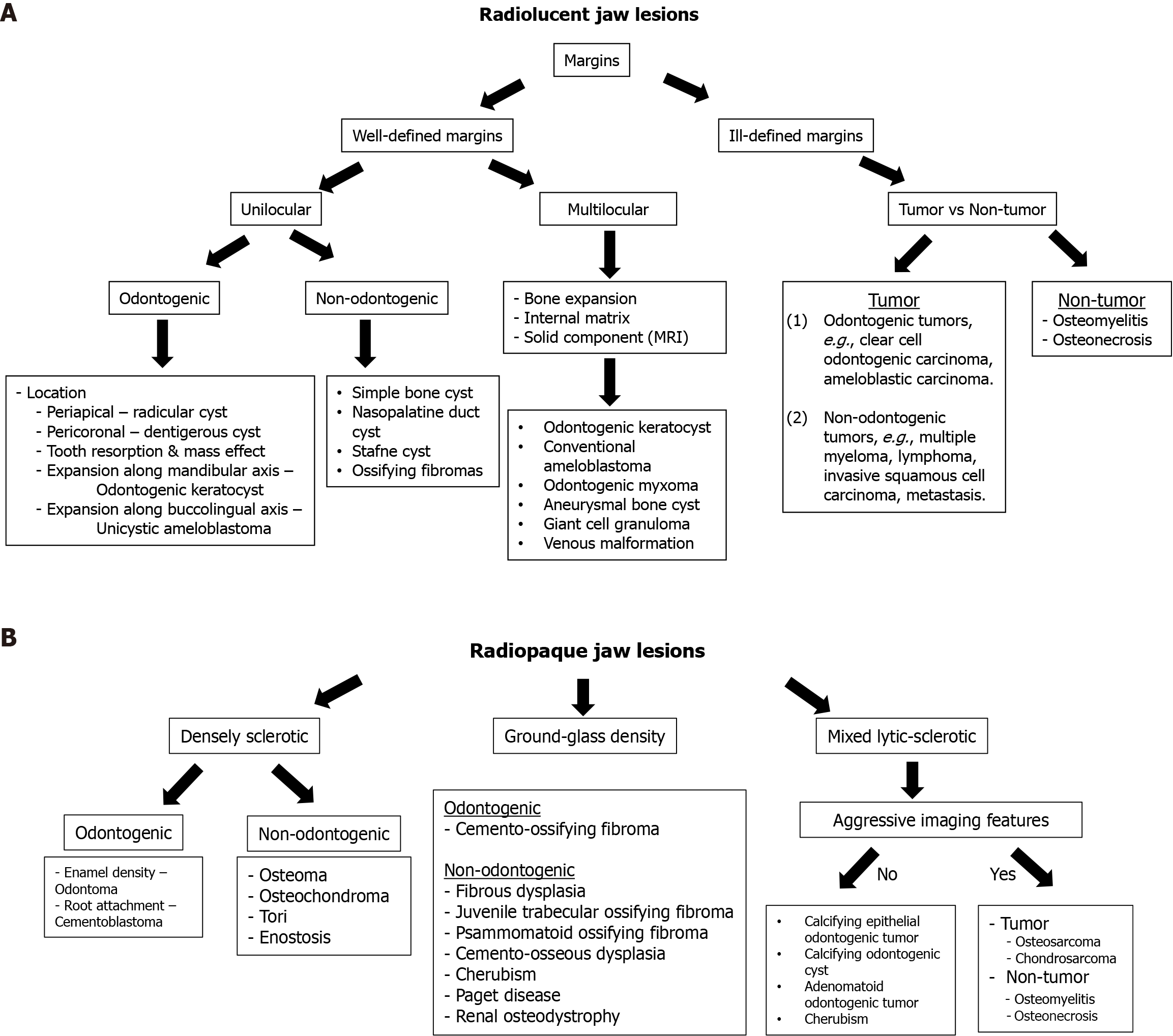
A meticulous assessment of imaging characteristics is essential for developing a comprehensive differential diagnosis of jaw lesions. Key imaging features that contribute to the diagnostic process include radiodensity, marginal definition, loculation pattern, relationship to adjacent teeth, erosion of teeth or bone, internal matrix appearance, patterns of osseous expansion, and the presence of soft tissue components (see Table 1). Among these, the evaluation of radiodensity is particularly critical in determining the nature of jaw lesions (Figure 4). Diagnoses can vary significantly based on the density characteristics of the lesion and are generally classified into two main categories: (1) Radiolucent; and (2) Radio-opaque lesions. The following section will provide a detailed discussion based on these categories.
| Key imaging characteristics | Description and/or spectrum of imaging findings |
| Radiodensity | Radiodensity is a crucial imaging feature for distinguishing between different jaw and maxillofacial bone lesions and is generally classified into 2 categories: (1) Radiolucent; and (2) Radio-opaque |
| Radiolucent lesions include cysts and radiolucent neoplasms. They can be classified based on margin definition into 2 categories: (1) Well-defined margins; and (2) Ill-defined margins | |
| Radiopaque lesions can be classified into 3 types: (1) Densely sclerotic; (2) Ground-glass; and (3) Mixed lytic-sclerotic patterns. Most densely sclerotic lesions are benign, including conditions such as odontoma and cementoblastoma | |
| Marginal definition | Assessing the margins of lesions indicates their aggressiveness and is crucial for differentiating between slow-growing benign tumors and more aggressive neoplasms |
| Well-defined margins are typically seen in benign, slow-growing lesions like dentigerous cysts, whereas aggressive, rapidly growing lesions, such as odontogenic carcinomas, often exhibit ill-defined margins | |
| Loculation pattern | Loculation patterns apply primarily to radiolucent lesions and are classified into two types: (1) Unilocular; and (2) Multilocular |
| For unilocular lesions with well-defined margins, the lesion’s location relative to a tooth can help differentiate diagnoses. For example, radicular cysts are found at the tooth apex, while dentigerous cysts are typically located around the crown of unerupted teeth | |
| Evaluating multilocular lesions on imaging can be challenging due to overlapping features among various pathologies. Accurate diagnosis often requires tissue sampling and histopathologic correlation. Ameloblastoma is a common odontogenic lesion that exhibits a multilocular pattern | |
| Relationship to adjacent teeth, erosion of the teeth or bone | The relationship of lesions to adjacent teeth is another important imaging clue, particularly when lesions are closely associated with or near teeth. Lesions closely related to a tooth or located above the inferior alveolar canal are more likely to be odontogenic in origin. Conversely, lesions centered below the inferior alveolar canal are likely non-odontogenic, while those within the canal may be vascular or neurogenic in origin |
| In lesions closely related to teeth, the specific location within tooth structures (i.e., root or crown) and their association with erupted or unerupted teeth can provide valuable diagnostic clues. For example, a dentigerous cyst typically attaches to the cemento-enamel junction of the crown of an unerupted tooth. In contrast, an odontogenic keratocyst generally attaches apically to the cemento-enamel junction of the crown | |
| The impact of lesions on surrounding structures, such as tooth displacement, tilting, or resorption, as well as bone erosion and destruction, may help distinguish between cystic and neoplastic lesions. Cystic lesions generally cause minimal tooth destruction and may tilt adjacent teeth, while neoplastic lesions often lead to resorption, destruction, and bodily movement of adjacent teeth | |
| Internal matrix appearance | Internal matrix patterns may help differentiate jaw lesions. Slowly growing tumors may deposit bone, creating a trabecular pattern, while some lesions, such as ameloblastomas, may display a “soap bubble” appearance. The presence of an internal chondroid matrix with a ring-and-arc pattern can suggest chondroid tumors, such as chondrosarcomas |
| Patterns of osseous expansion | Odontogenic keratocysts typically extend along the mandibular axis (the long axis of the mandible), while ameloblastomas tend to expand along the buccolingual axis (the short axis of the mandible) |
| Soft tissue component | The presence of an enhancing soft tissue component on contrast-enhanced computed tomography or magnetic resonance imaging indicates a higher likelihood of a true neoplasm rather than a cyst |
A wide array of radiolucent lesions can manifest within jaws, broadly categorized into cysts or radiolucent neoplasms. Imaging approach for radiolucent lesions has been extensively explored in the literature previously, and the imaging methodology below is derived from the prior analyses[1,6,14]. Initial assessment of lesion aggressiveness plays a crucial role in distinguishing between slow-growing benign tumors and more aggressive neoplasms, with particular attention paid to lesion margins[6,14]. For non-aggressive lucent lesions exhibiting well-defined borders, the lesion loculation pattern, whether unilocular or multilocular, assumes importance. Unilocular lesions can be subdivided into odontogenic or non-odontogenic cysts or tumors based on the origin. Differentiating between odontogenic or non-odontogenic tumors on imaging is challenging, although a few features can provide guidance[15]. Lesions intimately related to a tooth or situated above IAC are more likely odontogenic in origin, while lesions with their epicenter below the IAC, are more likely non-odontogenic. Lesions arising from the IAC are likely neural or vascular in origin[15]. Figure 5A presents a summary of the imaging approach to radiolucent jaw lesions. In the following discussion, radiolucent lesions will be further classified into two categories based on their marginal definition: (1) Radiolucent lesions with well-defined margins; and (2) Radiolucent lesions with ill-defined margins.
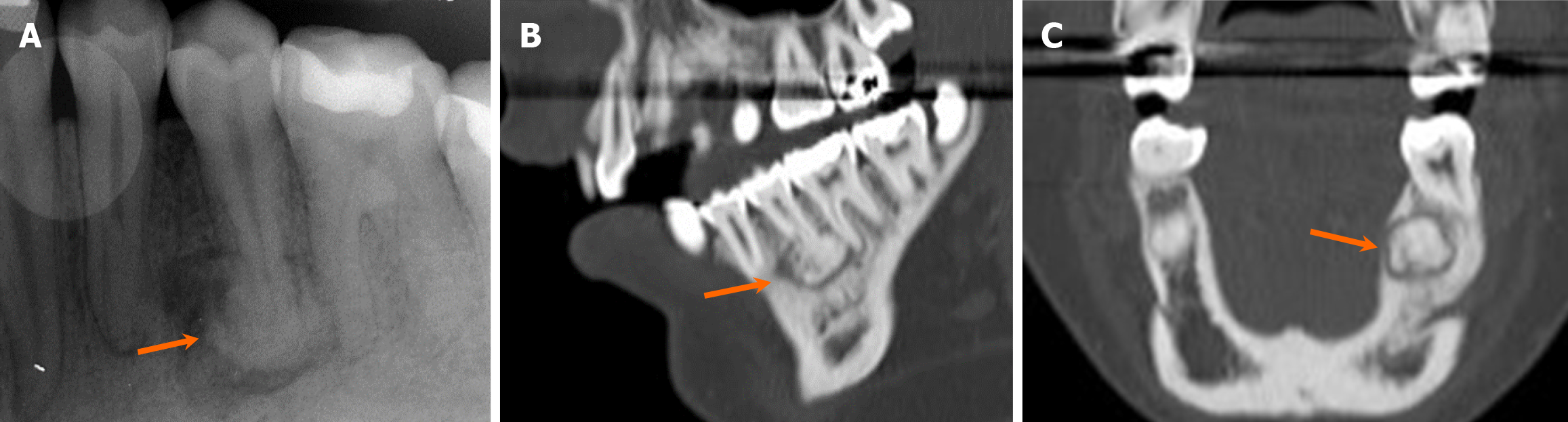
Unilocular radiolucent lesions: Several entities can present as unilocular lucent lesions with well-defined borders, and their location in relation to a tooth can serve as a valuable feature for distinguishing between different diagnoses. The most common of such lesions is a radicular cyst, situated periapically to a tooth[16]. Radicular cysts are related to dental caries, leading to pulp necrosis and subsequent spreading to the tooth apex[6]. This process is mediated by local bony destruction from inflammatory processes by osteoclasts and odontoclasts[17]. Initially, this inflammatory response results in the formation of an intraosseous granuloma and/or abscess. This may progress over time to form a cyst encapsulated by epithelium. Radiologically, radicular cysts demonstrate unilocular, well-defined margins, occasionally with dystrophic calcifications in long-standing cysts (Figure 6)[2]. In the instance where the associated non-vital tooth is removed but the cyst remains, it is called a residual cyst[18]. On MRI, radicular cysts often display thin-rim enhancement with T2 hyperintensity and no internal enhancement while periapical granuloma tend to be smaller with T2 hypointensity and central enhancement[19,20]. It is important to note that differentiating between radicular cysts and early-stage periapical cemento-osseous dysplasia (COD), a benign fibrous lesion, can be challenging due to overlapping imaging features. However, this differentiation can be made using a pulp vitality test: The tooth with periapical COD remains vital, while the tooth with a radicular cyst is not vital[21].
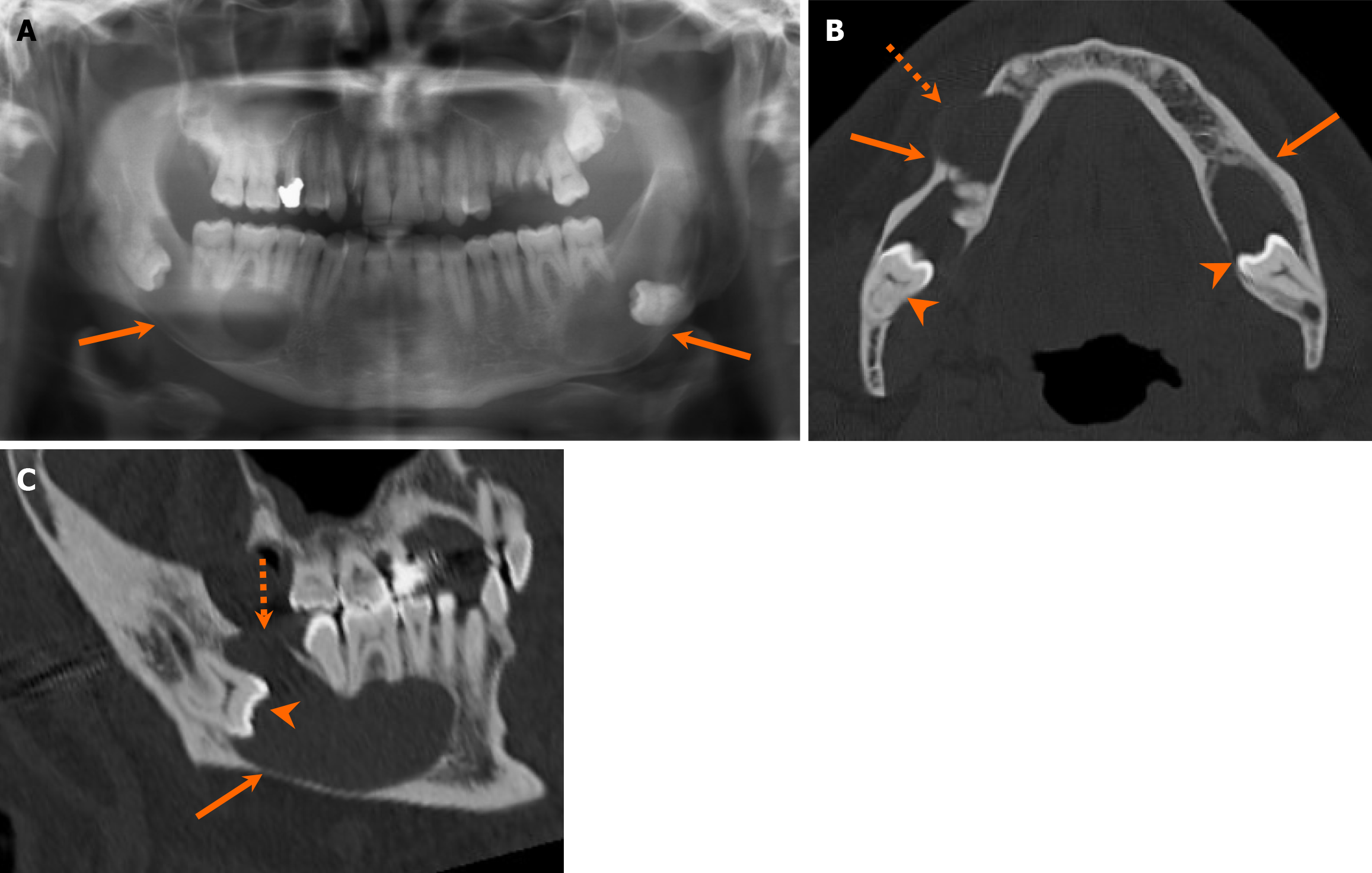
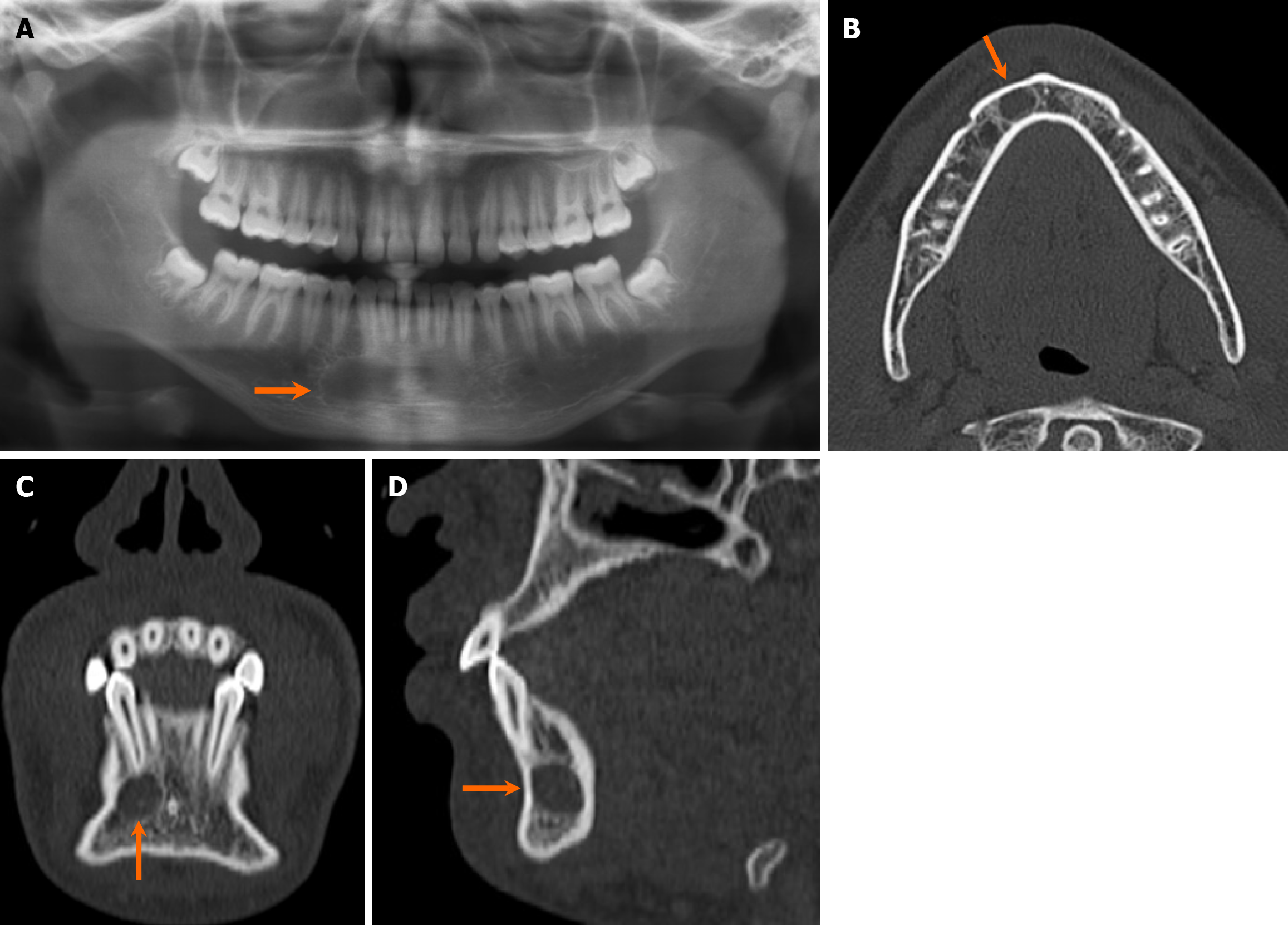
Dentigerous cysts are the second most prevalent radiolucent lesions with well-defined margins. They are classically associated with crowns of unerupted teeth (Figure 7). The pathogenesis of the dentigerous cyst is related to fluid accumulation between enamel remnant and the coronal aspect of the tooth. Resorption of adjacent tooth can be occasionally associated with progressively enlarging dentigerous cysts[22-24]. On MRI, the dentigerous cysts exhibit internal hyperintensity on T2-weighted images, accompanied by thin rim enhancement[20].
Lesions such as odontogenic keratocyst (OKC) and ameloblastoma, presenting with both unilocular and multilocular patterns, are often included in the differential considerations for various well-demarcated radiolucent lesions, given their relative prevalence. Detailed discussion of imaging features of OKC and ameloblastoma will be included below. In addition, several uncommon odontogenic tumors can present as unilocular radiolucent lesion in the jaws, including but not limited to cemento-ossifying fibroma (COF), glandular odontogenic cyst, calcifying odontogenic cyst, and adenomatoid odontogenic tumor[18].
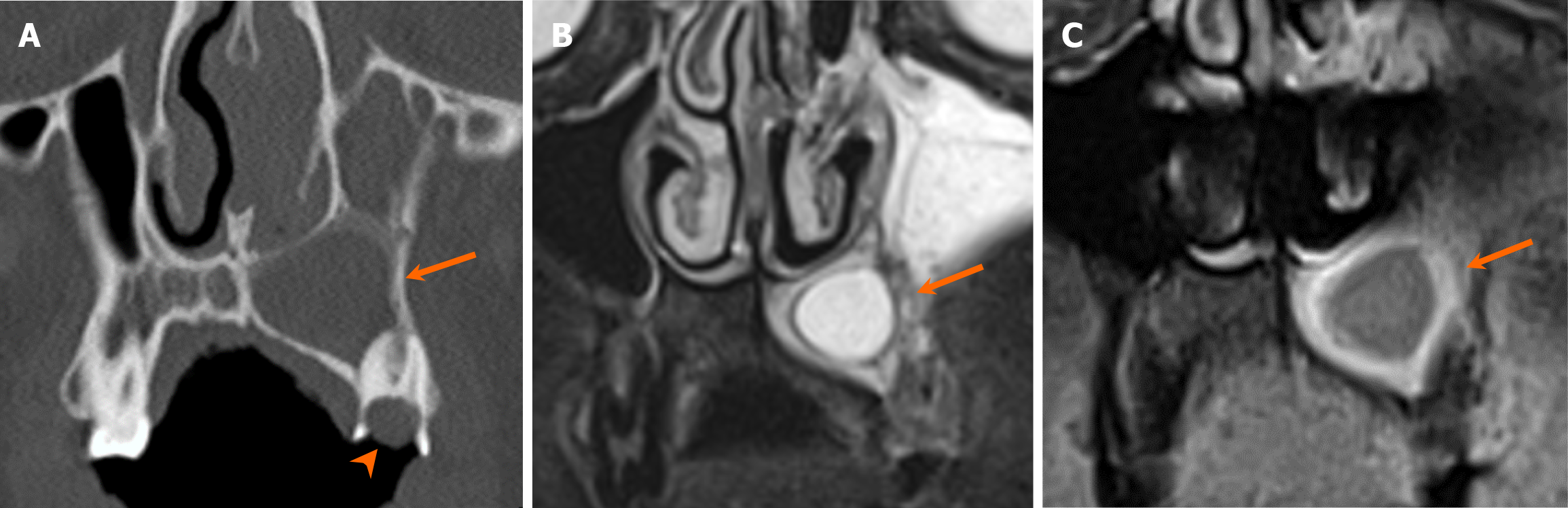
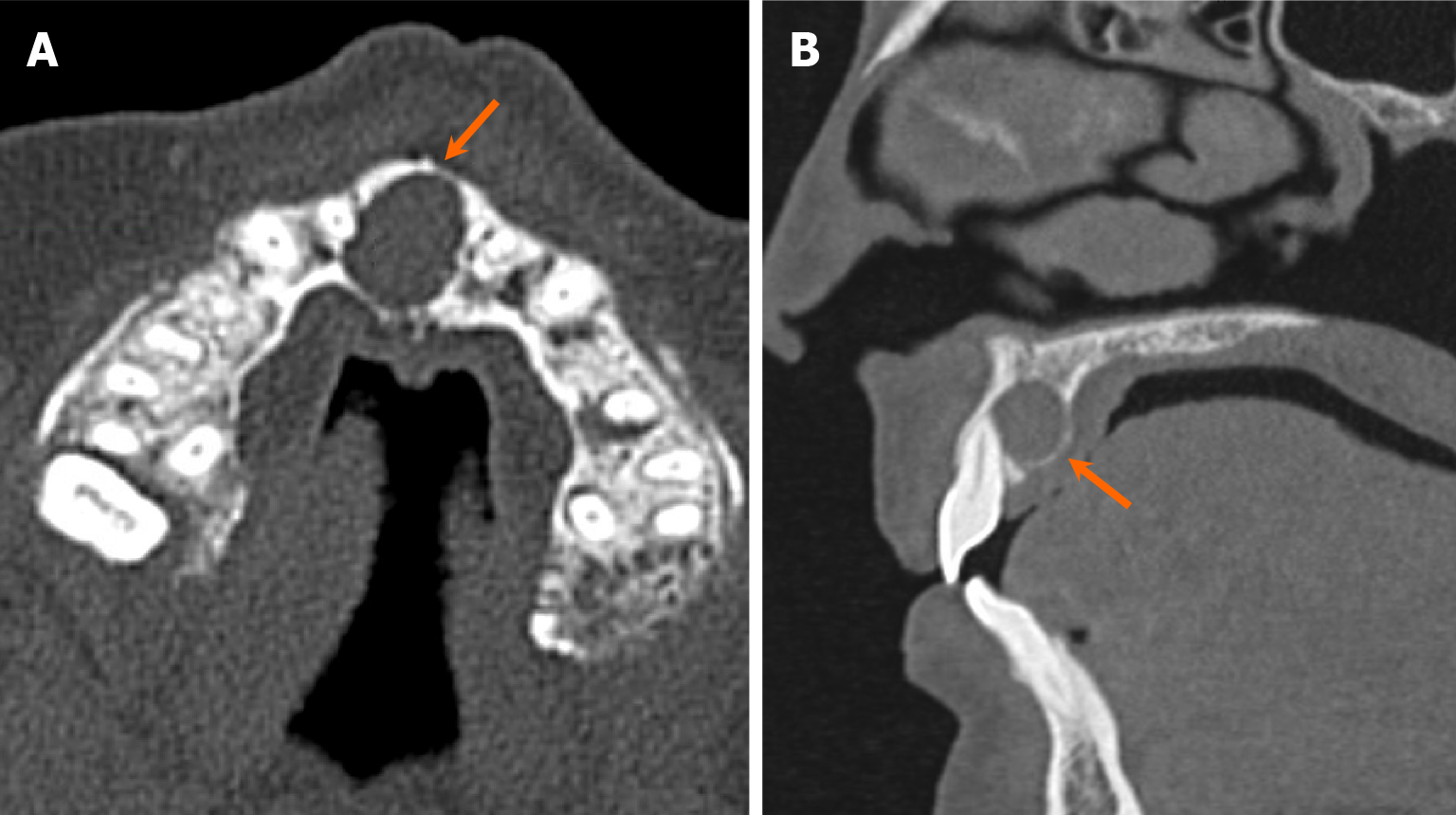
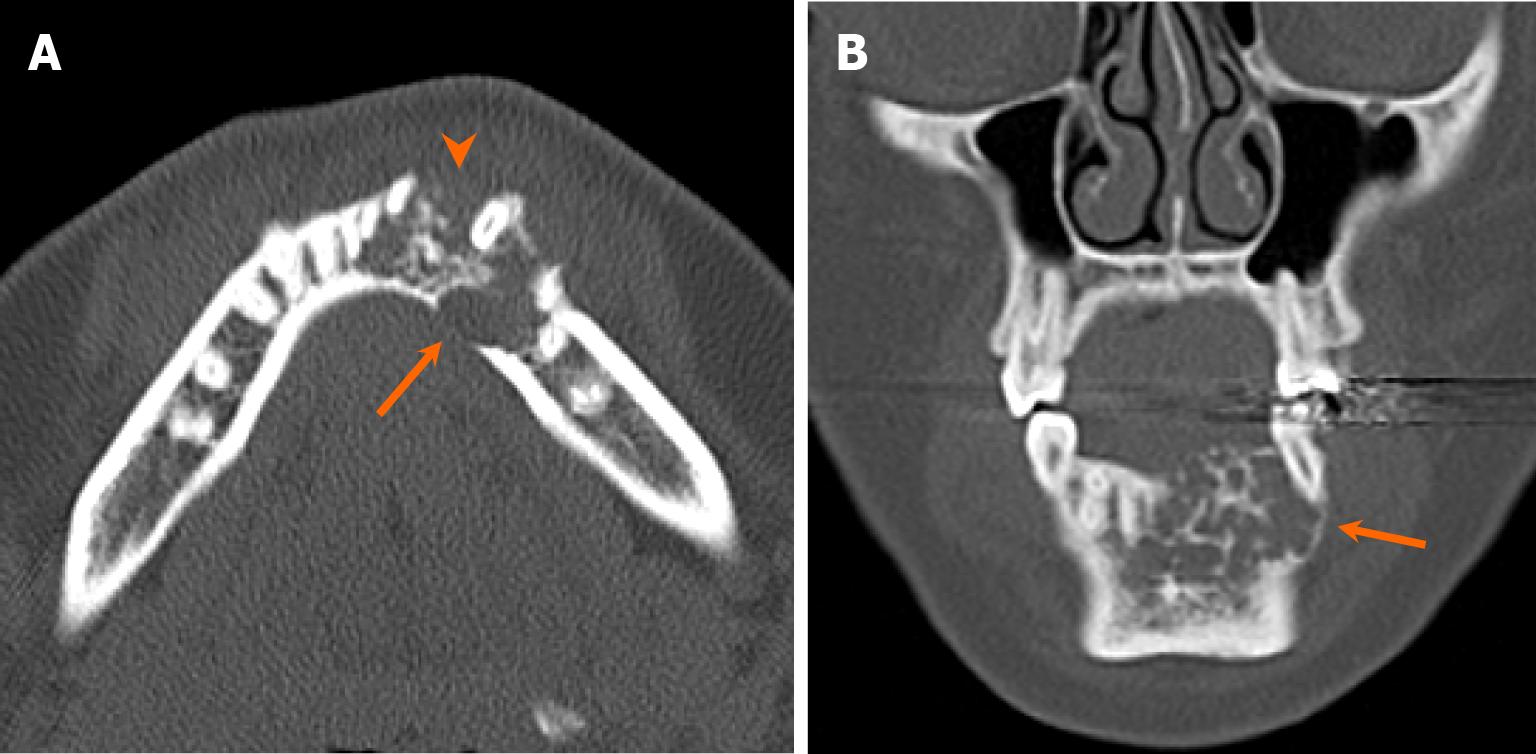
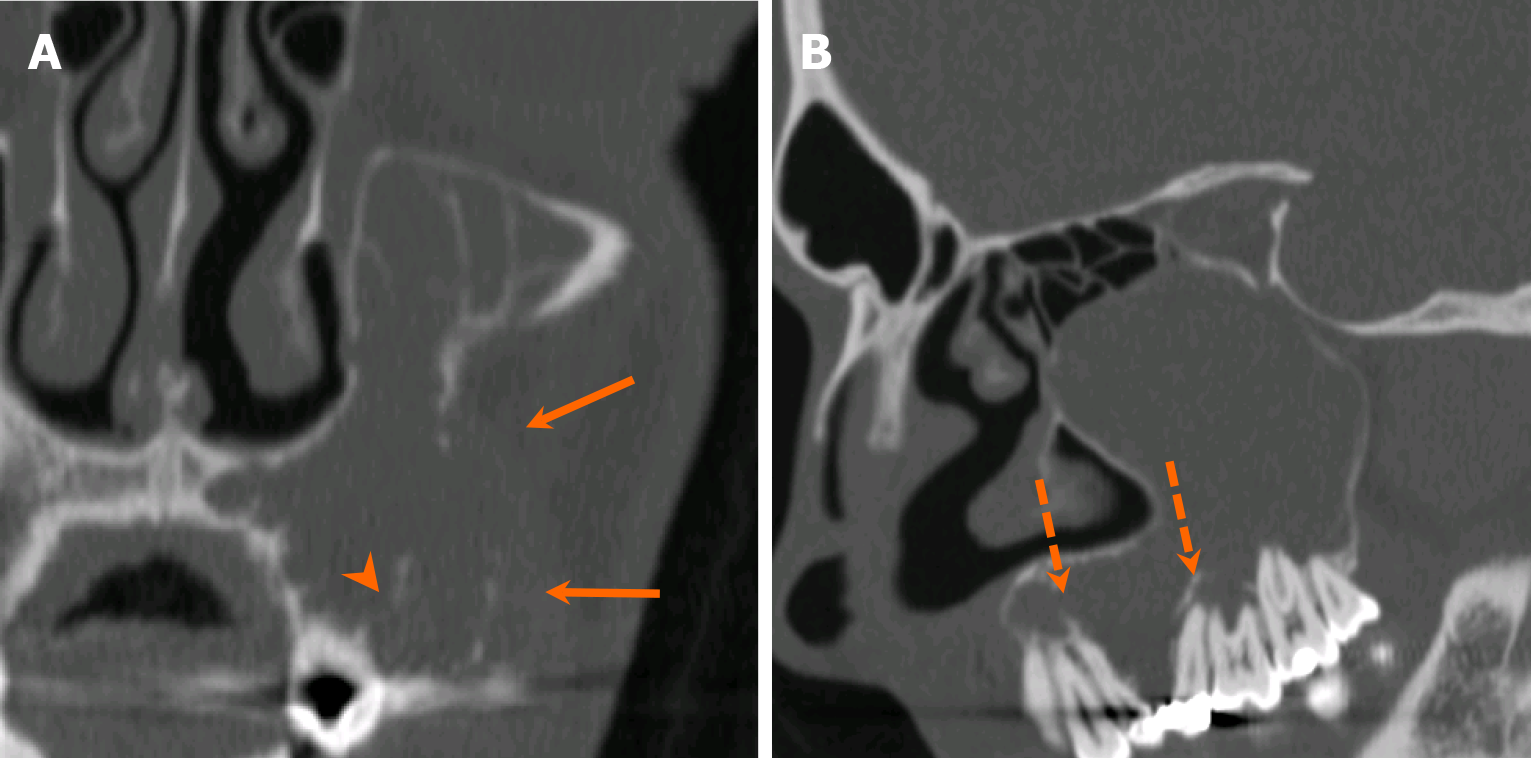
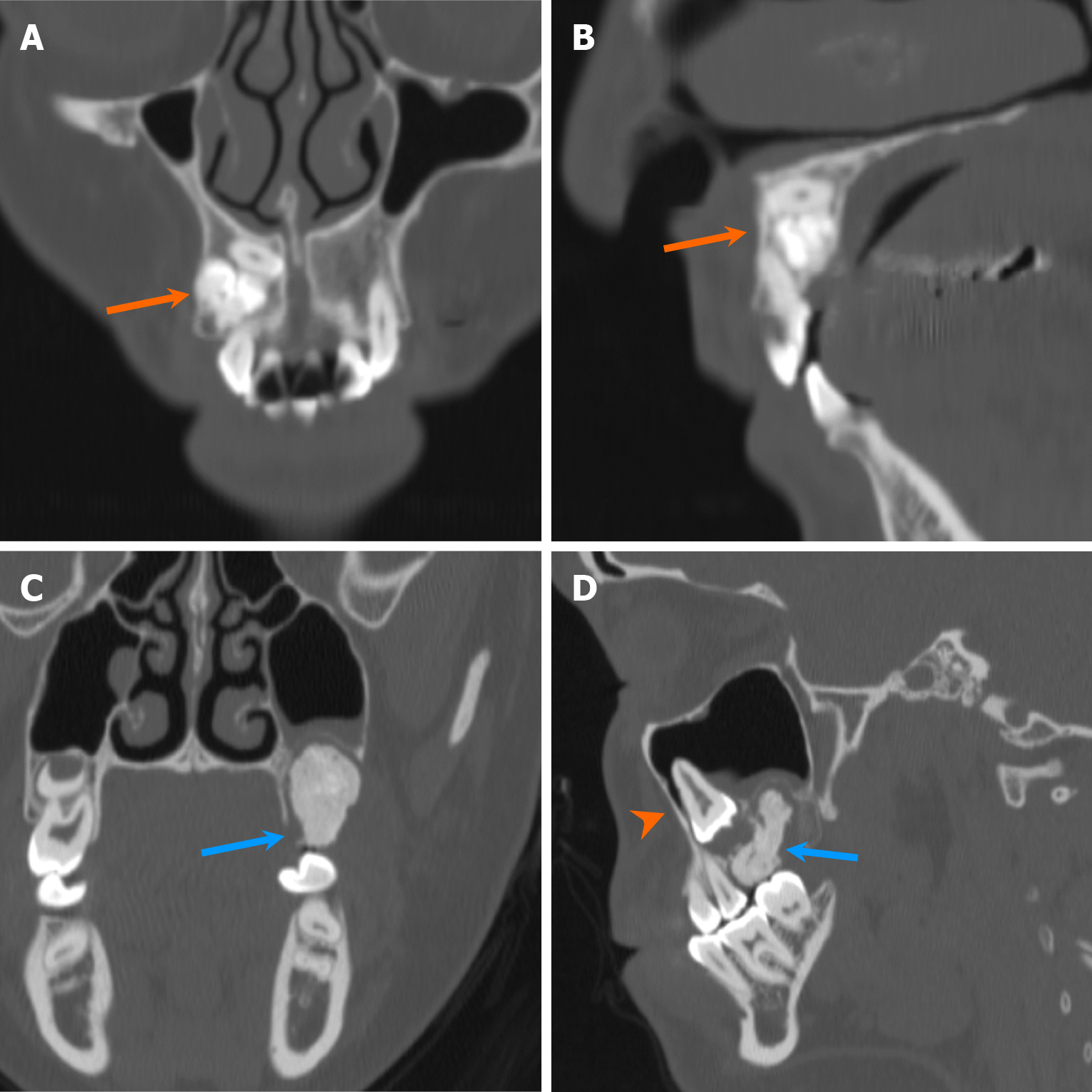
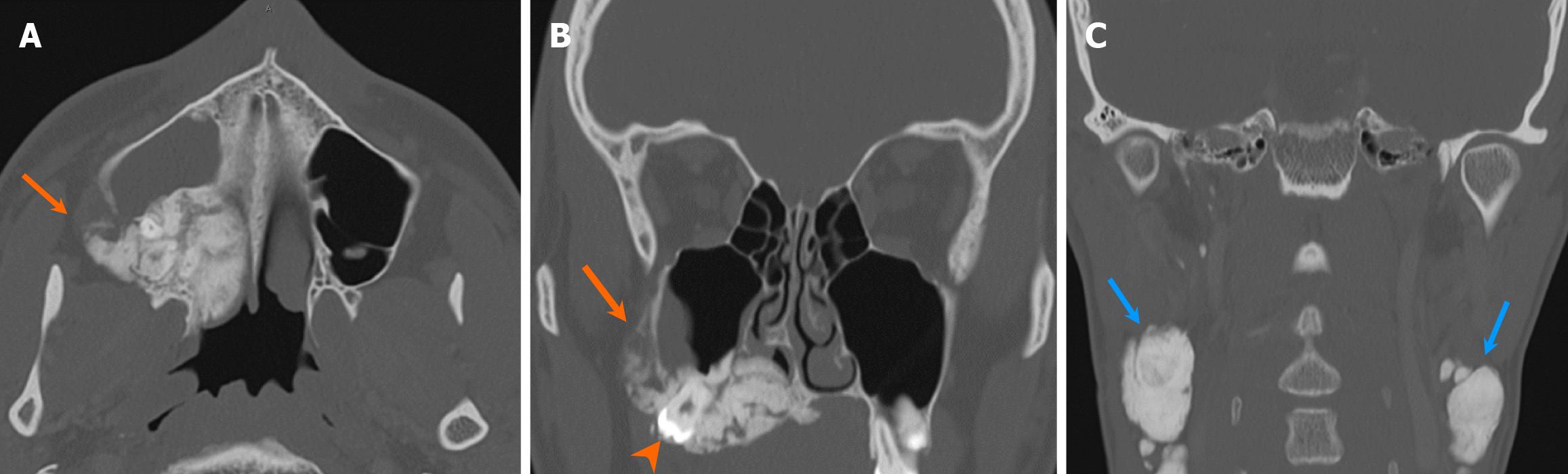
Non-odontogenic unilocular, radiolucent lesions may affect jaws, with one classic non-odontogenic cyst being the nasopalatine duct cyst, typically situated in the region of the incisive foramen (Figure 8). It is the most common developmental jaw cyst, and its morphology sometimes assumes a heart shape with the presence of anterior nasal spine, thus aiding in differentiation from radicular cyst and nasolabial cyst[25]. Moreover, non-odontogenic cystic lesions, such as simple bone cyst, often present as an unilocular, radiolucent lesion with or without association with teeth. Simple bone cysts are pseudocysts without intact epithelial lining. The etiology remains elusive, but purportedly developmental or traumatic in nature[26]. On imaging, it typically appears as unilocular, well-marginated lesions with thin, delicate borders and scalloping along the margins between teeth, while instances of mandibular bone expansion are less common[27] (Figure 9). Multilocular configurations of simple bone cysts are rare, thus mimicking other multilocular, radiolucent lesions[27]. Notably, within simple bone cysts, internal hemorrhagic components may be present, exhibiting hyperdensity on CT scans and variable signal intensities on MRI depending on the stage of degradation[1].
Multilocular radiolucent lesions: Evaluation of multilocular lesions on imaging poses challenges due to the variable appearance of pathologies, necessitating tissue sampling and histopathologic correlation for precise diagnosis. Internal trabecular patterns may provide ancillary diagnostic insights. Lesions such as OKC and ameloblastoma, which can manifest as both unilocular and multilocular, are often considered in the differentials for numerous well-circumscribed lucent lesions owing to their relative prevalence.
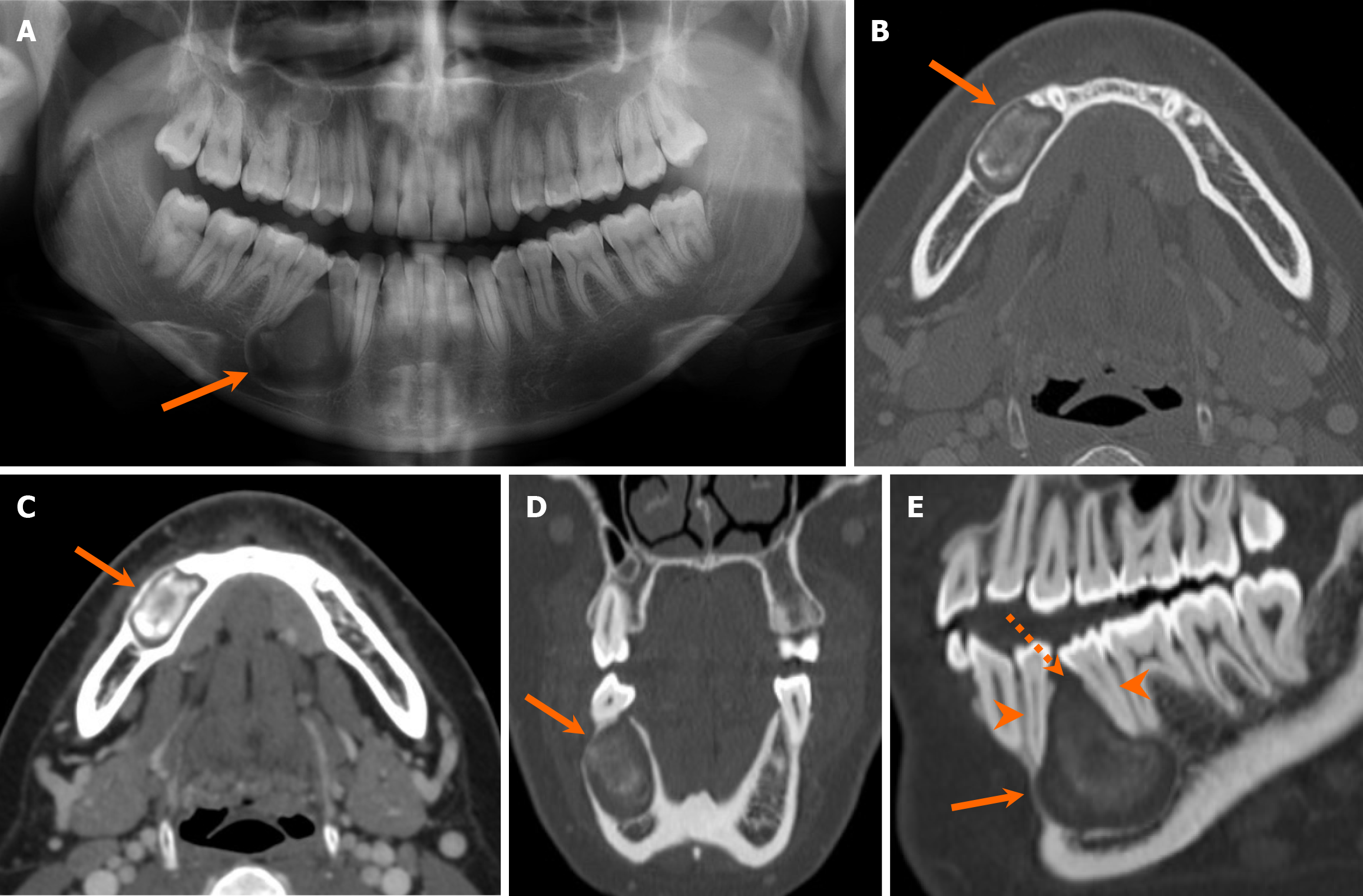
OKCs, characterized by epithelium-lined cystic lesions containing desquamated keratin, arise from dental lamina. On imaging, OKCs typically demonstrate well-defined margins with various sizes, loculation and trabeculation patterns (Figure 10). Notably, OKCs tend to expand along the medullary cavity, resulting in mandibular axis expansion rather than the buccolingual expansion often observed in ameloblastomas[28]. Multilocular OKCs contain thin septa between locules. Tooth displacement or tilting is common, although resorption is infrequent. Non-enhancing heterogenous attenuation within the lesion is atypical and is believed to be related to presence of concentrated keratin content[29]. On MRI, OKCs demonstrate heterogeneous signals on both T1- and T2-weighted sequences, along with enhancement of the wall and septa, and internal diffusion restriction in the region of keratin contents[30,31].
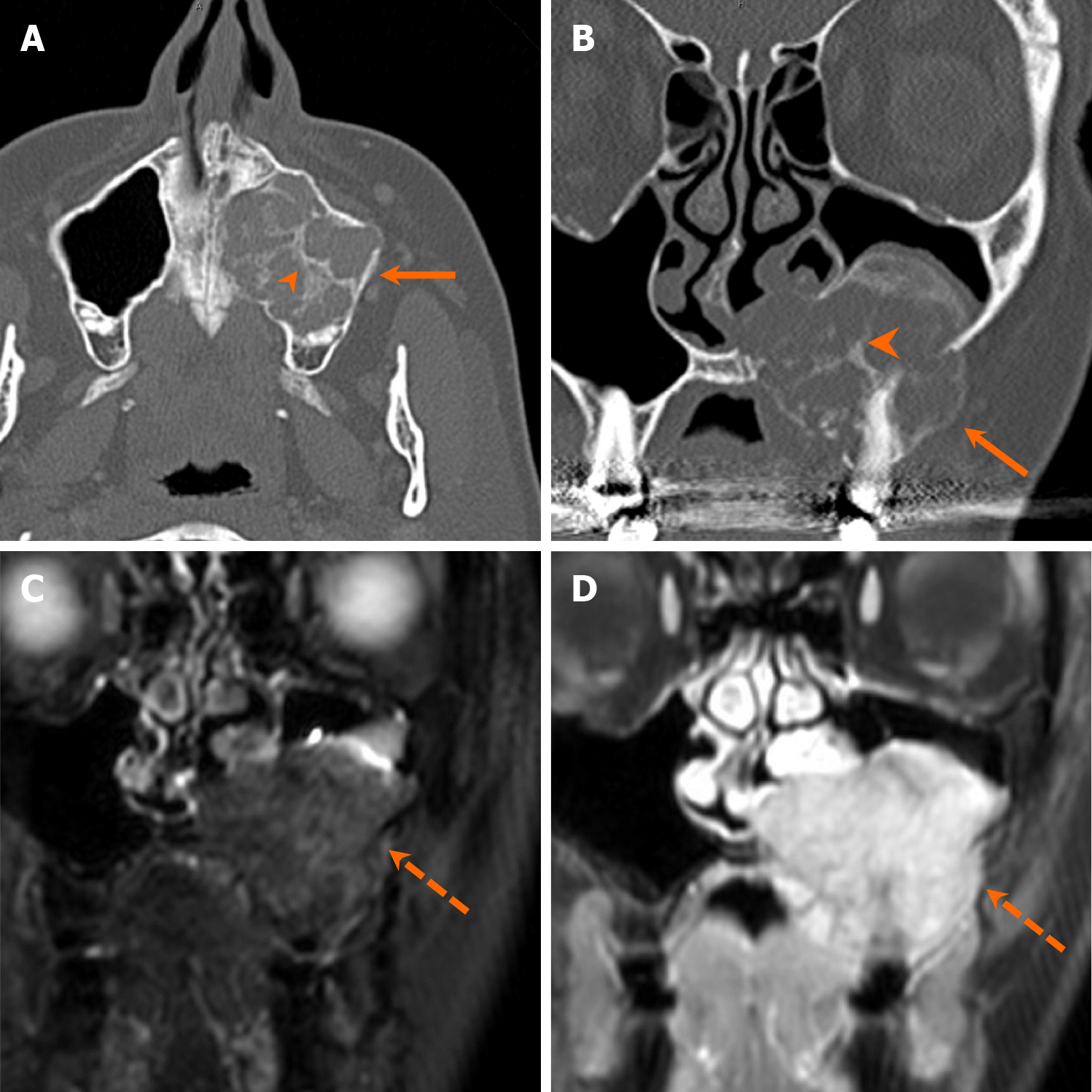
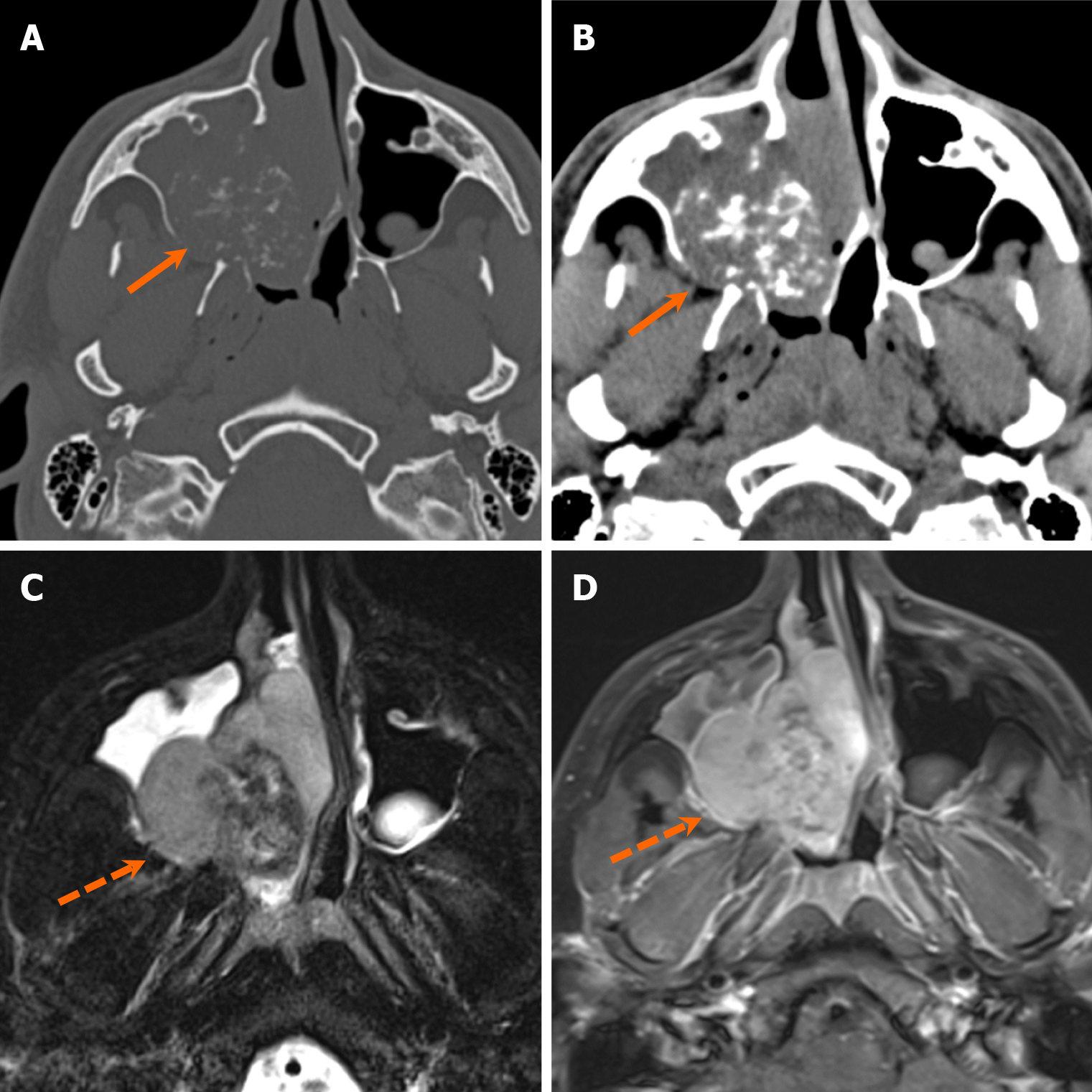
Ameloblastomas, benign odontogenic tumors arising from enamel forming epithelium, develop after failure to regress[22]. Ameloblastomas can exhibit various loculation patterns just like OKCs. There are multiple types of ameloblastomas, including conventional, unicystic, extra-osseous, and metastasizing variants. Unicystic ameloblastoma represents a less aggressive subtype (Figure 11), in contrast to the conventional, multilocular/multicystic ameloblastomas, which are more prevalent[18]. Radiologically, differentiating between ameloblastoma and OKC can be challenging, although certain features aid in distinction. Ameloblastomas typically exhibit a more multilocular appearance with increased septa, buccolingual expansion, and greater tooth displacement and resorption compared to OKCs[7,28,32] (Figure 12). On contrast-enhanced CT or MRI, the presence of enhancing mural nodule is suggestive of an ameloblastoma, as opposed to an OKC (Figure 13). The enhancing solid component is highly cellular and demonstrates reduced diffusivity on MRI, while the cystic component demonstrates facilitated diffusivity[20,31,33].
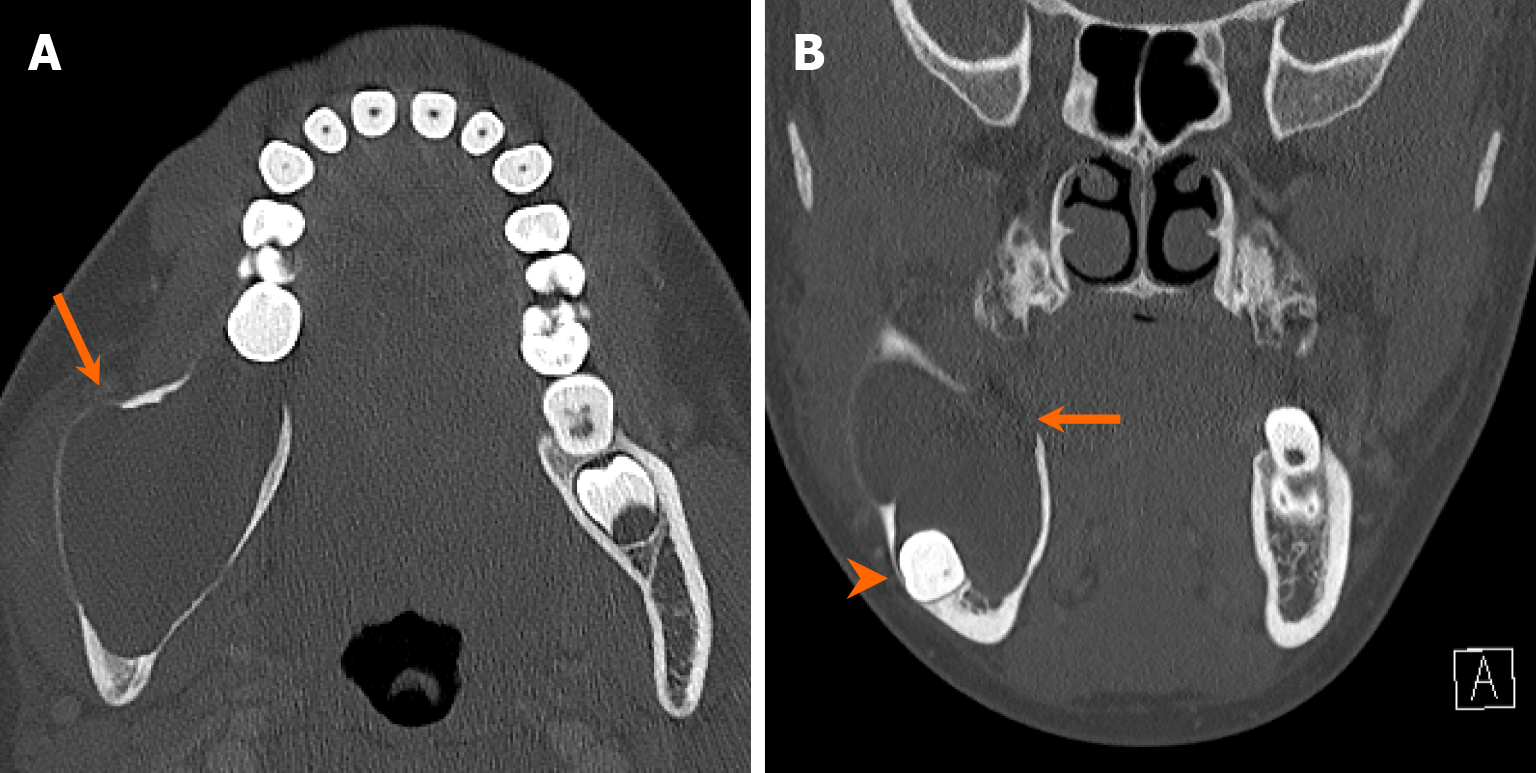
Odontogenic myxomas are locally invasive odontogenic tumors, thought to arise from odontogenic mesenchymal cells[34]. On imaging, odontogenic myxomas share similarities with ameloblastomas, presenting as either unilocular or multilocular expansile lesions with varying trabeculations, resembling “honeycomb” or “tennis-racket” appearances[35-37]. Margins can be either well or poorly-defined margination with locoregional mass effect that may cause tooth displacement and resorption[37] (Figure 14). Notably, tooth resorption is more commonly associated with ameloblastomas than with OKCs or odontogenic myxomas, and the presence or absence of tooth resorption may help differentiate these entities. On MRI, odontogenic myxomas typically show heterogeneous T2 hyperintensity with varying degrees of enhancement[35].
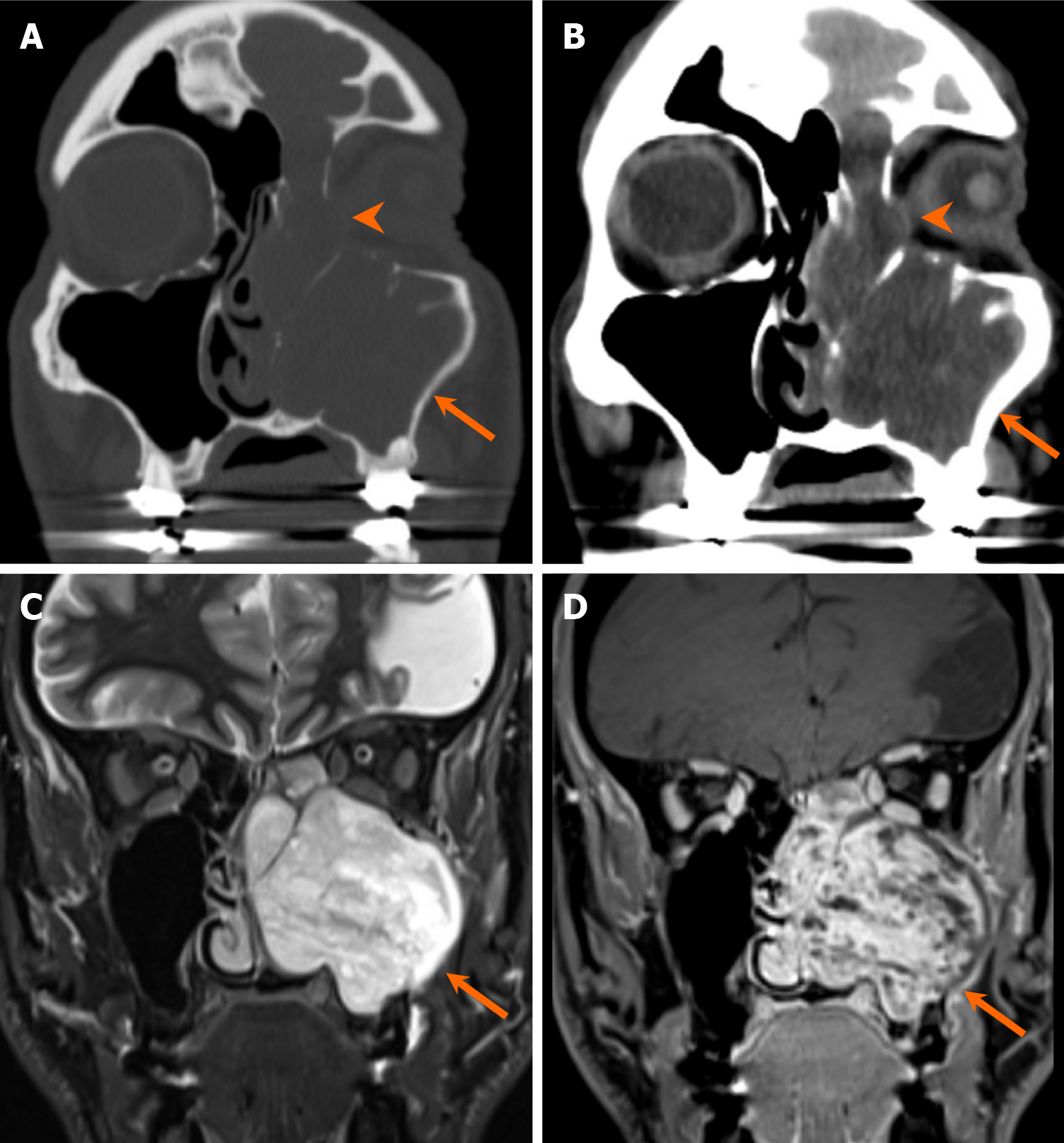
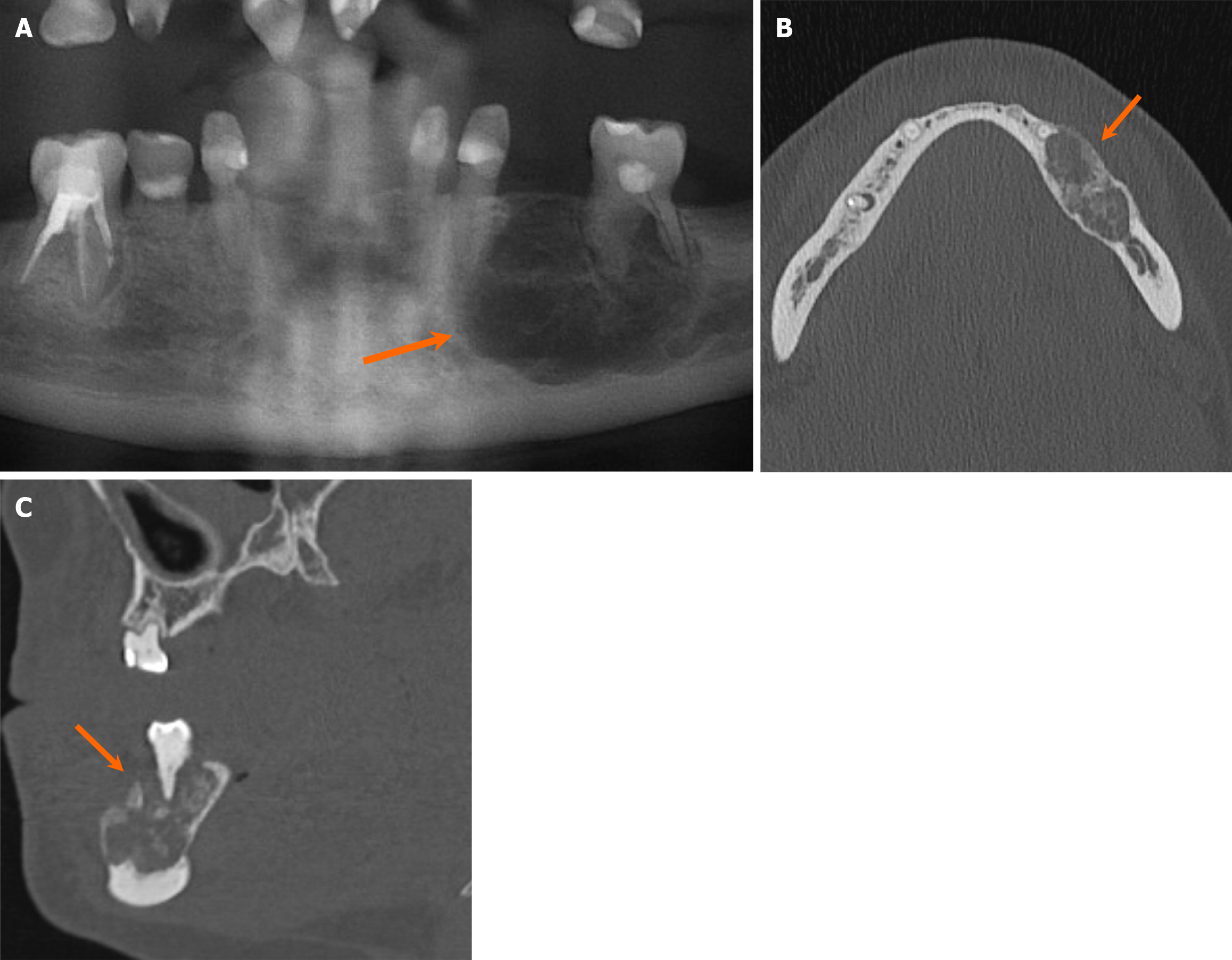
Non-odontogenic multilocular lesions include entities such as giant cell granuloma, aneurysmal bone cyst (ABC), venous malformation, among others. Giant cell granulomas are benign, but locally aggressive lesions, of uncertain etiology, comprised of fibrous tissue with scattered areas of hemorrhage and hemosiderin. They manifest in two forms: The peripheral form, arising from extraosseous tissues, and the central form, originating within bone[38]. Radiologically, giant cell granulomas are multilocular expansile lesions with well-defined but scalloped margins and internal septations, typically exhibiting less septation compared to ABC[38,39] (Figure 15). The septa in giant cell granulomas are usually finer and appear wispy or granular, in contrast to the sharper septa seen in ameloblastomas or odontogenic myxomas. On contrast-enhanced MRI, giant cell granulomas exhibit heterogeneous avid enhancement with areas of hemorrhage and hemosiderin[20,38]. ABCs are commonly observed as multilocular expansile lesions with blood-filled cavities separated by septa. They can develop secondary to various benign and malignant tumors[40]. While ABCs may resemble other multilocular lesions on imaging, their characteristic fluid-fluid levels on MRI are particularly helpful for diagnosis[41].
Primary considerations for radiolucent lesions with ill-defined margins include rapidly growing tumors or non-neoplastic infectious or inflammatory processes. Among benign neoplasms, odontogenic myxoma and osteoblastoma sometimes demonstrates ill-defined margins owing to rapid growth. Although some low-grade malignant neoplasms may lack ill-defined margins, they often exhibit poor-margination due to locally aggressive behavior. Malignant odontogenic tumors are diverse and challenging to distinguish without histopathologic correlation. While local invasion of oral cavity squamous cell carcinoma stands as the most common radiolucent jaw malignancy, discussion of this entity is beyond the scope of this review[6]. Malignant odontogenic tumors are categorized by the WHO into odontogenic carcinomas, sarcomas, and carcinosarcomas[42]. Odontogenic carcinomas comprise epithelial or clear cells, and manifest as radiolucent lesions with surrounding bony destruction[22] (Figure 16). Although rare, malignant transformation of benign odontogenic cysts has been documented in the literature[43]. Furthermore, certain malignant non-odontogenic radiolucent tumors affecting the jaws fall under the classification of bone and cartilage tumors, such as chondrosarcoma and rhabdomyosarcoma, often presenting with aggressive features. Common, radiolucent, non-neoplastic aggressive processes include osteomyelitis, osteoradionecrosis, or medication-induced osteonecrosis, although they are more prone to manifest as mixed lytic and sclerotic lesions[6].
The radiodensity pattern of radio-opaque jaw lesions can be categorized into three main types, as outlined by Curé et al[3] and Holmes et al[4]: Densely sclerotic, ground-glass, and mixed lytic-sclerotic patterns. The following discussion will be organized according to these patterns. Figure 5B provides a summary of the imaging approach to radio-opaque jaw lesions.
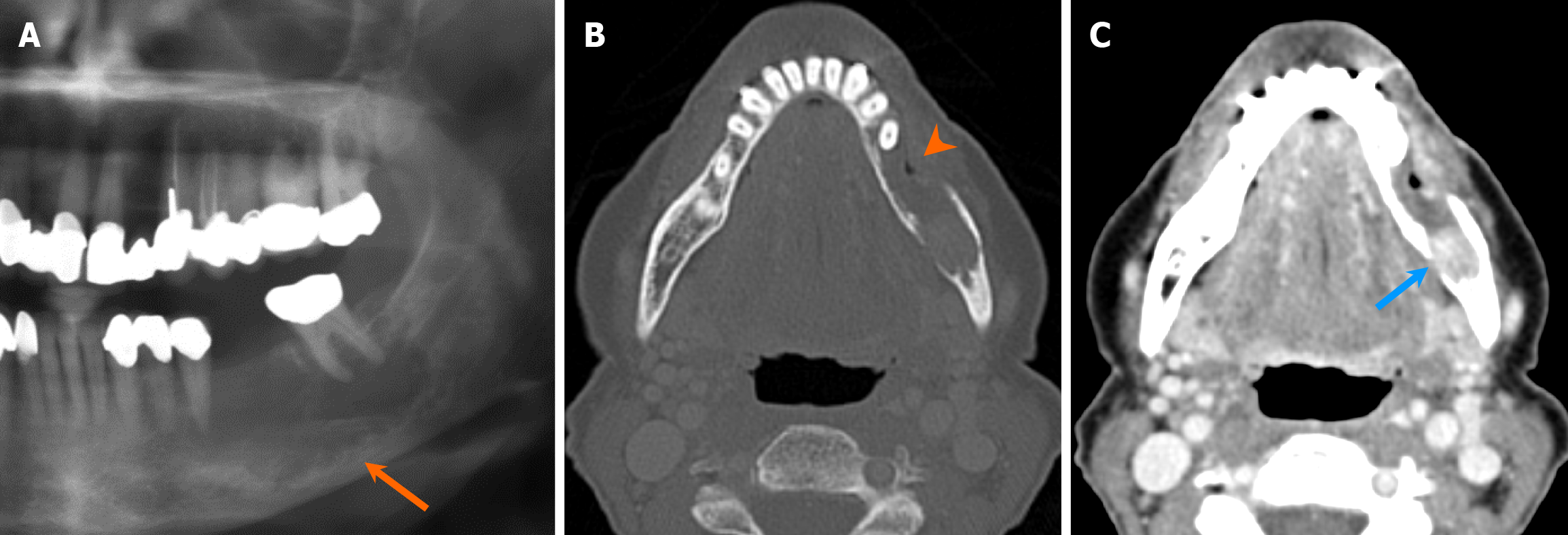
Most densely sclerotic lesions are benign. The location of the lesions plays a crucial role in distinguishing densely sclerotic lesions. Among odontogenic lesions, two notable entities presenting with dense sclerosis are cementoblastoma and odontoma. Cementoblastoma is a benign mesenchymal tumor composed of calcified matrix produced by cementoblasts. A hallmark feature of cementoblastoma is its attachment to the root of a tooth (Figure 17). Radiologically, they appear as densely sclerotic lesions with radiolucent halos, frequently associated with tooth resorption and bony expansion[44,45].
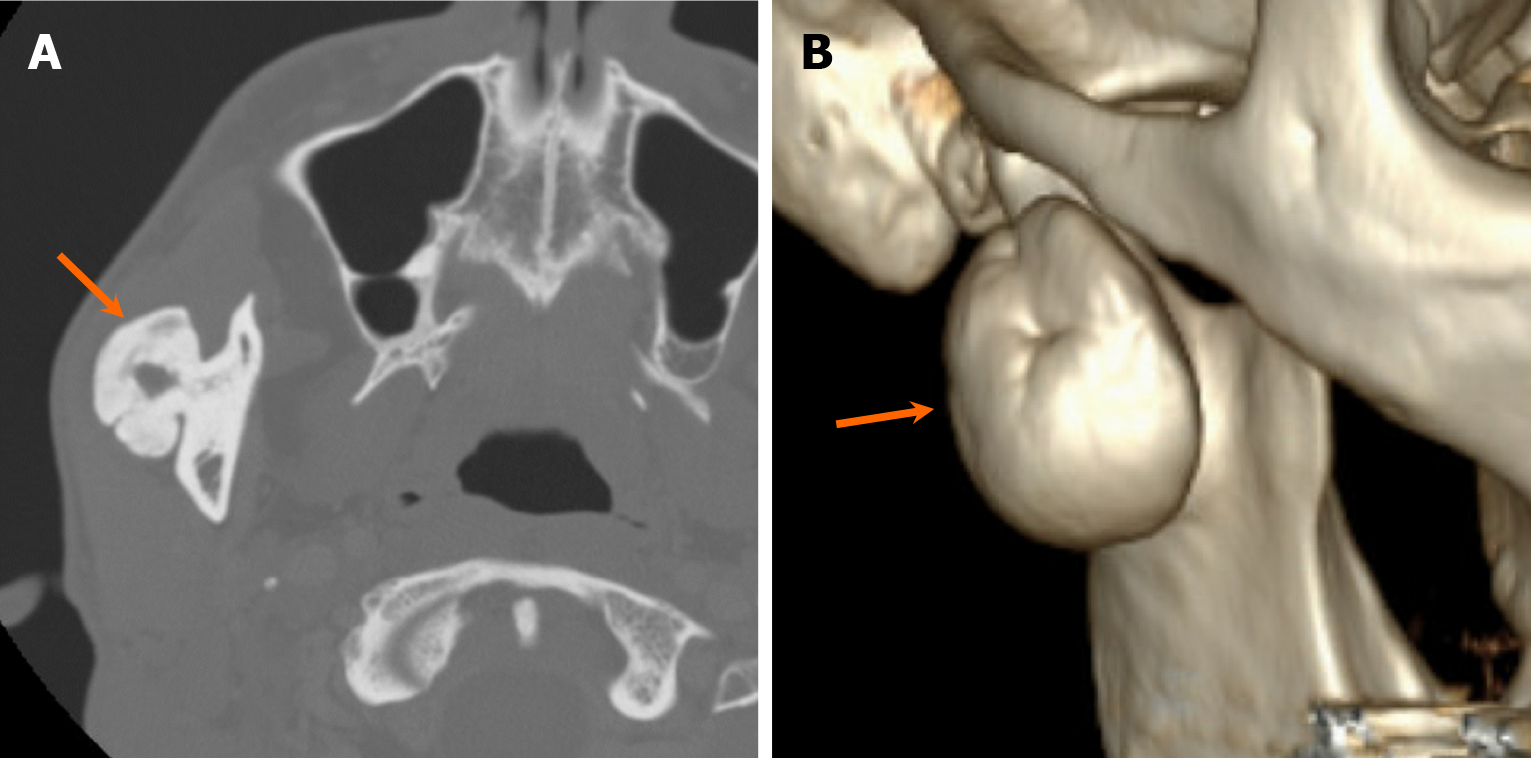
Odontomas are developmental hamartomatous lesions, characterized by the presence of dental structures. Odontomas exhibit areas with enamel density and are further classified into compound and complex odontomas. Compound odontomas consist of small denticles, the cumulative size of which grossly equals that of a tooth. On the other hand, complex odontomas are poorly differentiated lesions, characterized by a conglomerate mass of enamel and dentin[5] (Figure 18). A thin radiolucent fibrous capsule often covers odontomas. Occasionally, odontomas are associated with other odontogenic lesions, including dentigerous cyst and calcifying odontogenic cyst[46,47]. Developing odontomas, previously designated as ameloblastic fibroodontoma and ameloblastic fibrodentinoma, exhibit predominant radiolucency with varying degrees of mineralization[48]. This presentation poses challenges in differentiating them from other radiolucent lesions, such as ameloblastic fibromas, even histologically[49].
In cases where a densely sclerotic lesion is considered non-odontogenic, considerations may include osteoblastoma, osteoma, osteochondroma, tori, and idiopathic osteosclerosis. Osteoblastomas share histological similarities with cementoblastoma and are typically considered if they are not associated with a tooth root[4]. Osteomas can manifest as either endosteal or periosteal, leading to its central and peripheral form, respectively[4] (Figure 19). Osteochondromas, although rare in the jaw, demonstrate the similar imaging characteristics as osteochondromas found in other anatomic regions, presenting as exophytic lesions with corticomedullary continuity and hyalin cartilaginous caps. Tori of the maxilla and mandible are differential considerations for exophytic sclerotic lesions. Idiopathic osteosclerosis is a developmental dense bone island, typically not associated with a tooth and exhibiting no significant bony expansion[50].
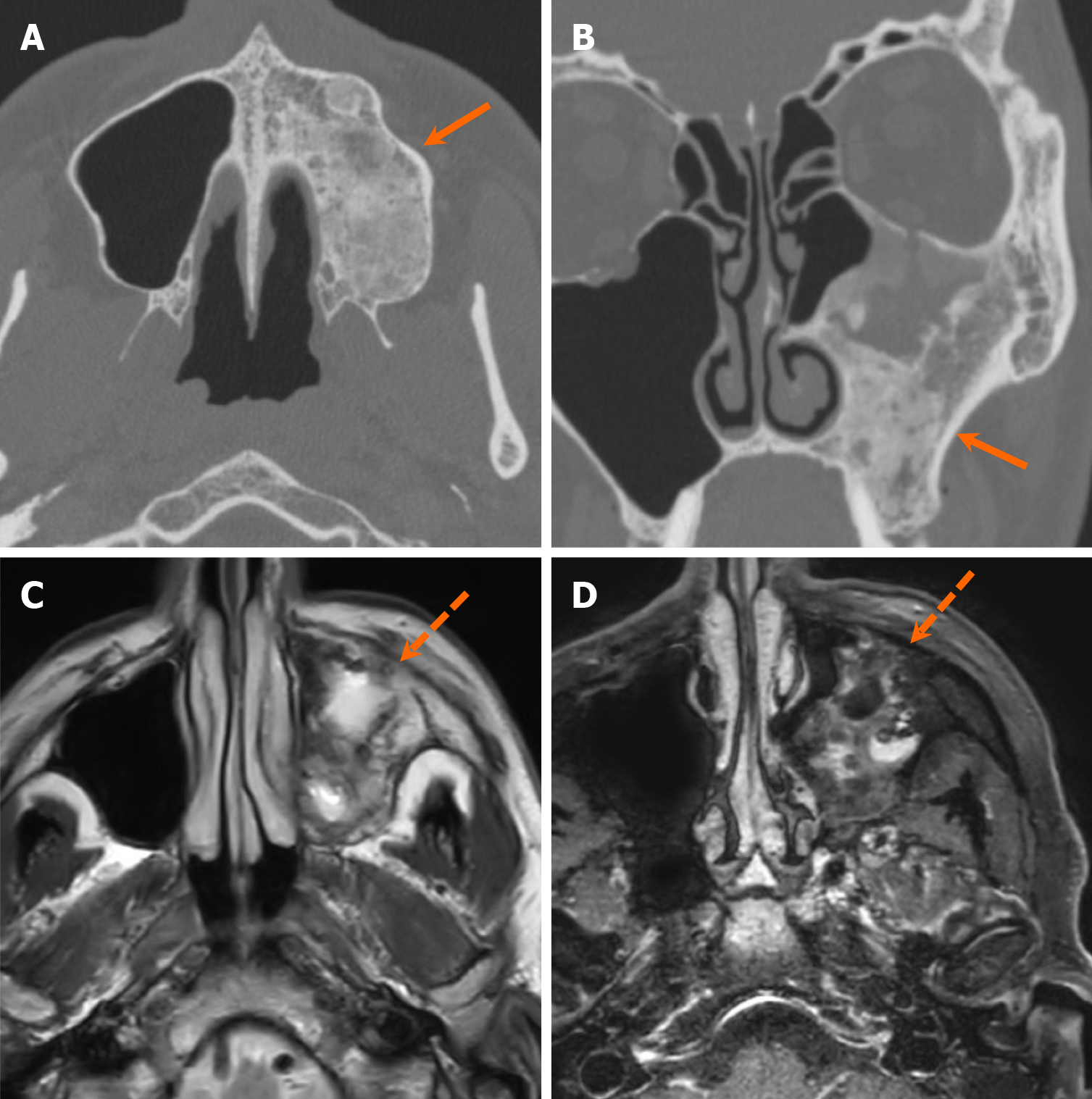
Ground-glass lesions can be classified into odontogenic and non-odontogenic categories. Many ground-glass lesions may demonstrate densely sclerotic appearance depending on the degree of mineralization. One odontogenic tumor frequently presenting with ground-glass density is COF. COFs are benign mesenchymal lesions, believed to originate from multipotent cells of periodontal membrane[51]. They exhibit well-defined borders, appearing either unilocular or multilocular, and demonstrate a centrifugally expansile growth pattern. Displacement and root resorption are commonly associated findings[51] (Figure 20). The term “ossifying fibroma” is traditionally used to indicate COF. Additionally, two other forms of ossifying fibromas exist: Juvenile trabecular ossifying fibroma and juvenile psammomatoid ossifying fibroma, both categorized as fibro-osseous tumors in the WHO classification[52]. These juvenile forms may occasionally display rapid growths and exhibit tendency to recur after treatment[52].
Non-odontogenic lesions displaying a ground-glass matrix are found in the fibro-osseous tumors according to the WHO classification. These include COD, fibrous dysplasia (FD), among others. CODs are fibro-osseous lesions that contains fibrous tissue with osteoid and cementoid matrix[53]. There are three forms of COD: Periapical, focal, and florid. Periapical CODs are confined to the apical regions of a few teeth in the anterior mandible, while focal CODs affect the apical regions of a single tooth typically in the posterior mandible. Florid CODs represent a more extensive form, affecting multiple regions of the jaw (Figure 21). The density of COD lesions can vary depending on the stage of deve
FD is also a frequently encountered fibro-osseous tumor. It can manifest as monostotic, craniofacial or polyostotic forms, with the latter potentially associated with syndromes, such as McCune-Albright and Mazabraud syndromes. FD often presents as an expansile lesion with poorly defined borders and overlying cortical thinning. While various attenuation patterns can be observed, the ground-glass pattern is more common[56] (Figure 22). Although infrequent, tooth resorption can be seen, while tooth displacement is more common[57]. Malignant transformation into sarcomas, particularly osteosarcoma, can occur, although rare. FD can be associated with ABC. Cherubism is considered a hereditary variant of FD, although it is categorized separately as a giant cell lesion and bone cyst according to the WHO classification. Radiologically, cherubism presents similarly to polyostotic FD[58]. Additionally, ground-glass lesions can arise from chronic metabolic or inflammatory conditions, such as renal osteodystrophy and Paget’s disease, which are crucial diagnostic considerations.
Mixed lytic and sclerotic lesions present with either non-aggressive or aggressive patterns, with non-aggressive lesions of odontogenic origin typically being benign. Sclerotic and ground-glass lesions, such as odontomas, CODs, COFs, or FD, gradually calcify during development and may exhibit a mixed density pattern in certain stages of development. A few additional odontogenic lesions demonstrate mixed radiolucent and sclerotic appearance. Calcifying epithelial odontogenic tumors are benign but occasionally can be locally aggressive lesions of odontogenic epithelial origin, characterized by the presence of calcifying amyloid-like component[59]. They exhibit various attenuation patterns, with a mixed lucent and sclerotic pattern being the most common. These tumors are typically found in the posterior mandible, and the calcified structures are often situated near the associated crown. They may be sometimes associated with impacted teeth, although resorption is rare[60]. The noncalcifying and Langerhans’s cell rich variant of the tumor can manifest as a unilocular or multilocular radiolucent lesion[59].
Both calcifying odontogenic cysts and adenomatoid odontogenic tumors primarily present as radiolucent lesions with internal calcifications, and they are frequently associated with an unerupted tooth, sharing similar radiologic characteristics. Calcifying odontogenic cysts are rare developmental cysts lined by ghost epithelial cells with a tendency to calcify. They are occasionally associated with an impacted tooth, which can lead to displacement. The calcified structures within these cysts are often present as discrete foci, unlike the more scattered flecks seen in calcifying epithelial odontogenic tumors[61]. Adenomatoid odontogenic tumors are rare odontogenic epithelial tumors that predominantly affect young patients. They are characterized by dystrophic calcific deposition and are often associated with an impacted canine. The lesion typically attaches to the impacted tooth at a site apical to the cemento-enamel junction[62].
The differential considerations for aggressive mixed lytic and sclerotic lesions are essentially similar to those for lucent lesions with aggressive features, but with the additional presence of mixed density appearance due to the deposition of bone/calcifications. Malignant bone and cartilage tumors such as osteosarcomas and chondrosarcomas are also examples of such lesions. Osteosarcomas of the jaw can be either primary or secondary to existing conditions, such as Paget’s disease, FD, or late sequela of radiation[63]. Imaging-wise, it shares similarities with osteosarcoma arising from other parts of the body, presenting with aggressive periosteal bone formation and a “sunburst” appearance[63] (Figure 23). The chondrosarcoma family of tumors consists of medullary tumors, characterized by the presence of chondroid matrix and may present as mostly radiolucent or mixed density lesion (Figure 24). Rhabdomyosarcoma with TFCP2 rearrangement is a unique subtype of rhabdomyosarcomas that exhibits a strong propensity for affecting the craniofacial bones[64]. It is an aggressive neoplasm composed of myogenic cells, typically presenting as an expansile, destructive lesion with an ill-defined border on imaging[64].
Tumors of the jaw and maxillofacial bone are categorized into four groups according to 2022 WHO classification, comprising: (1) Cysts of the jaw; (2) Odontogenic tumors; (3) Bone and cartilage tumors; and (4) Giant cell lesions and bone cysts (Table 2). Within the odontogenic tumors category, a further distinction is made between benign and malignant tumors. Benign odontogenic tumors are further classified into epithelial, mixed epithelial and mesenchymal, and mesenchymal tumors[65]. While there is no significant conceptual difference in the classification compared to previous editions, there have been considerable re-organization[5]. For example, fibro-osseous and osteochondromatous lesions, as well as benign and malignant bone and cartilage tumors, are grouped into one, bone and cartilage tumors. Additionally, the hematolymphoid tumor category is no longer included in the WHO classification. Another notable change is the reordering of the odontogenic tumor listings, with benign tumors now listed before malignant tumors.
| Classification of odontogenic and maxillofacial bone tumors | |
| Cyst of the jaws | Radicular cyst |
| Inflammatory collateral cyst | |
| Surgical ciliated cyst | |
| Nasopalatine duct cyst | |
| Gingival cyst | |
| Dentigerous cyst | |
| Orthokeratinized odontogenic cyst | |
| Lateral periodontal cyst and botryoid odontogenic cyst | |
| Calcifying odontogenic cyst | |
| Glandular odontogenic cyst | |
| Odontogenic keratocyst | |
| Odontogenic tumors | |
| Benign epithelial odontogenic tumors | Adenomatoid odontogenic tumor |
| Squamous odontogenic tumor | |
| Calcifying epithelial odontogenic tumor | |
| Ameloblastoma, unicystic | |
| Ameloblastoma, extraosseous/peripheral | |
| Ameloblastoma, conventional | |
| Adenoid ameloblastoma | |
| Metastasizing ameloblastoma | |
| Benign mixed epithelial and mesenchymal odontogenic tumors | Odontoma |
| Primordial odontogenic tumor | |
| Ameloblastic fibroma | |
| Dentinogenic ghost cell tumor | |
| Benign mesenchymal odontogenic tumor | Odontogenic fibroma |
| Cementoblastoma | |
| Cemento-ossifying fibroma | |
| Odontogenic myxoma | |
| Malignant odontogenic tumors | Sclerosing odontogenic carcinoma |
| Ameloblastic carcinoma | |
| Clear cell odontogenic carcinoma | |
| Ghost cell odontogenic carcinoma | |
| Primary intraosseous carcinoma, NOS | |
| Odontogenic carcinosarcoma | |
| Odontogenic sarcomas | |
| Giant cell lesions and bone cysts | Central giant cell granuloma |
| Peripheral giant cell granuloma | |
| Cherubism | |
| Aneurysmal bone cyst | |
| Simple bone cyst | |
| Bone and cartilage tumors | |
| Fibro-osseous tumors and dysplasia | Cemento-osseous dysplasia |
| Segmental odontomaxillary dysplasia | |
| Fibrous dysplasia | |
| Juvenile trabecular ossifying fibroma | |
| Psammomatoid ossifying fibroma | |
| Familial gigantiform cementoma | |
| Benign maxillofacial bone and cartilage tumors | Osteoma |
| Osteochondroma | |
| Osteoblastoma | |
| Chondroblastoma | |
| Chondromyxoid fibroma | |
| Desmoplastic fibroma of bone | |
| Malignant maxillofacial bone and cartilage tumors | Osteosarcoma of the jaw |
| The chondrosarcoma family of tumors | |
| Mesenchymal chondrosarcoma | |
| Rhabdomyosarcoma with TFCP2 rearrangement |
Since the last update in 2017, there has been significant advancement in molecular data availability. The common hotspot mutations seen in other neoplasms are absent in odontogenic cysts and tumors, reflecting their nature as hamartomas, benign neoplasms, or low-grade malignancies[65]. Despite significant advancements in molecular data, these changes have not yet led to substantial changes to the classification, except for the identification of rhabdomyosarcoma with TFCP2 gene rearrangement as a new disease entity in the updated version. The 2022 classification also introduces three new entities that, although long established in the literature, were previously unclassified, including adenoid ameloblastoma, surgical ciliated cyst, and segmental odontomaxillary dysplasia. Adenoid ameloblastomas, categorized as benign odontogenic tumors, are considered hybrid tumors, containing features of both ameloblastoma and adenoid odontogenic tumor. To date, there have been approximately 40 reported cases[66]. Radiologically, adenoid ameloblastomas typically present as aggressive radiolucent lesions with ill-defined borders[66]. Surgical ciliated cysts, although not a new entity, were introduced into the classification for the first time. These cysts are found in patients with a history of prior maxillary sinus surgery and are characterized as radiolucent lesions lined with respiratory epithelium[67]. Lastly, segmental odontomaxillary dysplasia is a non-hereditary developmental disorder characterized by segmental maxillary and soft tissue enlargement with dento-osseous abnormalities. While it is a relatively well-defined condition, it is often misdiagnosed as FD[65].
Some challenging or controversial aspects from 2017 edition remain, such as classifying metastasizing ameloblastoma as a benign entity and sclerosing odontogenic carcinoma as a malignancy despite its non-metastatic potential. Meta
In conclusion, the imaging approach to jaw and maxillofacial bone tumors is multifaceted and pivotal in accurately diagnosing these lesions. Achieving accurate diagnosis and effective management requires a comprehensive understanding of jaw and dental anatomy, coupled with a nuanced interpretation of imaging modalities. Meticulous assessment of various imaging characteristics, including radiodensity, marginal definition, loculation pattern, relationship to adjacent teeth, erosion of teeth or bone, internal matrix appearance, patterns of osseous expansion, and soft tissue component, serves as the foundation for formulating comprehensive differential diagnoses. However, imaging data for several entities is limited, partly due to the rarity of some conditions. Future research involving large-scale imaging databases could enhance our understanding of imaging characteristics and improve the differentiation of these lesions from others.
| 1. | Devenney-Cakir B, Subramaniam RM, Reddy SM, Imsande H, Gohel A, Sakai O. Cystic and cystic-appearing lesions of the mandible: review. AJR Am J Roentgenol. 2011;196:WS66-WS77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Yoshiura K, Weber AL, Runnels S, Scrivani SJ. Cystic lesions of the mandible and maxilla. Neuroimaging Clin N Am. 2003;13:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Curé JK, Vattoth S, Shah R. Radiopaque jaw lesions: an approach to the differential diagnosis. Radiographics. 2012;32:1909-1925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 4. | Holmes KR, Holmes RD, Martin M, Murray N. Practical Approach to Radiopaque Jaw Lesions. Radiographics. 2021;41:1164-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Soluk-Tekkesin M, Wright JM. The World Health Organization Classification of Odontogenic Lesions: A Summary of the Changes of the 2022 (5th) Edition. Turk Patoloji Derg. 2022;38:168-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 69] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 6. | Avril L, Lombardi T, Ailianou A, Burkhardt K, Varoquaux A, Scolozzi P, Becker M. Radiolucent lesions of the mandible: a pattern-based approach to diagnosis. Insights Imaging. 2014;5:85-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Harmon M, Arrigan M, Toner M, O'Keeffe SA. A radiological approach to benign and malignant lesions of the mandible. Clin Radiol. 2015;70:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Zohrabian VM, Poon CS, Abrahams JJ. Embryology and Anatomy of the Jaw and Dentition. Semin Ultrasound CT MR. 2015;36:397-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Scheinfeld MH, Shifteh K, Avery LL, Dym H, Dym RJ. Teeth: what radiologists should know. Radiographics. 2012;32:1927-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Husain MA. Dental Anatomy and Nomenclature for the Radiologist. Radiol Clin North Am. 2018;56:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Boeddinghaus R, Whyte A. Trends in maxillofacial imaging. Clin Radiol. 2018;73:4-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Koenig LJ. Imaging of the jaws. Semin Ultrasound CT MR. 2015;36:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Kurabayashi T, Ohbayashi N, Sakamoto J, Nakamura S. Usefulness of MR imaging for odontogenic tumors. Odontology. 2021;109:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kumar J, Vanagundi R, Manchanda A, Mohanty S, Meher R. Radiolucent Jaw Lesions: Imaging Approach. Indian J Radiol Imaging. 2021;31:224-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | White SC, Pharoah MJ. Oral Radiology: Principles and Interpretation. 6th ed. United States: Mosby, 2009. |
| 16. | Johnson NR, Gannon OM, Savage NW, Batstone MD. Frequency of odontogenic cysts and tumors: a systematic review. J Investig Clin Dent. 2014;5:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Chapman MN, Nadgir RN, Akman AS, Saito N, Sekiya K, Kaneda T, Sakai O. Periapical lucency around the tooth: radiologic evaluation and differential diagnosis. Radiographics. 2013;33:E15-E32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | McLean AC, Vargas PA. Cystic Lesions of the Jaws: The Top 10 Differential Diagnoses to Ponder. Head Neck Pathol. 2023;17:85-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Wamasing N, Yomtako S, Watanabe H, Sakamoto J, Kayamori K, Kurabayashi T. The magnetic resonance imaging characteristics of radicular cysts and granulomas. Dentomaxillofac Radiol. 2023;52:20220336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Mosier KM. Magnetic resonance imaging of the maxilla and mandible: signal characteristics and features in the differential diagnosis of common lesions. Top Magn Reson Imaging. 2015;24:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Park S, Jeon SJ, Yeom HG, Seo MS. Differential diagnosis of cemento-osseous dysplasia and periapical cyst using texture analysis of CBCT. BMC Oral Health. 2024;24:442. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Dunfee BL, Sakai O, Pistey R, Gohel A. Radiologic and pathologic characteristics of benign and malignant lesions of the mandible. Radiographics. 2006;26:1751-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 107] [Article Influence: 5.6] [Reference Citation Analysis (1)] |
| 23. | Struthers P, Shear M. Root resorption by ameloblastomas and cysts of the jaws. Int J Oral Surg. 1976;5:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Otonari-Yamamoto M, Nakajima K, Sato H, Wada H, Matsumoto H, Nishiyama A, Hoshino T, Matsuzaka K, Katakura A, Goto TK. Dentigerous cysts suspected the other odontogenic lesions on panoramic radiography and CT. Oral Radiol. 2024;40:319-326. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Kim SJ, Moon JW, Lee HM. Huge Nasopalatine Duct Cyst Treated by Transnasal Endoscopic Marsupialization: A Case Report and Literature Review. Ear Nose Throat J. 2023;1455613231177986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 26. | Kuhmichel A, Bouloux GF. Multifocal traumatic bone cysts: case report and current thoughts on etiology. J Oral Maxillofac Surg. 2010;68:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Sabino-Bezerra JR, Santos-Silva AR, Jorge J Jr, Gouvêa AF, Lopes MA. Atypical presentations of simple bone cysts of the mandible: a case series and review of literature. J Craniomaxillofac Surg. 2013;41:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Borghesi A, Nardi C, Giannitto C, Tironi A, Maroldi R, Di Bartolomeo F, Preda L. Odontogenic keratocyst: imaging features of a benign lesion with an aggressive behaviour. Insights Imaging. 2018;9:883-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Yonetsu K, Bianchi JG, Troulis MJ, Curtin HD. Unusual CT appearance in an odontogenic keratocyst of the mandible: case report. AJNR Am J Neuroradiol. 2001;22:1887-1889. [PubMed] |
| 30. | Minami M, Kaneda T, Ozawa K, Yamamoto H, Itai Y, Ozawa M, Yoshikawa K, Sasaki Y. Cystic lesions of the maxillomandibular region: MR imaging distinction of odontogenic keratocysts and ameloblastomas from other cysts. AJR Am J Roentgenol. 1996;166:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Srinivasan K, Seith Bhalla A, Sharma R, Kumar A, Roychoudhury A, Bhutia O. Diffusion-weighted imaging in the evaluation of odontogenic cysts and tumours. Br J Radiol. 2012;85:e864-e870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Weissman JL, Snyderman CH, Yousem SA, Curtin HD. Ameloblastoma of the maxilla: CT and MR appearance. AJNR Am J Neuroradiol. 1993;14:223-226. [PubMed] |
| 33. | Sumi M, Ichikawa Y, Katayama I, Tashiro S, Nakamura T. Diffusion-weighted MR imaging of ameloblastomas and keratocystic odontogenic tumors: differentiation by apparent diffusion coefficients of cystic lesions. AJNR Am J Neuroradiol. 2008;29:1897-1901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Colombo CS, Boivin Y. Myxoma of the jaws. Oral Surg Oral Med Oral Pathol. 1966;21:431-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Kheir E, Stephen L, Nortje C, van Rensburg LJ, Titinchi F. The imaging characteristics of odontogenic myxoma and a comparison of three different imaging modalities. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:492-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Peltola J, Magnusson B, Happonen RP, Borrman H. Odontogenic myxoma--a radiographic study of 21 tumours. Br J Oral Maxillofac Surg. 1994;32:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Wang K, Guo W, You M, Liu L, Tang B, Zheng G. Characteristic features of the odontogenic myxoma on cone beam computed tomography. Dentomaxillofac Radiol. 2017;46:20160232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Shrestha S, Zhang J, Yan J, Zeng X, Peng X, He B. Radiological features of central giant cell granuloma: comparative study of 7 cases and literature review. Dentomaxillofac Radiol. 2021;50:20200429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Jeyaraj P. Management of Central Giant Cell Granulomas of the Jaws: An Unusual Case Report with Critical Appraisal of Existing Literature. Ann Maxillofac Surg. 2019;9:37-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Devi P, Thimmarasa V, Mehrotra V, Agarwal M. Aneurysmal bone cyst of the mandible: A case report and review of literature. J Oral Maxillofac Pathol. 2011;15:105-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Asaumi J, Konouchi H, Hisatomi M, Matsuzaki H, Shigehara H, Honda Y, Kishi K. MR features of aneurysmal bone cyst of the mandible and characteristics distinguishing it from other lesions. Eur J Radiol. 2003;45:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Villegas KM, Paparella ML. Malignant odontogenic tumors. A report of a series of 30 cases and review of the literature. Oral Oncol. 2022;134:106068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 43. | Borrás-Ferreres J, Sánchez-Torres A, Gay-Escoda C. Malignant changes developing from odontogenic cysts: A systematic review. J Clin Exp Dent. 2016;8:e622-e628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Chrcanovic BR, Gomez RS. Cementoblastoma: An updated analysis of 258 cases reported in the literature. J Craniomaxillofac Surg. 2017;45:1759-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Huber AR, Folk GS. Cementoblastoma. Head Neck Pathol. 2009;3:133-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Vizuete-Bolaños MX, Salgado-Chavarria F, Ramírez-Martínez CM, Ramos-Nieto JJ, Vazquez-Dávalos NM. Compound odontoma associated with a calcifying odontogenic cyst. Case report and systematic review. J Stomatol Oral Maxillofac Surg. 2022;123:e97-e105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Kim KS, Lee HG, Hwang JH, Lee SY. Incidentally detected odontoma within a dentigerous cyst. Arch Craniofac Surg. 2019;20:62-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 48. | Watanabe M, Wakoh M, Nakajima K, Yoshida S, Sato H, Koyachi M, Odaka K, Koshimizu Y, Otonari-Yamamoto M, Takano M, Matsuzaka K, Katakura A, Shibahara T, Goto TK. Developing odontoma with an atypical radiological appearance: A case report. Oral Maxillofac Surg Cases. 2020;6:100138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Buchner A, Kaffe I, Vered M. Clinical and radiological profile of ameloblastic fibro-odontoma: an update on an uncommon odontogenic tumor based on a critical analysis of 114 cases. Head Neck Pathol. 2013;7:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Gamba TO, Maciel NAP, Rados PV, da Silveira HLD, Arús NA, Flores IL. The imaging role for diagnosis of idiopathic osteosclerosis: a retrospective approach based on records of 33,550 cases. Clin Oral Investig. 2021;25:1755-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Kaur T, Dhawan A, Bhullar RS, Gupta S. Cemento-Ossifying Fibroma in Maxillofacial Region: A Series of 16 Cases. J Maxillofac Oral Surg. 2021;20:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Zegalie N, Speight PM, Martin L. Ossifying fibromas of the jaws and craniofacial bones. Diagn Histopathol. 2015;21:351-358. [DOI] [Full Text] |
| 53. | Decolibus K, Shahrabi-Farahani S, Brar A, Rasner SD, Aguirre SE, Owosho AA. Cemento-Osseous Dysplasia of the Jaw: Demographic and Clinical Analysis of 191 New Cases. Dent J (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 54. | Günaçar DN, Köse TE, Arıcıoğlu B, Çene E. Retrospective radiological analysis of cemento-osseous dysplasia. Dent Med Probl. 2023;60:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Chadwick JW, Alsufyani NA, Lam EW. Clinical and radiographic features of solitary and cemento-osseous dysplasia-associated simple bone cysts. Dentomaxillofac Radiol. 2011;40:230-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Obermeier KT, Hartung JT, Hildebrandt T, Dewenter I, Smolka W, Hesse E, Fegg F, Otto S, Malenova Y, Abdullah A. Fibrous Dysplasia of the Jaw: Advances in Imaging and Treatment. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 57. | Wagner VP, Carlos R, Romañach MJ, Lopes MA, Speight PM, Vargas PA. Malignant transformation of craniomaxillofacial fibro-osseous lesions: A systematic review. J Oral Pathol Med. 2019;48:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | Beaman FD, Bancroft LW, Peterson JJ, Kransdorf MJ, Murphey MD, Menke DM. Imaging characteristics of cherubism. AJR Am J Roentgenol. 2004;182:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 59. | Lin HP, Kuo YS, Wu YC, Wang YP, Chang JY, Chiang CP. Non-calcifying and Langerhans cell-rich variant of calcifying epithelial odontogenic tumor. J Dent Sci. 2016;11:117-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Kaplan I, Buchner A, Calderon S, Kaffe I. Radiological and clinical features of calcifying epithelial odontogenic tumour. Dentomaxillofac Radiol. 2001;30:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 61. | Iida S, Fukuda Y, Ueda T, Aikawa T, Arizpe JE, Okura M. Calcifying odontogenic cyst: radiologic findings in 11 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:356-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | More CB, Das S, Gupta S, Bhavsar K. Mandibular adenomatoid odontogenic tumor: Radiographic and pathologic correlation. J Nat Sci Biol Med. 2013;4:457-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Chaudhary M, Chaudhary SD. Osteosarcoma of jaws. J Oral Maxillofac Pathol. 2012;16:233-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 64. | Gallagher KPD, Roza ALOC, Tager EMJR, Mariz BALA, Soares CD, Rocha AC, Abrahão AC, Romañach MJ, Carlos R, Hunter KD, Lopes MA, Vargas PA, Santos-Silva AR. Rhabdomyosarcoma with TFCP2 Rearrangement or Typical Co-expression of AE1/AE3 and ALK: Report of Three New Cases in the Head and Neck Region and Literature Review. Head Neck Pathol. 2023;17:546-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 65. | El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ. World Health Organization Classification of Head and Neck Tumours. [cited 15 March 2024]. Available from: https://www.iarc.who.int/news-events/who-classification-of-head-and-neck-tumours/. |
| 66. | Jayasooriya PR, Abeyasinghe WAMUL, Liyanage RLPR, Uthpali GN, Tilakaratne WM. Diagnostic Enigma of Adenoid Ameloblastoma: Literature Review Based Evidence to Consider It as a New Sub Type of Ameloblastoma. Head Neck Pathol. 2022;16:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 67. | Peighoun M, Samieirad S, Mohtasham N, Tohidi E, Moeini S. Surgical Ciliated Cyst of the Posterior Maxilla in an Old Male, Mimicking Residual Cyst or Odontogenic Keratocyst: A Case Report. World J Plast Surg. 2022;11:132-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 68. | Pandiar D, Anand R, Kamboj M, Narwal A, Shameena PM, Devi A. Metastasizing Ameloblastoma: A 10 Year Clinicopathological Review with an Insight Into Pathogenesis. Head Neck Pathol. 2021;15:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Vered M, Wright JM. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Odontogenic and Maxillofacial Bone Tumours. Head Neck Pathol. 2022;16:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (0)] |