Published online Jun 28, 2024. doi: 10.4329/wjr.v16.i6.232
Revised: April 29, 2024
Accepted: May 17, 2024
Published online: June 28, 2024
Processing time: 160 Days and 19.2 Hours
Langerhans cell histiocytosis (LCH) is characterized by diabetes insipidus and is an uncommon occurrence. Pathological biopsies still have a certain degree of diagnostic probability. We present a case in which LCH initially affected the pituitary gland. This resulted in a misdiagnosis of chronic inflammation upon pathological examination.
A 25-year-old female exhibited symptoms of diabetes insipidus. Magnetic resonance imaging revealed an enhanced foci in the pituitary gland. After surgical resection of the pituitary lesion, the pathological diagnosis was chronic inflammation. However, the patient later experienced bone destruction in the skull and lower limb bones. After the lower limb bone lesion was compared with the initial pituitary lesion, the final diagnosis was modified to LCH. The patient was treated with multiple chemotherapy courses. However, the patient’s condition gradually worsened, and she eventually passed away at home.
LCH should be considered when patients exhibit diabetes insipidus and absence of high signal intensity in the pituitary gland on sagittal T1-weighted image and abnormal enhancement in the pituitary region.
Core Tip: This case report details Langerhans cell histiocytosis (LCH) presenting in the pituitary gland of a 25-year-old female patient. The patient’s primary symptom was diabetes insipidus. The case was initially misdiagnosed as chronic inflammation despite postoperative pathological examination. Throughout the course of the disease multiple radiological examinations were conducted, and it was strongly indicated that LCH was the correct diagnosis. This case highlights the vital importance of imaging studies in the diagnosis of this condition.
- Citation: Zhang ZR, Chen F, Chen HJ. Multisystemic recurrent Langerhans cell histiocytosis misdiagnosed with chronic inflammation at the first diagnosis: A case report. World J Radiol 2024; 16(6): 232-240
- URL: https://www.wjgnet.com/1949-8470/full/v16/i6/232.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i6.232
Langerhans cell histiocytosis (LCH) is caused by the expansion of CD1a+/CD207+ cells. It is characterized by widespread organ involvement and dysfunction at all ages with varying degrees of systemic involvement[1]. LCH has specific affinities for the bones, skin, lungs, and pituitary gland[2]. The primary challenge in the treatment of LCH is establishing a correct diagnosis because it has a diverse presentation. Because LCH is often misdiagnosed, there are delays in initiating appropriate therapeutic interventions[3].
This report details the case of an adult female LCH patient with initial symptoms of polydipsia and polyuria. She was misdiagnosed with chronic inflammation. Based on the clinical manifestations, laboratory findings, and imaging results collected during the diagnostic and treatment processes of this case, we have summarized our experiences and provided insights to aid clinicians in enhancing their understanding of the disease herein. Early diagnosis of LCH is critical to facilitate timely initiation of appropriate treatment, thereby elevating the prognostic outcomes for patients.
A 25-year-old female patient was admitted to Hainan General Hospital on June 5, 2016. She presented with dry mouth, polydipsia, and polyuria that began 4 years prior to presentation. She also reported fatigue for 10 d prior to presentation.
The patient experienced dry mouth, polydipsia, and polyuria for 4 years prior to presentation without obvious inducement. The patient drank approximately 10000 mL of water per day and had nocturia approximately five times/night, without any obvious changes in food intake. However, the patient had gained 30 kg in weight in the past 4 years and had not received any treatment. Ten days prior, the patient experienced weakness, occasional dizziness, chest tightness, and shortness of breath. In the prior 3 d, the patient felt that her fatigue worsened, and she was unable to walk 1 d prior to presentation.
The patient underwent an abortion 4 years prior to presentation and was postoperatively menopausal.
Personal or family history was unremarkable.
Physical examination revealed that the patient had normal development with physical obesity, moderate nutritional status, and a dehydrated appearance without a full moon or buffalo hump. There was no obvious swelling of the lower limbs, the muscular strength was graded 1-2, and the dorsal pedis arterial pulsation weakened bilaterally. The patient’s bilateral knee reflexes were diminished, and ankle reflexes were present without abnormalities. The pressure sensation was absent in both feet (10 g nylon wire examination). There were no other significant abnormalities in the physical examination.
Laboratory examination revealed a blood glucose level of 38 mmol/L (normal range: 3.9-6.1 mmol/L), a potential hydrogen concentration of 7.3 mmol/L (normal range: 7.35-7.45 mmol/L), a pCO2 concentration of 24.4 mmol/L (normal range: 35-45 mmol/L), and a residual base excess of -11.9 mmol/L (normal range: -3-3mmol/L). Routine urine examination revealed occult blood (1+), protein (1+), and glucose (4+). Electrolyte examination revealed a sodium concentration of 127 mmol/L, potassium concentration of 2.73 mmol/L, and chlorine concentration of 128 mmol/L. Renal function examination revealed a uric acid level of 566 µmol/L, a creatinine level of 111 µmol/L, a cortisol level of 402 mmol/L, a C-peptide level (120’) of 0.633 nmol/L, and a C-peptide level (0’) of 0.410 nmol/L. Six tests for sex hormones showed luteinizing hormone of 1.08 IU/L, follicle-stimulating hormone of 1.48 IU/L, estradiol of 86 pmol/L, progesterone of 1.58 nmol/L, pituitary prolactin of 315.6 mIU/L, and testosterone of 1.22 nmol/L. Additionally, the examination showed a glycosylated hemoglobin level of 11.3%. The water deprivation test (stimulates the pituitary gland to secrete antidiuretic hormone) and the gonadotropin-releasing hormone excitability test (stimulates the pituitary gland to release follicle-stimulating hormone and luteinizing hormone) were normal.
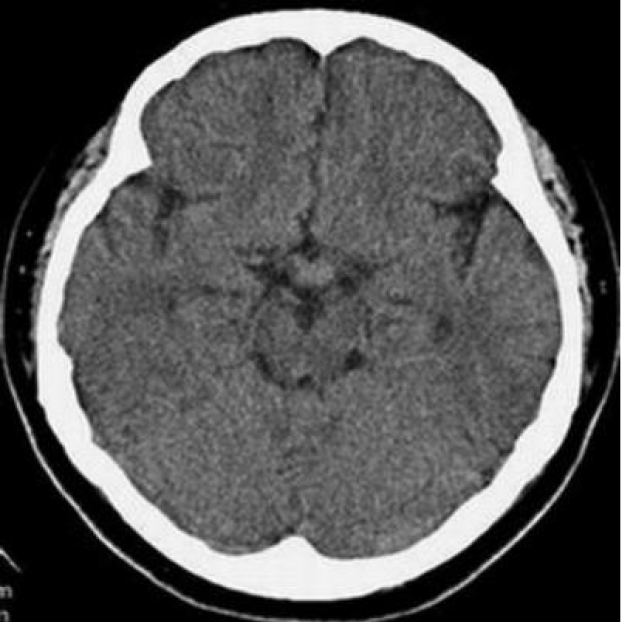
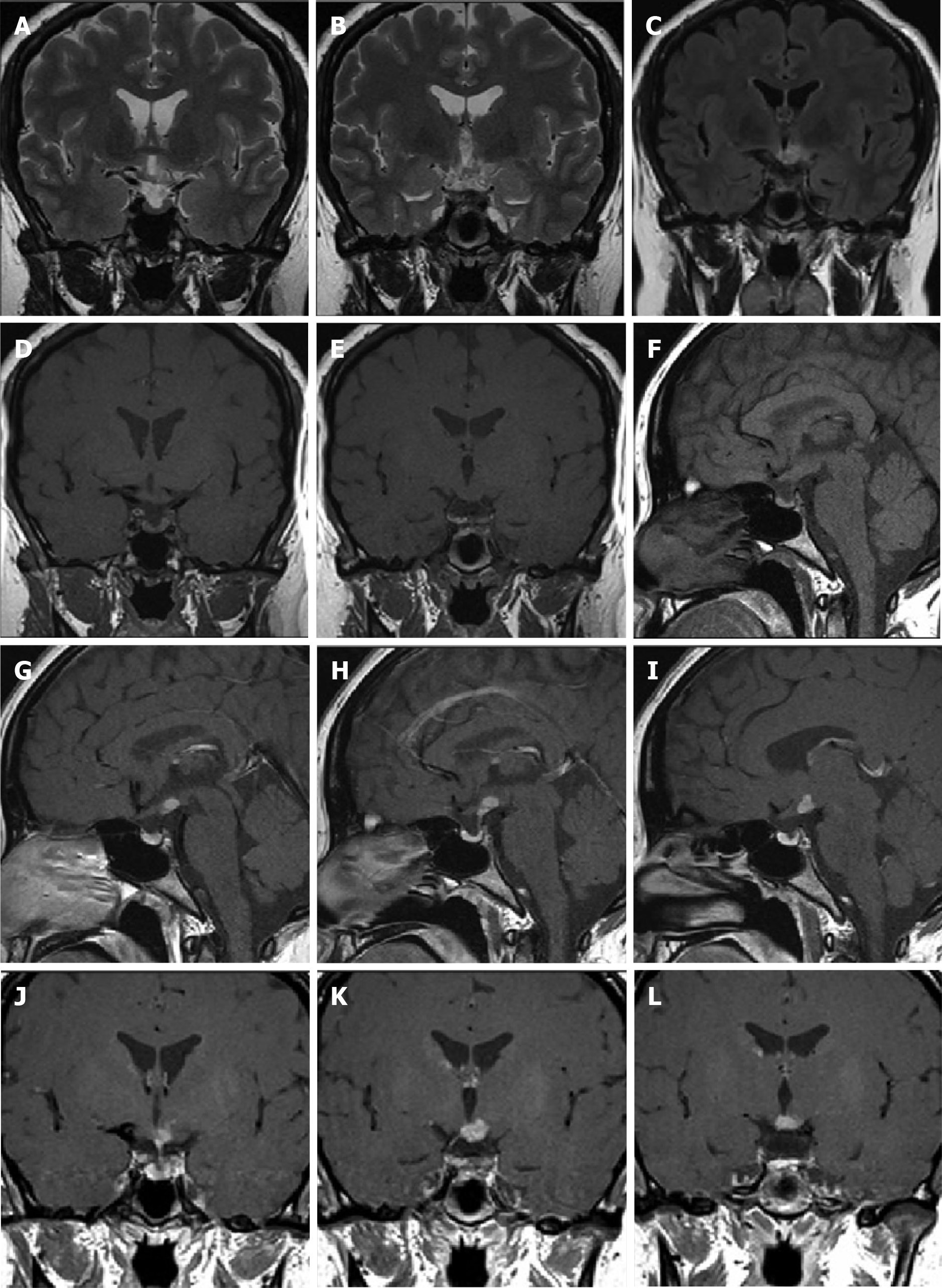
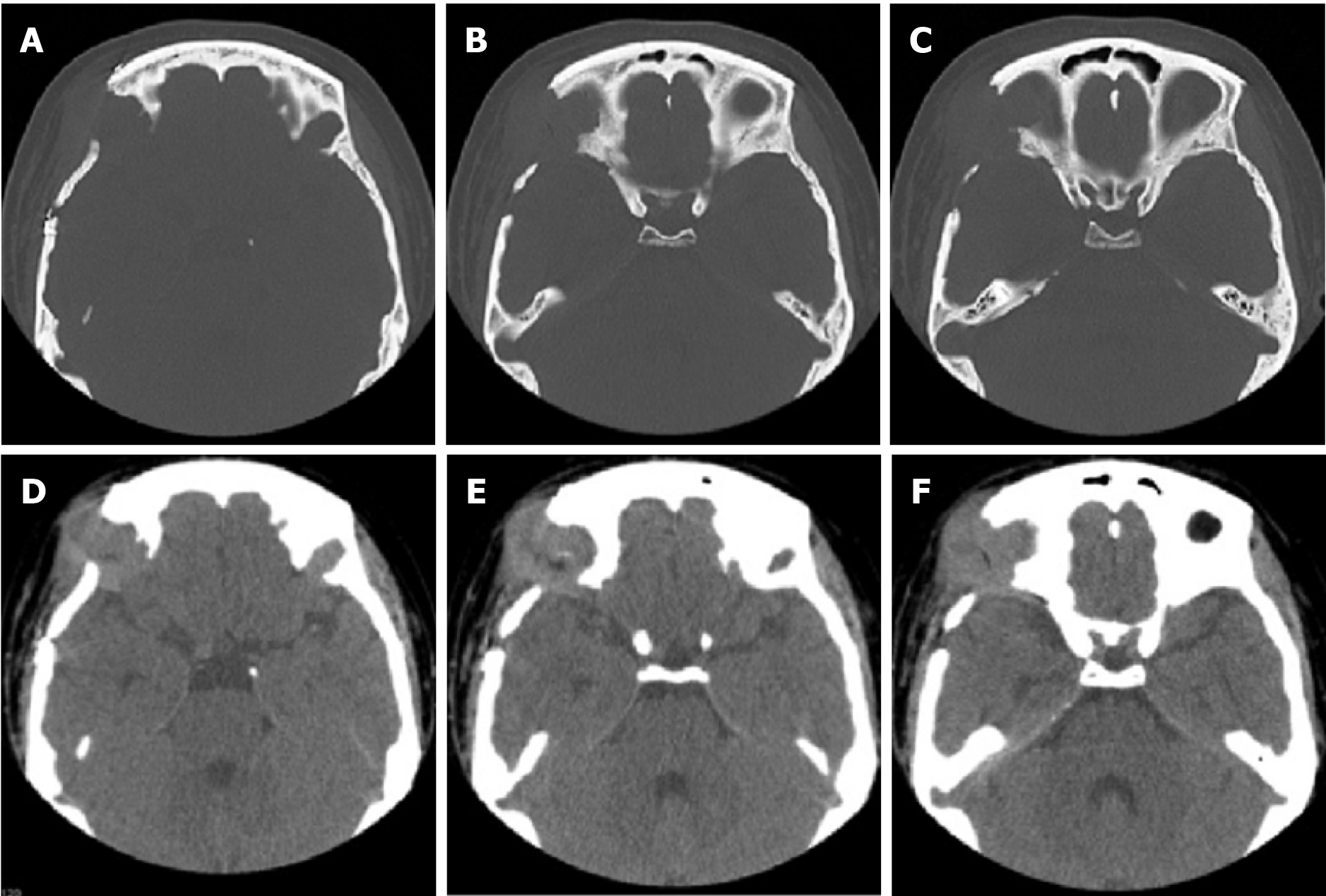
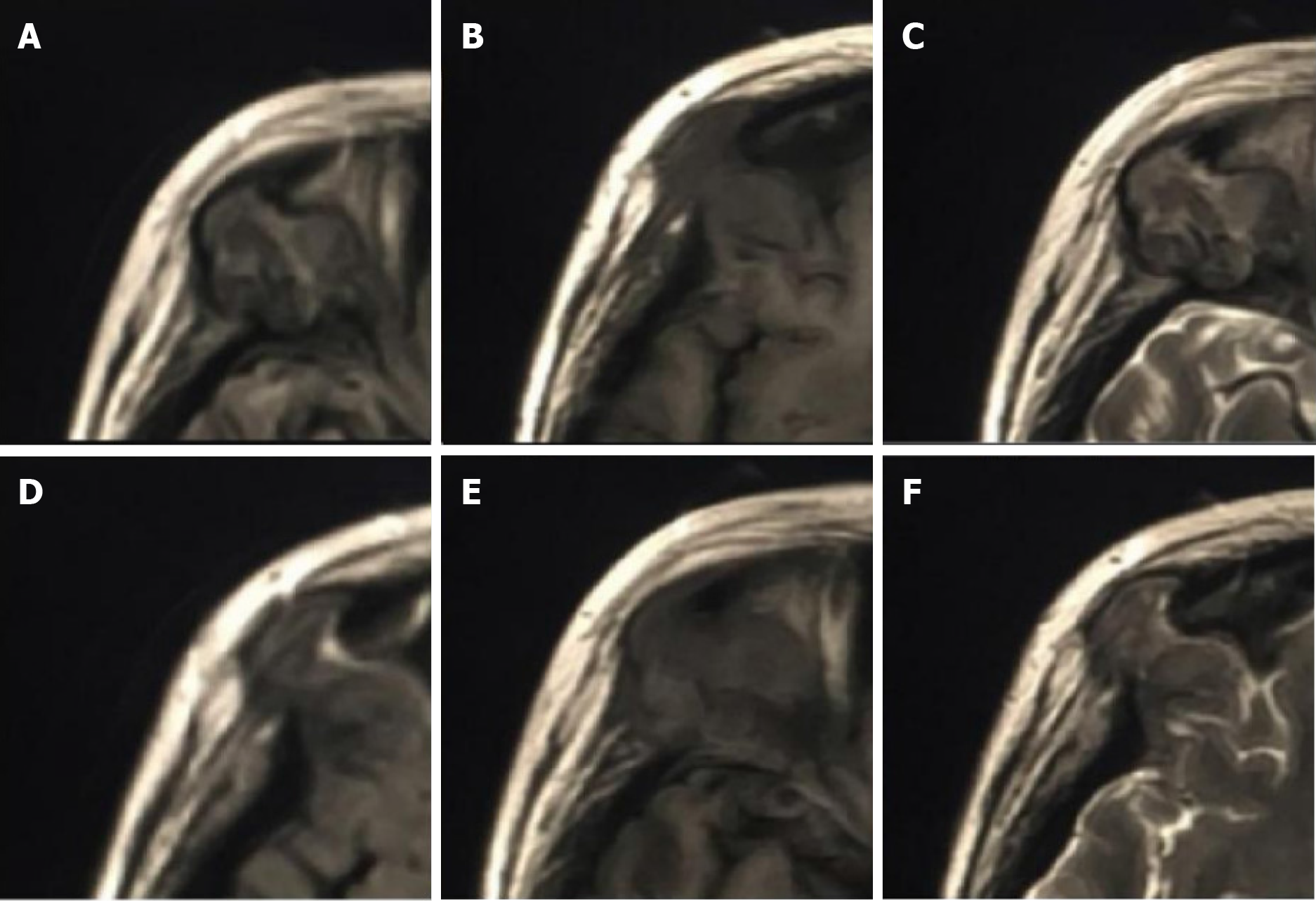
The patient underwent multiple imaging examinations in multiple areas. On June 5, 2016, the patient underwent a cranial computed tomography (CT) scan, which showed no significant abnormalities (Figure 1). On June 10, 2016, the patient underwent a cranial magnetic resonance imaging (MRI) enhance
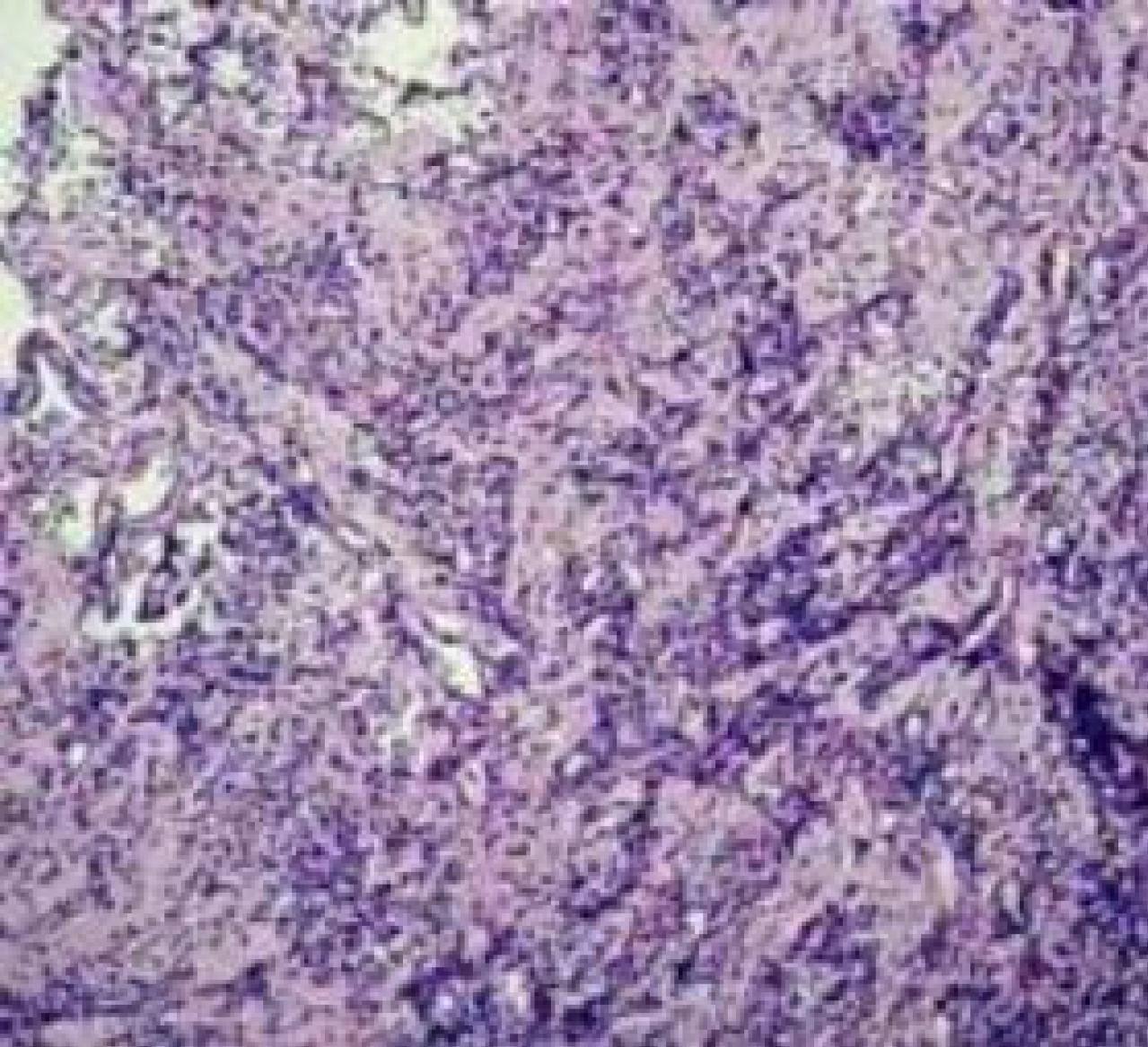
After surgical resection of the pituitary lesion, the pathological diagnosis was chronic inflammation (Figure 3). However, the patient later experienced multiple bone destruction in the skull and the femur. After the femur lesion was compared with the initial pituitary lesion, the final diagnosis was modified to LCH.
The patient subsequently underwent right frontotemporal craniotomy for tumor removal after consultation with neurosurgery experts from Beijing Tiantan Hospital on July 9, 2016. Intraoperative records showed that the tumor was located above the pituitary stalk, the hypothalamus was grayish-white in color, and the tumor was 1.0 cm × 0.6 cm × 0.8 cm in size with unclear borders, a general blood supply, and a slightly tough texture.
After surgery, the patient experienced an improvement in polydipsia and normalization of urine output. However, she experienced an onset of bilateral visual impairment (left visual field defect). Postoperatively, the patient received long-term treatment with insulin, metformin, acarbose, prednisone, and euthyrox. The patient was discharged after an adequate recovery.
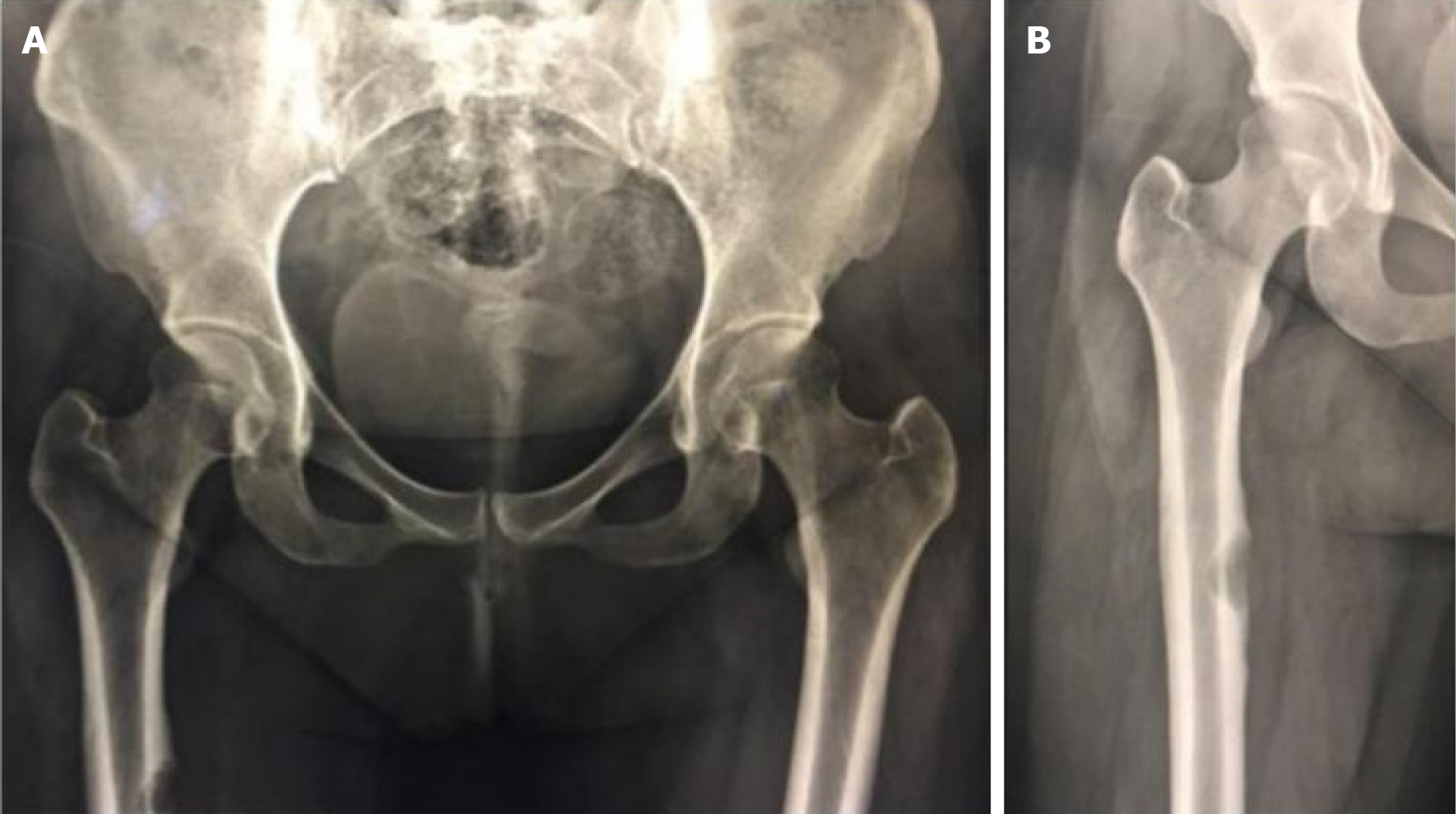
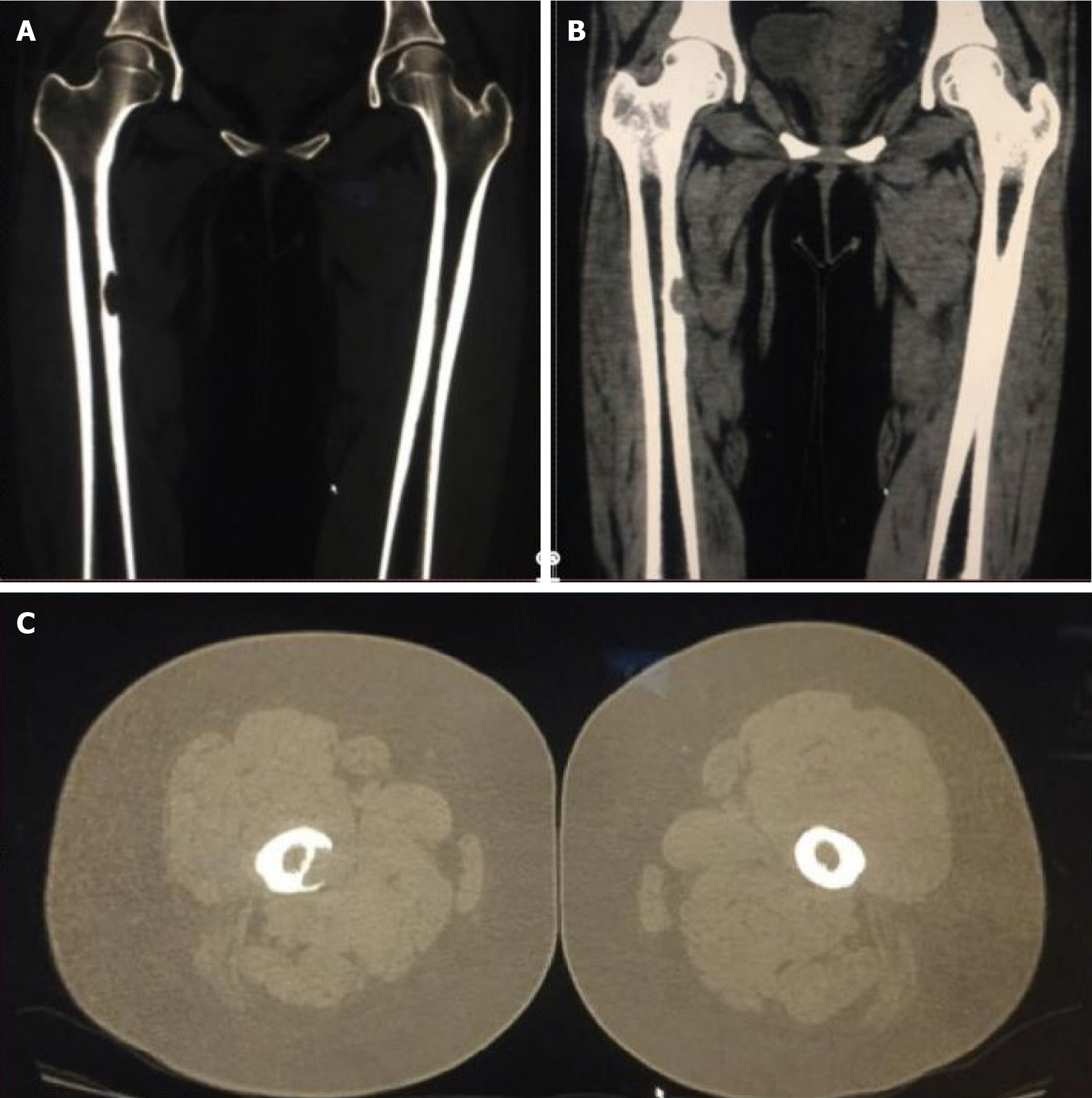
On July 22, 2016, the patient was readmitted to the hospital because of poor glycemic control, nausea, and vomiting for 1 d. She was discharged after her blood sugar was stabilized. On February 28, 2017, the patient was readmitted to the hospital for pain in the right hip, right thigh, and right knee for more than 6 months, which was aggravated by walking. Upon physical examination, the patient experienced pinprick-like pain at the site of the pain without localized redness, swelling, or fever. X-ray examination of the bilateral hip and right lower limb on February 28, 2017 revealed bone destruction and defects in the upper part of the right femur (Figure 4). Three-dimensional CT examination of both lower limbs on March 4, 2017 showed cortical destruction and soft tissue mass formation in the medial aspect of the right upper femur (Figure 5). The patient underwent a right femoral biopsy at an external hospital, that revealed fibroxanthoma.
On June 6, 2017, an excision of the right femoral mass was performed. Intraoperative records showed thickening and hardening of the medial cortex of the upper part of the right femur combined with bone destruction. A rust-like granulation tissue mass was present, and bone grafting was performed after removing the diseased tissue from the lesion site. Postoperative pathology results showed that the patient suffered from LCH. A retrospective review of the pathology of the pituitary lesion from 1 year prior led to a revised diagnosis of LCH.
After discharge, the patient returned to the hospital on July 19, 2017 with pain in both ears with pus, pain in the right orbital region, skin pain on the head, and multiple rashes all over the body. On July 26, 2017, orbital CT showed irregular bone destruction in the upper outer wall of the right eye orbit combined with the formation of a surrounding soft tissue mass (Figure 6). Orbital MRI showed irregular bone destruction in the upper outer wall of the right eye orbit. It was found with isotropic T1 and isotropic/slightly longer T2 signal shadows, with isotropic signals in the fluid attenuated inversion recovery sequence (Figure 7).
After the diagnosis was clarified, the patient was transferred to Sun Yat-Sen Memorial Hospital of Sun Yat-Sen University for inpatient treatment of vincristine + prednisone on August 16, 2017, August 23, 2017, and August 30, 2017. In addition, the patient received cyclophosphamide + vincristine + prednisone on October 2, 2017 and cyclophosphamide + adriamycin + vincristine + prednisone on November 3, 2017 and December 8, 2017. The patient was subsequently given a regimen of cyclophosphamide + adriamycin + vincristine + prednisone + etoposide on February 8, 2018.
During the course of chemotherapy, severe internal environmental disturbances occurred, leading to the premature termination of chemotherapy. The patient received supportive care, including fluid rehydration, glucose control, and other therapies. Electrolytes were rechecked, and sodium was 190 mmol/L, potassium was 2.38 mmol/L, and chlorine was 161 mmol/L. The patient’s general condition deteriorated significantly, and the prognosis was grave. After repeatedly explaining the critical condition to the family, they expressed understanding, and the patient was discharged on February 12, 2018. Subsequently, the family confirmed that the patient had passed away at home. Table 1 summarizes the timeline of the patient’s course of illness.
| Date | Symptoms onset | Imaging examination | Treatments administered | Follow-up outcomes |
| June 5, 2016 | Dry mouth, polydipsia, polyuria for 4 yr and fatigue for 10 d | Cranial CT scan, cranial MRI enhancement scan | Right frontotemporal craniotomy for tumor removal | Onset of bilateral visual impairment |
| July 22, 2016 | Poor glycemic control, nausea, and vomiting for 1 d | None | Received treatment to stabilize blood sugar levels | Discharge after blood sugar stabilized |
| February 28, 2017 | Pain in the right hip, right thigh, and right knee for more than 6 months, which was aggravated by walking | X-ray examination of the bilateral hip and right lower limb, three-dimensional CT examination of both lower limbs | Excision of the right femoral mass | Discharge after operation |
| July 19, 2017 | Pain in both ears with pus, pain in the right orbital region, skin pain on the head, and multiple rashes all over the body | Orbital CT and MRI | Multiple chemotherapy sessions over 7 months | Patient condition gradually worsened, and culminated with death at home |
LCH is defined as a clonally expansive inflammatory myeloid neoplasm. It is characterized by the infiltration of CD1a+/CD207+ myeloid dendritic cells and immune cells. The incidence of LCH is approximately 3-5 cases per million children and 1-2 cases per million adults[4]. The case of LCH initially occurring in the hypothalamic-pituitary region (HPR) is rare. The MRI features of HPR-LCH include absence of high signal intensity in the pituitary gland, significant enhancement on contrast scans[5], and thickening of the pituitary stalk[6]. These characteristics are not specific to LCH, making it challenging to differentiate from other saddle area diseases such as hypothalamic gliomas, hypothalamic hamartoma, and chronic hypothalamic inflammation.
In this case, the initial symptom was the clinical manifestation of pituitary invasion, and the MRI scan revealed a significant reduction in the height of the pituitary gland. A high signal shadow in the posterior lobe of the pituitary gland was not observed in the sagittal position of the T1-weighted image. Thickening of the pituitary stalk and funnel was observed via enhancement scanning, which was in accordance with the abovementioned features of the imaging manifestations of HPR-LCH and may be useful for diagnosing LCH.
In adults, LCH is prevalent in bones, with the skull being the most commonly involved bone, followed by the axial skeleton and the proximal tailbone. Manifestations usually include pain, lumps, and swelling at the affected sites. Bone involvement often manifests as a worm-eaten lesion on X-ray examination[3]. This type of lesion was also observed in the skull and femur of our patient. Imaging is critical for the early diagnosis of LCH. Our patient was misdiagnosed with chronic inflammation according to both in-hospital and outgoing pathology reports after the initial resection of the hypothalamic neoplasms.
The patient did not undergo further examinations such as immunohistochemistry and positron emission tomography-CT. The patient received symptomatic treatment for endocrine disorders and her other symptoms resulting from pituitary gland invasion. However, she was eventually diagnosed with LCH after undergoing surgical resection due to recurrence. LCH involving the pituitary gland can be prone to misdiagnosis due to its rarity and subtle clinical manifestations. Moreover, LCH lesions are often sparsely distributed in the involved organs and can be surrounded by fibrotic and inflammatory cells[3].
Approximately 40% of LCH patients have the BRAF V600E mutation[7], and patients with BRAF V600E, KRAS, and NRAS mutations tend to experience recurrence[4]. This leads to infiltration of neighboring tissues and destruction of their structures, which adversely affects the prognosis. Therefore, early diagnosis of LCH is crucial. Several studies have shown that in addition to traditional therapy, targeted therapy with BRAF inhibitors such as dabrafenib and trametinib[8,9] has beneficial effects, providing clinicians with new therapeutic options.
In the diagnosis of LCH, tissue biopsy, immunohistochemical staining[3], and 18F fluorodeoxyglucose PET/CT[10] can aid in the clinical treatment of LCH patients. In this particular case, the imaging findings and initial clinical manifestation of central diabetes insipidus strongly indicated the possibility of HPR-LCH. Unfortunately, the patient was first diagnosed with chronic inflammation. Even with negative pathological findings, the diagnosis of LCH could not be ruled out because the imaging findings and clinical manifestations strongly suggested its likelihood.
LCH can occur throughout the body but very rarely occurs in the pituitary gland. If a patient with diabetes insipidus is encountered and the pituitary imaging examination shows imaging features similar to this case, the possibility of LCH in the patient should be considered.
We would like to thank Huang WY of the Department of Radiology, Affiliated Hainan Hospital of Hainan Medical University (Hainan General Hospital) for collecting the medical data and for her guidance and constructive criticism.
| 1. | Rodriguez-Galindo C. Clinical features and treatment of Langerhans cell histiocytosis. Acta Paediatr. 2021;110:2892-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 2. | Allen CE, Merad M, McClain KL. Langerhans-Cell Histiocytosis. N Engl J Med. 2018;379:856-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 3. | Goyal G, Young JR, Koster MJ, Tobin WO, Vassallo R, Ryu JH, Davidge-Pitts CJ, Hurtado MD, Ravindran A, Sartori Valinotti JC, Bennani NN, Shah MV, Rech KL, Go RS; Mayo Clinic Histiocytosis Working Group. The Mayo Clinic Histiocytosis Working Group Consensus Statement for the Diagnosis and Evaluation of Adult Patients With Histiocytic Neoplasms: Erdheim-Chester Disease, Langerhans Cell Histiocytosis, and Rosai-Dorfman Disease. Mayo Clin Proc. 2019;94:2054-2071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 4. | Sato A, Kobayashi M, Yusa N, Ogawa M, Shimizu E, Kawamata T, Yokoyama K, Ota Y, Ichinohe T, Ohno H, Mori Y, Sakaida E, Kondo T, Imoto S, Nannya Y, Mitani K, Tojo A. Clinical and prognostic features of Langerhans cell histiocytosis in adults. Cancer Sci. 2023;114:3687-3697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Oda Y, Amano K, Seki Y, Kimura S, Yamashita K, Masui K, Komori T, Ichihara A, Kawamata T. Clinical features and difficulty in diagnosis of Langerhans cell histiocytosis in the hypothalamic-pituitary region. Endocr J. 2022;69:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Fan X, Liu T, Zhang Z, Sun J, Niu N, Mao C, Wang F, Li J, Zhou D, Cao X, Jin Z, Feng F. Comparison of neuroimaging features of histiocytic neoplasms with central nervous system involvement: a retrospective study of 121 adult patients. Eur Radiol. 2023;33:8031-8042. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Goyal G, Acosta-Medina AA, Abeykoon JP, Dai C, Ravindran A, Vassallo R, Ryu JH, Shah MV, Bennani NN, Young JR, Bach CR, Ruan GJ, Zanwar S, Tobin WO, Koster MJ, Davidge-Pitts CJ, Gruber LM, Dasari S, Rech KL, Go RS. Long-term outcomes among adults with Langerhans cell histiocytosis. Blood Adv. 2023;7:6568-6578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Suh JK, Kang S, Kim H, Im HJ, Koh KN. Recent advances in the understanding of the molecular pathogenesis and targeted therapy options in Langerhans cell histiocytosis. Blood Res. 2021;56:S65-S69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Cournoyer E, Ferrell J, Sharp S, Ray A, Jordan M, Dandoy C, Grimley M, Roy S, Lorsbach R, Merrow AC, Nelson A, Bartlett A, Picarsic J, Kumar A. Dabrafenib and trametinib in Langerhans cell histiocytosis and other histiocytic disorders. Haematologica. 2024;109:1137-1148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Prakash S, Shamim SA, Bakhshi S, Pushpam D, Sharma A. Langerhans Cell Histiocytosis of Bilateral Parotid and Submandibular Glands: Findings on 18F-FDG PET/CT. Clin Nucl Med. 2023;48:e200-e201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |