Published online Mar 28, 2024. doi: 10.4329/wjr.v16.i3.40
Peer-review started: December 27, 2023
First decision: January 5, 2024
Revised: January 19, 2024
Accepted: February 26, 2024
Article in press: February 26, 2024
Published online: March 28, 2024
Processing time: 90 Days and 3.7 Hours
Chronic pancreatitis (CP) is a fibroinflammatory disease characterized by irreversible destruction of pancreatic tissue. With the development of the disease, it may lead to exocrine and/or endocrine insufficiency. CP is one of the common diseases that cause abdominal pain, which will not get permanent spontaneous relief as the disease evolves. The American College of Gastroenterology clinical guidelines recommend computed tomography or magnetic resonance imaging as the first-line examination for the diagnosis of CP. CP common imaging findings include pancreatic atrophy, irregular dilatation of the pancreatic duct, calcification of pancreatic parenchyma, pancreatic duct stones, etc. In clinical practice, whether any correlations between CP-induced abdominal pain patterns (no pain/con
Core Tip: Chronic pancreatitis (CP) is a fibroinflammatory syndrome. On the one hand, pain is the most common clinical manifestation of CP. On the other hand, computed tomography (CT)/magnetic resonance imaging (MRI) is the most commonly used imaging examination for CP, and the American College of Gastroenterology clinical guidelines recommend CT or MRI as the first-line examination for the diagnosis of CP. However, there is no review on whether there is a cor
- Citation: Feng Y, Song LJ, Xiao B. Chronic pancreatitis: Pain and computed tomography/magnetic resonance imaging findings. World J Radiol 2024; 16(3): 40-48
- URL: https://www.wjgnet.com/1949-8470/full/v16/i3/40.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i3.40
Chronic pancreatitis (CP) is a fibroinflammatory syndrome caused by various causes. Long-term recurrent pancreatitis causes normal pancreatic parenchyma to be replaced by fibrotic tissues. With the development of the disease, pancreatic tissue and function undergo irreversible changes and destruction, which eventually lead to chronic abdominal pain, exocrine and endocrine pancreatic insufficiency[1,2].
The global incidence of CP is approximately 10 cases per 100,000 general population per year, and the incidence is increasing over time. Notably, the incidence of CP is twice as high in men as in women[3,4]. The general clinical manifestations of patients with CP are abdominal pain, nausea, vomiting, etc., among which abdominal pain is the most common (about 76% of CP patients)[5]. However, although it is not common, 10% of patients with CP are pain-free[6]. The pain manifestations of CP are highly variable and diverse, which can even be converted to each other. Unfortunately, the mechanism of pain is incompletely understood.
For the diagnosis of CP, the American College of Gastroenterology (ACG) clinical guidelines recommend computed tomography (CT) or magnetic resonance imaging (MRI) as the first-line examination[7].
The objective of the present review is to deeply investigate the pain, CT or MRI manifestations of CP patients, and to find out whether there are some change trends or relationships between pain and imaging findings, so as to build a cross-bridge between clinical manifestations and imaging manifestations of CP patients. Also, it can provide an imaging basis and foundation for the classification and diagnosis of abdominal pain types in clinical CP patients, which may improve the diagnostic accuracy and the prognosis of CP patients.
The first definition of CP was proposed in 1946[8], and a new mechanistically derived definition of CP was published by Whitcomb et al[9] in 2016, that is, CP is characterized by pathologic fibro-inflammatory syndrome of the pancreas in individuals with genetic, environmental and other risk factors such as hypercalcemia, hypertriglyceridemia, autoimmune disorders and so forth. Advanced CP is featured with pancreatic atrophy, fibrosis, chronic pain, ductal distortion and strictures, calcifications, dysplasia, pancreatic exocrine dysfunction, and endocrine dysfunction[9,10].
CP is a fibroinflammatory disease of the pancreas. Its pathophysiology is very complex and the pathogenesis is not completely understood. Although the mechanism of CP is complex, a large number of studies have shown a similar development trend, that is, various causes lead to progressive irreversible damage to the pancreatic parenchyma, and the pancreatic enzymes release following the injury of the exocrine tissue leading to inflammation. Long-term recurrent pancreatitis and injury can activate pancreatic stellate cells, leading to the formation of fibers and scars. Finally, the pancreas shrinks and hardens, resulting in exocrine and endocrine insufficiency of the pancreas[11-14].
The clinical manifestations of CP are mainly upper abdominal pain, which is complex and multimodal. The pain pattern varies according to the temporal nature and severity of CP. According to the temporal nature of pain, it can be divided into constant or intermittent pain[15]. According to the severity of pain, it can be divided into mild, moderate, or severe. Previous studies[10,16,17] used five types of pain patterns (A-E) (Table 1), and patients were required to choose from five pain patterns according to the type and severity of their pain. The most common pain pattern is the 'D' type, which is characterized by constant mild to moderate pain plus episodes of severe pain. In addition, in a retrospective study of 54 CP patients, Bahuva et al[18] found that the presence or absence of pain was not significantly related to the severity of CP structural changes, regardless of the structural changes. Therefore, this study showed that pancreatic morphological abnormalities had a poor predictive ability for CP pain.
| Pattern | Implication |
| A | Usually, pain-free with episodes of mild to moderate pain |
| B | Constant mild to moderate pain |
| C | Usually, pain-free with episodes of severe pain |
| D | Constant mild to moderate pain plus episodes of severe pain |
| E | Constant severe pain |
| Frequency of pain | |
| Intermittent | Pain pattern A or C |
| Constant | Pain pattern B, D or E |
| Severity of pain | |
| Mild-moderate | Pain pattern A or B |
| Severe | Pain pattern C, D or E |
CP not only have a variety of pain patterns but also have a complex mechanism of pain. Including multiple factors, such as pancreatitis; the pancreatic duct is compressed and narrowed due to stones or strictures, which may lead to duct hypertension or pancreatic ischemia and further cause pain; and complications such as pseudocysts, local inflammatory masses, duodenal and bile duct obstruction[19,20]. At present, the common CP pain assessment tools recommended by the international consensus guidelines include the Visual Analogue scale, Izbiki pain score, McGill Pain Questionnaire, and so on[21].
In the early years, some scholars[22,23] believed that the spontaneous relief of pain depended on the progressive development of CP, and believed that the pain of CP would improve with progressive pancreatic insufficiency (such as severe exocrine/endocrine insufficiency, severe duct abnormalities, more pancreatic calcification, etc.), especially for alcoholic CP, which is the so-called "burn-out" hypothesis.
However, most studies[16,24,25] have shown that the pain of CP did not seem to be significantly improved over time, and it was not uncommon for patients to experience pain recurrence during follow-up.
Combined with the study of Dimcevski et al[26], it has been shown that there are neurological components in CP-induced pain, and cortical reorganization is a pathogenesis of CP patients, indicating that the pain of CP may be largely independent of pancreatic fibrosis and progressive pancreatic insufficiency. The possible phenomenon is that as the disease progresses, the pain of CP patients may be alleviated, but it cannot be significantly relieved permanently. Therefore, waiting for spontaneous pain relief seems to be inaccurate and not worthy of praise.
The diagnosis of CP is to combine its clinical features with characteristics of CT or MRI, endoscopic ultrasonography (EUS), and pancreatic function examination[7,19]. Clinically, CT or MRI recommended by the ACG clinical guidelines are often used as the first-line examination for the diagnosis of CP[7]. The diagnostic criteria for CP can be traced back to the Cambridge classification of pancreatic morphology in 1984 (Table 2)[27], and the M-Annheim classification of CP proposed by Schneider et al[14] in 2007 (Table 3), as detailed in the tables.
| To evaluate chronic pancreatitis from the aspects of pancreatic parenchyma, pancreatic duct morphology, local changes, and so on by CT and US | ||
| Normal | Quality study visualising the whole gland without abnormal features | |
| Equivocal | One sign only | Main duct enlarged (< 4 mm) |
| Gland enlarged (up to 2 × normal) | ||
| Cavities (< 10 mm) | ||
| Irregular ducts | ||
| Focal acute pancreatitis | ||
| Parenchymal heterogeneity | ||
| Mild changes | Two or more signs | Duct wall echoes increased |
| Moderate changes | Irregular head/body contour | |
| Marked changes | As above, and with one or more of: Large cavities (> 10 mm), gross gland enlargement (> 2 × normal), intraductal filling defects or calculi, duct obstruction, structure or gross irregularity, contiguous organ invasion | |
| At least one of the following four items should be met |
| Pancreatic calcifications |
| Moderate or marked ductal lesions (according to the Cambridge classification) |
| Marked and persistent exocrine insufficiency defined as pancreatic steatorrhea markedly reduced by enzyme supplementation |
| Typical histology of an adequate histological specimen |
The progression of CP can be divided into early and advanced stages. The early stage especially refers to the period before the development of morphological changes in the pancreas. Because the morphology of the pancreas is mostly normal at this time, it is difficult to diagnose by conventional imaging methods. In the advanced stage, the morphology of the pancreas changes, which often manifests as pancreatic atrophy, pancreatic parenchyma calcification, irregular dilatation and distortion of the pancreatic duct, intraductal calculi, etc., and it can also be accompanied by complications, such as pseudocyst, common bile duct stricture, duodenal obstruction, etc. These manifestations can be detected by conventional radiological imaging (CT or MRI)[28]. CT scan is easily available, noninvasive, and relatively cheaper compared to other modalities. MRI is superior to CT for the evaluation of pancreatic parenchymal and ductal changes, with better resolution than CT, but it is more expensive and requires more time.
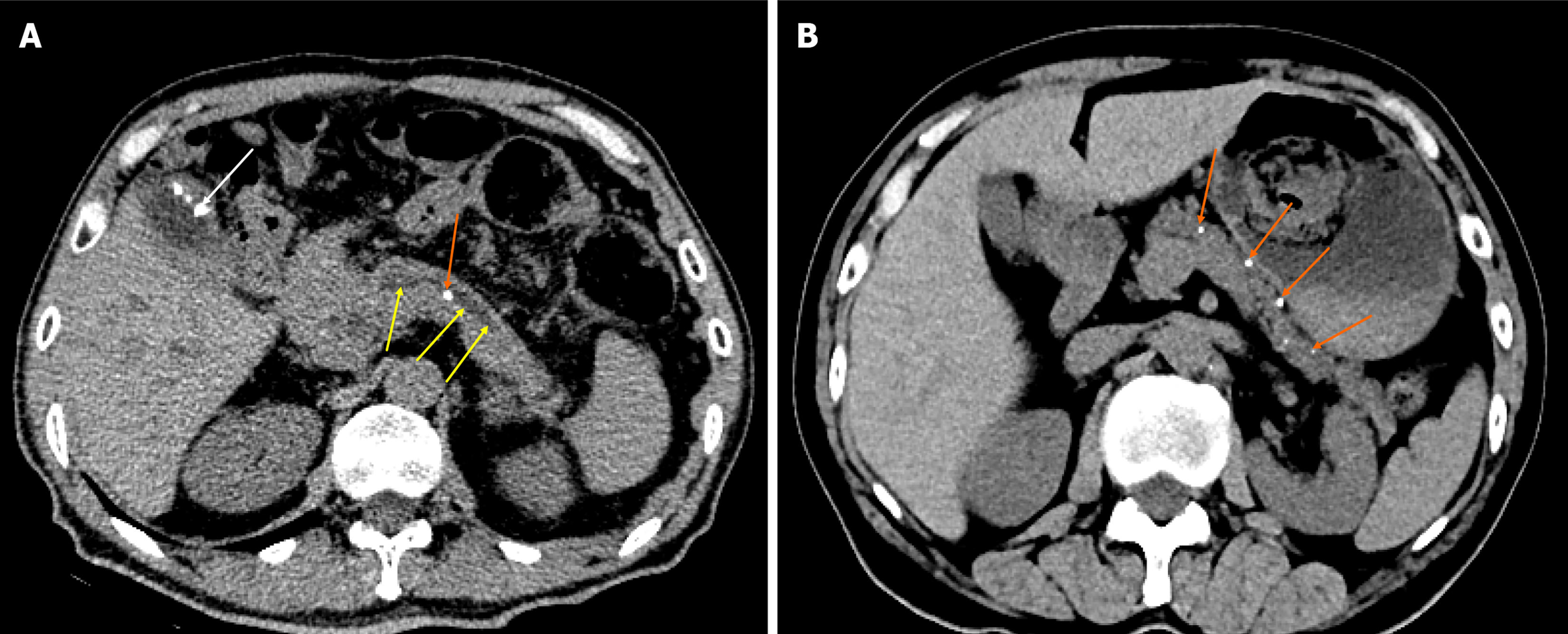
Three common CT findings of CP are pancreatic ductal dilatation (68%; Figure 1A), parenchymal atrophy (54%; Figure 1B), and pancreatic calcification (50%; Figure 1B)[19]. MRI shows pancreatic duct stones better than CT, while CT shows calcification better than MRI.
In recent years, most of the literature and guidelines[29,30] emphasize the importance of early diagnosis of CP, and achieve the purpose of early detection and early diagnosis before irreversible changes in the pancreas, so as to avoid late complications and improve the prognosis and clinical outcomes of CP patients.
Early diagnosis of pancreatic fibrosis can provide a valuable opportunity to prevent disease progression[11]. Khatkov et al[11] believed that multidetector CT (MDCT) can be used as a non-invasive diagnostic tool for detecting pancreatic fibrosis, and its post-processing indicators are related to the degree of pancreatic fibrosis, so it is expected to be used for early diagnosis of CP. The results of a study involving 74 patients showed that compared with mild pancreatic fibrosis, the normalized contrast enhancement ratio during the venous phases and the pancreatic late/early attenuation ratio increased in patients with severe pancreatic fibrosis, while the unenhanced pancreatic density decreased. Therefore, the application value of MDCT in the early diagnosis of CP is further verified.
A prospective study by Liu et al[31] showed that pancreatic stiffness and T1 relaxation time in multimodal functional MRI can be used as independent predictors of pancreatic fibrosis grading and showed significant associations with fibrosis extent. And substantial changes in mild CP can be detected by T1-mapping, which is manifested by a significant increase in the T1 relaxation time of pancreatic parenchyma in patients with mild CP[32]. Therefore, T1-mapping can also be used as a new MRI technique to evaluate early CP. In addition, magnetic resonance elastography (MRE)-derived stiffness, which can be used to measure tissue mechanical properties and provide information on soft tissue stiffness in vivo, is the highest diagnostic indicator for detecting CP without secretory insufficiency (reflecting early CP), and there is no difference in the diagnostic effect between T1 relaxation time and MRE-derived stiffness[28]. Furthermore, the research of Wang et al[33] showed that multitasking dynamic contrast enhanced MRI technology can not only achieve early detection and early diagnosis of CP because changes in microcirculation characteristics are usually preceded by morphological changes, but also it can identify and diagnose CP and pancreatic ductal adenocarcinoma by estimating microcirculation parameters (such as tissue plasma flow, fractional plasma volume, transfer constant, etc.).
Before this, there were many studies on the correlation between the pain of CP and its imaging morphological manifestations, but they were scattered and not concentrated. On one hand, pain is the most common clinical manifestation of CP. On the other hand, CT/MRI is the most commonly used imaging examination for CP. There is no review to summarize the correlation and connection between the two. Here, it is summarized in the form of tables (Table 4) and text, in order to find something valuable.
| Ref. | n | Research type | Imaging techniques | Results |
| Bornman et al[34], 1980 | 47 | A prospective study | ERCP | The incidence of pancreatic duct obstruction or stricture in patients with painless and painful CP was comparable, indicating that the morphological changes of the pancreatic duct are not related to the occurrence of pain |
| Jensen et al[35], 1984 | 101 | A comparative study | ERP | There was no correlation between the degree of pain in CP (no pain, light pain, moderate pain, severe pain) and the dilatation of the main pancreatic duct measured by ERP (the diameter of the main pancreatic duct in the pancreatic body exceeding 5 mm was defined as dilatation) |
| Malfertheiner et al [37], 1987 | 64 | A prospective study | CT/ERP | There was a poor correlation between the severity of pain and abdominal imaging features in CP patients, but it was also found that patients with large pancreatic cysts were most often associated with severe pain (62%), while enlargement of pancreatic gland, small cysts, and duct dilatation were roughly the same as different degrees of pain. Most (89%) patients with calcification still had pain and some of them (39%) showed severe pain |
| Morgan et al[36], 2003 | 25 | A retrospective study | ERCP | There was a poor correlation between the morphological changes of the main pancreatic duct (whether the duct was dilated or blocked) and pain |
| Mullady et al[16], 2011 | 414 | A prospective cohort study | CT/ERCP | The duration of disease in CP patients was not related to either the frequency of pain (intermittent vs constant) or the severity of pain (mild, moderate or severe) |
| Bahuva et al[18], 2013 | 54 | A retrospective study | CT/MRCP/EUS | The presence or absence of visceral pain is not significantly related to the severity of CP structural changes, whether the structural changes are severe, mild, or absent |
| Frøkjær et al[39], 2013 | 40 | A controlled study | MRCP/DWI | The pancreatic pathological imaging findings of the fibrotic changes as well as atrophy and ductal pathology were not associated with pain |
| Wilcox et al[17], 2015 | 518 | A retrospective study | CT/MRI/MRCP/EUS/ERCP | CP patients with different pain patterns, temporal nature of pain (no pain, intermittent, constant) or pain severity (no pain, mild-moderate, severe) were very similar in the distribution of imaging findings |
| Madzak et al[2], 2017 | 82 | A prospective cohort study | s-MRI | There was no correlation between pancreatic parenchyma and ductal changes, pain severity, and pain interference scores |
It can be traced back to a study of 47 patients with CP by Bornman et al[34] in 1980. The ERCP examination technique used in the study showed that the incidence of pancreatic duct obstruction or stricture was comparable between painless and painful CP patients, indicating that the morphological changes of the pancreatic duct were not related to the occurrence of pain. Along this line, it can be found in subsequent studies that Jensen et al[35] found there was no correlation between the expansion of the main pancreatic duct and the severity of pain in patients with CP in a comparative study of 101 patients. Similarly, Morgan et al[36] also confirmed that in patients with CP, whether the main pancreatic duct was dilated or blocked, the pain performance was similar. Therefore, it is not hard to see that there is no significant correlation between the presence or severity of pain in CP patients and the morphological changes (dilatation or stricture) of the pancreatic duct.
In addition, further studies[18] also found that there was no significant correlation between the presence or absence of abdominal pain and the severity of CP morphological structural changes. A retrospective study of 518 CP patients was conducted by Wilcox et al[17] using CT/MRI/MRCP and other imaging methods. It was found that CP patients with different pain patterns, temporal nature of pain (no pain, intermittent, constant), or severity of pain (no pain, mild-moderate, severe) were very similar in the distribution of imaging findings.
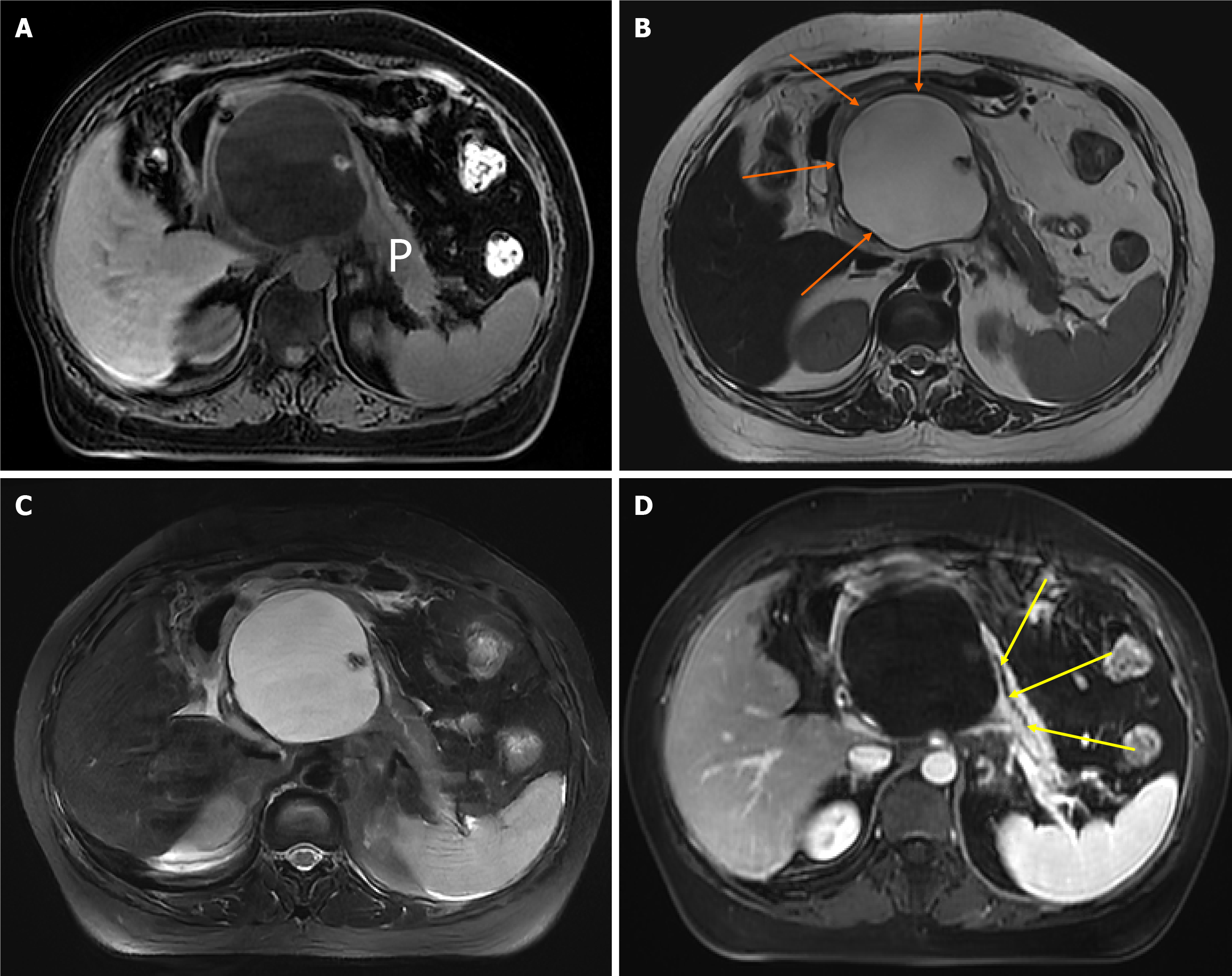
In summary, the severity and duration of abdominal pain symptoms caused by CP are not significantly correlated with the degree of damage to pancreatic anatomical structures (such as pancreatic parenchymal atrophy, pancreatic duct dilatation or stricture, and pancreatic parenchymal fibrosis). However, although the correlation between pain and imaging morphological changes is poor, some other meaningful things have been found. For example, CP patients with large pancreatic cysts are most often associated with severe pain (Figure 2) The correlation between pancreatic en
Moreover, it was found that the duration of disease in CP patients was not related to either the frequency of pain (intermittent vs constant) or the severity of pain (mild, moderate, or severe)[16]. Combined with CP-induced pancreatic neural alterations, such as intrapancreatic nerves increase in size (neural hypertrophy) and number (increased neural density), related studies have shown that these neural alterations are related to the severity of neuropathic pain. Therefore, the neural alterations of CP are active shapers of disease evolution and progression[38]. This means that a series of pancreatic morphological imaging findings (such as parenchymal atrophy and pancreatic duct dilatation) with the progression of CP may not be related to the severity of pain in patients, and the presence or absence of visceral pain has nothing to do with the severity of CP because most of the pain drivers of advanced CP are neurological rather than pancreatic. Therefore, it is not difficult to explain why the correlation between pain symptoms and imaging findings is poor[18,39].
In summary, CP is a fibroinflammatory syndrome caused by a variety of causes. The most common clinical manifestation is abdominal pain and the mechanism of abdominal pain is not fully understood. CT/MRI is usually used as the first-line imaging diagnosis of CP. The duration and severity of abdominal pain caused by CP are poorly correlated with pancreatic imaging morphological changes. There is a correlation between pain caused by CP and neural alterations and related complications. Therefore, the next research should further explore the relationship between neural alterations or related complications caused by CP and pain, in order to have a deeper understanding of CP.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Machado MC, Brazil S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499-512. [PubMed] [DOI] [Full Text] |
| 2. | Madzak A, Olesen SS, Lykke Poulsen J, Bolvig Mark E, Mohr Drewes A, Frøkjær JB. MRI assessed pancreatic morphology and exocrine function are associated with disease burden in chronic pancreatitis. Eur J Gastroenterol Hepatol. 2017;29:1269-1275. [PubMed] [DOI] [Full Text] |
| 3. | Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, Petrov MS. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45-55. [PubMed] [DOI] [Full Text] |
| 4. | Petrov MS, Yadav D. Global epidemiology and holistic prevention of pancreatitis. Nat Rev Gastroenterol Hepatol. 2019;16:175-184. [PubMed] [DOI] [Full Text] |
| 5. | Thompson BS, Philcox S, Devereaux B, Metz AJ, Croagh D, Gray A, Hamarneh Z, Windsor JA, Neale RE. Prodromal Signs and Symptoms of Chronic Pancreatitis: A Systematic Review. J Clin Gastroenterol. 2022;56:e1-e10. [PubMed] [DOI] [Full Text] |
| 6. | Cohen SM, Kent TS. Etiology, Diagnosis, and Modern Management of Chronic Pancreatitis: A Systematic Review. JAMA Surg. 2023;158:652-661. [PubMed] [DOI] [Full Text] |
| 7. | Gardner TB, Adler DG, Forsmark CE, Sauer BG, Taylor JR, Whitcomb DC. ACG Clinical Guideline: Chronic Pancreatitis. Am J Gastroenterol. 2020;115:322-339. [PubMed] [DOI] [Full Text] |
| 8. | Comfort MW, Gambill EE, Baggenstoss AH. Chronic relapsing pancreatitis; a study of 29 cases without associated disease of the biliary or gastrointestinal tract. Gastroenterology. 1946;6:376-408. [PubMed] |
| 9. | Whitcomb DC, Frulloni L, Garg P, Greer JB, Schneider A, Yadav D, Shimosegawa T. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218-224. [PubMed] [DOI] [Full Text] |
| 10. | Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA. 2019;322:2422-2434. [PubMed] [DOI] [Full Text] |
| 11. | Khatkov IE, Bordin DS, Lesko KA, Dubtsova EA, Karnaukhov NS, Kiriukova MA, Makarenko NV, Dorofeev AS, Savina IV, Salimgereeva DA, Shurygina EI, Vinokurova LV. Contrast-Enhanced Computed Tomography and Laboratory Parameters as Non-Invasive Diagnostic Markers of Pancreatic Fibrosis. Diagnostics (Basel). 2023;13. [PubMed] [DOI] [Full Text] |
| 12. | Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, Mayerle J, Drewes AM, Rebours V, Akisik F, Muñoz JED, Neoptolemos JP. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. [PubMed] [DOI] [Full Text] |
| 13. | Apte M, Pirola RC, Wilson JS. Pancreatic stellate cell: physiologic role, role in fibrosis and cancer. Curr Opin Gastroenterol. 2015;31:416-423. [PubMed] [DOI] [Full Text] |
| 14. | Schneider A, Löhr JM, Singer MV. The M-Annheim classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101-119. [PubMed] [DOI] [Full Text] |
| 15. | Tuck NL, Teo K, Kuhlmann L, Olesen SS, Johnson M, Bean DJ, Rashid U, MacCormick AD, Srikumar G, Drewes AM, Windsor JA. Pain patterns in chronic pancreatitis and chronic primary pain. Pancreatology. 2022;22:572-582. [PubMed] [DOI] [Full Text] |
| 16. | Mullady DK, Yadav D, Amann ST, O'Connell MR, Barmada MM, Elta GH, Scheiman JM, Wamsteker EJ, Chey WD, Korneffel ML, Weinman BM, Slivka A, Sherman S, Hawes RH, Brand RE, Burton FR, Lewis MD, Gardner TB, Gelrud A, DiSario J, Baillie J, Banks PA, Whitcomb DC, Anderson MA; NAPS2 Consortium. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut. 2011;60:77-84. [PubMed] [DOI] [Full Text] |
| 17. | Wilcox CM, Yadav D, Ye T, Gardner TB, Gelrud A, Sandhu BS, Lewis MD, Al-Kaade S, Cote GA, Forsmark CE, Guda NM, Conwell DL, Banks PA, Muniraj T, Romagnuolo J, Brand RE, Slivka A, Sherman S, Wisniewski SR, Whitcomb DC, Anderson MA. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol. 2015;13:552-60; quiz e28. [PubMed] [DOI] [Full Text] |
| 18. | Bahuva R, Walsh RM, Kapural L, Stevens T. Morphologic abnormalities are poorly predictive of visceral pain in chronic pancreatitis. Pancreas. 2013;42:6-10. [PubMed] [DOI] [Full Text] |
| 19. | Mann R, Boregowda U, Vyas N, Gajendran M, Umapathy CP, Sayana H, Echavarria J, Patel S, Saligram S. Current advances in the management of chronic pancreatitis. Dis Mon. 2021;67:101225. [PubMed] [DOI] [Full Text] |
| 20. | Anderson MA, Akshintala V, Albers KM, Amann ST, Belfer I, Brand R, Chari S, Cote G, Davis BM, Frulloni L, Gelrud A, Guda N, Humar A, Liddle RA, Slivka A, Gupta RS, Szigethy E, Talluri J, Wassef W, Wilcox CM, Windsor J, Yadav D, Whitcomb DC. Mechanism, assessment and management of pain in chronic pancreatitis: Recommendations of a multidisciplinary study group. Pancreatology. 2016;16:83-94. [PubMed] [DOI] [Full Text] |
| 21. | Drewes AM, van Veldhuisen CL, Bellin MD, Besselink MG, Bouwense SA, Olesen SS, van Santvoort H, Vase L, Windsor JA. Assessment of pain associated with chronic pancreatitis: An international consensus guideline. Pancreatology. 2021;21:1256-1284. [PubMed] [DOI] [Full Text] |
| 22. | Ammann RW, Akovbiantz A, Largiader F, Schueler G. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology. 1984;86:820-828. [PubMed] |
| 23. | Ammann RW, Buehler H, Muench R, Freiburghaus AW, Siegenthaler W. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas. 1987;2:368-377. [PubMed] [DOI] [Full Text] |
| 24. | Lankisch PG, Löhr-Happe A, Otto J, Creutzfeldt W. Natural course in chronic pancreatitis. Pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54:148-155. [PubMed] [DOI] [Full Text] |
| 25. | Lankisch PG, Seidensticker F, Löhr-Happe A, Otto J, Creutzfeldt W. The course of pain is the same in alcohol- and nonalcohol-induced chronic pancreatitis. Pancreas. 1995;10:338-341. [PubMed] [DOI] [Full Text] |
| 26. | Dimcevski G, Sami SA, Funch-Jensen P, Le Pera D, Valeriani M, Arendt-Nielsen L, Drewes AM. Pain in chronic pancreatitis: the role of reorganization in the central nervous system. Gastroenterology. 2007;132:1546-1556. [PubMed] [DOI] [Full Text] |
| 27. | Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756-759. [PubMed] [DOI] [Full Text] |
| 28. | Olesen SS, Steinkohl E, Hansen TM, Drewes AM, Frøkjær JB. Single- and multiparameter magnetic resonance imaging for diagnosing and severity grading of chronic pancreatitis. Abdom Radiol (NY). 2023;48:630-641. [PubMed] [DOI] [Full Text] |
| 29. | Whitcomb DC, Shimosegawa T, Chari ST, Forsmark CE, Frulloni L, Garg P, Hegyi P, Hirooka Y, Irisawa A, Ishikawa T, Isaji S, Lerch MM, Levy P, Masamune A, Wilcox CM, Windsor J, Yadav D, Sheel A, Neoptolemos JP; Working Group for the International (IAP – APA – JPS – EPC) Consensus Guidelines for Chronic Pancreatitis. International consensus statements on early chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with The International Association of Pancreatology, American Pancreatic Association, Japan Pancreas Society, PancreasFest Working Group and European Pancreatic Club. Pancreatology. 2018;18:516-527. [PubMed] [DOI] [Full Text] |
| 30. | Shimizu K, Ito T, Irisawa A, Ohtsuka T, Ohara H, Kanno A, Kida M, Sakagami J, Sata N, Takeyama Y, Tahara J, Hirota M, Fujimori N, Masamune A, Mochida S, Enomoto N, Shimosegawa T, Koike K. Evidence-based clinical practice guidelines for chronic pancreatitis 2021. J Gastroenterol. 2022;57:709-724. [PubMed] [DOI] [Full Text] |
| 31. | Liu C, Shi Y, Lan G, Xu Y, Yang F. Evaluation of Pancreatic Fibrosis Grading by Multiparametric Quantitative Magnetic Resonance Imaging. J Magn Reson Imaging. 2021;54:1417-1429. [PubMed] [DOI] [Full Text] |
| 32. | Tirkes T, Lin C, Fogel EL, Sherman SS, Wang Q, Sandrasegaran K. T(1) mapping for diagnosis of mild chronic pancreatitis. J Magn Reson Imaging. 2017;45:1171-1176. [PubMed] [DOI] [Full Text] |
| 33. | Wang N, Gaddam S, Xie Y, Christodoulou AG, Wu C, Ma S, Fan Z, Wang L, Lo S, Hendifar AE, Pandol SJ, Li D. Multitasking dynamic contrast enhanced magnetic resonance imaging can accurately differentiate chronic pancreatitis from pancreatic ductal adenocarcinoma. Front Oncol. 2022;12:1007134. [PubMed] [DOI] [Full Text] |
| 34. | Bornman PC, Marks IN, Girdwood AH, Clain JE, Narunsky L, Clain DJ, Wright JP. Is pancreatic duct obstruction or stricture a major cause of pain in calcific pancreatitis? Br J Surg. 1980;67:425-428. [PubMed] [DOI] [Full Text] |
| 35. | Jensen AR, Matzen P, Malchow-Møller A, Christoffersen I. Pattern of pain, duct morphology, and pancreatic function in chronic pancreatitis. A comparative study. Scand J Gastroenterol. 1984;19:334-338. [PubMed] |
| 36. | Morgan DE, Smith JK, Hawkins K, Wilcox CM. Endoscopic stent therapy in advanced chronic pancreatitis: relationships between ductal changes, clinical response, and stent patency. Am J Gastroenterol. 2003;98:821-826. [PubMed] [DOI] [Full Text] |
| 37. | Malfertheiner P, Büchler M, Stanescu A, Ditschuneit H. Pancreatic morphology and function in relationship to pain in chronic pancreatitis. Int J Pancreatol. 1987;2:59-66. [PubMed] [DOI] [Full Text] |
| 38. | Demir IE, Friess H, Ceyhan GO. Neural plasticity in pancreatitis and pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2015;12:649-659. [PubMed] [DOI] [Full Text] |