Published online Jul 28, 2024. doi: 10.4329/wjr.v16.i7.241
Revised: May 30, 2024
Accepted: July 1, 2024
Published online: July 28, 2024
Processing time: 93 Days and 15.6 Hours
Lymphoscintigraphy is a nuclear medicine procedure that uses a small quantity of radioactive particles for visualizing the lymphatic system. Traditionally, the radiotracer was injected subcutaneously, but the quality of lymphatic path imaging was scarce due to high background. Intradermal radiotracer injection is considered the modern-day intralymphatic injection. We propose rest/stress intradermal lymphoscintigraphy for the diagnosis, staging and surgical planning of lymphedema. Major and minor findings were described in primary and secondary lymphedema. Based on the in-depth information of the lymphatic pathways, physiotherapists and microsurgeons can obtain important functional information in patients’ selection to treat with physical treatments and/or undergo microsurgery.
Core Tip: Intradermal lymphoscintigraphy is a helpful functional nuclear imaging test that evaluates the lymphatic flow of the superficial and deeper lymphatic pathways at rest as well as following short and prolonged muscular activity, thus visualizing draining lymph nodes in a single 1-hour examination. This diagnostic method may help physiotherapists to evaluate the effects of muscular exercise and physical therapy on lymph drainage and surgeons to plan microsurgical treatments in advanced clinical stages of lymphedema.
- Citation: Tartaglione G. Advantages of the intradermal lymphoscintigraphy. World J Radiol 2024; 16(7): 241-246
- URL: https://www.wjgnet.com/1949-8470/full/v16/i7/241.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i7.241
Lymphoscintigraphy is a nuclear medicine procedure based on the injection of a little amount of radiotracer in an area of the body to visualize lymph drainage and regional lymph nodes. In the past, the radiotracers available had an acidic pH and physicians prefer subcutaneous, subfascial or submucosal injections to avoid the burning sensation at the injection site due to the presence of pH receptors in the dermis. Subcutaneous injections resulted in a slow injection site clearance, an intense liver and bladder uptake, a higher background, and a scarce quality of imaging of lymphatic pathways due to the passage of tracer in bloodstream.
When injected intradermally, the radiotracer is quickly absorbed by lymphatic capillary networks which are in the reticular and papillary dermis, offering a large surface for uptake and a better visualization of the superficial and deep lymphatic system in an amount of shorter time. Both the epidermis and subcutaneous tissue lack lymphatic capillaries. Nowadays, intradermal tracer injection is considered the modern-day intralymphatic injection for lymphatic mapping.
The ideal imaging agent should have an adequate particle size, a fast clearance, a rapid lymph node accumulation, a favourable safety profile, and a neutral injected solution pH. Currently, 99mTc-HSA nanocolloids in Europe and 99mTc-tilmanocept in ultrasound show these favorable characteristics and can thus be injected intradermally without causing any pain at the injection site. In the present paper, we describe the technical aspects and advantages of rest/stress intradermal lymphoscintigraphy in lymphedema assessment. After removing elastic sleeves or stockings, we injected intradermally two doses of 50 MBq in 0.3 mL of 99mTc-nanocolloid fractioned in strategic points based on anatomical distribution of main lymphatic collectors of the affected limb. The intermetatarsal space and the external and internal peri malleolar areas were used for the lower limb, while the first and fourth intermetacarpal space for upper limb. A resting scan is acquired at once following tracer injection. Afterward, the patient performed a two-minute muscular exercise (light weightlifting for upper limb, stepping for lower limbs) followed by a stress scan. Subsequently, the patient performed a limited by symptoms muscular exercise (squeezing for upper limb, walking for lower limbs) and a delayed scan can be acquired one hour after tracer injection. According to the severity, the test may visualize several patterns: The minor findings in lymphatic insufficiency are: (1) Delayed lymphatic drainage from injection site. When using an intradermal injection, the normal tracer appearance time in regional lymph nodes is < 10 minutes. Tracer stagnation area. At delayed scan, the radiotracer may remain along the course of the lymphatic pathway also following a prolonged muscular exercise; (2) Little tracer stagnation areas at the elbow or along lymphatic vessels in proximity of the valves might appear in clinical stage 0-1 as a subclinical sign of developing secondary lymphedema; (3) Deviation of the main lymphatic pathway. The lymph may deviate toward collectors around small saphenous or cephalic vein, instead of great saphenous or basilic vein; (4) Collateral lymphatic vessel development (< 3). The most frequent compensatory mechanism in the first stages of lymphedema; (5) Crossover drainage. In secondary lymphedema, after unilateral inguinal lymph node dissection or in breast cancer patients after axillary lymph node clearance, the lymph may cross the midline onto the contralateral side; (6) Deep vessels. Progressive functional damage to the main superficial lymphatic vessels due to genetic causes or lymph node dissection is common in lymphoedema patients. The deep lymphatic vessels can support lymphatic drainage and can be visualized in a delayed scan after prolonged exercise. The deep lymphatics can be recognized by the uptake of tracers in the popliteal or epitrochlear nodes; and (7) Tortuous course of lymph vessel. Tortuous vessels are more often seen in early-stage lipedema and filariasis.
The major findings in lymphatic insufficiency are: (1) Lymphangiectasia is a pathologic dilation of lymph vessels with tracer stagnation that may appear in both primary and secondary lymphedema; (2) Extravasation of tracer (or lymphatic leaking) is more commonly observed following an interruption of the lymphatic system due to increased resistance to lymphatic flow and partial leakage of lymph. This pattern may be an early sign of secondary lymphedema if it is located near the elbow, groin or middle third of the legs; (3) Dermal backflow is a consequence of restricted or largely obstructed lymphatic flow. It can be seen more often in delayed scans after prolonged muscular activity. The mechanism of dermal backflow is that muscular exercise increases intraluminal pressure within the lymphatic vessel, pushing lymph ahead. However, in patients with lymphedema, the increased resistance to lymph flow may determine an incompetence of anti-reflux valves, and the lymph exits from lymphatic collectors returning into the cutaneous surface of the limb. Dermal backflow may be proximal or distal. In advanced clinical stages after an extensive surgical lymphadenectomy and/or radiation treatment, the entire limb may be involved. In areas affected by dermal backflow, lymphatic pathways visualization may be difficult or hardly recognizable; (4) Absent number of vessels. In patients with primary lymphedema, imaging of the main superficial pathways may be difficult due to damage or compromised functionality; (5) Absent number of nodes. Fewer or no lymph nodes following surgical dissection and/or radiation therapy can cause an accumulation of excess lymph fluid in the tissues with damaged drainage; and (6) No flow. No lymphatic flow may develop in patients with acute lymphangitis or in severe clinical stages of lymphedema. To better define the extension of the damage, we recommend reinjecting small doses of radiotracer in various parts of the limb.
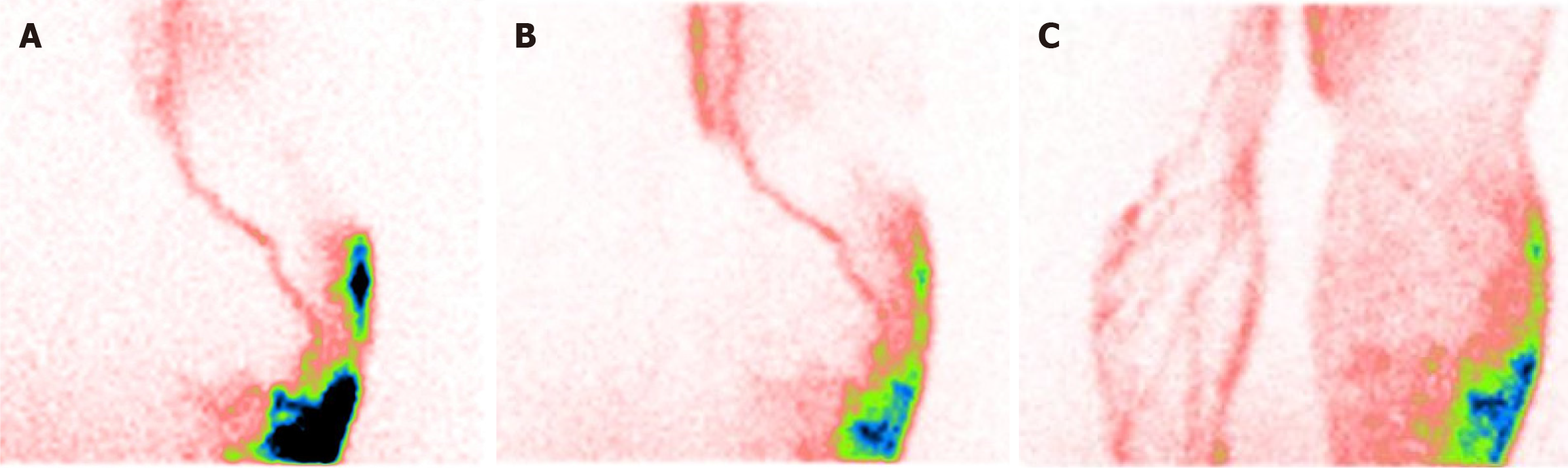
These findings can appear or change after a prolonged muscular exercise (delayed scan). In any case, radiotracer extravasation from lymphatic collectors is the most significant sign of lymphedema (Figure 1). Lymphoscintigraphy can be useful for detecting lymphatic leakage as a frequent complication in vascular surgery or after kidney transplantation. It is due to the trauma of lymphatic system. Indocyanine green lymphangiography with embolization can both be used in diagnosis and therapy to close the leakage.
Lymphedema is a chronic condition caused by a damage of lymphatic system characterized by progressive swelling of the limbs or other area of the body due to impaired lymphatic drainage. Lymphedema is most often a complication of cancer surgical or radiation treatment or parasitic infections, but it can also be a consequence of genetic disorders. Surgical removal or injury to lymph nodes and lymph vessels and radiation treatment for cancer radiation may result in secondary lymphedema. Lymphedema usually develops within 1-3 years after surgery, or 30 days after radiotherapy. It is important to note that lymphedema can progress through these stages over time, so early detection and treatment are key to managing the condition and preventing complications. Physical treatments for lymphedema may include physical therapy, compression garments, multilayer compression bandaging, compression devices, and exercise.
The muscle movement from exercise and a progressive resistance training improve the flow of lymph, which may help treat lymphedema. The American College of Sports Medicine recommends that a supervised exercise program is safe for patients with or at risk for lymphedema after cancer treatments. Muscular exercise as first-line treatment is a part of a healthy lifestyle and is essential for effective secondary lymphedema management. Before starting any exercise program, individuals should be cleared for the program of activity. Rest/stress intradermal lymphoscintigraphy may be performed in patients at risk to develop a secondary lymphedema to evaluate the direct effects of muscular exercise on lymph drainage. The scan may show subclinical findings of lymphedema as little tracer stagnation areas near valves of main lymphatic collector, at elbow or at middle third of legs. The evidence of draining lymph nodes of axilla or groins is an essential element to consider before starting muscular exercise program safely.
Today, lymphedema treatment is a combination of conservative and operative methods. Complex physical therapy is the first-line treatment and can be divided into two phases: A first, intensive phase composed of manual lymphatic drainage, skin care, muscular activity and/or compression, followed by a second phase to stabilize and conserve the results obtained through daily elastic compression garments. Surgical treatment options, in conjunction with conservative treatments, may provide clinical improvement and limb volume reduction[1]. Surgical therapies can be divided into reconstructive lymphatico-venous anastomosis (LVA) and vascular lymph node transfer, and ablatives (liposuction and dermolipectomy). LVA creates a bypass though which excess interstitial fluid excess may be drained directly into the venous system. Vascular lymph node transfer comprises the transplant of vascularized autologous lymph nodes from an unaffected region anatomized to recipient vessels in the lymphedematous area. Although the precise mechanism is still unknown, it is hypothesized that the transferred lymph nodes establish an effective shunting system via lymph angiogenesis to drain interstitial fluid into the venous system[2].
Diagnosis is based on anamnesis, clinical findings, and imaging. Imaging of the lymphatic system plays a fundamental role in diagnosing of lymphedema. Various imaging modalities may image the lymphatic system such as lymphography, lymphoscintigraphy, magnetic resonance imaging, computed tomography (CT), and ultra-high frequency ultrasound (UHFUS). A strict cooperation between surgeon, physiotherapist, radiologist, and nuclear physician is strongly recommended for a better use of technologies available[3].
Usually, there is no specific preparation for a patient receiving lymphoscintigraphy. However, in advanced clinical stage we prefer imaging patients only after an intensive decongestive treatment. Radiotracer injection sites may vary due to discrepancies among protocols[4-8]. In our experience, we performed intradermal radiotracer injections in specific points previously agreed with the microsurgeon, at the distal part of the affected limb. We recommend injecting in areas of skin where the dermis is well represented, avoiding scars or areas with dermatitis. Rest/stress intradermal lympho
With a detailed knowledge of the lymphatic pathway, the visual assessment of lymphoscintigraphy provides information regarding patent lymphatic drainage patterns, lymph nodes, collateral circulation, as well as the direction of lymph flow and potential disruptions in the affected limb[10]. In secondary lymphedema, the uptake of draining lymph nodes in atypical sites are indicative of rearrangement of the lymphatic flow. The transport index (TI) is a semi- quantitative evaluation of lymphatic flow that considers five factors noted when performing lymphoscintigraphy: Transport kinetics, distribution pattern of the radiotracer, time to visualization of lymph nodes, quality of visualization of lymph nodes, and visualization of lymphatic vessels[11]. A score ranging from 0 to 9 indicates a normal score while a score greater than 10 signals a pathological finding. In 2022, we described how pathological elements seen at rest/stress intradermal lymphoscintigraphy can be subdivided into major and minor findings[12]. In patients with secondary lymphoedema, a significantly higher percentage of serious findings such as lymph congestion, tracer extravasation and dermal reflux were seen. While a significantly higher percentage of minor findings, such as deep lymphatic vessels and radiotracer uptake at the epitrochlear and popliteal lymph nodes were observed in patients with primary lymphedema. Extensive dermal backflow and the absence of a draining lymph node are signs of severe clinical stages of lymphoedema. In our experience, we found a significantly higher incidence of dermal backflow in secondary lymphoedema as a result of medical procedures such as radiotherapy and surgical treatments for cancer. A scarce or absent visualization of lymphatic vessels in all phases of study, or a wide extent of dermal-backflow, and no evidence of lymph nodes (also after superficial and deeper re-injection in other sites) are related to more severe clinical stages of lymphedema.
The information obtained from lymphoscintigraphy renders it a reference exam in the decision- making process of selecting lymphedema patient who are microsurgical candidates and the development of personalized surgical treatment plans. Vaqueiro et al[13] report the use of lymphoscintigraphy for diagnosis and patient selection to undergo microvascular procedures, such as LVA, in patients with patent lymphatic channels at imaging. Campisi et al[14] describe the use of lymphatic microsurgery in patients with early-stage lymphedema with a lymphoscintigraphy that shows a low inguinal or axillary lymph nodal uptake and a minimal or absent passage of the tracer beyond this proximal lymph nodal area. Maegawa et al[15] indicate a relationship between lymphoscintigraphic findings and indications for lymphatic microsurgery in patients with secondary lower limb lymphedema. According to their observations, lymphoscintigraphic images can be divided into five types and suggest type 3 (no inguinal lymph nodes detected, dermal backflow in the thigh and/or leg, lymph stasis is observed) is the most suitable for LVA. A similar relationship was described by Mikami et al[16] between lymphoscintigraphic findings and indications for lymphatic microsurgery in patients with secondary upper limb lymphedema. Their results revealed lymphoscintigraphic images can be divided into five major types and three subtypes and suggest LVA should be considered as a treatment option for lymphedema types II-V. Boccardo et al[17] report a clinical and instrumental selection of 74 women with a history of axillary lymph node dissection for breast cancer with the use of lymphoscintigraphy. Patients with a body mass index less than 30 kg/m2 and a TI score greater than 10 on lymphoscintigraphy were indications for Lymphatic Microsurgical Preventing Healing technique. Gentileschi et al[18] underline the feasibility of single photon emission CT/CT combined with ultrasound fusion imaging for the preoperative identification of groin efferent lymph node in patients scheduled for peripheric lymphaticovenular anastomosis. In severe clinical stages of lymphedema[19], Visconti et al[20] suggest an integration of nuclear imaging with UHFUS, that may help detecting the lymphatic vessels and evaluating their degeneration status, including wall and lumen characteristics.
Lymphoscintigraphy plays a leading role in the early diagnosis, may help evaluate the effects of muscular exercise and physical therapy on lymphedema, and plan microsurgical treatments such as lymphatic-venous anastomosis or vascularized lymph node transfer. Thanks to a suitable energy of radioactive signal the lymphoscintigraphy is the best modality for lymphatic imaging in obese and lipedema patients or for identification of sentinel node in unexpected site. However, it has a few limitations. First, there is no universally standardized imaging protocol, and each lymphedema center performs a different and personalized imaging modality. The imaging protocol can vary according to the extension of lymphedema, the radiotracer, radioactivity dose and injected volume, number of injections, injection site and plane, and if performed at rest or in conjunction with a stress activity. Secondly, lymphoscintigraphy is characterized by a limited spatial resolution making it more difficult to find lymphatic pathways in the three-dimensional space. In the lymphoscintigraphic images of lymphedema patients, pathological findings such as collateral lymphatic pathways and dermal backflow can impede the visualization of patent lymphatic vessels. In our experience, dorsal lymphatic pathways of the arm can overlap the lymphatic vessels that follow the cephalic vein in upper limb lymphedema. Likewise, anterior collateral lymphatic pathways can overlap the lymphatic vessels that follow the small saphenous vein in lower limb lymphedema. Nevertheless, the limited three-dimensional anatomic definition with lymphoscintigraphy can be overcome by taking anterior and posterior scans of the affected limb. By seeing the lymphatic pathways from multiple points of view, the two-dimensional lymphoscintigraphy images provide information of a three-dimensional space. Moreover, lymphoscintigraphy does not provide direct information about the quality of the lymphatic vessels. However, a qualitative analysis of lymphoscintigraphic findings may correlate to lymphatic vessel quality as seen intraoperatively. In our experience, patent lymphatic channels are more commonly found in patients with a lymphoscintigraphy that displays a linear pattern, ectasia, or radiotracer stasis. On the other hand, lymphatic channels tend to be sclerotic if dermal backflow and radiotracer extravasation are seen with lymphoscintigraphy. Nevertheless, in a surgical perspective an integration with UHFUS imaging may be suggested.
Lymphoscintigraphy plays a leading role in the early diagnosis, may help evaluate the effects of muscular exercise and physical therapy on lymphedema, and plan microsurgical treatments such as lymphatic-venous anastomosis or vascularized lymph node transfer. Thanks to a suitable energy of radioactive signal the lymphoscintigraphy is the best modality for lymphatic imaging in obese and lipedema patients or for identification of sentinel node in unexpected site. However, it has a few limitations. First, there is no universally standardized imaging protocol, and each lymphedema center performs a different and personalized imaging modality. The imaging protocol can vary according to the extension of lymphedema, the radiotracer, radioactivity dose and injected volume, number of injections, injection site and plane, and if performed at rest or in conjunction with a stress activity. Secondly, lymphoscintigraphy is characterized by a limited spatial resolution making it more difficult to find lymphatic pathways in the three-dimensional space. In the lymphoscintigraphic images of lymphedema patients, pathological findings such as collateral lymphatic pathways and dermal backflow can impede the visualization of patent lymphatic vessels. In our experience, dorsal lymphatic pathways of the arm can overlap the lymphatic vessels that follow the cephalic vein in upper limb lymphedema. Likewise, anterior collateral lymphatic pathways can overlap the lymphatic vessels that follow the small saphenous vein in lower limb lymphedema. Nevertheless, the limited three-dimensional anatomic definition with lymphoscintigraphy can be overcome by taking anterior and posterior scans of the affected limb. By seeing the lymphatic pathways from multiple points of view, the two-dimensional lymphoscintigraphy images provide information of a three-dimensional space. Moreover, lymphoscintigraphy does not provide direct information about the quality of the lymphatic vessels. However, a qualitative analysis of lymphoscintigraphic findings may correlate to lymphatic vessel quality as seen intraoperatively. In our experience, patent lymphatic channels are more commonly found in patients with a lymphoscintigraphy that displays a linear pattern, ectasia, or radiotracer stasis. On the other hand, lymphatic channels tend to be sclerotic if dermal backflow and radiotracer extravasation are seen with lymphoscintigraphy. Nevertheless, in a surgical perspective an integration with UHFUS imaging may be suggested.
| 1. | Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology. 2020;53:3-19. [PubMed] |
| 2. | Liu HL, Pang SY, Lee CC, Wong MM, Chung HP, Chan YW. Orthotopic transfer of vascularized groin lymph node flap in the treatment of breast cancer-related lymphedema: Clinical results, lymphoscintigraphy findings, and proposed mechanism. J Plast Reconstr Aesthet Surg. 2018;71:1033-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Tartaglione G, Pagan M, Ieria FP, Visconti G, Tartaglione T. Imaging the Lymphatic System. Radiology-Nuclear Medicine Diagnostic Imaging. 2023;. [DOI] [Full Text] |
| 4. | Yoon JA, Shin MJ, Shin YB, Kim K, Park H, Kang T, Kong IJ, Kim H, Park MS, Kim JH. Correlation of ICG lymphography and lymphoscintigraphy severity stage in secondary upper limb lymphedema. J Plast Reconstr Aesthet Surg. 2020;73:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Akita S, Mitsukawa N, Kazama T, Kuriyama M, Kubota Y, Omori N, Koizumi T, Kosaka K, Uno T, Satoh K. Comparison of lymphoscintigraphy and indocyanine green lymphography for the diagnosis of extremity lymphoedema. J Plast Reconstr Aesthet Surg. 2013;66:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Imai H, Yoshida S, Mese T, Roh S, Fujita A, Sasaki A, Nagamatsu S, Koshima I. Correlation between Lymphatic Surgery Outcome and Lymphatic Image-Staging or Clinical Severity in Patients with Lymphedema. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Sherman AI, Ter-Pogossian M. Lymph-node concentration of radioactive colloidal gold following interstitial injection. Cancer. 1953;6:1238-1240. [PubMed] [DOI] [Full Text] |
| 8. | Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med. 2003;44:43-57. [PubMed] |
| 9. | Shinaoka A, Koshimune S, Yamada K, Kumagishi K, Suami H, Kimata Y, Ohtsuka A. Correlations between Tracer Injection Sites and Lymphatic Pathways in the Leg: A Near-Infrared Fluorescence Lymphography Study. Plast Reconstr Surg. 2019;144:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Villa G, Campisi CC, Ryan M, Boccardo F, Di Summa P, Frascio M, Sambuceti G, Campisi C. Procedural Recommendations for Lymphoscintigraphy in the Diagnosis of Peripheral Lymphedema: the Genoa Protocol. Nucl Med Mol Imaging. 2019;53:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Gale RP, Graze PR, Wells J, Ho W, Hershko C, Lowenberg B, Feig S, Cline MJ. Autologous bone marrow transplantation in patients with cancer. Exp Hematol. 1979;7 Suppl 5:351-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Tartaglione G, Ieria FP, Visconti G, Bartoletti R, Tarantino G, Aloisi D, Gentileschi S, Salgarello M. Rest/Stress Intradermal Lymphoscintigraphy for the Functional Imaging of the Lymphatic System. Clin Nucl Med. 2022;47:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Vaqueiro M, Gloviczki P, Fisher J, Hollier LH, Schirger A, Wahner HW. Lymphoscintigraphy in lymphedema: an aid to microsurgery. J Nucl Med. 1986;27:1125-1130. [PubMed] |
| 14. | Campisi C, Eretta C, Pertile D, Da Rin E, Campisi C, Macciò A, Campisi M, Accogli S, Bellini C, Bonioli E, Boccardo F. Microsurgery for treatment of peripheral lymphedema: long-term outcome and future perspectives. Microsurgery. 2007;27:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Maegawa J, Mikami T, Yamamoto Y, Satake T, Kobayashi S. Types of lymphoscintigraphy and indications for lymphaticovenous anastomosis. Microsurgery. 2010;30:437-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Mikami T, Hosono M, Yabuki Y, Yamamoto Y, Yasumura K, Sawada H, Shizukuishi K, Maegawa J. Classification of lymphoscintigraphy and relevance to surgical indication for lymphaticovenous anastomosis in upper limb lymphedema. Lymphology. 2011;44:155-167. [PubMed] |
| 17. | Boccardo F, Fulcheri E, Villa G, Molinari L, Campisi C, Dessalvi S, Murdaca G, Campisi C, Santi PL, Parodi A, Puppo F, Campisi C. Lymphatic microsurgery to treat lymphedema: techniques and indications for better results. Ann Plast Surg. 2013;71:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Gentileschi S, Albanese R, Pino V, Stefanizzi G, Fragomeni S, Zagaria L, Ieria FP, Salgarello M, Scambia G, Garganese G. SPECT/CT and fusion ultrasound to target the efferent groin lymph node for lymphatic surgery. Microsurgery. 2019;39:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Falcão RP, Ismael SJ. Leu 7+, Leu 11a- acute T-lymphoblastic leukemia having low K cell activity and no NK cell activity. Am J Hematol. 1987;24:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Visconti G, Bianchi A, Hayashi A, Cina A, Maccauro G, Almadori G, Salgarello M. Thin and superthin perforator flap elevation based on preoperative planning with ultrahigh-frequency ultrasound. Arch Plast Surg. 2020;47:365-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |