Published online Nov 28, 2024. doi: 10.4329/wjr.v16.i11.657
Revised: September 6, 2024
Accepted: September 12, 2024
Published online: November 28, 2024
Processing time: 163 Days and 0.3 Hours
Patent foramen ovale (PFO)-related right-to-left shunts (RLSs) have been impli
To investigate the accuracy of multimodal ultrasound to improve diagnostic efficiency in detecting PFO-related RLSs.
We prospectively enrolled four patients with cryptogenic stroke (n = 1), migraine (n = 2), and unexplained dizziness (n = 1) who underwent synchronized cTCD combined with cTTE. The participants were monitored and followed-up for 24 months.
cTTE identified moderate and large RLSs in patients with recurrent cryptogenic stroke and migraines, whereas cTCD revealed only small RLSs. Moderate and large RLS were confirmed on combined cTTE and cTCD. After excluding other causes, both patients underwent PFO occlusion. At 21- and 24-month follow-up examinations, neither stroke nor migraine had recurred. cTTE revealed a small RLS in a third patient with unexplained dizziness and a fourth patient with migraines; however, simultaneous cTCD detected a large RLS. These patients did not undergo interventional occlusion, and dizziness and headache recurred at the 17- and 24-month follow-up examinations.
Using cTTE or cTCD may underestimate RLS, impairing risk assessments. Combining synchronized cTCD with cTTE could enhance testing accuracy and support better diagnostic and therapeutic decisions.
Core Tip: Synchronized multimodal ultrasonography, combining contrast transcranial Doppler (cTCD) with contrast transthoracic echocardiography (cTTE), enhances the accuracy of right-to-left shunt (RLS) detection in patients with cryptogenic stroke, migraine, and dizziness. Traditional methods relying solely on cTCD or cTTE may result in false-negative or underestimated RLS results. Synchronized testing offers a more comprehensive assessment, enabling the identification of inherently large RLS and supporting precise etiological analyses. Incorporating synchronized multimodal ultrasonography into clinical practice can enhance patient outcomes by facilitating more accurate diagnosis and informed treatment decision-making in individuals with cryptogenic stroke, migraine, and dizziness.
- Citation: Yao MJ, Zhao YY, Deng SP, Xiong HH, Wang J, Ren LJ, Cao LM. Right-to-left shunt detection via synchronized contrast transcranial Doppler combined with contrast transthoracic echocardiography: A preliminary study. World J Radiol 2024; 16(11): 657-667
- URL: https://www.wjgnet.com/1949-8470/full/v16/i11/657.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i11.657
Right-to-left shunt (RLS), caused by a patent foramen ovale (PFO), accounts for > 90% of RLS cases[1] and has been implicated in cryptogenic stroke[2], transient ischemic attack[3], unexplained dizziness[4,5], and migraine with aura[6,7]. The larger the RLS, the higher the risk of these events[8,9]. Accurate detection of RLS is crucial to determine the cause and guide treatment decisions. Currently, contrast transcranial Doppler (cTCD) and contrast transthoracic echocardiography (cTTE) are the primary methods to screen for PFO[10]. cTCD is more sensitive than cTTE in detecting RLS, making it the preferred initial screening tool for PFO and RLS[11,12]. However, Liu et al[13] proposed that cTCD should not be used as the sole screening method for PFO, suggesting that additional techniques, such as cTTE and contrast transesophageal ultrasonography (TEE), should be employed. The accuracy of cTCD or cTTE results can be affected by factors such as the acoustic window, preparation of the contrast saline solution, execution of the Valsalva maneuver (VM), and examiner expertise. Therefore, relying only on cTTE or cTCD for RLS detection can result in underestimation or false-negative results. The quality of test results is vital for accurate etiological assessment and treatment planning. To enhance the accuracy and reliability of RLS detection, a combination of non-synchronous cTCD and cTTE has been implemented[14,15]. However, this method was shown to be inefficient. Nonsynchronous testing requires patients to undergo multiple contrast echocardiography sessions, which increases costs, risks, and hospitalization duration. We propose the adoption of synchronous multimodal ultrasound for more efficient and precise RLS detection. Synchronous multimodal ultrasound offers complementary benefits through mutual synergistic interactions and result verification and may accurately assess RLS and enhance diagnostic efficiency[15,16].
The synchronized use of cTCD and cTTE may offer benefits that exceed the advantages of each method used individually. Therefore, we aimed to assess the accuracy of synchronized multimodal ultrasound (cTCD combined with cTTE) in detecting RLS. This study addresses a significant literature gap by enhancing diagnostic efficiency through a synchronized approach that has been previously underexplored.
We performed synchronized cTCD combined with cTTE in four patients to assess its impact on etiological analysis and treatment decision-making. Multimodal ultrasonography involves the participant receiving cTCD and cTTE simultaneously, with shared ultrasound contrast. This study was approved by the Ethics Review Board of the First Affiliated Hospital of Shenzhen University (Approval No. 20220413006-XZ2022) and performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its subsequent amendments. Written informed consent was obtained from the participants.
We prospectively recruited four patients admitted with cryptogenic stroke, migraine, and dizziness at the First Affiliated Hospital of Shenzhen University from February to August 2020. Diagnoses of cryptogenic stroke[17] and migraine[18] were based on relevant criteria. The inclusion criteria were age 18–80 years and satisfactory image quality during multimodal ultrasonography. The exclusion criteria were congenital heart diseases, such as atrial and ventricular septal defects, and inability to perform VM.
For the contrast saline solution, we used a mixture of saline solution (8 mL), air (1 mL), and blood (1 mL). The resulting fluid was vigorously stirred at least 20 times in two 10-mL syringes using a sterile three-way connector[16]. Subsequently, the contrast saline solution bolus was rapidly injected into the left antecubital vein for 2–3 s using an 18-G needle to ensure that it reached the right atrium[19]. The solution was injected following VM initiation[20].
The participants were trained on VM performance before the tests. They were instructed to blow into a specialized mouthpiece connected to a manometer and sustain a pressure of 40 mmHg for at least 5 s. Simultaneously, we evaluated VM effectiveness by measuring the middle cerebral artery peak systolic velocity, which should decrease by at least 25% compared to the baseline value[21].
cTCD was performed using a TCD machine (Doppler-BoxX; Compumedics, Singen, Germany) equipped with a 2-MHz probe, which supported single-probe and double-depth monitoring. The participants were placed in a left-lateral posture, and the blood flow in the middle cerebral artery was observed through the temporal window. If the sound transmission in the temporal window was inadequate, a suboccipital window was selected to monitor the vertebral or basilar arteries. The contrast saline solution was administered at rest and during the two VMs.
The grading criteria for RLS in cTCD were as follows[22]: Grade 0 (negative), no microbubbles; Grade I, small shunt (1–10 microbubbles); Grade II, moderate shunt (10–30 microbubbles, but no curtain); and Grade III, large shunt (> 30 microbubbles with a curtain pattern). The RLS grade was determined based on the highest microbubble count observed during cTCD.
cTTE was performed using an EPIQ 7C Color Doppler Ultrasound (Philips Healthcare, Best, Netherlands) equipped with a 1.0–5.0-MHz probe. TTE was performed to exclude congenital heart disease before administration of the ultrasound contrast agent. The apical four-chamber view was continuously recorded during the contrast injections. The grading criteria for RLS in cTTE were as follows[22]: Grade 0 (negative) = no microbubbles; Grade I = 1–10 microbubbles; Grade II = 11–30 microbubbles; Grade III = more than 30 microbubbles, with the left atrium nearly filled with microbubbles, or the presence of left atrial opacity. The RLS grade was determined based on the highest number of microbubbles observed in the left atrium.
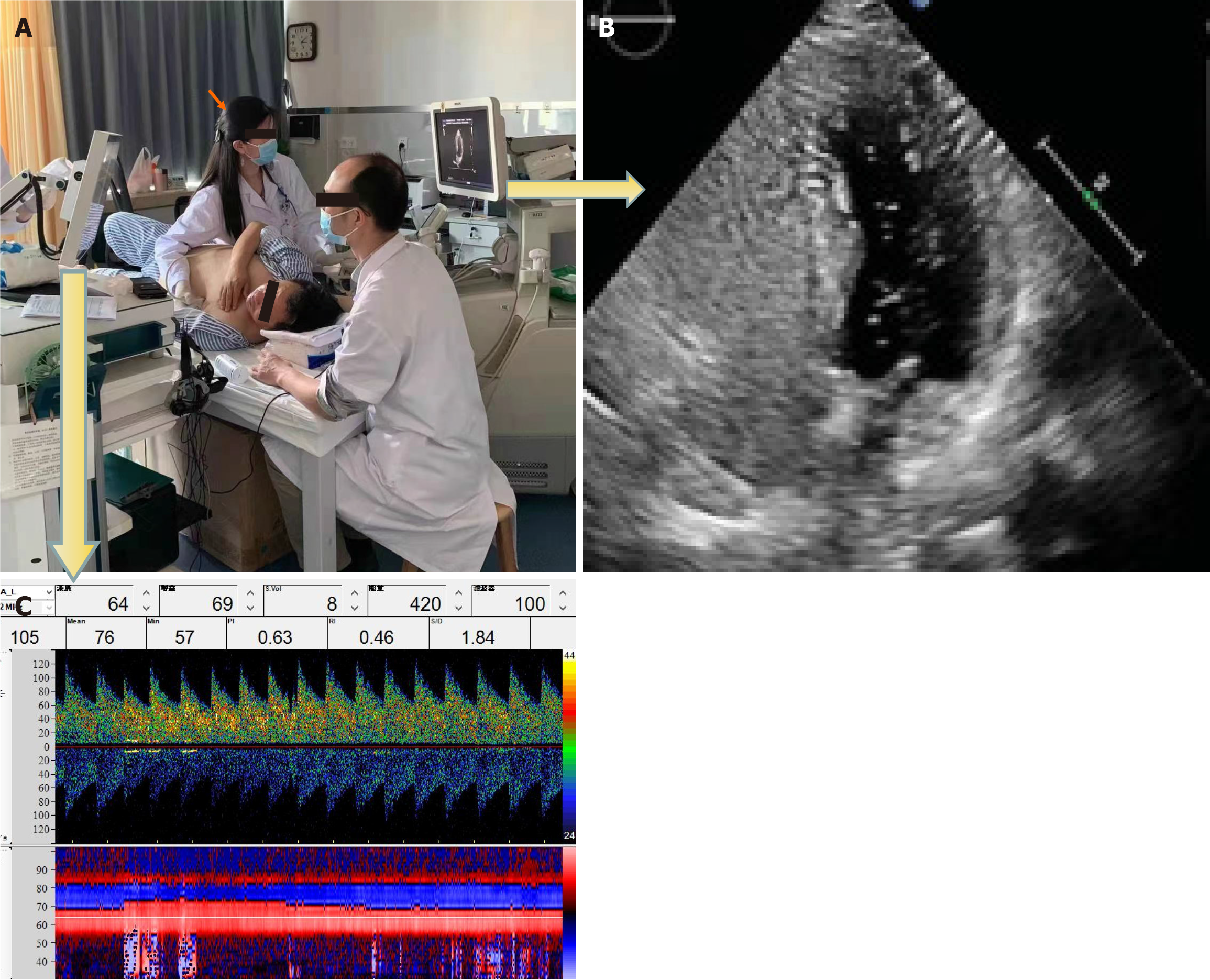
The cTCD and cTTE results were evaluated by two experienced technicians who were blinded to each other’s results. The technicians were not permitted to view or inquire concerning the other party’s test results until test completion. We simultaneously conducted cTCD and cTTE during the same contrast-enhanced ultrasound to observe the microbubble signals in the brain and heart using TCD and TTE, respectively. Hence, the cTCD and cTTE shared the same contrast saline solution, posture, and VM (Figure 1). The final outcome of synchronized joint testing was based on the highest number of microemboli signals identified.
A 39-year-old man was admitted because of numbness in his right upper limb persisting for 7 months, accompanied by unclear speech for 5 months, with worsening symptoms intensifying over the previous month. His medical history included renal calculi, but no hereditary diseases.
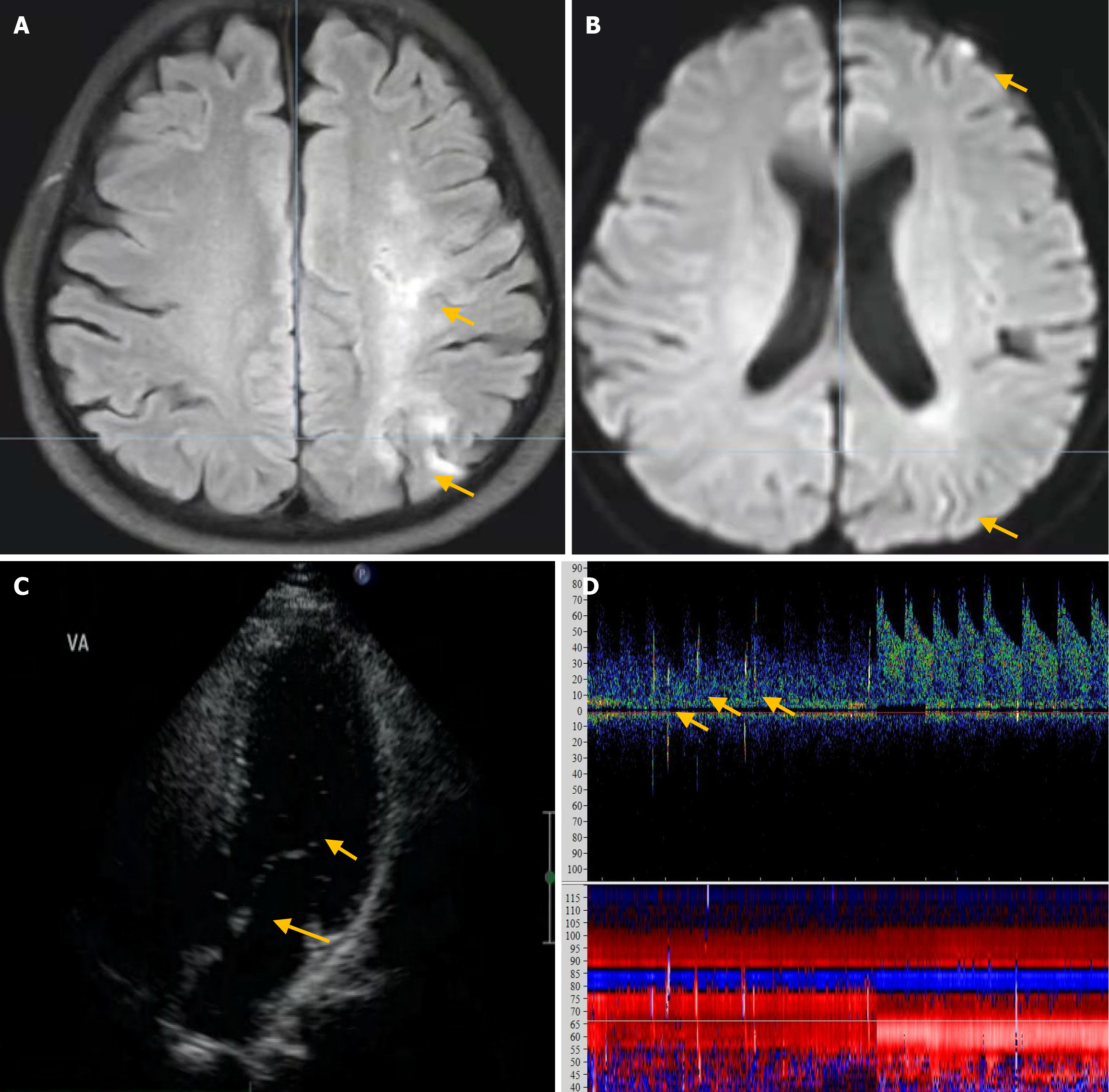
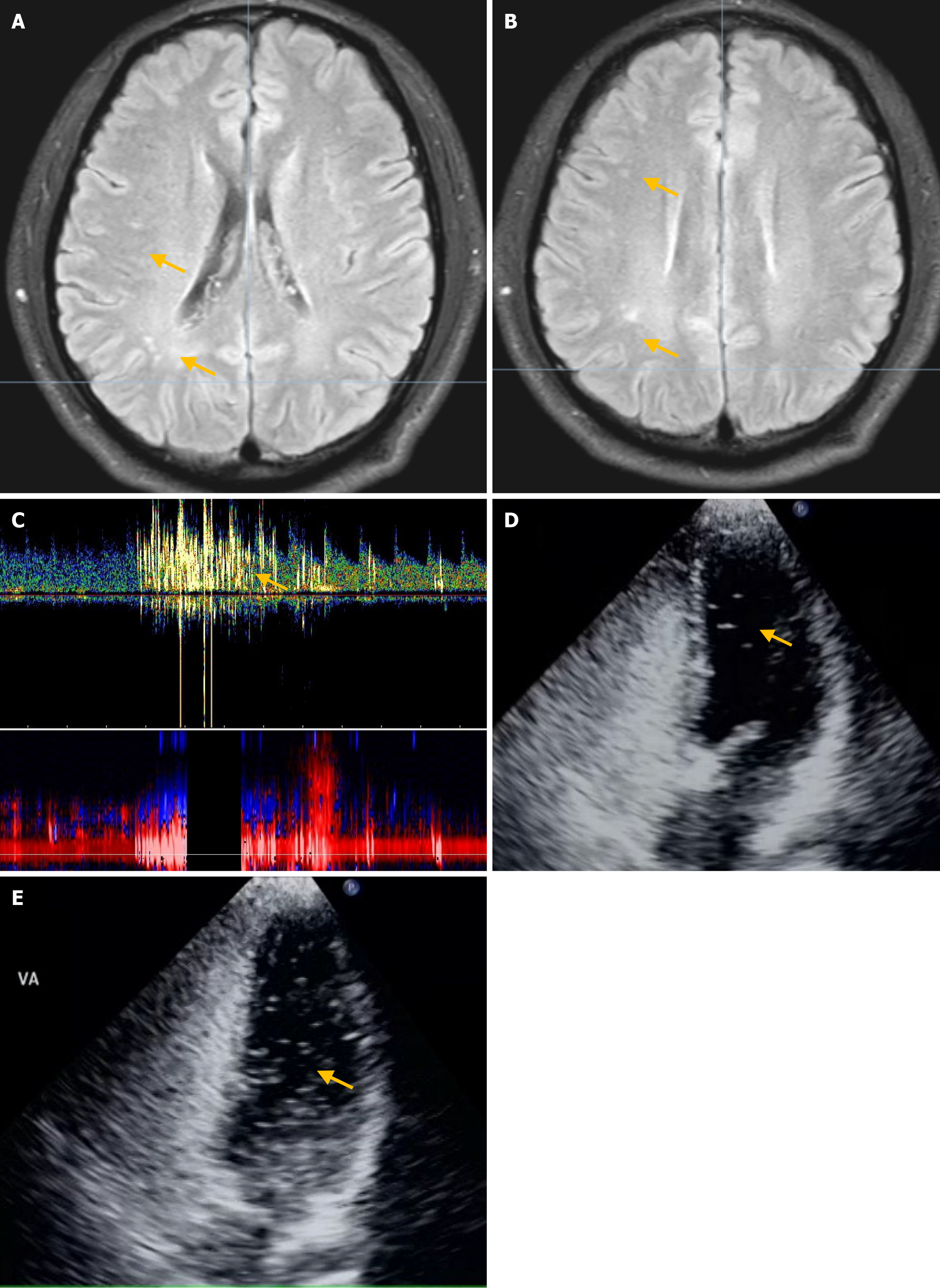
Upon physical examination, the patient displayed dysarthria, reduced muscle strength in the right upper limb (5-/5), impaired finger-nose, and alternating movement tests on the right side. No other neurological abnormalities were detected. Blood tests revealed normal levels of low-density lipoprotein cholesterol, glycated hemoglobin, electrolytes, carcinoembryonic antigen, alpha-fetoprotein, carbohydrate antigen (CA)-125, CA-199, troponin I, and N-terminal pro-brain natriuretic peptide (NT-proBNP), in addition to normal liver, kidney, coagulation, and thyroid function results (Table 1). The patient’s antibody test results for anticardiolipin, antinuclear, hepatitis B virus, syphilis virus, and human immunodeficiency virus were negative. The results of tests for protein S, protein C, and antithrombin III were within the normal limits. Echocardiography revealed no significant abnormality, but 24-hour dynamic electrocardiogram revealed frequent premature ventricular beats. Brain magnetic resonance imaging (MRI) revealed multiple infarcts in the frontal and occipital lobes and subcortical white matter, including some subacute infarcts (Figure 2A and B). Magnetic resonance angiography showed no abnormalities. cTTE detected moderate RLS at rest and during VM (Figure 2C), whereas concurrent cTCD detected no RLS at rest and a small RLS during VM (Figure 2D).
| Parameter | Result | Reference range | Interpretation |
| Patient 1 | |||
| Homocysteine (μmol/L) | 17.3 | 0–15.0 | Increased |
| High-density lipoprotein cholesterol (mmol/L) | 0.86 | 1.03–2.07 | Decreased |
| Manual platelet count (× 109/L) | 550 | 100–300 | Increased |
| Lactic acid (mmol/L) | 2.6 | 0.7–2.5 | Increased |
| JAK2 V617F | Positive | Negative | Abnormal |
| Patient 2 | |||
| Glycated hemoglobin (%) | 6.3 | 4.2–6.2 | Increased |
| Uric acid (μmol/L) | 447.7 | 208–428 | Increased |
| Thyroid-stimulating hormone, (μIU/mL) | 0.287 | 0.55–4.78 | Decreased |
| 2-hour postprandial blood glucose, (mmol/L) | 10.6 | 3.89–7.8 | Increased |
| Antinuclear antibodies | 1: 100 | < 1: 100 | Increased |
| Patient 3 | |||
| High-density lipoprotein cholesterol (mmol/L) | 0.76 | 1.03–2.07 | Decreased |
| Patient 4 | |||
| Total bilirubin (μmol/L) | 35.8 | 3.0–22 | Increased |
| Non-conjugated bilirubin (μmol/L) | 36.4 | 0–19 | Increased |
| Hepatitis B surface antigen (IU/mL) | > 2500.00 | < 0.08 | Increased |
Based on the combined results of cTTE and cTCD, the patient was diagnosed with moderate intrinsic RLS. Treatment included aspirin, clopidogrel, pantoprazole, atorvastatin, edaravone, and Erigeron breviscapus to enhance cerebral circulation, along with oxiracetam to boost cerebral metabolism. The patient was discharged after 12 days and exhibited significant improvement in symptoms. At 4 months after discharge, the patient was diagnosed with PFO based on the TEE results, leading to successful PFO occlusion. No stroke recurrence was observed during the 21-month follow-up period.
A 25-year-old man was admitted to our hospital with a history of recurrent headaches that had persisted for > 6 years and had intensified in the previous 2 weeks. The patient experienced severe, paroxysmal right temporal pulsating headaches that were alleviated with painkillers. His mother had a history of migraines. The patient had a 6-month history of smoking, with no history of infectious diseases or toxic exposure.
Physical examinations conducted on the patient revealed no neurological abnormalities. Blood test results for D-dimer, troponin I, creatine kinase isoenzyme, carcinoembryonic antigen, alpha-fetoprotein, CA-125, and CA-199 were all normal. Tests for antinuclear and soluble antigen antibodies were negative, and the red blood cell count, erythrocyte sedimen
A 50-year-old man presented with recurrent dizziness for 1 year, triggered by changes in body position or strenuous exercise. The episodes occurred approximately once per week, and the patient reported syncope 1 month prior to admission. The patient had no history of genetic disease, smoking, or alcohol abuse.
Physical examination revealed no abnormal neurological findings. Blood test results for red cell counts, coagulation, as well as liver, kidney, and thyroid functions were normal. The electrolyte, carcinoembryonic antigen, glycosylated hemoglobin, CA-125, CA-199, troponin I, NT-proBNP, and fasting glucose levels were all within normal limits. Electroencephalography revealed mild abnormalities; 24-hour dynamic electrocardiography showed frequent premature ven
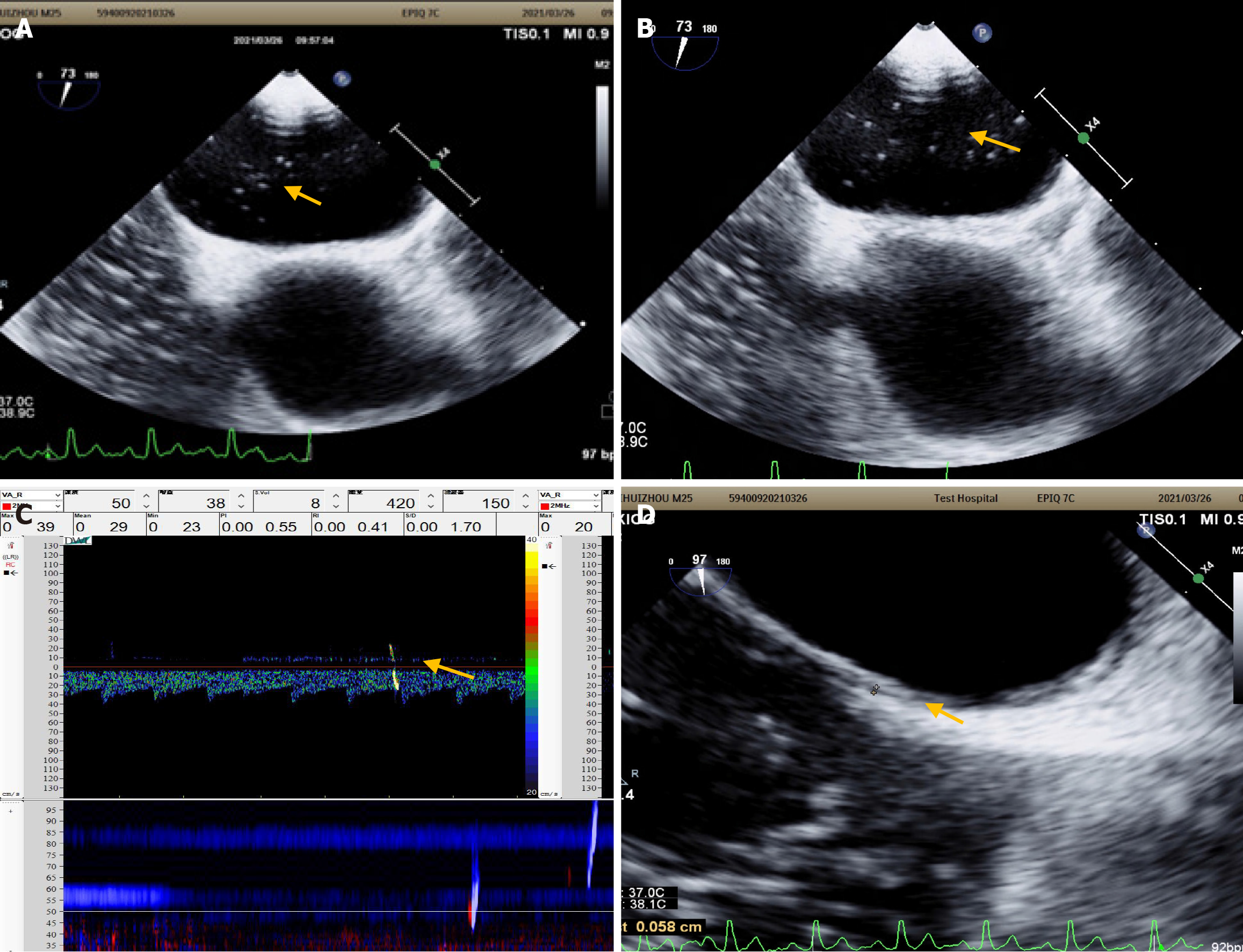
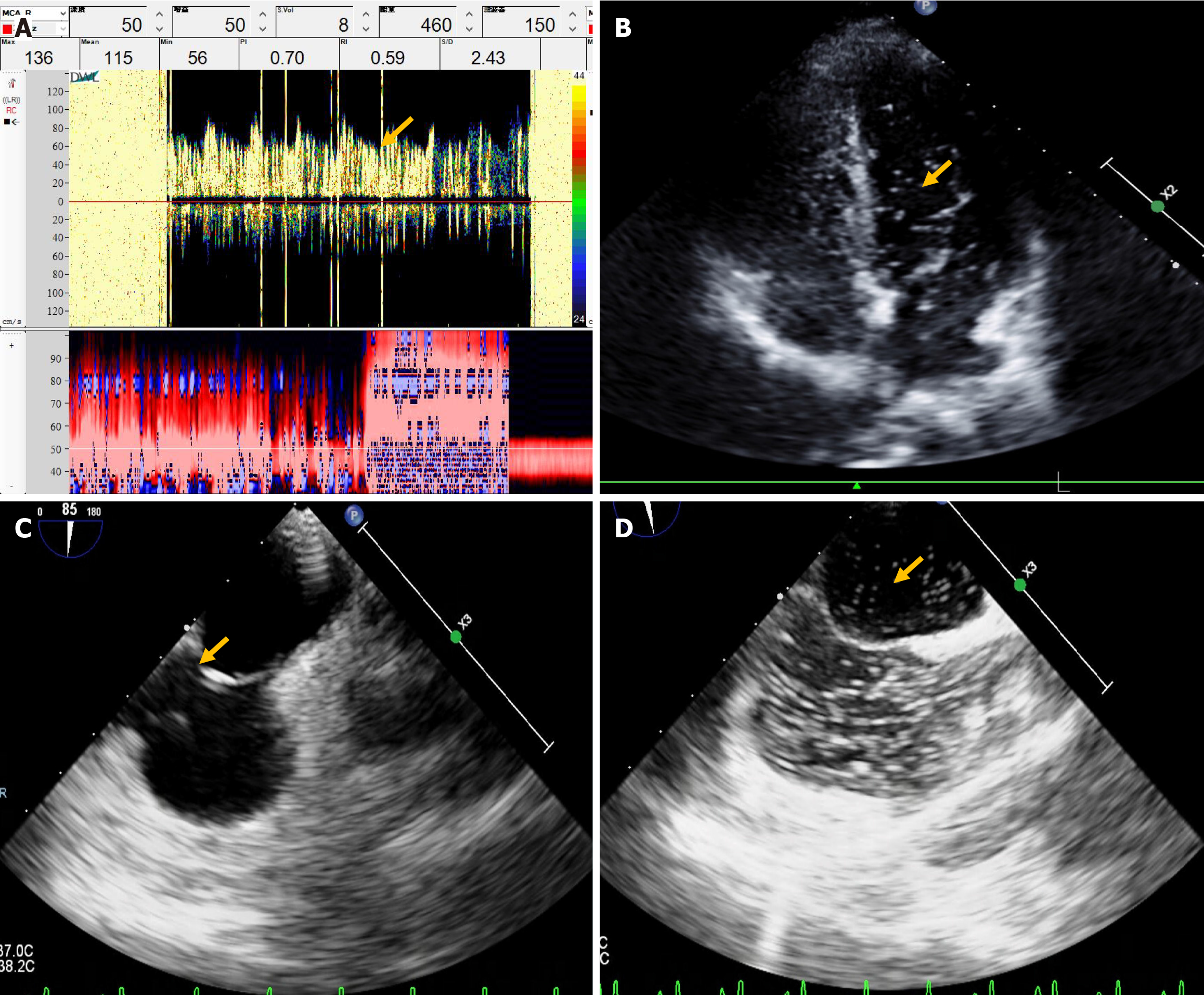
Head and neck CT angiography, Dix–Hallpike test, roll test, pure-tone hearing threshold testing, and upright tilt table testing revealed no significant abnormalities. Echocardiography revealed left ventricular enlargement, wall motion abnormalities, and decreased diastolic function. cTTE detected a small and moderate RLS at rest and during VM, respectively (Figure 4C and D); synchronous cTCD detected a small and large RLS during rest and VM, respectively (Figure 4E). Considering these findings, an inherently large RLS was identified. The patient was treated with betahistine mesylate for 8 days to alleviate dizziness, and Erigeron breviscapus was administered to enhance cerebral circulation, resulting in improved dizziness. The patient did not undergo PFO occlusion and reported recurrent dizziness at the 2-year follow-up visit.
A 30-year-old woman was admitted to our hospital with recurring headaches that had persisted for more than a decade. The headaches had occurred three to four times per month during the past year and were accompanied by nausea and fatigue. Each headache lasted for 7–8 hour and was alleviated with painkillers. The patient had a history of pyelonephritis but had no hereditary diseases or toxic exposure.
Physical examination revealed no abnormal neurological findings. Blood tests revealed that the glycated hemoglobin, creatinine, cholesterol, and D-dimer levels were within the normal limits. The patient’s red blood cell counts and thyroid and coagulation functions were also normal. Brain CT, electrocardiography, and echocardiography revealed no abnor
Our study demonstrated that the use of cTCD or cTTE individually may yield false-negative results or underestimate the presence of RLS. In contrast, combining the two techniques using synchronized cTCD and cTTE can help mitigate this issue and enhance the analysis of potential causes, thereby supporting more informed treatment decisions.
cTCD and cTTE have inherent limitations that directly contribute to the underestimation of RLS. The specific reasons for this are as follows: First, cTTE is influenced by a range of factors including body position, obesity, breast tissue, gas in the lungs, and the VM[22]. Obesity and breast tissue can obstruct the heart, making it difficult for ultrasound waves to penetrate, which affects the quality of cardiac ultrasound imaging. When gas is present in the lungs, ultrasonic waves cause reflections and scattering, thereby impacting the quality of cardiac ultrasound images. Increased thoracic pressure from the VM can affect the shape and position of the heart, affecting the stability of microbubble monitoring during cTTE. However, the cTCD detection process is not affected by these interfering factors[11].
Second, patients with poor temporal bone windows (10%–20% of patients with stroke possess a poor temporal window)[23] or suboccipital window may encounter challenges. Additionally, severe narrowing or occlusion of the carotid artery or middle cerebral artery can also affect the results of the investigation, making it challenging to complete cTCD at such times. Therefore, cTTE can serve as an alternative. It is rare for the acoustic windows in the chest and head to be simultaneously deficient. During cTTE, we observed that the time for the contrast agent injected into the vein to reach the right atrium varied among the patients.
Third, in cTCD, patients are instructed to stop the VM at a fixed time, which may result in the right atrium not being fully filled with the contrast agent, potentially reducing RLS detection. By using a synchronized approach that combines cTCD with cTTE, cTTE enables clear observation of whether microbubbles fully occupy the right atrium. This accurate guidance of VM cessation enhances both the likelihood and accuracy of detecting RLS.
Finally, cTCD only detects intracranial shunts, which are a subset of cardiogenic RLS[24], whereas cTTE can roughly determine whether a detected RLS is of cardiac or pulmonary origin by analyzing the relationship between the timing of microbubble appearance and the cardiac cycle[20]. It can even directly observe the blood flow crossing the atrial septum, thereby confirming the presence of a PFO.
Synchronous cTCD and cTTE combines the strengths of both tests while minimizing their limitations, enhancing the detection rate and accuracy of RLS assessment. This synchronized approach involves shared use of contrast agents, patient positioning, VM, and simultaneous testing, creating optimal conditions for comparing cTCD and cTTE, and resulting in high comparability. Compared to asynchronous testing, synchronous multimodal ultrasound reduces the use of contrast agents and workload, improving test safety and patient compliance. Additionally, operators can cross-verify results, gaining valuable experience. Synchronized cTCD combined with cTTE offers a one-stop, efficient, multidisciplinary collaboration that is particularly valuable for precise PFO screening in individuals with cryptogenic stroke and migraine.
Multimodal ultrasonography enhances diagnostic and treatment decisions. In Patients 1 and 2, multimodal ultrasonography revealed a large RLS, which facilitated the identification of its cause. Brain MRI, particularly in Patient 1, revealed multiple small subcortical infarct foci, corroborating the association with PFO-related stroke[19,25]. Based on these findings, PFO occlusion was performed, and long-term follow-up indicated no stroke recurrence.
In patients 3 and 4, a large RLS detected using multimodal ultrasound provided a clearer explanation of the etiology. Laboratory analyses, brain MRI, and other tests in these patients did not elucidate the etiology, except for findings from multimodal ultrasound. Our findings suggest that synchronous multimodal ultrasound is a feasible and accurate screening method for PFO and RLS. Relying solely on cTTE or cTCD results may complicate etiology identification and affect subsequent treatment decisions.
This study has some limitations. This was a small-sample exploratory study; therefore, the generalizability of the findings is limited. This multimodal joint ultrasound technique places higher demands on multidisciplinary collaboration. In future studies, the sample size should be expanded and strategies should be explored to streamline multi
Accurate assessment of the PFO/RLS is crucial for evaluating the risk of onset, conducting etiological analyses, and making treatment decisions in patients with stroke or migraine. The synchronized use of cTCD and cTTE can enhance their respective efficiencies, offering benefits beyond their individual contributions. This approach holds significant potential for clinical applications and merits further investigation.
We wish to thank the “Double-First Class” Application Characteristic Discipline of Hunan Province (Pharmaceutical Science) for the support.
| 1. | Guo R, Zhang SY, Yin LL, Wang K, Ding MY, Cui CS. [Comparative enhancement of transcranial Doppler ultrasound in the correlation between cryptogenic stroke and right-to-left shunt: A single-center study in Liaoning province]. Zhongfen Yu Shenjingjibing Zazhi. 2020;37:31-34. |
| 2. | Mojadidi MK, Zaman MO, Elgendy IY, Mahmoud AN, Patel NK, Agarwal N, Tobis JM, Meier B. Cryptogenic Stroke and Patent Foramen Ovale. J Am Coll Cardiol. 2018;71:1035-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 3. | Mazzucco S, Li L, Binney L, Rothwell PM; Oxford Vascular Study Phenotyped Cohort. Prevalence of patent foramen ovale in cryptogenic transient ischaemic attack and non-disabling stroke at older ages: a population-based study, systematic review, and meta-analysis. Lancet Neurol. 2018;17:609-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Cao Q, Shen Y, Hou Z, Li D, Tang B, Xu L, Li Y. The Relationship Between Patent Foramen Ovale and Unexplained Dizziness: A Prospective Analysis in China. Neuropsychiatr Dis Treat. 2022;18:1495-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 5. | Li Y, Shen Y, Cao Q, Cheng Y, Xu L, Tang Z. Effect of Interventional Therapy Unexplained Dizziness and Relationship Between Dizziness Handicap Inventory and Right-to-Left Shunt Grading. Int J Gen Med. 2023;16:803-811. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Kumar P, Kijima Y, West BH, Tobis JM. The Connection Between Patent Foramen Ovale and Migraine. Neuroimaging Clin N Am. 2019;29:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | He Q, Zhang Y, Wang F, Li C, Guo R, Li X, Luan B, Zhao H, Meng L, Chen H, Meng L. Impact of right-to-left shunt and transcatheter closure on the clinical features of migraine. Int J Neurosci. 2020;130:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Scavasine VC, Chamma JF, Bazan R, Braga GP, Lange MC, Zétola VHF. Comparison of right-to-left shunt characteristics in cryptogenic embolic ischemic stroke and non-cardioembolic ischemic stroke. Arq Neuropsiquiatr. 2021;79:859-863. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 9. | Zhao Q, Liu R, Zhou J, Dong Z, Chen Y. Prevalence and grade of RLS in migraine: A prospective study of 251 migraineurs by synchronous test of c-TTE and c-TCD. Medicine (Baltimore). 2021;100:e24175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yang X, Wang H, Wei Y, Zhai N, Liu B, Li X. Diagnosis of Patent Foramen Ovale: The Combination of Contrast Transcranial Doppler, Contrast Transthoracic Echocardiography, and Contrast Transesophageal Echocardiography. Biomed Res Int. 2020;2020:8701759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Maillet A, Pavero A, Salaun P, Pibourdin A, Skopinski S, Thambo JB, Sibon I, Constans J, Boulon C. Transcranial Doppler to Detect Right to Left Communication: Evaluation Versus Transesophageal Echocardiography in Real Life. Angiology. 2018;69:79-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Tian L, Zhang M, Nie H, Zhang G, Luo X, Yuan H. Contrast-enhanced transcranial doppler versus contrast transthoracic echocardiography for right-to-left shunt diagnosis. J Clin Monit Comput. 2023;37:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Liu F, Kong Q, Zhang X, Li Y, Liang S, Han S, Li G. Comparative analysis of the diagnostic value of several methods for the diagnosis of patent foramen ovale. Echocardiography. 2021;38:790-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Ma J, Liao HJ, Zhang Y, Li LY, Zhang JQ, Zhao SG, Song XJ. [Comparison of the application of cTTE and cTEE combined with cTCD in the diagnosis and intervention of patent foramen ovale]. Zhongguo Xiandai Yixue Zazhi. 2022;32:13-17. |
| 15. | Yao Q, Xiong H, Zhang D, Ren S, Qi W, Zou X, Zhao Y, Huang S, Wang J, Cao L. Synchronous multimode ultrasound for assessing right-to-left shunt: a prospective clinical study. Front Neurol. 2023;14:1148846. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Li RB, Cao L, Fu M, Cai XD. Detection rate and shunt grading with synchronous testing of contrast transcranial Doppler and contrast transthoracic echocardiography: Preliminary findings. Medicine (Baltimore). 2023;102:e33928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 17. | Bray EP, McMahon NE, Bangee M, Al-Khalidi AH, Benedetto V, Chauhan U, Clegg AJ, Georgiou RF, Gibson J, Lane DA, Lip GYH, Lightbody E, Sekhar A, Chatterjee K, Watkins CL. Etiologic workup in cases of cryptogenic stroke: protocol for a systematic review and comparison of international clinical practice guidelines. Syst Rev. 2019;8:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, Bisdorff A, Versino M, Evers S, Kheradmand A, Newman-Toker D. Vestibular migraine: Diagnostic criteria1. J Vestib Res. 2022;32:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 19. | Kim BJ, Sohn H, Sun BJ, Song JK, Kang DW, Kim JS, Kwon SU. Imaging characteristics of ischemic strokes related to patent foramen ovale. Stroke. 2013;44:3350-3356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Li HZ, He YF, Wang QG, Hu HB, Zhou Q, Zhang WQ, Liu YC, Chen WB, Xu JZ, Shen QS, Zhang HW, Fei HW, Chen XB, Li YS, Wang ZC, Zhang GC, Li Y, Zhang CJ. [Clinical operation specifications for echocardiography and right heart acoustic imaging of patent foramen ovale]. Zhongguo Shiyong Neike Zazhi. 2022;42:376-380. [DOI] [Full Text] |
| 21. | Zhang YS, Zhu XY. [Chinese experts' recommendations on the management of patent foramen ovale closure]. Xinzang Zazhi. 2015;27:373-379. [DOI] [Full Text] |
| 22. | Mahmoud AN, Elgendy IY, Agarwal N, Tobis JM, Mojadidi MK. Identification and Quantification of Patent Foramen Ovale-Mediated Shunts: Echocardiography and Transcranial Doppler. Interv Cardiol Clin. 2017;6:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Seidel G, Kaps M, Gerriets T. Potential and limitations of transcranial color-coded sonography in stroke patients. Stroke. 1995;26:2061-2066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 24. | Cao L, Huang X, Wang H. Relevance of small right-to-left shunt in contrast-enhanced transcranial Doppler in young and middle-aged patients with cryptogenic stroke: a report of two cases and literature review. Int J Neurosci. 2022;132:1118-1122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Schuchlenz HW, Weihs W, Horner S, Quehenberger F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. 2000;109:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |