Published online Jul 28, 2024. doi: 10.4329/wjr.v16.i7.265
Revised: June 8, 2024
Accepted: July 2, 2024
Published online: July 28, 2024
Processing time: 76 Days and 16.9 Hours
Bone is one of the common sites of metastasis from prostate carcinoma. Bone scintigraphy (BS) is one of the most sensitive imaging modalities currently used for bone metastatic work-up. Skeletal metastasis in prostate carcinoma commonly involves pelvic bones but rarely involves extrapelvic-extraspinal sites.
To retrospectively analyze the BS data to determine the pattern of skeletal metastases in the prostate carcinoma.
This retrospective observational study involves patients with biopsy-proven prostate carcinoma referred for BS for staging assessment. Patients with abnormal BS were evaluated for the pattern of skeletal involvement and data were pre
A total of 150 patients with biopsy-proven prostate cancer who were referred for staging were included in the study. Thirteen of 150 patients (8.67%) had no abnormal uptake on planar images, ruling out metastatic disease. Twenty-four patients (16%) had heterogeneous uptake in the spine with distribution characteristic of degenerative disease and no scan pattern of metastatic disease. Thirty patients (20%) had multifocal uptake involving both pelvic and extra pelvic bones on planar images typical for skeletal metastasis and were considered metastatic. Eighty-three out of 150 patients (55.3%) had increased tracer uptake, which was indeterminate, thus, single photon emission computed tomography-computed tomography (SPECT-CT) was acquired, which showed 51 with metastatic disease, 31 benign lesions, and one indeterminate finding. Seven of 150 patients had exclusive pelvic bone uptake, which was found to be metastatic in 4/7 patients in SPECT-CT. Fifty six out of 150 patients showed exclusive extrapelvic tracer uptake, of which only 3 had vertebral metastatic disease. None of the patients with increased uptake exclusively in the extrapelvic-extraspinal location was metastatic.
The incidence of exclusive extrapelvic skeletal metastatic disease in prostate carcinoma is 2% (excluding one patient with indeterminate findings). Further, none of the patients in the current study had exclusive extrapelvic-extraspinal metastasis. Thus, exclusive extrapelvic-extraspinal focal abnormality on planar BS carries a very low probability of metastatic disease and hence, further imaging or SPECT-CT can be safely avoided in such cases.
Core Tip: The current study analyzed bone scintigraphy (BS) data from 150 patients with biopsy-proven prostate carcinoma to determine skeletal metastasis patterns. The most common site of skeletal metastasis was pelvis. The incidence of exclusive extrapelvic skeletal metastatic disease was 2%, excluding one indeterminate case. Additionally, no patients in the study had exclusive extrapelvic-extraspinal metastasis. Therefore, exclusive extrapelvic-extraspinal focal abnormalities on planar BS have a very low likelihood of being metastatic, making further imaging or single photon emission computed tomography-computed tomography often unnecessary.
- Citation: Singh P, Agrawal K, Rahman A, Singhal T, Parida GK, Gnanasegaran G. Incidence of exclusive extrapelvic skeletal metastasis in prostate carcinoma on bone scintigraphy. World J Radiol 2024; 16(7): 265-273
- URL: https://www.wjgnet.com/1949-8470/full/v16/i7/265.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i7.265
Prostate carcinoma (PCa) is one of the most common cancers worldwide and stands as the second most commonly diagnosed cancer in men. It constitutes the 5th most common cause of cancer-related death[1]. The prevalence of PCa increases with age. About less than 5% of individuals under the age of 30 years harbor PCa, while this rises to greater than 59% in those with age above 80 years[2-4]. Skeleton is the most common site for metastatic disease involvement in PCa. Radionuclide bone scintigraphy (BS) is one of the most sensitive investigations for screening of skeletal metastases in PCa with the added advantage of low cost and the ability to screen the entire skeleton in a single study[5,6].
In PCa, pelvic bones, followed by the spine, represent the most common site of metastatic bone disease. This can be attributed to the low resistance venous connection between the periprostatic venous plexus and valveless vertebral venous plexus of Batson and the presence of a highly conducive microenvironment due to abundant red marrow, particularly in the pelvic bones providing the ideal “soil” for metastatic disease development[7-9]. Involvement of other bones without involvement of pelvic bones and spine is rarely encountered. We retrospectively analyzed the BS data to determine the pattern of bone metastases in PCa[5,10,11].
The study involved a retrospective analysis of BS data of prostate cancer patients referred to the Department of Nuclear Medicine who underwent whole-body skeletal scintigraphy between August 2016 to June 2023. All patients with histopathologically proven PCa referred for whole-body BS for metastatic work-up were reviewed. Patients’ details, clinical history, result of other imaging modalities and follow-up of the patients were recorded. The study was performed in accordance with the Declaration of Helsinki and after receiving approval from the institutional ethical committee (No. T/IM-NF/Nucl.Med/23/187).
(1) Histopathologically proven PCa; (2) Patient referred for initial staging work-up; and (3) No history of prior hormonal therapy, radiotherapy, chemotherapy, or any other form of systemic therapy.
(1) Histopathological diagnosis not available; or (2) History of prior systemic therapy.
BS imaging protocol: Whole body BS was performed after intravenous administration of 20-25 mCi (740-925 MBq) of Tc-99m methylene diphosphonate (MDP) following SNMMI Procedure Standard for BS 4.0. Images were acquired on a dual-head gamma camera (Discovery NM/CT 670, GE Healthcare) using a low energy high-resolution parallel hole collimator with an energy window width of 20% centered at 140 KeV. Whole body planar images were acquired 3 hours after tracer injection in anterior and posterior views with a matrix size of 1024 × 256[12].
Regional single-photon emission computed tomography (SPECT)-computed tomography (CT) was acquired in patients with indeterminate and suspicious lesions on planar images. The SPECT was acquired in a step-and-shoot manner with 60 stops, 25 seconds/stop, and angular movement of 3 degrees/head/stop using a matrix size of 128 × 128 and co-registered with CT (low-dose non-contrast 16 slice CT acquired keeping 120 KVp, 50 mAs tube energy setting). The images were analyzed using the Xeleris 4.0 workstation. SPECT-CT images were processed with 8 iterations and sub-sets and co-registration with CT was done on Volumetric MI software to form fused 3D images[12].
The scans were interpreted individually by two experienced nuclear medicine physicians. The scans were categorized as positive, negative, or suspicious/indeterminate for skeletal metastases on planar studies. The tracer uptake on planar BS was reported positive for metastasis when there is a classical pattern for metastatic disease involvement, like multiple foci of uptake. The scan was reported as negative when it conforms to the physiological distribution of the tracer or to the typical pattern of benign disease viz degenerative changes, arthritis, etc. In suspicious/indeterminate uptake, SPECT/CT of the corresponding region was available in all cases. SPECT/CT was interpreted as positive, negative or indeterminate for metastasis. In case of discordancy of results, the help of a third nuclear medicine physician was sought, and the final result was made on the basis of consensus.
The data were analysed on a per-patient basis, and the incidence of multiple metastases, including pelvic and extrapelvic, exclusive extrapelvic, and exclusive extrapelvic/extraspinal metastasis, was recorded. The data are presented in descriptive format in the form of percentages.
A total of 150 patients with biopsy-proven prostate cancer for staging were included in the study. The median age of included patients was 68.7 years (range: 42-86 years). A total of 81/150 patients showed metastatic disease.
Thirteen of 150 patients (8.67%) had no abnormal uptake on planar images ruling out metastatic disease. Twenty-four patients (16%) had heterogenous uptake in the spine with distribution characteristic of degenerative disease and no scan pattern of metastatic disease. Thirty patients (20%) had multifocal uptake involving both pelvic and extra pelvic bones on planar images typical for skeletal metastasis and were considered metastatic (Table 1).
| Pattern of uptake on bone scan | Number of patients among, n = 150 |
| No abnormal increased osteoblastic activity | 13 |
| Increased uptake with pattern typical of degenerative changes/benign uptake | 24 |
| Multifocal increased uptake typical for metastatic disease | 30 |
| Indeterminate or suspicious for metastatic uptake where SPECT-CT was performed | 83 |
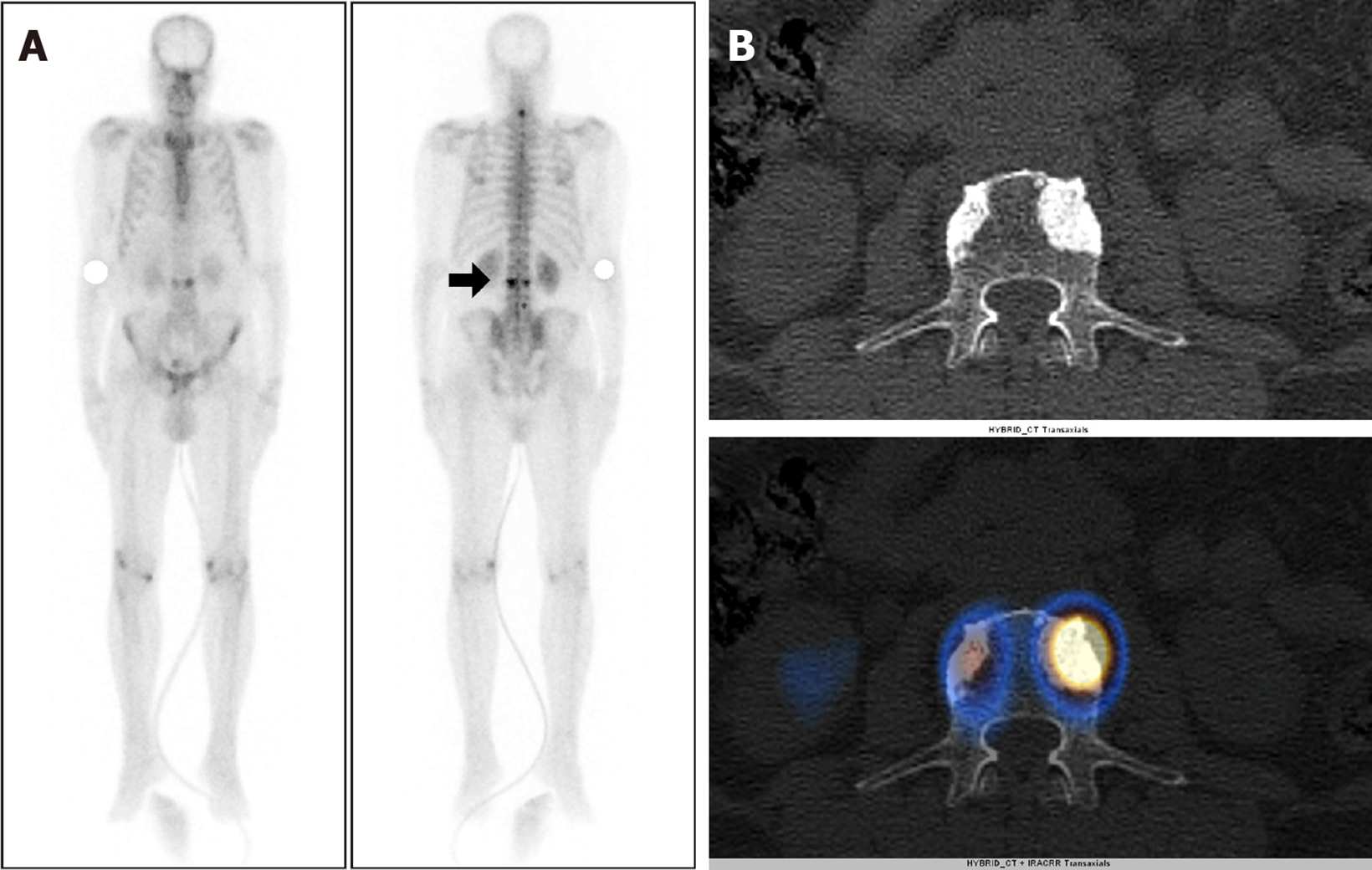
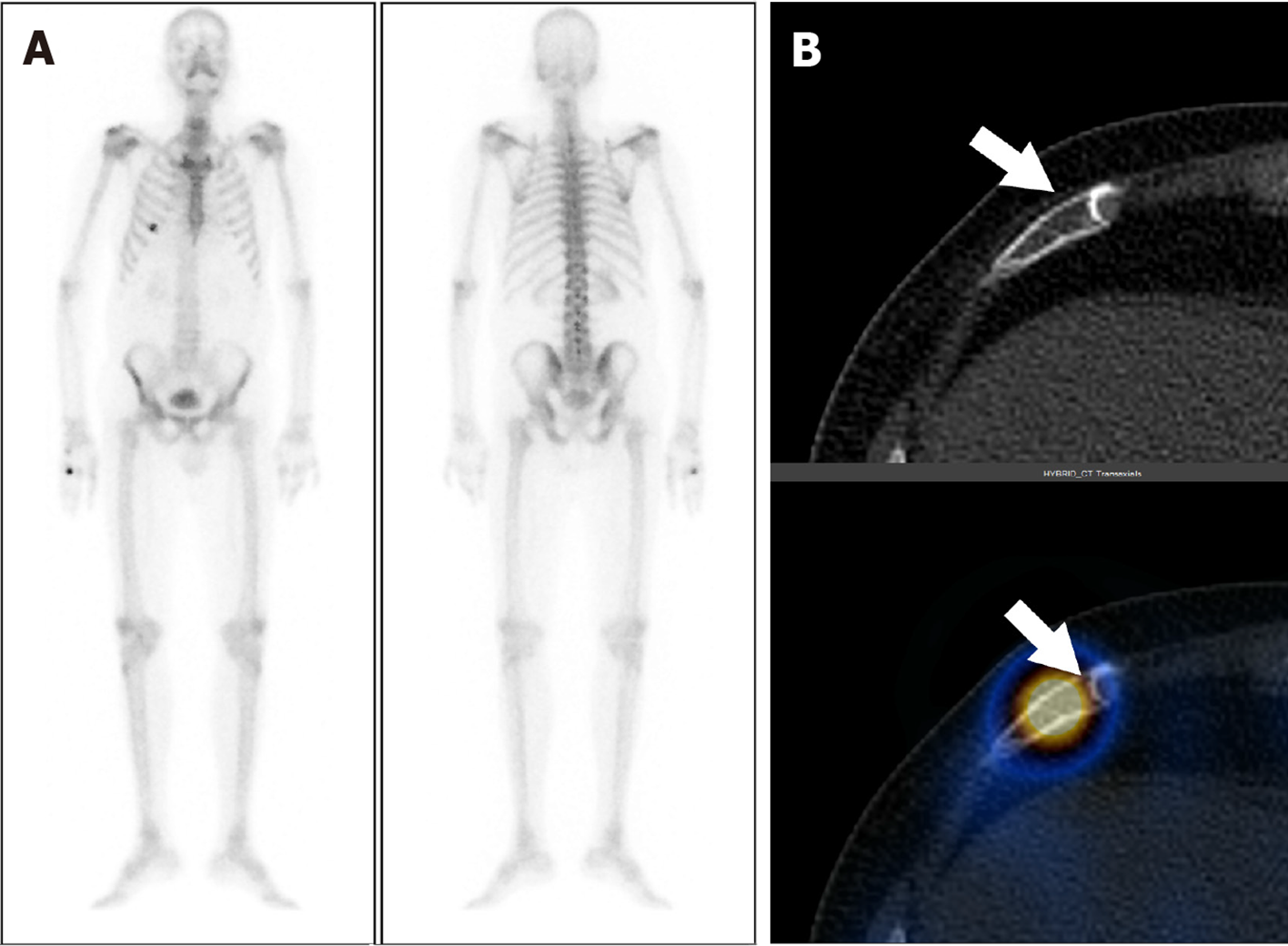
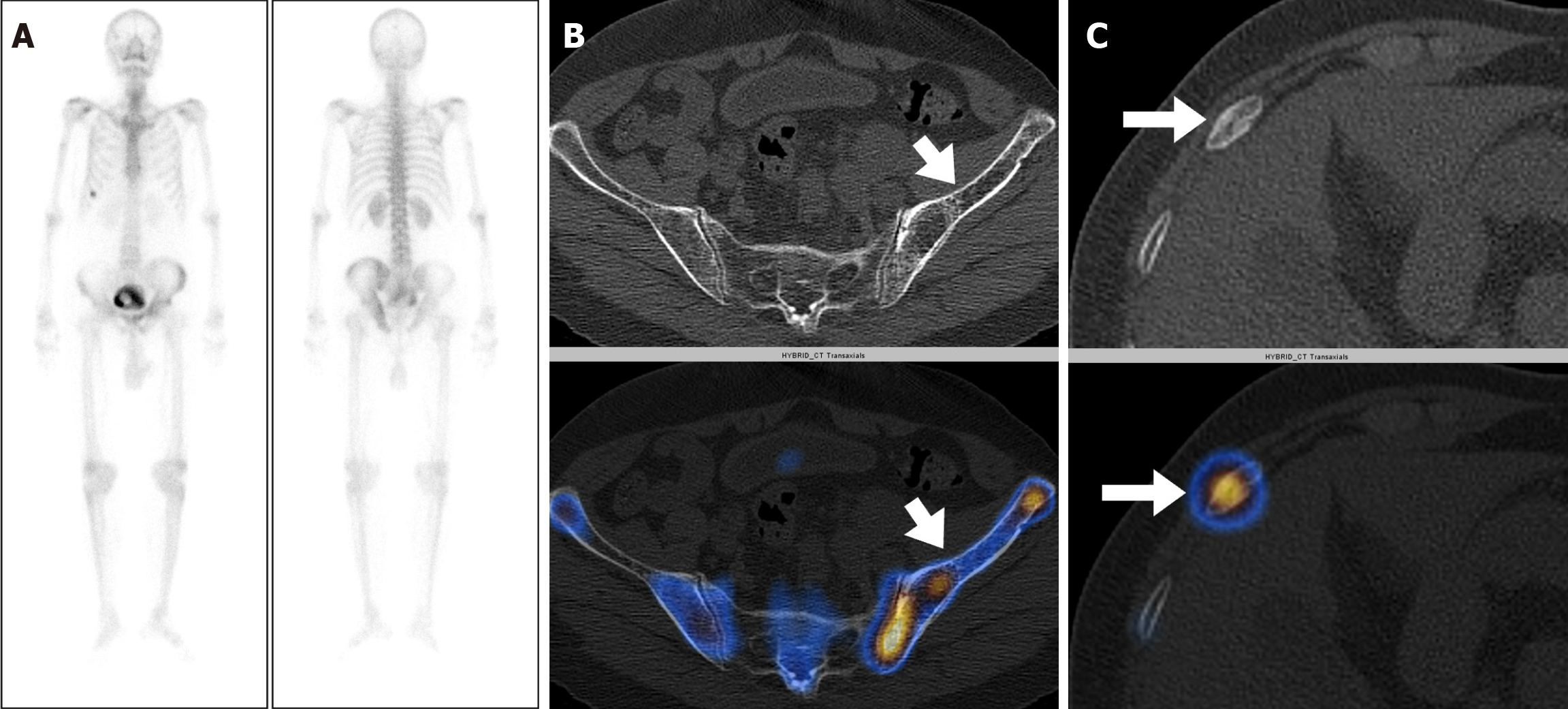
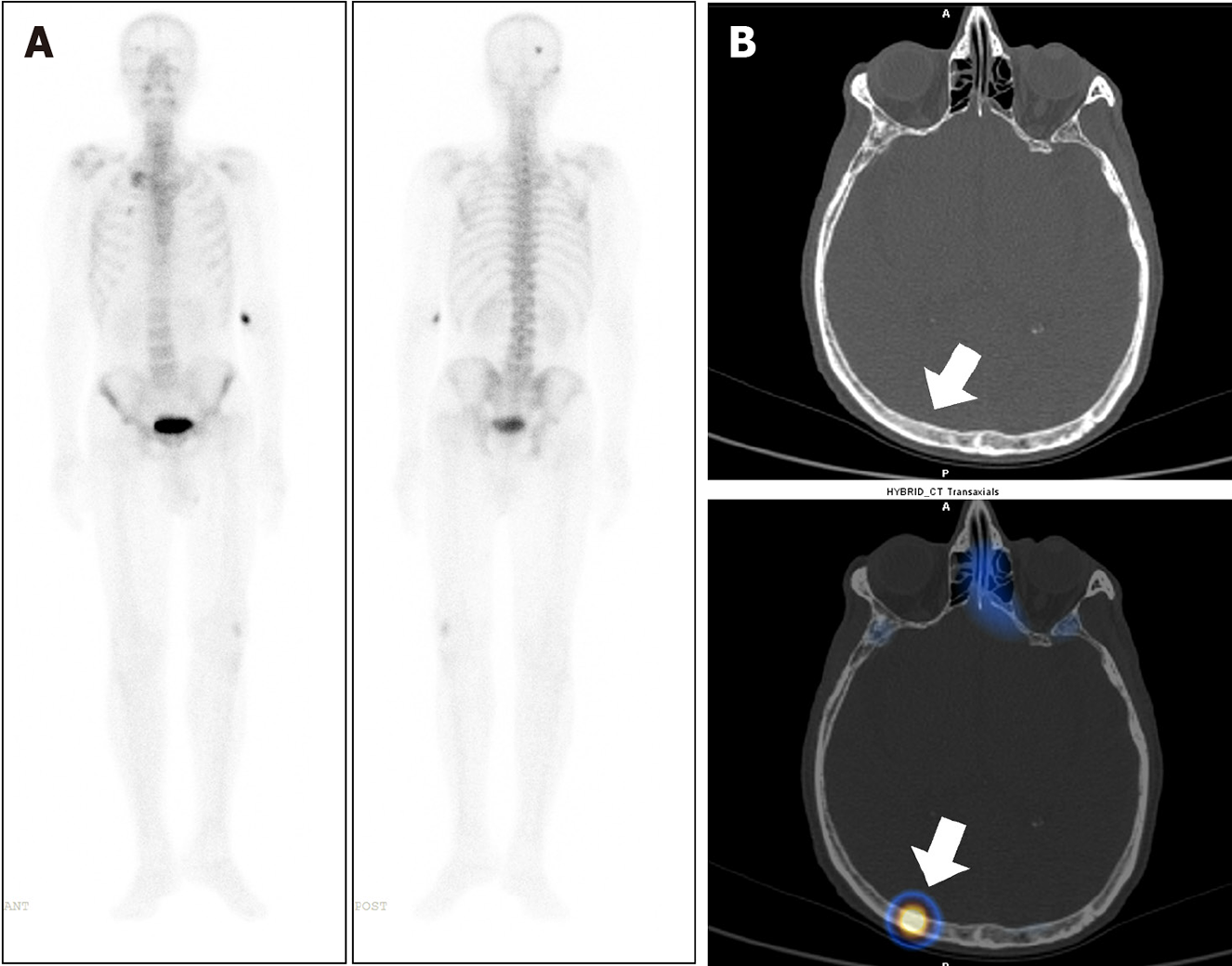
Eighty-three out of 150 patients (55.3%) had increased tracer uptake on whole-body planar images, which were indeterminate or suspicious for metastatic disease. Thus, SPECT-CT was acquired to characterize the uptake further (Table 2). Forty-four of 83 patients (53%) had more than five foci of abnormally increased osteoblastic activity involving all pelvic, spinal, and extraspinal sites, and SPECT-CT was performed to confirm the metastatic disease. All of these patients were found to be metastatic on SPECT-CT. Focal osteoblastic activity localizing exclusively to the pelvis was seen in 7 patients, with 4 patients proven to be metastatic, and 3 patients having benign uptake on SPECT-CT. Thus, pondering a very high relative risk of isolated pelvic focal osteoblastic activity being malignant. The remaining 32 out of 83 patients showed abnormal exclusive extra pelvic tracer uptake. These included 20 patients with vertebral (17 benign and 3 metastatic) as shown in Figure 1, and 12 patients with exclusive extrapelvic extraspinal uptake (11 benign and 1 indeterminate) as shown in Figures 2-4. None of the patients with osteoblastic abnormality exclusively in the extrapelvic-extraspinal regions on planar BS was metastatic. One patient had focal uptake in the skull and was deemed indeterminate on SPECT-CT.
| Lesion | Number of patients among, n = 83 |
| Benign | 31 |
| Metastatic | 51 |
| Indeterminate | 1 |
Thus, in total, of all patients showing exclusive extrapelvic uptake (56 patients), only three were metastatic (all vertebral metastatic disease) (Table 3). Exclusive extrapelvic/extraspinal uptake was found in a total of 12 patients, of which locations of uptake were ribs (7/12), sternum and manubriosternal joint (2/12), and skull (3/12). None of these exclusive extra pelvic-extraspinal sites of abnormal tracer uptake was malignant on SPECT-CT. Thus, pondering a negligible risk of metastatic skeletal disease in absence of pelvic and spinal bones involvement. Table 4 elaborates the SPECT-CT findings in solitary extra pelvic uptake. Most common site for metastatic disease involvement were pelvic bones followed by vertebrae and ribs (Table 5).
| Site of uptake | Benign | Metastatic | Indeterminate |
| Vertebra | 41 | 3 | 0 |
| Ribs | 7 | 0 | 0 |
| Sternum and manubriosternal region | 2 | 0 | 0 |
| Skull | 2 | 0 | 1 |
| Serial No. | Age in years | Site of uptake | SPECT-CT findings | Final diagnosis |
| 1 | 67 | Left 1st rib | Arthritis | Benign |
| 2 | 83 | Right 5th rib | Fibrous dysplasia | Benign |
| 3 | 66 | Right 6th rib | Fracture line likely post traumatic | Benign |
| 4 | 63 | Right 11th rib | Fracture line likely post traumatic | Benign |
| 5 | 72 | Right 6th rib | Fracture line likely post traumatic | Benign |
| 6 | 60 | Sternum | Manubriosternal joint | Benign |
| 7 | 76 | Right parietal bone | Subtle sclerosis not typical for metastatic disease | Indeterminate |
| Site | Number of patients |
| Super-scan | 10 |
| Pelvic bones | 68 |
| Vertebrae | 63 |
| Ribs | 55 |
| Sternum | 42 |
| Femur | 41 |
| Scapula | 41 |
| Skull | 32 |
| Clavicle | 29 |
| Humerus | 23 |
| Others | 7 (4 tibia, 2 forearm bones, 1 maxilla) |
In this study, we found pelvic bones and vertebrae to be the most common sites of metastatic disease in PCa. Further, this study demonstrates that the risk of a metastatic disease of an extra-pelvic/extraspinal uptake in PCa is negligible. Exclusive extrapelvic osteoblastic activity localizing to the spine has a less but significant risk of metastatic disease (15%).
PCa is relatively indolent and has slow growth. Thus, PCa has a favorable prognosis. The 5-year survival rates approach nearly 100% for localized as well as locoregional disease. However, this reduces to almost 34% once the patient has metastatic disease. Thus, marking the importance of early diagnosis of metastatic disease in PCa. With the implementation of early detection strategies like prostate-specific antigen screening programs, the majority of the cases are diagnosed in the early stages[2,6,13-15]. At diagnosis, about 78% of the patients have localized disease, while 16% of patients have locoregional lymph nodal involvement, and only 6% of cases have metastatic disease.
Bone and bone marrow, providing the ideal site for metastatic disease development, constitute the most common site for metastatic disease involvement of PCa. Lymph nodes, lung and liver follow this. Approximately 60% of patients progress to metastatic disease throughout management, and approximately 80% of patients with fatal progressive PCa harbor bone metastasis[16,17]. Bone metastasis commonly localizes to pelvic bones. This is hypothesized to be secondary to the retrograde spread of the tumor via the venous communication between the low resistance periprostatic venous plexus and Batson’s plexus. BS is the most commonly used imaging modality for the assessment of bone metastasis in PCa staging. BS can be performed using 99m-Tc MDP and 99m-Tc HDP (hydroxymethylene diphosphonate). These radiopharmaceuticals, when injected intravenously, get rapidly chemisorbed onto the hydroxyapatite crystals and more so at the sites of increased osteoblastic activity, thus highlighting the skeletal involvement with high sensitivity[8,18-20].
However, not all areas of increased osteoblastic activity are metastatic, and they may represent some benign pathology. Thus, emanating its limited specificity. The use of targeted SPECT/CT offers more specific diagnostic options in this subgroup of patients. It adds anatomical information of CT with functional information of SPECT and can help rule out benign causes of increased tracer uptake and confirm the metastatic disease. SPECT/CT, however, is associated with increased patient radiation burden by its CT component and adds to the total scan time. It adds approximately 0.5 to 2.6 mSv of radiation dose to the patient and approximately ≥ 12 mins to the acquisition time, depending on the area imaged. Thus, employing the need for caution while performing SPECT/CT[21-23]. Further, it is not always available in all Nuclear Medicine departments.
Skeletal metastasis from PCa commonly localizes to pelvic bones followed by vertebrae. Isolated focal areas of uptake, apart from these regions, are rarely due to skeletal metastasis[13,14,17]. Wang et al[24] studied the distribution of skeletal metastasis in PCa and found vertebrae and pelvis to be the most frequent sites to harbor metastasis. Only 1% of patients had exclusive extrapelvic-extraspinal metastasis. The present study revealed metastatic disease involvement only in 3 patients (2%) with extra-pelvic skeletal disease in the absence of pelvic bone involvement. All three patients had involvement of vertebrae. None of the 150 patients had exclusive extrapelvic-extraspinal skeletal metastasis.
In an autopsy study by Mintz and Smith[25], including 100 patients of PCa, 21 patients had bone metastasis, and all the cases had involvement of the axial skeleton, with no incidence of isolated appendicular skeleton involvement. The pelvis was the most common site for metastatic disease and was involved in 13/21 cases. Similar results were observed by Roth et al[14] where axial skeleton involvement was found in all the patients (n = 54), while pelvic involvement was observed in 92.5% (n = 50) of patients. Also, in another autopsy study by Bubendorf et al[6], the axial skeleton was the predominant site of skeletal metastasis, with exclusively extrapelvic-extraspinal metastasis skeleton being rarely involved. Thus, fortifying the hypothesis of the current study.
The current study's limitations include a retrospective design, limited sample size, lack of survival data, and lack of histopathology confirmation of the BS findings.
The incidence of exclusive extrapelvic skeletal metastatic disease in PCa is 2% (excluding one patient with indeterminate findings). None of the patients in the current study had exclusive extrapelvic-extraspinal metastasis. Thus, exclusive extrapelvic-extraspinal focal abnormality on planar BS carries a very low probability of metastatic disease, and hence, further imaging or SPECT/CT can be safely avoided in such cases.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020-2070. Nat Rev Clin Oncol. 2021;18:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 461] [Article Influence: 115.3] [Reference Citation Analysis (35)] |
| 3. | Schaeffer EM, Srinivas S, Adra N, An Y, Barocas D, Bitting R, Bryce A, Chapin B, Cheng HH, D'Amico AV, Desai N, Dorff T, Eastham JA, Farrington TA, Gao X, Gupta S, Guzzo T, Ippolito JE, Kuettel MR, Lang JM, Lotan T, McKay RR, Morgan T, Netto G, Pow-Sang JM, Reiter R, Roach M, Robin T, Rosenfeld S, Shabsigh A, Spratt D, Teply BA, Tward J, Valicenti R, Wong JK, Shead DA, Snedeker J, Freedman-Cass DA. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21:1067-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 238] [Reference Citation Analysis (16)] |
| 4. | World Health Organization. Global Health Estimates 2020: Prostate Source: Globocan 2020 Number of new cases in 2020, both sexes, all ages. 2020. [cited 27 June 2024]. Available from: https://gco.iarc.fr/today. |
| 5. | San Francisco IF, Rojas PA, Bravo JC, Díaz J, Ebel L, Urrutia S, Prieto B, Cerda-Infante J. Can We Predict Prostate Cancer Metastasis Based on Biomarkers? Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (35)] |
| 6. | Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1179] [Cited by in RCA: 1263] [Article Influence: 50.5] [Reference Citation Analysis (35)] |
| 7. | Clarke NW, Hart CA, Brown MD. Molecular mechanisms of metastasis in prostate cancer. Asian J Androl. 2009;11:57-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (35)] |
| 8. | Akin O, Hricak H. Imaging of prostate cancer. Radiol Clin North Am. 2007;45:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (18)] |
| 9. | Brook R, Tung K, Oeppen R. Batson’s plexus and retrograde venous spread of malignancy – a pictorial review. Cancer Imaging. 2014;14:P40. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (35)] |
| 10. | Manna F, Karkampouna S, Zoni E, De Menna M, Hensel J, Thalmann GN, Kruithof-de Julio M. Metastases in Prostate Cancer. Cold Spring Harb Perspect Med. 2019;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (16)] |
| 11. | Roudier MP, Vesselle H, True LD, Higano CS, Ott SM, King SH, Vessella RL. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin Exp Metastasis. 2003;20:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (37)] |
| 12. | Beheshti M, Langsteger W, Fogelman I. Prostate cancer: role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:396-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | McDavid K, Lee J, Fulton JP, Tonita J, Thompson TD. Prostate cancer incidence and mortality rates and trends in the United States and Canada. Public Health Rep. 2004;119:174-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Roth AR, Harmon SA, Perk TG, Eickhoff J, Choyke PL, Kurdziel KA, Dahut WL, Apolo AB, Morris MJ, Perlman SB, Liu G, Jeraj R. Impact of Anatomic Location of Bone Metastases on Prognosis in Metastatic Castration-Resistant Prostate Cancer. Clin Genitourin Cancer. 2019;17:306-314. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Hariharan K, Padmanabha V. Demography and disease characteristics of prostate cancer in India. Indian J Urol. 2016;32:103-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Deek MP, Taparra K, Dao D, Chan L, Phillips R, Gao RW, Kwon ED, Deville C, Song DY, Greco S, Carducci MA, Eisenberger M, DeWeese TL, Denmeade S, Pienta K, Paller CJ, Antonarakis ES, Olivier KR, Park SS, Stish BJ, Tran PT. Patterns of Recurrence and Modes of Progression After Metastasis-Directed Therapy in Oligometastatic Castration-Sensitive Prostate Cancer. Int J Radiat Oncol Biol Phys. 2021;109:387-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part I: international comparisons. BJU Int. 2002;90:162-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Hayward SJ, McIvor J, Burdge AH, Jewkes RF, Williams G. Staging of prostatic carcinoma with radionuclide bone scintigraphy and lymphography. Br J Radiol. 1987;60:79-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Berish RB, Ali AN, Telmer PG, Ronald JA, Leong HS. Translational models of prostate cancer bone metastasis. Nat Rev Urol. 2018;15:403-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Wong SK, Mohamad NV, Giaze TR, Chin KY, Mohamed N, Ima-Nirwana S. Prostate Cancer and Bone Metastases: The Underlying Mechanisms. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Tulik M, Tulik P, Kowalska T. On the optimization of bone SPECT/CT in terms of image quality and radiation dose. J Appl Clin Med Phys. 2020;21:237-246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Alrehily FA, Alqahtani KS, Aljohani MH, Alharbi NS, Alharbi RM, Abdulaal OM, Alshamrani AF, Alsaedi AS, Al-Murshedi SH, Alhazmi FH. Establishing local diagnostic reference levels for computed tomography examinations using size-specific dose estimates. Saudi Med J. 2023;44:761-766. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Gupta SK, Trethewey S, Brooker B, Rutherford N, Diffey J, Viswanathan S, Attia J. Radionuclide bone scan SPECT-CT: lowering the dose of CT significantly reduces radiation dose without impacting CT image quality. Am J Nucl Med Mol Imaging. 2017;7:63-73. [PubMed] |
| 24. | Wang C, Shen Y, Zhu S. Distribution Features of Skeletal Metastases: A Comparative Study between Pulmonary and Prostate Cancers. PLoS One. 2015;10:e0143437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Mintz ER, Smith GG. Autopsy Findings in 100 Cases of Prostatic Cancer. N Engl J Med. 1934;211:479-487. [DOI] [Full Text] |