Published online May 28, 2024. doi: 10.4329/wjr.v16.i5.128
Revised: April 23, 2024
Accepted: May 8, 2024
Published online: May 28, 2024
Processing time: 107 Days and 10.5 Hours
In cases of coronavirus disease 2019 (COVID-19), favipiravir is commonly included to the therapy regimen. Drug interactions between favipiravir and other COVID-19 therapy drugs are frequently researched. However, no research on possible drug interactions between Favipiravir and radiocontrast agents, which have become almost crucial in diagnostic processes while not being part of the treatment, has been found.
To determine potential medication interactions between Favipiravir and radio
The study comprised patients who were taking Favipiravir for COVID-19 therapy and underwent a contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) test while taking the medicine. The computerized patient files of the cases included in the study, as well as the pharmacovigilance forms in the designated hospital, were evaluated for this purpose.
The study included the evaluation of data from 1046 patients. The study sample's mean age was 47.23 ± 9.48 years. The mean age of cases with drug interactions was statistically significant greater than that of cases with no drug interactions (P = 0.003). When evaluated with logistic regression analysis, a 1-year raises in age increases the risk of developing drug interactions by 1.63 times (P = 0.023). There was no statistically significant difference in the occurrence of medication interactions between the sexes (P = 0.090). Possible medication interactions were discovered in 42 cases (4%).
The findings of this study revealed that the most notable findings as a result of the combined use of contrast agents and favipiravir were increased creatinine and transaminase values, as well as an increase in the frequency of nausea and vomiting. The majority of drug interactions discovered were modest enough that they were not reflected in the clinic. Drug interactions become more common as people get older.
Core Tip: In cases of coronavirus disease 2019, favipiravir is commonly included to the therapy regimen. The aim of this study is to identify potential medication interactions between Favipiravir and radiocontrast agents. Possible medication interactions were discovered in 42 cases (4%). The mean age of cases with drug interactions was statistically substantially greater than that of cases with no drug interactions. There was no statistically significant difference in the occurrence of medication interactions between genders. The majority of drug interactions discovered were modest enough that they were not reflected in the clinic.
- Citation: Aydin S, Aydin OC, Yazar MF, Aydemir H, Kantarci M, Barun S. Assessment of the potential interactions between favipiravir and radiocontrast agents. World J Radiol 2024; 16(5): 128-135
- URL: https://www.wjgnet.com/1949-8470/full/v16/i5/128.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i5.128
With the coronavirus disease 2019 (COVID-19) pandemic, the use of Favipiravir has increased rapidly. Parallel to this, the need for and use of imaging methods is increasing exponentially. As a natural consequence of this situation, Favipiravir and computed tomography (CT) or magnetic resonance imaging (MRI) contrast agents are frequently used together[1].
Radiological imaging methods are widely used in the diagnosis of COVID-19, monitoring of treatment, and determination of complications and comorbidities. It is known that COVID-19 disease causes microvascular damage and hypercoagulation. CT examinations, especially angiography performed with contrast agent injection or CT examinations in the triple exclusion protocol, have a very important place in the investigation of thromboembolic and other comor
The basic building blocks of contrast agents used in CT and MRI examinations are Iodine and Gadolinium, respectively. Both iodinated and gadolinium contrast agents are usually eliminated by the kidneys. An exception to this situation is that Gadoxetate disodium (Primovist-Bayer) is eliminated equally by the renal (approximately 50%) and hepatobiliary (approximately 50%) routes. CT and MRI contrast agents generally cause drug interactions via the renal elimination pathway. As an exception, hepatic elimination may be included in this mechanism for Gadoxetate disodium[6].
Favipiravir, an agent frequently used in the treatment of COVID-19, is metabolized in the liver and its inactive metabolite T705M1 is excreted by the kidneys[7].
When the literature is searched in detail, there is no study investigating the drug interactions between radiocontrast agents, which have become almost indispensable in diagnostic processes, and Favipiravir, which is frequently added to the treatment regimen. In this study, possible drug interactions between radiocontrast agents and Favipiravir and the effects of age and gender on interactions were investigated.
It is a retrospective study designed in an observational-analytical character. Our institutional review board gave its approval to this retrospective study (Decision number: E-1845362.11.ED.28451). Electronic patient files and pharmacovigilance forms of the cases included in the study were examined. The data collected covers the period between January 2020 and June 2022.
(1) Molecular name of the contrast agent used in CT and MRI examination; (2) Favipiravir and contrast agent doses, drug interactions; (3) If there is a drug interaction, the possible mechanism and the results of the interaction; (4) The total number of drugs/pharmacological agents used by the patients included in the study; and (5) The age and gender of the cases were recorded as data.
Inclusion criteria: Patients using Favipiravir for the treatment of COVID-19 and who had contrast-enhanced CT or MRI while using this drug were included.
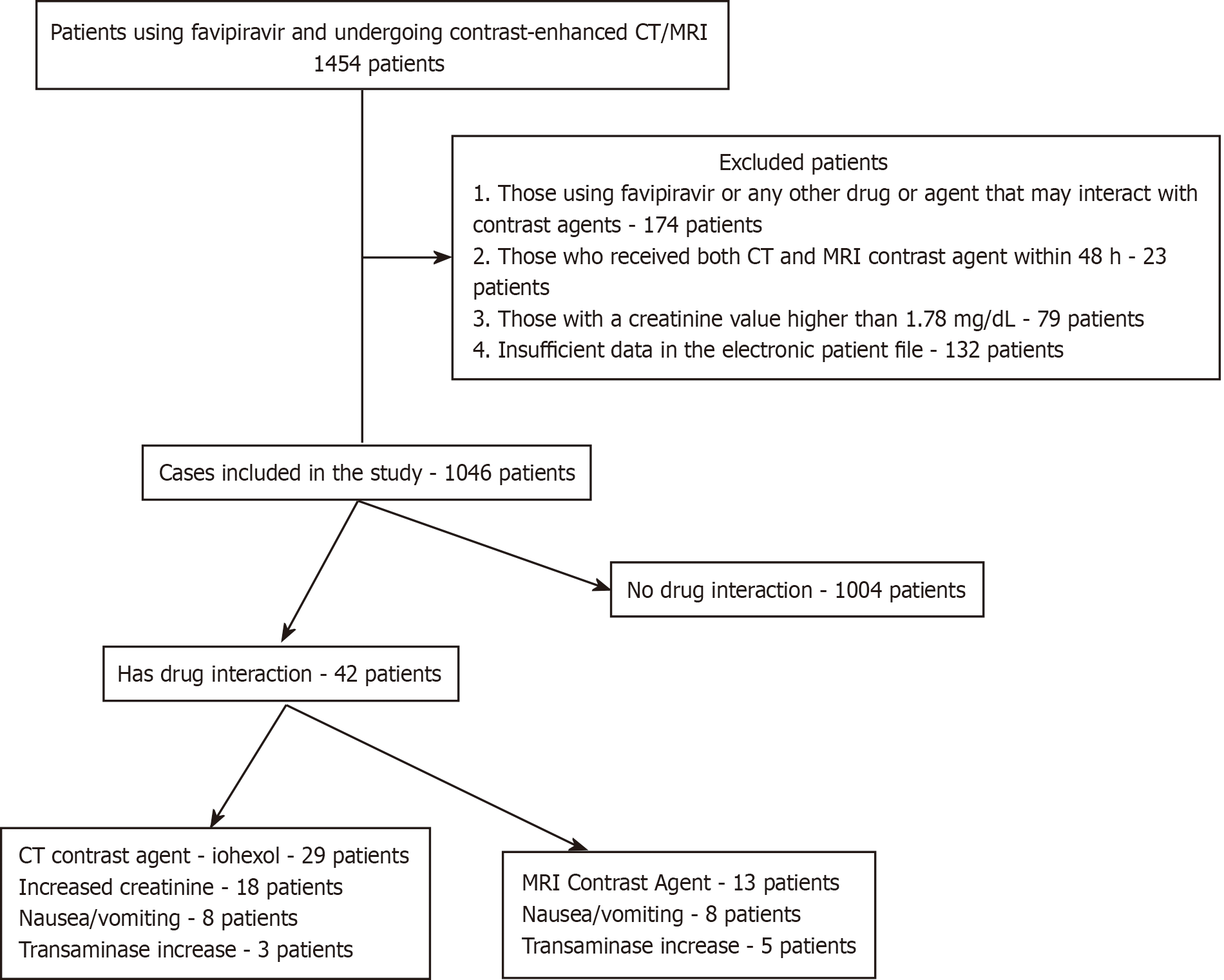
Exclusion criteria: (1) Electronic patient files that do not contain sufficient and/or necessary information recorded as study data (132 patients); (2) Patients using favipiravir or another drug or agent that may interact with contrast agents (174 patients); (3) Cases in which both CT and MRI contrast material were applied within 48 h (23 patients); and (4) As a natural additional exclusion criterion, cases with a creatinine value higher than 1.78 mg/dL were excluded (79 patients).
Within the scope of the study, 1454 patients were examined, and 408 patients were excluded in accordance with the stated exclusion criteria. The study sample consisted of 1046 patients. The flow chart of the cases examined within the scope of the study is presented in Figure 1.
In cases where favipiravir and CT or MRI contrast agents are used together, a change in the effect level of any of the agents mentioned or the occurrence of an adverse reaction due to the mentioned agents is defined as the presence of drug interaction[8]. The presence of drug interaction was determined by looking at the electronic patient file data of the cases included in the study or, if available, the pharmacovigilance forms of the cases.
In order to investigate the effect of the presence of drug interaction on the contrast agent function, CT and MRI images of the subjects included in the study were evaluated for the presence/absence of abnormality in organ enhancement by two radiologists who were not aware of the drug interaction development data. What is meant by the definition of abnormality in organ enhancement is the situation in which intra-abdominal solid organ images do not comply with the visual criteria accepted as standard by radiologists; although it is a relatively subjective evaluation in this respect, it is the only method that can be used since there is no standard numerical value determined for intra-abdominal solid organ enhancement. The presence/absence of abnormality was determined by consensus by radiologists.
Statistical analyzes were carried out using the IBM SPSS v.24.0 program. Normal distribution fit of the data was evaluated with the Kolmogorov-Smirnov test. Numerical data with normal distribution are shown as mean ± SD (mean age). Categorical variables are expressed as frequency and percentage. Differences between categorical variables were evaluated with the Chi-Square test. The differences between the mean ages of the cases with and without drug interaction were evaluated with the independent groups t test (student t test). Logistic regression analysis was used to examine the effect of age and gender on the development of drug interactions.
The study sample consists of 1046 patients. 651 (62.2%) patients were female and 395 (37.7%) patients were male. The mean age of the study sample was 47.23 ± 9.48 years (min.-max. 13-87 years). In total, possible drug interactions were detected in 42 cases (4%). Of these drug interactions, 29 (29/1046, 2.7%) were observed with CT contrast agent (Iohexol), and 13 (13/1046, 1.2%) were observed with MRI contrast agents. The mean age of the cases with drug interactions was 58.28 ± 7.44 years. The mean age of the cases with drug interactions with CT contrast agents was 56.60 ± 6.43 years. The mean age of the cases with drug interaction with MRI contrast agents was 62.05 ± 3.18 years. The mean age of the subjects with drug interactions detected was statistically significantly higher than those without any drug interaction (58.28 ± 7.44 vs 46.77 ± 8.16; P = 0.003) (Table 1).
| Patients with drug interactions | Patients without drug interactions | Whole population | χ2 P value | |
| Age (yr) | 58.28 ± 7.44 | 46.77 ± 8.16 | 47.23 ± 9.48 | 0.003a |
There was no statistically significant difference in the incidence of drug interactions between male and female genders (P = 0.090) (Table 2).
| Gender | Patients with drug interactions | Patients without drug interaction | Whole population | χ2 P value |
| Female | 26 (3.9) | 625 (96.1) | 651 | 0.090 |
| Male | 16 (4) | 379 (96) | 395 |
Favipiravir was used for 5 d of treatment at a maintenance dose of 1200 mg/day, after a loading dose of 3200 mg in all included subjects. CT contrast material was applied to 765 (73%) cases and MRI contrast material was applied to 281 (26.8%) cases. The CT contrast agent administered was Iohexol (OPAXOL 350 mg I/mL IA/IV solution for injection). The application dose is 1 mL/kg. The distribution of drug interactions thought to develop with Iohexol is as follows: Increase in creatinine values in 18 patients (62%) (1 acute renal failure), nausea-vomiting in 8 patients (27.5%), increase in transaminase values in 3 patients (10.3%).
Meglumine Gadoterate (Clariscan, 0.5 mmol/mL injectable solution 20 mL, vial) and Gadoxetic Acid Disodium (Primovist, pre-filled syringe 1 × 10 mL containing 0.25 mmol/mL injectable solution) were used as MRI contrast agents. The number of patients using MRI contrast agents is presented in detail in Table 3.
| MRI contrast agent | Number of patients |
| Meglumine gadoterate | 158 (56.2) |
| Gadoxetic acid disodium | 123 (43.8) |
| Total | 281 (100) |
Of the 13 drug interactions that developed as a result of MRI contrast agents, 10 (10/13, 76.9%) were detected as a result of use with Gadoxetic Acid Disodium and 3 (3/13, 23%) with Meglumine Gadoterate. The distribution of drug interactions thought to develop with MRI contrast material is as follows: Increased transaminase values in 8 patients (61.5%), nausea-vomiting in 5 patients (38.4%).
The rate of drug interactions resulting from the combined use of CT contrast agents and Favipiravir is statistically significantly lower than those that develop as a result of the use of MRI contrast agents (CT, 29/765, 3.7%-MRI, 13/281, 4.6%; P = 0.001) (Table 4).
The rate of drug interactions that occur with the use of Gadoxetic Acid Disodium is statistically significantly higher than those that occur as a result of the use of Meglumine Gadoterate (P = 0.001) (Table 5).
| MRI radiocontrast agent type | Patients with drug interactions | Patients without drug interaction | Whole population | χ2 P value |
| Gadoxetic acid disodium | 10 (8.1) | 113 (91.9) | 123 | 0.001a |
| Meglumine gadoterate | 3 (1.8) | 155 (98.2) | 158 |
In the image contrast quality examinations carried out by radiologists, no abnormality was observed in the images after contrast agent injection in any of the cases in which possible drug interaction was detected.
When favipiravir and radiocontrast agents were used together, when the effect of age and gender on drug interaction was evaluated by logistic regression analysis, it was found that gender had no effect on the development of drug interaction (P = 0.090). A 1-year increase in age increases the risk of developing drug interaction 1.63 times (Nagelkerke R2 = 0.508, OR = 1.63, P = 0.023).
The main purpose of our research is to detect drug interactions between Favipiravir, which is widely used during the COVID-19 pandemic, and CT and MRI contrast agents, which are effectively used in diagnostic and treatment examinations in COVID-19 cases.
According to the data of this study, there is a significant and positive relationship between age and drug interaction rates between Favipiravir and contrast agents. The risk of drug interactions increases with age. Current studies show that the risk of drug interactions and the severity of developing drug interactions increase with age[9,10]. In this respect, our data is compatible with the literature. Increasing polypharmacy rates and decreasing metabolization-elimination capacity with age are shown as the main reasons for the increased risk and severity of drug interactions with age.
It is emphasized in the literature that women face more adverse drug reactions than men[9,11]. According to our data, there is no difference between men and women in terms of drug interactions between Favipiravir and CT or MRI contrast agents. In this respect, a different result was obtained from the literature. We think that the possible reasons for the mentioned difference are only the investigation of drug interactions between limited pharmacological agents and the relatively limited number of the research population.
In our study, the rate of drug interaction between Iohexol, a CT contrast agent, and Favipiravir was found to be 2.7%. Adverse reaction rates with CT contrast agents vary between 1%-23% in the literature[12]. It is seen that our data is very close to the lower limit. The most common adverse reaction detected is an increase in creatinine values. Nausea-vomiting, another adverse reaction, is a frequently described complaint for both CT contrast agents and Favipiravir[7,12]. As a result of the combined use of two pharmacological agents, it can be thought that the aforementioned adverse reaction develops more frequently or more severely due to the common elimination pathways. No elevation in transaminase values as an adverse reaction was reported in previous studies after the use of CT contrast agents; however, this is a known and common adverse reaction for Favipiravir[7,12]. We think that the transaminase elevation detected in the results of our study is an aggravated adverse reaction caused by Favipiravir, and in this respect, our data are compatible with the literature. According to the data of our study transaminase elevations resulting from the combined use of CT contrast agents and Favipiravir do not have a clinical effect and were detected during routine biochemistry follow-ups. Only one of the cases of elevated creatinine occurred with acute renal failure, and the other cases were detected during routine biochemistry follow-ups. This can be interpreted as drug interactions that occur as transaminase and creatinine elevations are generally mild and do not reach the severity that will be reflected in the clinic. Another situation that we found within the scope of our study is that drug interactions do not reduce the effect of CT contrast agents on imaging. This is data that supports our conclusions that the drug interactions that occur are subclinical-mild.
All of the drug interactions that we have detected with MRI contrast agents are in the form of adverse reactions and the incidence is determined as 1.2%. According to literature data, the rate of adverse reaction development as a result of the use of MRI contrast material varies between 0.5%-2.5%[13]. It is seen that our rates are at the medium frequency level according to the literature. Within the scope of the study, drug interactions for two different types of MRI contrast agents were investigated. The first of these agents is Meglumine Gadoterate, since it is a contrast agent that is eliminated from the kidneys without being metabolized, drug interactions that develop as a result of joint use with Favipiravir are thought to occur as a result of the cooperation in the elimination phase from the kidneys, just like with CT contrast agents. The second MRI contrast agent is Gadoxetic Acid Disodium. Unlike Meglumine Gadoterate, this contrast agent enters the hepatocytes and is eliminated from there by being secreted into the bile. This contrast agent is excreted both by the kidneys and the elimination rates are equal to each other by the kidneys and the biliary tract. Due to the stated pharmacokinetic properties, Gadoxetic Acid Disodium is similar to Favipiravir in both the renal elimination phase and hepatic metabolism phase. Considering these mechanisms, it can be predicted that concomitant use with Gadoxetic Acid Disodium will be more risky in terms of drug interactions. Our study data support this prediction. The rate of drug interactions that occur with the use of Gadoxetic Acid Disodium is statistically significantly higher than those that occur as a result of the use of Meglumine Gadoterate.
Another remarkable study result is that the rate of drug interactions that develop as a result of the combined use of CT contrast agents and Favipiravir is statistically significantly lower than those that develop as a result of the use of MRI contrast agents. This can be explained by the mechanism proposed for MRI contrast agents; The increase in drug interaction rates of Gadoxetic Acid Disodium makes MRI contrast agents more risky in the whole population.
Drug interactions with MRI contrast agents include elevated transaminase values, nausea and vomiting. Favipiravir causes both adverse reactions. Nausea-vomiting is a well-known adverse reaction to MRI contrast agents, while elevated transaminases have not been identified[7,13]. When the literature data and study results are evaluated together, it can be thought that the combined use of Favipiravir and MRI contrast agents increases the transaminase-increasing property of Favipiravir. Again, we think that the risk of nausea and vomiting of both agents increases as a result of concomitant use. Transaminase elevations occurring within the scope of the study did not have a clinical effect and were detected during routine biochemistry follow-ups. In this case, it can be interpreted that drug interactions that occur in the form of transaminase elevation are generally mild and do not reach the severity that will be reflected in the clinic. Another fact that emerged according to the results of the study is that drug interactions do not reduce the effect-diagnostic power of MRI contrast agents in imaging. This finding is in line with our inferences that the drug interactions described are subclinical-mild.
A very low rate of adverse effects in the combined use of favipiravir and radiological contrast agents, and the fact that the adverse effects observed are predominantly at subclinical levels, are findings that encourage the safe use of the mentioned substances.
Although the COVID-19 pandemic is over, Favipiravir is an antiviral drug used against RNA virus infections that is used in different viral infections and has promising results for its use in the future[14,15].
The study has some limitations worth highlighting. Increasing the population size will provide more accurate data. The study was carried out in a single center. Our study was designed as a preliminary study, mainly aimed at detecting prevalence data; Therefore, it does not include a control group. Further studies with a well-matched control group may reveal the presence of drug interactions more objectively. Only Iohexol was evaluated as a CT contrast agent. It is accepted that Iohexol, an ionic contrast agent, and other ionic contrast agents exhibit similar behavior[12,13]. However, the collection of information on different contrast agents may lead to changes in data. For the same reason, data on non-ionic contrast agents could not be presented. Meglumine Gadoterate and Gadoxetic Acid Disodium were studied as MRI contrast agents. It is emphasized in the literature that contrast agents other than Gadoxetic Acid Disodium exhibit similar behaviors and adverse effect profiles[12,13]; however, increasing the number of contrast agents examined may increase data reliability. The study omitted cases involving medicines that are known to interact with favipiravir or contrast agents. However, it still included participants involving the use of numerous drugs. The utilization of only Favipiravir and contrast media in the treatment of a patient group may have an impact on the outcomes.
In conclusion, the results obtained from this study showed that the most prominent findings that emerged as a result of the combined use of contrast agents and favipiravir were an increase in creatinine and transaminase values and an increase in the frequency of nausea and vomiting. It was observed that the majority of the drug interactions detected were mild so that they were not reflected in the clinic. While the frequency of drug interactions increased significantly with age, no significant change was recorded by gender. It has been revealed that the most risky contrast agent for use with favipiravir is Gadoxetic Acid Disodium.
| 1. | Aydın S, Karavaş E, Ünver E, Şenbil DC, Kantarcı M. Long-term lung perfusion changes related to COVID-19: a dual energy computed tomography study. Diagn Interv Radiol. 2023;29:103-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 2. | Aydin S, Unver E, Karavas E, Yalcin S, Kantarci M. Computed tomography at every step: Long coronavirus disease. Respir Investig. 2021;59:622-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Bahadir S, Aydın S, Kantarci M, Unver E, Karavas E, Şenbil DC. Triple rule-out computed tomography angiography: Evaluation of acute chest pain in COVID-19 patients in the emergency department. World J Radiol. 2022;14:311-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Guven F, Ogul H, Eren S, Aydin S, Kantarci M. The Role of Computerized Tomography in Diagnosis and Follow-Up in COVID-19 Pneumonia. Arch Basic Clin Res. 2021;3:111-119. [DOI] [Full Text] |
| 5. | Caruso D, Zerunian M, Pucciarelli F, Lucertini E, Bracci B, Polidori T, Guido G, Polici M, Rucci C, Iannicelli E, Laghi A. Imaging of abdominal complications of COVID-19 infection. BJR Open. 2021;2:20200052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Çelik Aydin Ö, Aydin S, Güney H. Kontrast Ajanlar; Farmakolojik Özellikleri, Genel Advers Reaksiyonları ve İlaç Etkileşimleri. Ankara Eğitim ve Araştırma Hastanesi Tıp Dergisi. 2020;53:61-67. [DOI] [Full Text] |
| 7. | Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-Hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021;11:11022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 8. | Ivanyuk A, Livio F, Biollaz J, Buclin T. Renal Drug Transporters and Drug Interactions. Clin Pharmacokinet. 2017;56:825-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 9. | Hughes JE, Russo V, Walsh C, Menditto E, Bennett K, Cahir C. Prevalence and Factors Associated with Potential Drug-Drug Interactions in Older Community-Dwelling Adults: A Prospective Cohort Study. Drugs Aging. 2021;38:1025-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Zerah L, Henrard S, Wilting I, O'Mahony D, Rodondi N, Dalleur O, Dalton K, Knol W, Haschke M, Spinewine A. Prevalence of drug-drug interactions in older people before and after hospital admission: analysis from the OPERAM trial. BMC Geriatr. 2021;21:571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Zucker I, Prendergast BJ. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol Sex Differ. 2020;11:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 375] [Article Influence: 75.0] [Reference Citation Analysis (0)] |
| 12. | Andreucci M, Faga T, Pisani A, Sabbatini M, Michael A. Acute kidney injury by radiographic contrast media: pathogenesis and prevention. Biomed Res Int. 2014;2014:362725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Neeley C, Moritz M, Brown JJ, Zhou Y. Acute side effects of three commonly used gadolinium contrast agents in the paediatric population. Br J Radiol. 2016;89:20160027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Shiraki K, Daikoku T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 15. | Łagocka R, Dziedziejko V, Kłos P, Pawlik A. Favipiravir in Therapy of Viral Infections. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |