Published online May 28, 2024. doi: 10.4329/wjr.v16.i5.115
Revised: March 19, 2024
Accepted: April 16, 2024
Published online: May 28, 2024
Processing time: 115 Days and 5 Hours
Gastrointestinal bleeding (GIB) is a severe and potentially life-threatening condition, especially in cases of delayed treatment. Computed tomography angio
To determine whether a volumetric estimation of the extravasated contrast at CTA in GIB may be a predictor of subsequent positive angiographic findings.
In this retrospective single-centre study, 35 patients (22 men; median age 69 years; range 16-92 years) admitted to our institution for active GIB detected at CTA and further submitted to catheter angiography between January 2018 and February 2022 were enrolled. Twenty-three (65.7%) patients underwent endoscopy before CTA. Bleeding volumetry was evaluated in both arterial and venous phases via a semi-automated dedicated software. Bleeding rate was obtained from volume change between the two phases and standardised for unit time. Patients were divided into two groups, according to the angiographic signs and their concordance with CTA.
Upper bleeding accounted for 42.9% and lower GIB for 57.1%. Mean haemoglobin value at the admission was 7.7 g/dL. A concordance between positive CTA and direct angiographic bleeding signs was found in 19 (54.3%) cases. Despite no significant differences in terms of bleeding volume in the arterial phase (0.55 mL vs 0.33 mL, P = 0.35), a statistically significant volume increase in the venous phase was identified in the group of patients with positive angiography (2.06 mL vs 0.9 mL, P = 0.02). In the latter patient group, a significant increase in bleeding rate was also detected (2.18 mL/min vs 0.19 mL/min, P = 0.02).
In GIB of any origin, extravasated contrast volumetric analysis at CTA could be a predictor of positive angiography and may help in avoiding further unnecessary procedures.
Core Tip: Computed tomography angiography (CTA) plays a pivotal role in the diagnostic-therapeutic management of gastrointestinal bleeding (GIB). Since a large portion of GIB cases detected by CTA are occult on catheter angiography, our study aimed to evaluate whether the volumetric assessment of extravasated contrast through a semiautomated software could be identified as a predictive factor for positive angiography. The bleeding volume in the venous phase and the bleeding rate were significantly associated with positive angiographic findings. The reproducibility of the volumetric analysis was also demonstrated by a significant inter-observer agreement between two radiologists with different levels of experience.
- Citation: Cacioppa LM, Floridi C, Bruno A, Rossini N, Valeri T, Borgheresi A, Inchingolo R, Cortese F, Novelli G, Felicioli A, Torresi M, Boscarato P, Ottaviani L, Giovagnoni A. Extravasated contrast volumetric assessment on computed tomography angiography in gastrointestinal bleeding: A useful predictor of positive angiographic findings. World J Radiol 2024; 16(5): 115-127
- URL: https://www.wjgnet.com/1949-8470/full/v16/i5/115.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i5.115
Gastrointestinal bleeding (GIB) is a severe and potentially life-threatening condition leading to high mortality and morbidity rates, especially in those patients with delayed treatment or multiple comorbidities[1,2]. Due to the increasing life expectancy and the ever more frequent employment of oral anticoagulant agents, GIB has become a greater health concern in the Western world[3-5].
According to the bleeding location, GIB is traditionally divided into upper and lower, proximal to the ligament of Treitz and downstream of the duodenum-jejunal junction, respectively[6-10].
The detection of GIB is challenging due to the long length and motility of the gastrointestinal tract, the intermittent course of blood extravasation, and the frequent occurrence in patients with multiple comorbidities[11]. Endoscopy and computed tomography angiography (CTA) play a pivotal role in the diagnostic-therapeutic management of GIB. Both the diagnostic methods aim to early identify the bleeding source and to action a prompt treatment of the haemorrhage[12,13]. According to the gastrointestinal tract involved, endoscopy is the first choice for upper GIB, due to its capability to localize the bleeding site, and to allow biopsies and therapeutic procedures[13]. In lower GIB, emergent endoscopy may be strongly limited by the poor bowel preparation, the presence of coarse blood clots hiding the bowel mucosae, and the difficult exploration of the small bowel tracts[14]. In emergent settings, especially those with lower bleeding and a previous negative endoscopy, CTA is considered the first choice of radiological imaging. Due to its high accessibility and reproducibility, the low bleeding detection threshold, and the ability to localize the bleeding source and to provide information on vascular anatomy, CTA has become crucial for the planning of subsequent angiographic procedures[15-20].
Over the years, transcatheter arterial embolization (TAE) has become a valid treatment option for acute GIB refractory to conservative management, with excellent technical and clinical success rates and a low risk of complications, even if no consensus guidelines on indication, timing, and technical issues still exist[14].
Despite that CTA has been reported to be more sensitive than catheter angiography in the detection of active GIB signs, the need for angiography should not be exclusively based on a positive CTA as several patient related factors have to be considered[19]. Furthermore, a large portion of GIB cases detected by CTA are occult on catheter angiography[21]. The identification of GIB cases which are more likely to benefit from TAE based on CTA parameters other than positivity could thereby be beneficial for patient management[22].
Volumetric analyses of arterial extravasation at CTA have been previously reported in several settings and through different softwares, with most of them applied to pelvic and intracranial districts[23,24].
Our aim was to determine whether the volumetric assessment of extravasated contrast at CTA in upper and lower GIB could be identified as a standardized and reproducible method to predict positive angiographic findings.
The study was approved by the Internal Review Board and conducted in conformity to the ethics guidelines of the 1990 Declaration of Helsinki and its amendments. Due to the retrospective design, this study did not require the approval by the Ethics Committee of the University Politecnica delle Marche. All patients provided informed written consent to the procedure.
This single-centre retrospective study analysed all the consecutive patients admitted to the emergency department of our institution for upper or lower GIB with direct signs of bleeding and active arterial contrast extravasation at CTA from January 2018 to December 2022. Within 12 h from the initial CTA, all the included patients received a diagnostic confirmation by catheter angiography and subsequent TAE treatment if an ongoing GIB was documented by the presence of active contrast extravasation.
Files and images were extracted from the Radiology Information System and from the Picture Archiving and Communication System (PACS) of our institutional hospital.
The searched key words were “gastrointestinal and arterial extravasation of contrast medium” and “gastrointestinal bleeding”. Demographic, clinical, and laboratory data were obtained from digital medical records.
Additional characteristics including bleeding site, vascular territory involved in the haemorrhage, time to catheter angiography after the initial CTA, and the necessity for further treatments after catheter angiography were also collected.
The exclusion criteria were age < 18 years, missing or incomplete clinical and imaging data, bleeding arising from oesophageal varices or gastrointestinal malignancies. Patients with indirect bleeding signs or signs suggestive for visceral pseudoaneurysm revealed at CTA or angiography were also excluded.
All computed tomography (CT) scans were acquired using a 128-slice multi detector CT scanner (Revolution CT, General Electric Medical Systems, Milwaukee, WI, USA). The principal scanning parameters are summarized in Table 1. An 18-gauge cannula was placed into a superficial vein of the antecubital fossa or forearm for the injection of intravenous contrast medium. All images were acquired in craniocaudal direction with a scan length ranging from the diaphragm to the ischial tuberosities. All patients were positioned supine, feet first.
| CTA acquisition protocol (128 MDCT-GE medical system) | |
| KVp | 100-120 kVp |
| mAs | 200-700 (smart mA) |
| Collimation | 80 mm |
| Rotation time | 0.28 s |
| Pitch | 0.992 |
| FOV | Large body |
| Acquisition thickness | 2.5 mm |
| Reconstruction thickness | 0.625 mm |
| Contrast medium volume | 1.2-1.5 mL/kg |
| Contrast medium infusion rate | ≥ 4 mL/s |
| IDR | 1.4-1.8 |
The standardized institutional CT protocol for acute GIB included an initial non-contrast phase, followed by contrast-enhanced arterial and venous phases. A volume of 1.2-1.5 mL/kg of iodinated contrast medium (370 mg/mL of iodine concentration) followed by 40 mL of saline solution, both at a flow rate ≥ 4 mL/s, was intravenously administered to reach an iodine delivery rate of 1.4-1.8. The arterial phase was acquired approximately 20-30 s after the injection using bolus tracking technology with the region of interest (ROI) positioned at the level of the descending aorta. The subsequent venous phase was obtained approximately 45 s after the injection. Axial 0.625 mm reconstructions and multi-planar reformations (coronal and sagittal for both the arterial and venous phases) were available on the PACS.
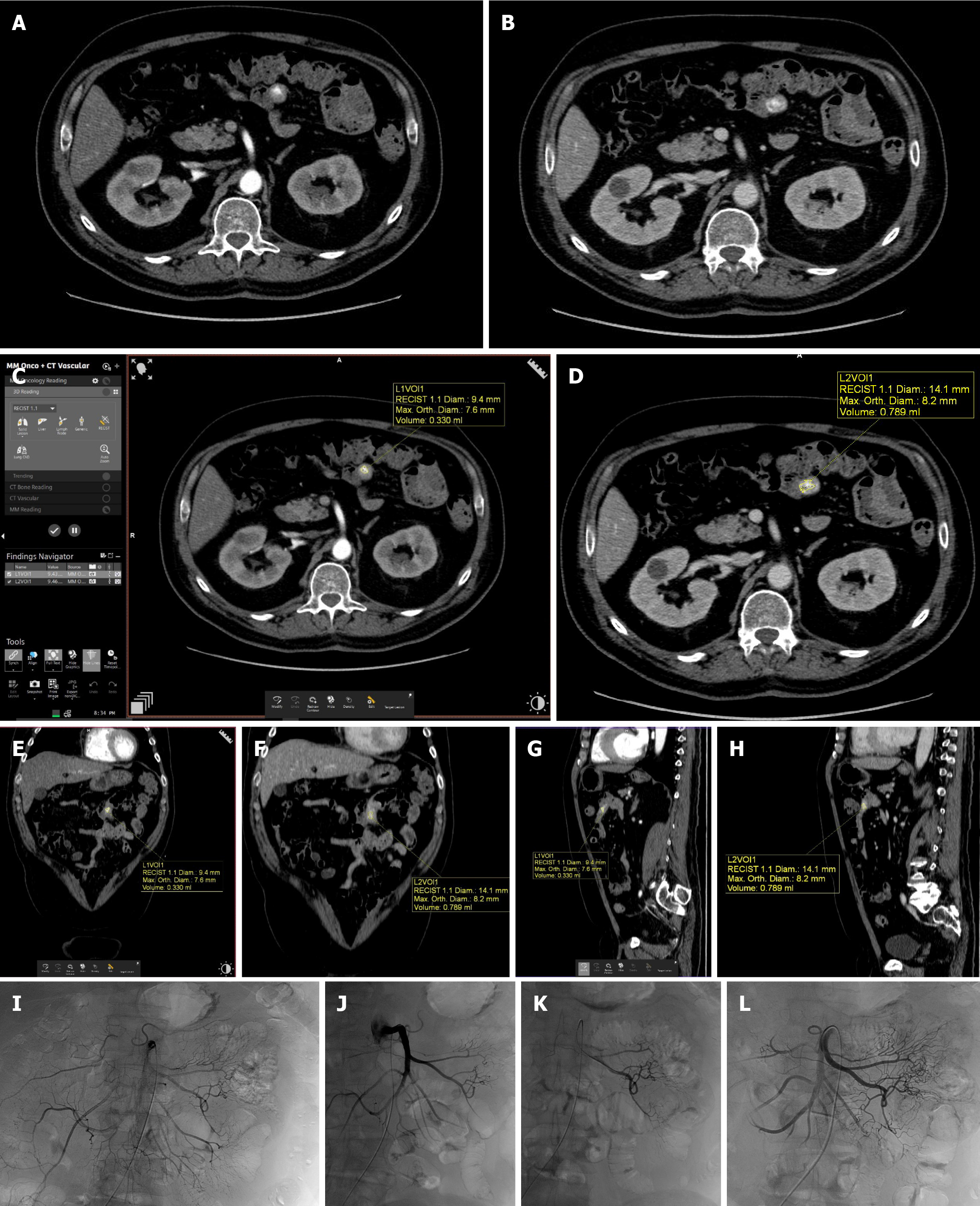
After a visual inspection of plain and contrast axial and reformatted images, the volumetric analysis was performed via a semiautomated software on a dedicated advanced workstation (Syngo MMWP version VA 20; Siemens Healthcare, Forchheim, Germany).
The measurement of haemorrhage size was performed by the "MM Oncology + CT Vascular" application using a “ROI method” consisting in a semiautomatic selection of contrast extravasated areas according to a preset density threshold (≥ 90 HU). The application relies on different semiautomatic segmentation tools such as edge tracking and threshold recognition, as well as a manual pixel selection tool. After the initial pixel selection, the software automatically connects pixels with similar density. The ROI can be modified in each slice by "edge-tracing" or "limit-tracing" tools. Bleeding volumetry was evaluated in both arterial and venous phases. Bleeding rate was obtained by the volume change elapsed between the two phases and standardized for unit time.
Image analyses and volumetric measurements were performed by two independent radiologists with > 10 and < 3 years of experience in emergency and vascular radiology (Reader A and Reader B, respectively). Both the investigators were blind to sex, age, patient history, and radiology reports. The images were anonymised and displayed in random order to reduce reader bias.
All the endovascular procedures were performed in the two fully equipped angio-suites of our institution (Allura Xper R7, Philips and Axiom Artis Zee, Siemens Healthcare) by one of the six fellowship-trained interventional radiologists.
Patients were prepared with trichotomy, skin disinfection, and sterile draping. Right or left transfemoral access was obtained and a 4 Fr introducer sheath was placed.
Preliminary selective angiograms were obtained by automated contrast agent injection by placing 4-5 Fr diagnostic catheters, mostly Cobra-2 and Simmons-1 (Cordis, Santa Clara, CA, United States), in celiac trunk and superior and inferior mesenteric arteries. Contrast agent dose and injection rate were determined by the individual operator based on patient and vessels characteristics.
Super-selective angiographies of vessels suspected or identified on CTA examination as responsible for GIB were performed using 2.4 to 2.7 microcatheter systems (Progreat, Terumo Medical, Tokyo, Japan) to visualize their course, caliber, and branches, and to evaluate the best embolization strategy.
The embolization of target vessels was exclusively performed in case of angiographic direct (contrast blush) findings suggestive of active GIB.
In all cases, mechanical embolic agents consisting in 0.018” platinum microcoils with a deployed diameter of 2-5 mm and with pushable (Boston scientific, Natick, MA, United States) or detachable (Azur Hydrocoils, Terumo Medical, Tokyo, Japan) delivering systems were employed.
Super-selective catheterisations and angiographies of adjacent and collateral vessels around the bleeding site were also performed. Access sites were closed by manual compression.
Clinical success was defined as the presence of haemodynamic (blood pressure > 90 mmHg or responsive to fluid resuscitation) and laboratory stability after TAE procedure, as previously reported[25].
Procedure-related complications were recorded as suggested by the Society of Interventional Radiology[26,27]. According to the concordance of the angiographic findings to CTA, two groups of patients (concordance and non-concordance group) were considered.
The correlation between bleeding volumetry evaluated at CTA and the presence of positive angiographic signs of GIB was considered as primary outcome.
As secondary outcome, the reproducibility of the volumetric assessment was evaluated through the inter-observer agreement.
Data were analysed using STATA BE - Basic Edition 17.0 (StataCorp LLC, 4905 Lakeway Drive College Station, TX, United States) statistical software. The distribution of categorical variables is expressed as absolute frequency and percentage; numerical variables are presented as the mean value ± SD, if normally distributed, and as median (interquartile range) if not normally distributed. For comparison of categorical variables between two groups, the Chi-square test was used, as appropriate. For comparison of numerical variables between two groups, Student's t test or Mann-Whitney U test was used, depending on the distribution of variables. P < 0.05 was considered statistically significant.
Student's t tests were performed to compare the bleeding volume between the positive angiography group and the negative angiography group.
Inter-observer agreement analysis was conducted using Bland-Altman plot method for both arterial phase and venous phase volume measurements (95% limit of agreement).
A total of 35 patients with active bleeding signs detected at CTA were included. The mean age was 69 ± 18.5 years, with a range of 16-92 years. Twenty-two (62.9%) patients were male whereas 13 were female (37.1%).
In 15 (42.9%) patients the bleeding site was in the upper gastrointestinal tract whereas in 20 (57.1%) of cases the lower tract was involved. The main causes of GI bleeding were diverticular disease (10 patients, 28.5%) and peptic ulcer (8 patients, 22.8%). The most involved vascular territories were terminal branches of the sigmoid artery (6 cases,17.1%) and branches of the gastroduodenal artery (5 cases; 14.2%). The mean haemoglobin value at the admission was 7.5 ± 1.7 g/dL.
Demographic, etiological, laboratory, and clinical characteristics of the study population are summarized in Table 2.
| Characteristic | Value |
| Demographic | n (range) |
| Patients (n) | 35 |
| Age (yr) | 67 (19-92) |
| Male/female | 22/13 |
| Weight (kg) | 86 (67-118) |
| Height (cm) | 175 (155-188) |
| BMI (kg/m2) | 25.7 (17-33.5) |
| Etiological | |
| Bleeding site | |
| Upper GI tract | 15 (42.9) |
| Lower GI tract | 20 (57.1) |
| Bleeding cause | |
| Peptic ulcer | 8 (22.8) |
| Diverticular disease | 10 (28.5) |
| Gastritis or duodenitis | 3 (8.5) |
| Angiodysplasia | 3 (8.5) |
| Pancreatitis | 2 (5.7) |
| Spontaneous | 4 (11.4) |
| Iatrogenic | 5 (14.2) |
| Vascular territory | |
| Gastroduodenal artery | 5 (14.2) |
| Left gastric artery | 3 (8.5) |
| Short gastric artery | 1 (2.8) |
| Pancreaticoduodenal artery | 4 (11.4) |
| Superior mesenteric artery (terminal branches) | 1 (2.8) |
| Right colic artery (distal branches) | 2 (5.7) |
| Ileo-colic artery | 3 (8.5) |
| Marginal artery | 2 (5.7) |
| Jejunal artery | 2 (5.7) |
| Ileal artery | 3 (8.5) |
| Inferior mesenteric artery (terminal branches) | 6 (17.1) |
| Sigmoid artery (terminal branches) | 2 (5.7) |
| Inferior epigastric artery | 1 (2.8) |
| Laboratory | |
| Pre-CTA haemoglobin (g/dL), median (interquartile range) | 7.5 (4-13) |
| Clinical | |
| Hematemesis | 9 (25.7) |
| Coffee ground emesis | 4 (11.4) |
| Melena | 5 (14.2) |
| Haematochezia | 14 (40) |
| Haemodynamic instability | 3 (8.5) |
Twenty-three (65.7%) patients had been preliminarily submitted to endoscopy/colonoscopy before CTA. In 15 (42.9%) cases, endoscopic evidence of active bleeding was present and in 2 (5.7%) cases, endoscopic haemostasis by clip positioning was unsuccessfully attempted.
All patients, due to the presence of active signs of GIB at CTA, were submitted to catheter angiography for bleeding evaluation with a mean time interval between CTA and angiography of 4 (2-9) h.
In 19 (54.3%) patients, an active GIB was detected at angiographic examination, and they were therefore considered as a concordance group. In all those cases, a TAE procedure was further performed with a technical success of 100% and clinical success of 84.2%. No major complications were registered. Three (15.8%) cases of recurrent GIB after the first TAE were recorded, all managed conservatively, without the need of re-intervention.
In 16 (45.7%) cases, the direct signs of GIB detected at CTA were not confirmed by catheter angiography, and they were thus considered as a non-concordance group. Due to the persistence of symptoms or low haemoglobin values, the absence of ongoing GIB was confirmed by a second catheter angiography in 3 (8.6%) cases and by CTA follow-up within the next 24 h in 13 (37.1%) cases.
Arterial and venous bleeding volumes were calculated in all the included patients as previously described, with a mean evaluation time of 6 ± 1 min.
At volumetric analysis, a median arterial volume of 0.46 (0.21-0.76) mL, median venous volume of 1.83 (0.66-2.83) mL, and median bleeding rate of 2.04 (0.05-3.09) mL/min were obtained (Table 3).
| Parameter | General population |
| Time interval between CTA and DSA, median (interquartile range) (h) | 4 (2-9) |
| Bleeding arterial volume, median (interquartile range) (mL) | 0.46 (0.21-0.76) |
| Bleeding venous volume, median (interquartile range) (mL) | 1.83 (0.66-2.83) |
| Bleed rate, median (interquartile range) (mL/min) | 2.04 (0.05-3.09) |
In 24 (68.6%) cases the bleeding rate was > 1 mL whereas in the residual 11 (31.4%) cases it was < 1 mL.
No significant differences in terms of pre-procedural haemoglobin value (P = 0.07), bleeding arterial volume (P = 0.35), and upper vs lower bleeding site (P = 0.55) were found between the concordance and the non-concordance groups.
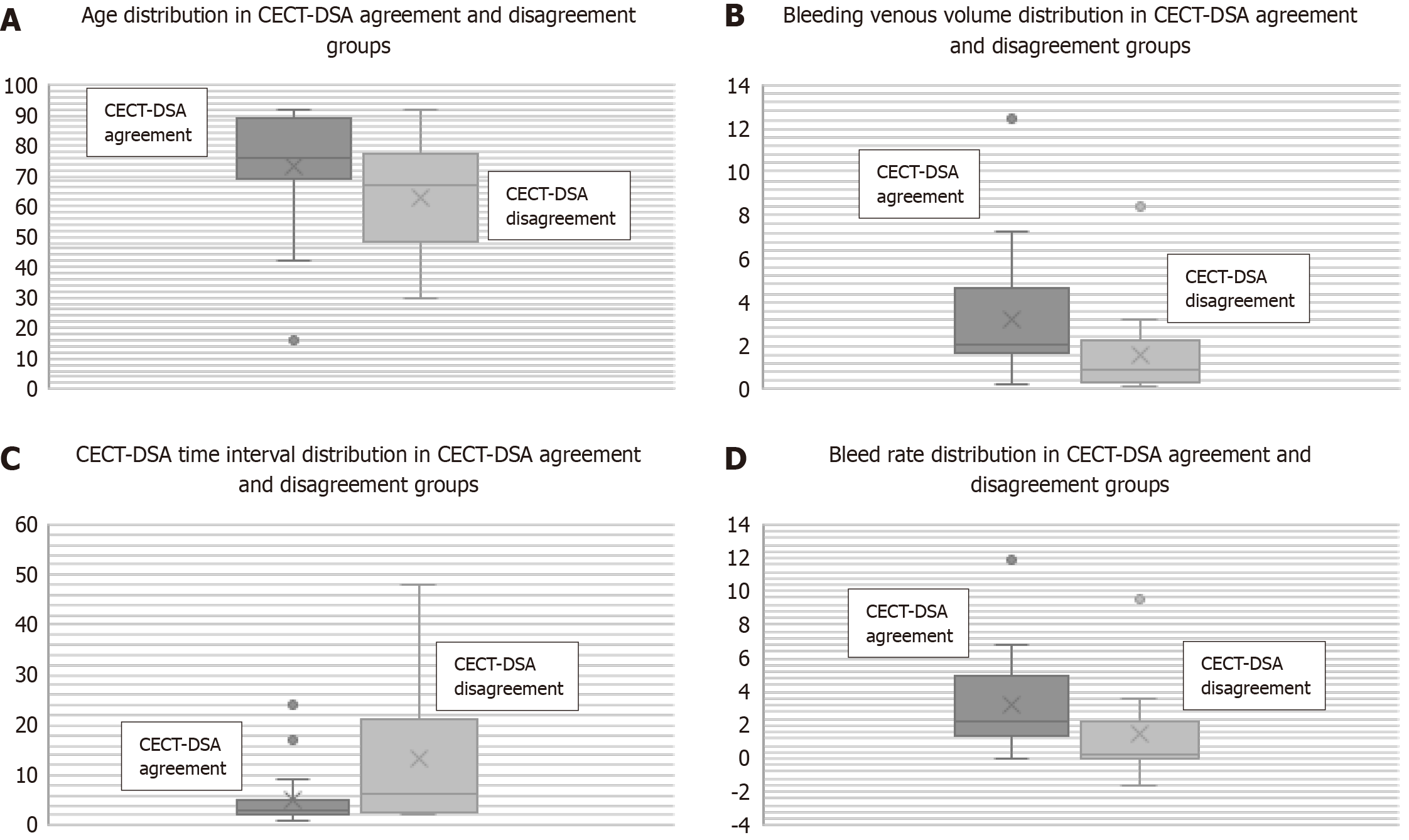
Conversely, significant differences were recorded between the concordance and the non-concordance groups in terms of median age [76 (69-89) years vs 67 (51-75.5) years, respectively; P = 0.05], bleeding volume in venous phase (2.06 mL vs 0.9 mL, respectively; P = 0.02), CTA-angiography time interval [3 (2-5) h vs 6.5 (2.7-20) h, respectively; P = 0.02], and bleeding rate (2.18 mL/min vs 0.19 mL/min, respectively; P = 0.02) (Figure 1). Regarding the concordance group, higher values of mean age, bleeding venous volume, and bleeding rate and a shorter CTA-angiography time interval were registered. The described results are displayed in Table 4.
| Parameter | Concordance group (n = 19) | Non-concordance group (n = 16) | P value, Chi-square test | P value, Mann-Whitney U test |
| Patients | 19 (54.2) | 16 (45.7) | ||
| Age (yr) | 73.3 (± 18.0) | 62.8 (± 17.3) | 0.05 | |
| Male/female (ratio) | 11/8 | 10/6 | NS | |
| Haemoglobin, median (interquartile range) (g/dL) | 7.2 (6-8.2) | 8 (7.5-8.3) | NS | |
| Upper bleeding | 9 (47.4) | 6 (37.5) | NS | |
| Lower bleeding | 10 (52.6) | 10 (62.5) | NS | |
| Time interval between CTA and DSA, median (interquartile range) (h) | 3 (2-5) | 6.5 (2.7-20) | 0.02 | |
| Bleeding arterial volume, median (interquartile range) (mL) | 0.55 (0.25-0.80) | 0.33 (0.18-0.66) | NS | |
| Bleeding venous volume, median (interquartile range) (mL) | 2.06 (1.67-4.66) | 0.9 (0.27-2.15) | 0.02 | |
| Bleed rate, median (interquartile range) (mL/min) | 2.18 (1.30-5.01) | 0.19 (0.02-2.21) | 0.02 |
The results of volumetric analyses of the arterial and venous phases of the two readers with inter-observer agreement are reported in Table 5. The Bland–Altman plots revealed mean difference values (± SD) of -0.05 ± 0.52 (95% limits of agreement: -1.98 to 0.98) in the arterial volume analysis and -0.56 ± 2.85 (95% limits of agreement: -6.15 to 5.03) in the venous volume analysis (Figure 2). The 95% of the measurements of reader A and reader B fell between the two lines of limits with few outliers (two for arterial phase and one for venous phase), showing a significant agreement between the two readers. In addition, the zero value on the vertical axis was within the confidence interval, showing the absence of significant systematic error. Two cases of GIB detected at CTA and subsequently submitted to angiography are illustrated in Figures 3 and 4.
| Variable | Reader A | Reader B | Average difference (95%CI) | DS of average difference | 95% upper agreement limit (95%CI) | 95% lower agreement limit (95%CI) |
| Volume in the arterial phase | 0.70 ± 0.83 | 0.75 ± 1.01 | -0.05 (-0.23 to 0.13) | ± 0.52 | 0.98 (0.73–1.36) | -1.98 (-1.46 to -0.83) |
| Volume in the venous phase | 2.50 ± 2.70 | 3.05 ± 3.88 | -0.56 (-1.54 to 0.42) | ± 2.85 | 5.03 (3.67 to 7.09) | -6.15 (-8.20 to -0.79) |
Due to its higher incidence in elderly population and to the significant hospitalization, complications, and death rates, GIB has become a great socioeconomic burden[14].
About 80% of GIB cases resolve conservatively with supportive measures alone, although the remaining cases require further interventions and a multidisciplinary approach including different specialized professionals[20,28]. Since CT angiography imaging technology has advanced over the years, the time to diagnosis as well as the sensitivity and specificity in the detection of life-threatening GIB has considerably improved, with undeniable advantages in terms of prognosis and emergency room workflow[29]. CTA has nowadays replaced tagged red blood cell scans and lower endoscopy in poorly prepared patients with lower GIB. It is also frequently performed in upper GIB cases in whom endoscopy is inconclusive[30]. The most important advantages of CTA lie in its availability, the rapid acquisition time, and the capability to clarify the bleeding source prior to submitting patients to further invasive procedures, thus reducing the overall time from the decision to catheter-directed embolization[30-32].
In agreement with several previous studies and meta-analyses, our results have confirmed TAE as a valuable therapeutic option for GIB, with high technical and clinical success and a low complication rate[33,34]. More specifically, a high technical success and favourable clinical outcomes were previously reported for TAE procedure in about 80% of patients with detected upper GIB and in lower GIB unresponsive to systemic therapy that cannot be managed in any other way[2].
In order to perform an effective TAE, CTA plays a critical role in the detection of bleeding site, delineating the underlying vascular anatomy and providing information about possible vascular variants, with a reported sensitivity of nearly 100%[2,35]. Furthermore, due to the relatively high rate of intestinal ischemia following TAE in lower GIB (up to 4% of cases), especially in cases of blind embolization, CTA positivity is crucial before performing TAE[19].
The main diagnostic paradigms of CTA have so far only been focused on the binary analysis of positive vs negative bleeding signs as well as bleeding localization, while other potential predictive factors for future positive catheter angiography and resolutive TAE have been poorly explored[23]. Nevertheless, the volumetric assessment of bleeding or hematoma entity has been previously investigated in the literature. Chikamatsu et al[36] proposed its employment as a prognostic factor in the context of post-biopsy renal bleeding. Other studies have also considered the hematoma size as a predictor of successful interventional management in pelvic trauma[36,37].
Regarding GIB, in a series of three cases, P et al[38] described the potential of volumetric estimation of GIB on CTA for illustrative purposes rather than showing population-based results[38]. In another recent study exclusively focused on lower GIB[23], a similar semiautomatic dedicated software was adopted to determine the active hemorrhage size both with axial and volumetric measurements. Similarly to our series, this study obtained promising results, suggesting that such objective imaging characteristics detected at CTA may be successfully used to predict the likelihood of positive catheter angiography[23].
Furthermore, there is still debate on safety and utility of empiric embolization regardless of positive catheter angiography, primarily in lower GIB. In this regard, the GIB volumetric assessment enhances the role of CTA in improving patient selection and risk stratification, to better identify which patients would mostly benefit from (target or prophylactic) TAE, avoiding the adjunctive risks of an invasive procedure[23,38].
The implications of our volumetric analysis may be easily understood since bleeding volume in the venous phase and bleeding rate were comprehensibly associated with positive findings at catheter angiography. As already hypothesized and adopted in the everyday practice, the growing of bleeding over time, detected at the venous phase, is a more reliable marker of bleeding entity than the initial size in the arterial phase.
The correlation of CTA-angiography time interval and positive angiographic findings confirms that CTA offers a significant time advantage over other modalities. Furthermore, image analyses should be performed by means of rapid and practical at-the-workstation softwares to avoid time-consuming measurements and further treatment delays.
In this regard, the feasibility and reproducibility of the adopted software and the volumetric measurements are of paramount importance. These aspects have frequently been evaluated in studies involving dual-energy CT postprocessing techniques, where the performance of the readers in visual analysis and the readers’ interpretation times have been taken into consideration[39-41].
Our series was the first in the literature to evaluate the reproducibility of the volumetric assessment in GIB, reaching a significant inter-observer agreement between two blind radiologists with different levels of experience.
The authors acknowledge several limitations. First, the study is retrospective and single-centered, and the sample size is small. In addition, the limitations of CTA in the detection of low-rate or intermittent bleeding, in anatomically unfavourable regions, and in presence of metallic artifacts in the bowel lumen must be considered. The volumetric assessment of extravasated contrast is subjected to discrepancies between operators with different experience and familiarity with the applied software. An intra-observer agreement evaluation is lacking, as well as the evaluation of the learning-curve of the individual operators. Lastly, other limitations stem from the variable diagnosis to catheter angiography time and from several procedure-related factors such as contrast injection rate, the use of power injector, and the variable selectivity of vessel catheterisations.
In conclusion, bleeding volumetry estimation obtained by reproducible measurements on CTA and through a semiautomated software is a feasible and useful tool in predicting subsequent positive angiographic findings in both upper and lower GIB. Larger populations and multicentric studies are necessary to confirm these results and to identify threshold bleeding volumes below which catheter angiography could be avoided.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Hassan SA, United States S-Editor: Li L L-Editor: Wang TQ P-Editor: Zheng XM
| 1. | Nable JV, Graham AC. Gastrointestinal Bleeding. Emerg Med Clin North Am. 2016;34:309-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Ini' C, Distefano G, Sanfilippo F, Castiglione DG, Falsaperla D, Giurazza F, Mosconi C, Tiralongo F, Foti PV, Palmucci S, Venturini M, Basile A. Embolization for acute nonvariceal bleeding of upper and lower gastrointestinal tract: a systematic review. CVIR Endovasc. 2023;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Hreinsson JP, Gumundsson S, Kalaitzakis E, Björnsson ES. Lower gastrointestinal bleeding: incidence, etiology, and outcomes in a population-based setting. Eur J Gastroenterol Hepatol. 2013;25:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 4. | Kim JS, Kim BW, Kim DH, Park CH, Lee H, Joo MK, Jung DH, Chung JW, Choi HS, Baik GH, Lee JH, Song KY, Hur S. Guidelines for Nonvariceal Upper Gastrointestinal Bleeding. Gut Liver. 2020;14:560-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 5. | Lanas A, Dumonceau JM, Hunt RH, Fujishiro M, Scheiman JM, Gralnek IM, Campbell HE, Rostom A, Villanueva C, Sung JJY. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018;4:18020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Nelms DW, Pelaez CA. The Acute Upper Gastrointestinal Bleed. Surg Clin North Am. 2018;98:1047-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Aoki T, Hirata Y, Yamada A, Koike K. Initial management for acute lower gastrointestinal bleeding. World J Gastroenterol. 2019;25:69-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (4)] |
| 8. | Whitehurst BD. Lower Gastrointestinal Bleeding. Surg Clin North Am. 2018;98:1059-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Gerson LB, Fidler JL, Cave DR, Leighton JA. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol. 2015;110:1265-87; quiz 1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 10. | Oakland K. Changing epidemiology and etiology of upper and lower gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2019;42-43:101610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | McPherson SJ, Sinclair MT, Smith NCE. Severe Gastrointestinal Haemorrhage: Summary of a National Quality of Care Study with Focus on Radiological Services. Cardiovasc Intervent Radiol. 2017;40:223-230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Orpen-Palmer J, Stanley AJ. Update on the management of upper gastrointestinal bleeding. BMJ Med. 2022;1:e000202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Triantafyllou K, Gkolfakis P, Gralnek IM, Oakland K, Manes G, Radaelli F, Awadie H, Camus Duboc M, Christodoulou D, Fedorov E, Guy RJ, Hollenbach M, Ibrahim M, Neeman Z, Regge D, Rodriguez de Santiago E, Tham TC, Thelin-Schmidt P, van Hooft JE. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:850-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 14. | Loffroy R, Favelier S, Pottecher P, Estivalet L, Genson PY, Gehin S, Cercueil JP, Krausé D. Transcatheter arterial embolization for acute nonvariceal upper gastrointestinal bleeding: Indications, techniques and outcomes. Diagn Interv Imaging. 2015;96:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Sengupta N, Feuerstein JD, Jairath V, Shergill AK, Strate LL, Wong RJ, Wan D. Management of Patients With Acute Lower Gastrointestinal Bleeding: An Updated ACG Guideline. Am J Gastroenterol. 2023;118:208-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 81] [Reference Citation Analysis (33)] |
| 16. | Wells ML, Hansel SL, Bruining DH, Fletcher JG, Froemming AT, Barlow JM, Fidler JL. CT for Evaluation of Acute Gastrointestinal Bleeding. Radiographics. 2018;38:1089-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 17. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:459-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 18. | Carney BW, Khatri G, Shenoy-Bhangle AS. The role of imaging in gastrointestinal bleed. Cardiovasc Diagn Ther. 2019;9:S88-S96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Di Serafino M, Iacobellis F, Schillirò ML, Dell'Aversano Orabona G, Martino A, Bennato R, Borzelli A, Oliva G, D'Errico C, Pezzullo F, Barbuto L, Ronza R, Ponticiello G, Corvino F, Giurazza F, Lombardi G, Niola R, Romano L. The Role of CT-Angiography in the Acute Gastrointestinal Bleeding: A Pictorial Essay of Active and Obscure Findings. Tomography. 2022;8:2369-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 20. | Cellina M, Cè M, Rossini N, Cacioppa LM, Ascenti V, Carrafiello G, Floridi C. Computed Tomography Urography: State of the Art and Beyond. Tomography. 2023;9:909-930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 21. | Newman C, Nandurkar R, Holcdorf D, Gerstenmaier J, Tagkalidis P, Clements W. Role of CT angiography and therapeutic anticoagulation in patients presenting to the emergency department with acute gastrointestinal bleeding. J Med Imaging Radiat Oncol. 2023;67:37-44. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Hsu M, Shah N, Bernal-Fernandez M, HonShideler C, Soto J, Anderson S, Ramalingam V. CTA measurements of acute lower gastrointestinal bleeding size predict subsequent positive catheter angiography. Abdom Radiol (NY). 2020;45:615-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Wu TC, Chen TY, Shiue YL, Chen JH, Hsieh TJ, Ko CC, Lin CP. Added value of delayed computed tomography angiography in primary intracranial hemorrhage and hematoma size for predicting spot sign. Acta Radiol. 2018;59:485-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Pini R, Faggioli G, Gargiulo M, Gallitto E, Cacioppa LM, Vacirca A, Pisano E, Pilato A, Stella A. The different scenarios of urgent carotid revascularization for crescendo and single transient ischemic attack. Vascular. 2019;27:51-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Karuppasamy K, Kapoor BS, Fidelman N, Abujudeh H, Bartel TB, Caplin DM, Cash BD, Citron SJ, Farsad K, Gajjar AH, Guimaraes MS, Gupta A, Higgins M, Marin D, Patel PJ, Pietryga JA, Rochon PJ, Stadtlander KS, Suranyi PS, Lorenz JM; Expert Panel on Interventional Radiology. ACR Appropriateness Criteria® Radiologic Management of Lower Gastrointestinal Tract Bleeding: 2021 Update. J Am Coll Radiol. 2021;18:S139-S152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: The Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 558] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 27. | Vacirca A, Faggioli G, Pini R, Gallitto E, Mascoli C, Cacioppa LM, Gargiulo M, Stella A. The Outcome of Technical Intraoperative Complications Occurring in Standard Aortic Endovascular Repair. Ann Vasc Surg. 2019;56:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 28. | Nozawa Y, Michimoto K, Ashida H, Baba A, Fukuda T, Ojiri H. Inferior vena cava diameter on CT angiography predicts mesenteric angiography positive for extravasation in colonic diverticular bleeding. Radiol Med. 2022;127:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Hsu MJ, Dinh DC, Shah NA, Bernal-Fernandez MC, Soto JA, Anderson SW, Ramalingam V. Time to conventional angiography in gastrointestinal bleeding: CT angiography compared to tagged RBC scan. Abdom Radiol (NY). 2020;45:307-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Hawks MK, Svarverud JE. Acute Lower Gastrointestinal Bleeding: Evaluation and Management. Am Fam Physician. 2020;101:206-212. [PubMed] |
| 31. | Muhammad A, Awais M, Sayani R, Saeed MA, Qamar S, Rehman A, Baloch NU. Empiric Transcatheter Arterial Embolization for Massive or Recurrent Gastrointestinal Bleeding: Ten-year Experience from a Single Tertiary Care Center. Cureus. 2019;11:e4228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16:3957-3963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Lau LHS, Sung JJY. Treatment of upper gastrointestinal bleeding in 2020: New techniques and outcomes. Dig Endosc. 2021;33:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Shi ZX, Yang J, Liang HW, Cai ZH, Bai B. Emergency transcatheter arterial embolization for massive gastrointestinal arterial hemorrhage. Medicine (Baltimore). 2017;96:e9437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Geffroy Y, Rodallec MH, Boulay-Coletta I, Jullès MC, Ridereau-Zins C, Zins M. Multidetector CT angiography in acute gastrointestinal bleeding: why, when, and how. Radiographics. 2011;31:E35-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Chikamatsu Y, Matsuda K, Takeuchi Y, Kagaya S, Ojima Y, Fukami H, Sato H, Saito A, Iwakura Y, Nagasawa T. Quantification of bleeding volume using computed tomography and clinical complications after percutaneous renal biopsy. Clin Kidney J. 2017;10:9-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Anderson SW, Soto JA, Lucey BC, Burke PA, Hirsch EF, Rhea JT. Blunt trauma: feasibility and clinical utility of pelvic CT angiography performed with 64-detector row CT. Radiology. 2008;246:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | P AP, Gadabanahalli K, Bhat V, N KKB. A novel concept on volumetric assessment and quantification of gastrointestinal bleed on computed tomography angiography: Observations based on a case series. Ann Hepatobiliary Pancreat Surg. 2021;25:160-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Dane B, Gupta A, Wells ML, Anderson MA, Fidler JL, Naringrekar HV, Allen BC, Brook OR, Bruining DH, Gee MS, Grand DJ, Kastenberg D, Khandelwal A, Sengupta N, Soto JA, Guglielmo FF. Dual-Energy CT Evaluation of Gastrointestinal Bleeding. Radiographics. 2023;43:e220192. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 40. | Cacioppa LM, Pini R, Longhi M, Vacirca A, Gallitto E, Faggioli G, Gargiulo M, Stella A. The Value of Carotid Endarterectomy as a Learning Tool for Trainees. Ann Vasc Surg. 2018;47:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Trabzonlu TA, Mozaffary A, Kim D, Yaghmai V. Dual-energy CT evaluation of gastrointestinal bleeding. Abdom Radiol (NY). 2020;45:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |