Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.621
Revised: September 3, 2024
Accepted: September 11, 2024
Published online: October 28, 2024
Processing time: 102 Days and 4.5 Hours
Difficulties in making an accurate preoperative diagnosis of cystic pancreatic lesions pose a challenge for radiologists. It would be helpful to report rare cases and review the literature.
In the present report, a case of a patient with a pancreatic cystic lesion initially misdiagnosed as a pseudocyst by radiologist was documented, which was later pathologically confirmed as pancreatic ductal adenocarcinoma with neuroendo
Careful evaluation of the characteristics revealed by multimodal imaging tech
Core Tip: A case of cystic pancreatic ductal adenocarcinoma with neuroendocrine involvement that was initially misdiagnosed as a pseudocyst was reported. The lack of specific symptoms and distinctive radiological features poses challenges in the preoperative diagnosis of pancreatic cystic lesions, particularly in differentiating between atypical and rare lesions.
- Citation: Zou DM, Shu ZY, Cao X. Cystic ductal adenocarcinoma of pancreas complicated with neuroendocrine tumor: A case report and review of literature. World J Radiol 2024; 16(10): 621-628
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/621.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.621
Pancreatic neoplasms typically have severe health implications. They can be categorized as either exocrine or endocrine tumors based on their cellular origin and histopathology. Exocrine tumors account for > 95% cases of pancreatic neo
In the present case report, a patient with pancreatic ductal adenocarcinoma with neuroendocrine tumor showing a cystic lesion was documented. Combined with the naked eye findings of the resected specimens and the pathological changes under the microscope, it seems difficult to explain the imaging and pathological features of this case through the well-known cystic mechanism of pancreatic ductal adenocarcinoma. On this basis, discussions are provided and related literature was reviewed to improve understanding into the pathophysiology of PCLs. This may be particularly helpful in providing more comprehensive insight into pancreatic ductal adenocarcinoma.
A 66-year-old male patient was referred to the hospital due to persistent abdominal distension for > 1 months.
Previous gastroscopy examinations in other hospitals had indicated a large submucosal projection in the gastric body.
No special notes.
No special notes.
Physical examination revealed no abnormalities.
Laboratory tests revealed elevated levels of carbohydrate antigen 19-9 (CA 19-9) in the serum (51.49 U/mL; normal value, < 37 U/mL), whilst the levels of lipase (34 U/L; normal value, 0-60 U/L) and amylase (38 U/L; normal value, 0-140 U/L) were within the normal range.
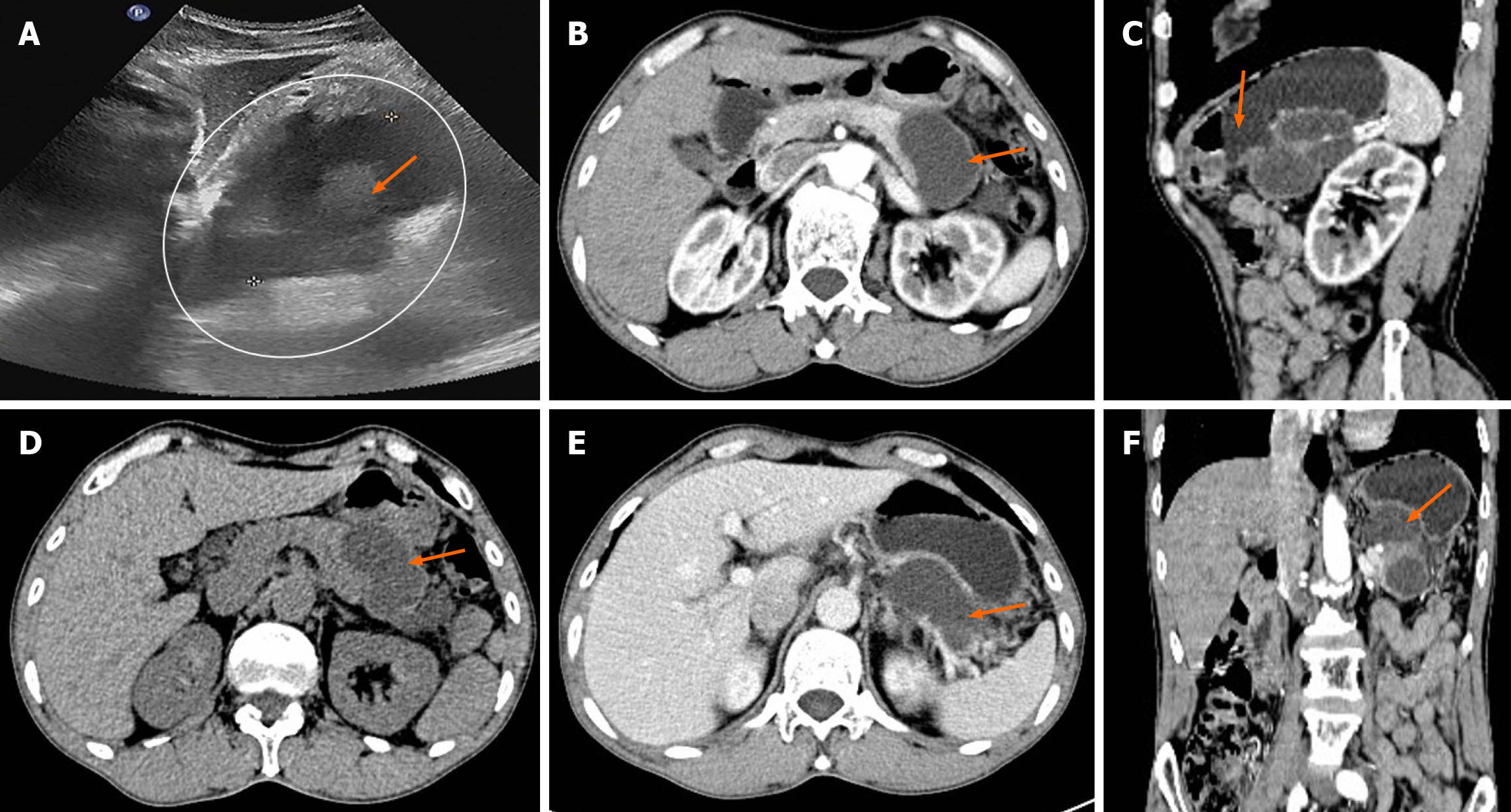
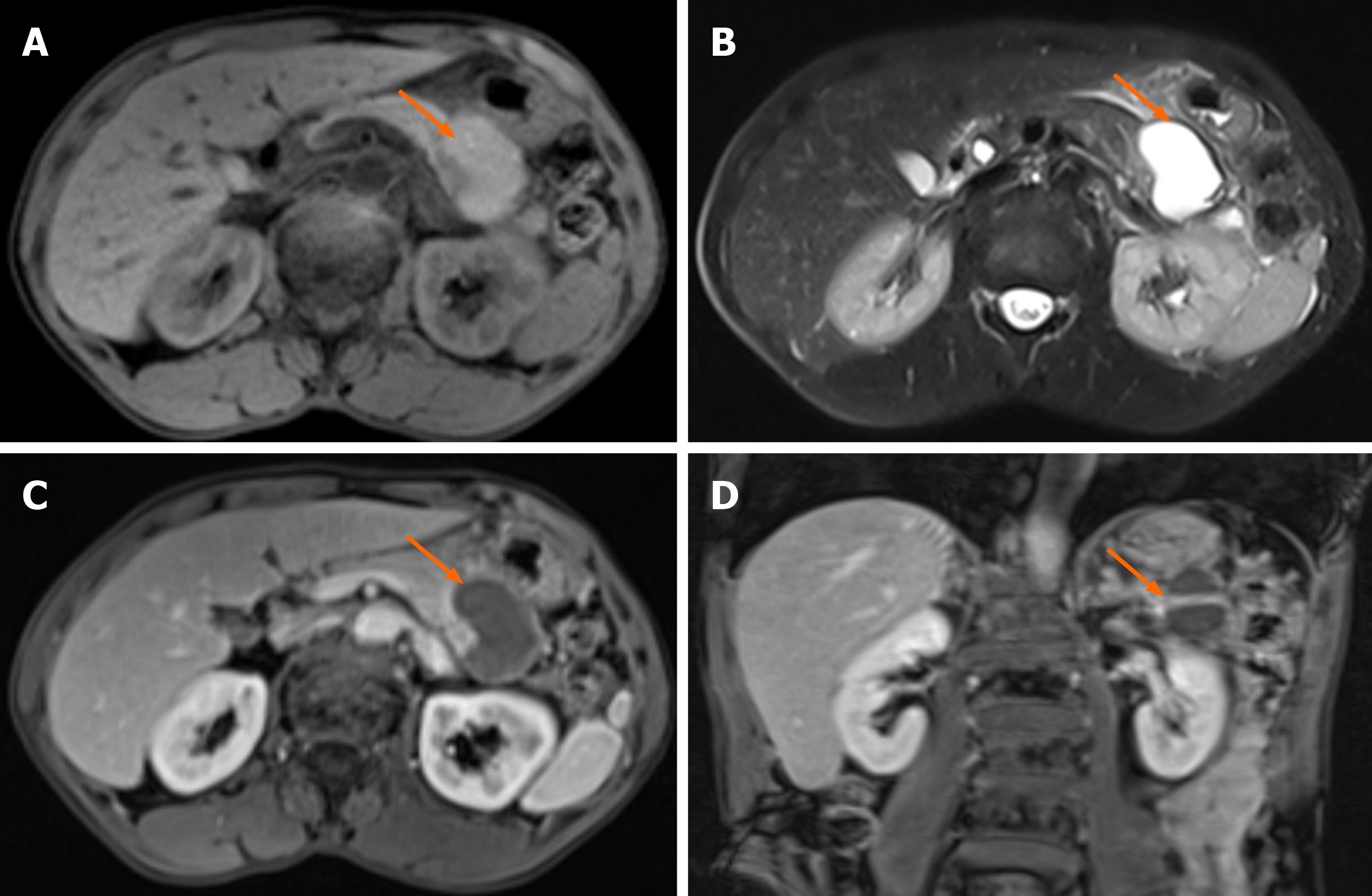
Ultrasonography revealed the presence of heterogenous hypoechoic cystic lesions (Figure 1). Computed tomography (CT) scans of the abdomen, both non-enhanced and three-phase enhanced, revealed irregular cysts located in the tail of the pancreas. These cysts protruded outside the border of the pancreas, were in close proximity to the gastric wall and partially encased the blood vessels at the hilum of the spleen (Figure 1). magnetic resonance imaging (MRI) revealed a high signal intensity in the T1-weighted images (T1WI) and clear high signal intensity in T2-weighted images (T2WI). Additionally, delayed enhancement of the cyst wall was observed during the enhanced scan (Figure 2).
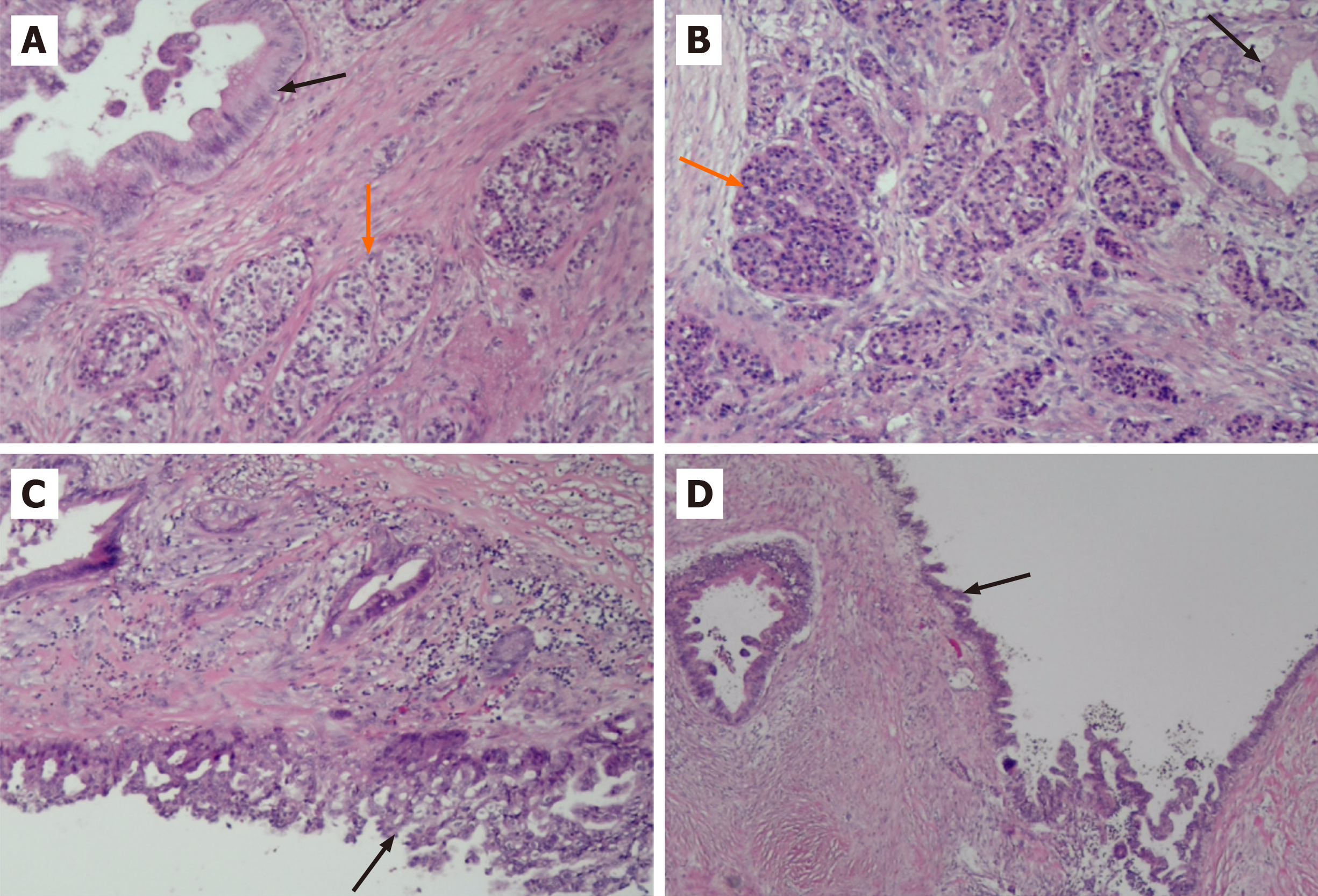
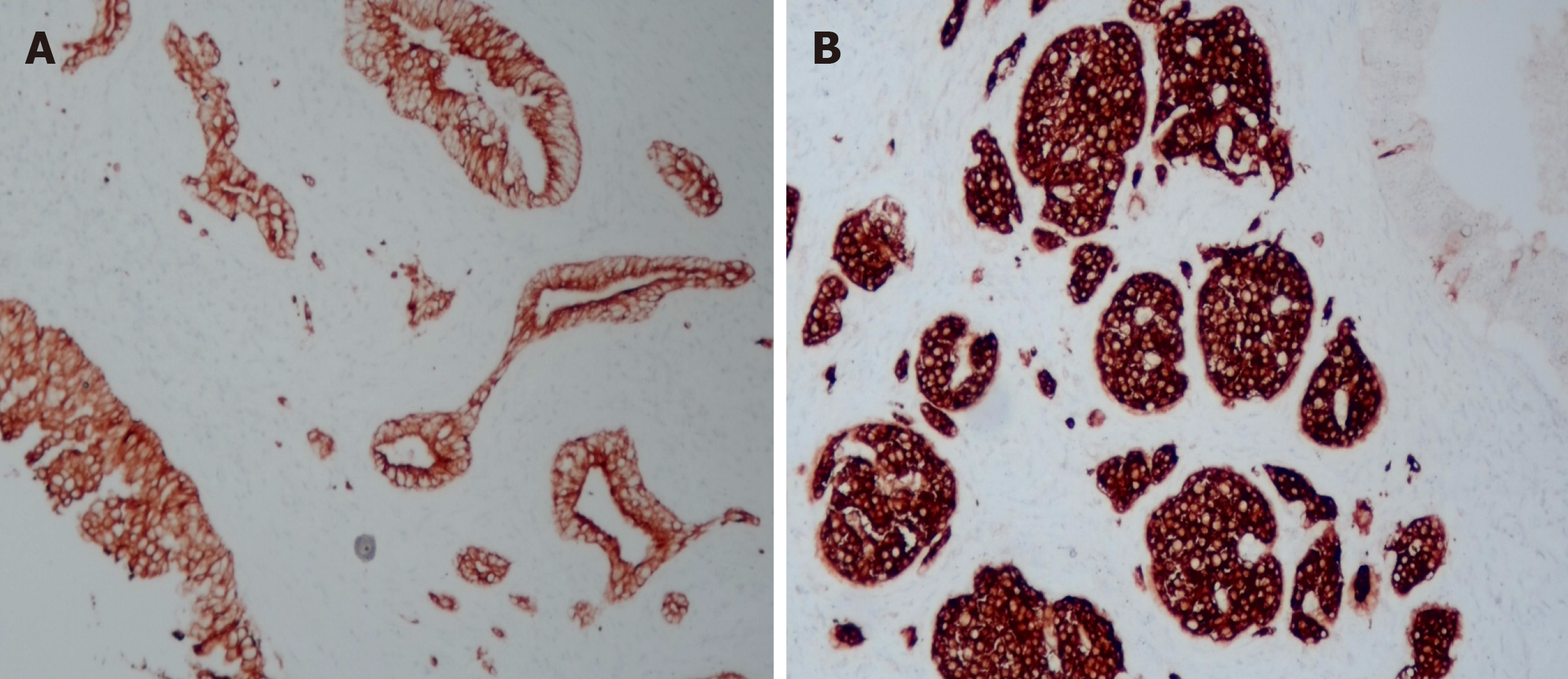
Pathological examination of the resected mass from the body and tail of the pancreas, spleen and regional lymph nodes revealed a moderately differentiated ductal adenocarcinoma with a G1 neuroendocrine tumor. The neuroendocrine tumor component accounted for approximately 10% of the tumor (Figure 3). No tumor involvement was observed in the pancreatic capsule, the stump end of the pancreas or the spleen. Additionally, no tumor metastasis was detected in the lymph nodes. Immunohistochemistry confirmed the origin of the tumor cells from epithelial and neuroendocrine cells (Figure 4). Ultimately, the clinical diagnosis was established as moderately differentiated ductal adenocarcinoma, stage IIB (T3N1M0pm), with neuroendocrine tumor involvement.
The patient underwent resection of the body and tail of the pancreas and splenectomy followed by two cycles of chemotherapy.
Subsequent CT examinations conducted 3 months later revealed no residual tumor, recurrence, or complications. Currently, the patient has been followed up for 7 months and remains in good health.
PCL with malignant or malignant potential include cystic ductal adenocarcinoma, intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasm (MCN), solid pseudopapillary tumors (SPT) and cystic neuroendocrine tumors (CNET). By contrast, benign pancreatic lesions can include serous cystadenoma (SCA), pseudocysts and squa
In total, approximately 7% pancreatic ductal adenocarcinoma (PDAC) exhibit cystic lesions on imaging[4-7]. According to the histopathological subtypes, cystic lesions caused by PDAC can be divided into neoplastic and non-neoplastic subtypes[8]. Neoplastic cysts in PDAC include large-duct type cysts, neoplastic mucin cysts, colloid carcinoma and degenerative cystic changes. Non-neoplastic cystic lesions in PDAC include retention cyst changes caused by duct obstruction and pseudocysts caused by tumor-associated pancreatitis[4,9-11]. PDAC with large-duct type cysts tend to remain solid, with several small intratumoral cysts, which can exhibit the ‘honeycomb’ appearance in the images. Therefore, they are prone to misdiagnosis as SCA or branched pancreatic duct type IPMN[9,12]. PDAC with neoplastic mucin cysts are caused by mucin-producing tumor cells that are typically solitary with cysts ≤ 7 cm in size. However, they do occasionally present in multiple cysts. PDAC with neoplastic mucin cysts should be differentiated from IPMN with invasive carcinoma, since the former will more likely present as a solid mass, whilst the latter will more likely appear around the solid mass[10]. Colloid carcinoma is a histological variant of PDAC and has a superior prognosis compared with that of other PDAC subtypes. Because of its rich extracellular mucus, it frequently presents as high-signal intensity on T2WI and internal reticular and marginal progressive enhancement on MRI[13]. The high-signal intensity on both T1WI and T2WI in our case may be caused by intracapsular hemorrhage[14], which can be inferred from the blood clots found after operation. Pathologically, PDAC with degenerative cystic changes are formed by tumor necrosis, with necrotic and hemorrhagic tissues in the cavity. It typically presents as a large single cystic lesion in the tumor, where the edge and center of the tumor is irregular[4,10]. PDAC with retention cysts is caused by tumor obstruction of the pan
CNET account for 13%-17% of all pancreatic neuroendocrine tumors[15]. The mechanism of neuroendocrine tumor cyst formation is proposed to be caused by the slow and expansive growth of the tumor, leading to the formation of fibrous capsules and cystic degeneration, characterized by hemorrhage and necrosis in the tumor or tumor growth in the duct[16-18]. Although cystic changes do occur, their solid components will typically show a strong arterial enhancement, enabling its distinguishment from PDAC[19]. The present case does not satisfy this diagnosis of mixed tumor because the neuroendocrine tumor accounted for only 10% of the total tumor. It may also be because the enhanced scan showed delayed enhancement of the cyst wall, which is different from the features shown by the early enhancement of CNET. Although a number of reports of mixed tumors of the pancreas have been previously documented[20-22], completely cystic lesions lined with epithelium remained scarce, which was the case in the present report.
IPMN accounts for 21%-33% of all PCL and typically occurs in the head of pancreas[23,24]. This form of lesions will progress into invasive cancer 27.6%-68% of the time, which is the highest potential of all precancerous lesions of PDAC known. It can be subdivided into main duct, branch duct and mixed type[25-27]. Thin-slice enhanced CT and 3D-mag
PLEC is a rare cystic lesion of the pancreas, which mainly originates from the residual epithelium of the peripancreatic lymph nodes and is most frequently observed in middle-aged and elderly male patients[35,36]. PLEC can present as unilocular or multilocular cystic lesions. On CT plain scans, highly invasive cysts that are thin and uniform with high density protruding beyond the outline of the pancreas and septum of the cyst wall can be seen, coupled with rare calcification[37]. By contrast, MRI typically reveals low signal on T1WI and high signal on T2WI. Contrast-enhanced scan will show slight enhancement of the cyst wall or septum[38]. A small number of cases have previously reported that their cellular morphological features may overlap with other benign and malignant pancreatic lesions[39]. EDC is a rare non-neoplastic true cyst of the pancreas. Pancreatic EDC originates from an intrapancreatic accessory spleen, normally as a cystic lesion at the edge of the pancreatic tail[40]. If imaging features similar to normal spleen are identified in the pan
Due to the overlapping imaging features among the various pancreatic cystic lesions, non-invasive diagnosis based solely on imaging examination is both critical and challenging. Accurate diagnosis requires the consideration of multiple complex factors, such as patient age, tumor location, composition of cystic lesions, relationship with the pancreatic duct and findings around the cystic lesions. Additional cellular and molecular investigations will be required to obtain precise diagnostic, treatment and prognostic information.
In the present particular case, the radiologist initially misdiagnosed the lesion as a pseudocyst. This could be attributed to similarities in imaging features between pseudocysts and the absence of solid components. Furthermore, uncertainty regarding whether the patient had experienced pancreatitis prior to admission and the lack of a significant increase in CA199 levels may have contributed to the misdiagnosis. Subsequent postoperative diagnosis confirmed pancreatic ductal adenocarcinoma with a neuroendocrine tumor which, to the best of our knowledge, is a rarely reported pathological diagnosis for pancreatic cystic lesions.
The lack of specific symptoms and distinctive radiological features poses challenges in the preoperative diagnosis of pancreatic cystic lesions, particularly in differentiating between atypical and rare lesions. The present case, along with a review of the literature, highlights the importance of carefully assessing the characteristics of each type of these lesions by using multimodal imaging techniques and integrating them with the patient's medical history, laboratory examinations and follow-up observations. This comprehensive approach is crucial in determining the necessity for surgical resection and obtaining accurate diagnostic and prognostic information. Our report on a rare case provides valuable insights into the diagnosis of pancreatic cystic disease.
| 1. | Pokrzywa CJ, Abbott DE, Matkowskyj KA, Ronnekleiv-Kelly SM, Winslow ER, Weber SM, Fisher AV. Natural History and Treatment Trends in Pancreatic Cancer Subtypes. J Gastrointest Surg. 2019;23:768-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Klimstra DS, Adsay V. Acinar neoplasms of the pancreas-A summary of 25 years of research. Semin Diagn Pathol. 2016;33:307-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Pusateri AJ, Krishna SG. Pancreatic Cystic Lesions: Pathogenesis and Malignant Potential. Diseases. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Kosmahl M, Pauser U, Anlauf M, Klöppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18:1157-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Kosmahl M, Pauser U, Peters K, Sipos B, Lüttges J, Kremer B, Klöppel G. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 253] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Martin I, Hammond P, Scott J, Redhead D, Carter DC, Garden OJ. Cystic tumours of the pancreas. Br J Surg. 1998;85:1484-1486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Brugge WR, Lauwers GY, Sahani D, Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 490] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 8. | Youn SY, Rha SE, Jung ES, Lee IS. Pancreas ductal adenocarcinoma with cystic features on cross-sectional imaging: radiologic-pathologic correlation. Diagn Interv Radiol. 2018;24:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Cho HW, Choi JY, Kim MJ, Park MS, Lim JS, Chung YE, Kim KW. Pancreatic tumors: emphasis on CT findings and pathologic classification. Korean J Radiol. 2011;12:731-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Yoon SE, Byun JH, Kim KA, Kim HJ, Lee SS, Jang SJ, Jang YJ, Lee MG. Pancreatic ductal adenocarcinoma with intratumoral cystic lesions on MRI: correlation with histopathological findings. Br J Radiol. 2010;83:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Luchini C, Capelli P, Scarpa A. Pancreatic Ductal Adenocarcinoma and Its Variants. Surg Pathol Clin. 2016;9:547-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Hori S, Shimada K, Ino Y, Oguro S, Esaki M, Nara S, Kishi Y, Kosuge T, Hattori Y, Sukeda A, Kitagawa Y, Kanai Y, Hiraoka N. Macroscopic features predict outcome in patients with pancreatic ductal adenocarcinoma. Virchows Arch. 2016;469:621-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yoon MA, Lee JM, Kim SH, Lee JY, Han JK, Choi BI, Choi JY, Park SH, Lee MW. MRI features of pancreatic colloid carcinoma. AJR Am J Roentgenol. 2009;193:W308-W313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Hu S, Hu CH, Yang L, Xing JM, Chen JH, Ge ZL, Liu JS. Atypical imaging observations of branchial cleft cysts. Oncol Lett. 2014;7:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Hurtado-Pardo L, Cienfuegos JA, Ruiz-Canela M, Panadero P, Benito A, Hernández Lizoain JL. Cystic pancreatic neuroendocrine tumors (cPNETs): a systematic review and meta-analysis of case series. Rev Esp Enferm Dig. 2017;109:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Kamisawa T, Fukayama M, Koike M, Tabata I, Okamoto A. A case of malignant cystic endocrine tumor of the pancreas. Am J Gastroenterol. 1987;82:86-89. [PubMed] |
| 17. | Buetow PC, Parrino TV, Buck JL, Pantongrag-Brown L, Ros PR, Dachman AH, Cruess DF. Islet cell tumors of the pancreas: pathologic-imaging correlation among size, necrosis and cysts, calcification, malignant behavior, and functional status. AJR Am J Roentgenol. 1995;165:1175-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Iacono C, Serio G, Fugazzola C, Zamboni G, Bergamo Andreis IA, Jannucci A, Zicari M, Dagradi A. Cystic islet cell tumors of the pancreas. A clinico-pathological report of two nonfunctioning cases and review of the literature. Int J Pancreatol. 1992;11:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Kalb B, Sarmiento JM, Kooby DA, Adsay NV, Martin DR. MR imaging of cystic lesions of the pancreas. Radiographics. 2009;29:1749-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Sabol M, Donát R, Macháleková K, Šintál D, Durdík S. Collision of a neuroendocrine tumor and ductal adenocarcinoma of the pancreas: a rare case report. Neuro Endocrinol Lett. 2020;41:60-68. [PubMed] |
| 21. | Ohno A, Fujimori N, Miki M, Oono T, Igarashi H, Matsuda R, Koga Y, Oda Y, Ohtsuka T, Nakamura M, Ito T, Ogawa Y. Collision of a pancreatic ductal adenocarcinoma and a pancreatic neuroendocrine tumor associated with multiple endocrine neoplasm type 1. Clin J Gastroenterol. 2021;14:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Serafini S, Da Dalt G, Pozza G, Blandamura S, Valmasoni M, Merigliano S, Sperti C. Collision of ductal adenocarcinoma and neuroendocrine tumor of the pancreas: a case report and review of the literature. World J Surg Oncol. 2017;15:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | de Wilde RF, Hruban RH, Maitra A, Offerhaus GJA. Reporting precursors to invasive pancreatic cancer: pancreatic intraepithelial neoplasia, intraductal neoplasms and mucinous cystic neoplasm. Diagn Histopathol. 2012;18:17-30. [RCA] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Rezaee N, Barbon C, Zaki A, He J, Salman B, Hruban RH, Cameron JL, Herman JM, Ahuja N, Lennon AM, Weiss MJ, Wood LD, Wolfgang CL. Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB (Oxford). 2016;18:236-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 25. | Fernández-del Castillo C, Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 2010;139:708-713, 713.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Adsay NV, Longnecker DS, Klimstra DS. Pancreatic tumors with cystic dilatation of the ducts: intraductal papillary mucinous neoplasms and intraductal oncocytic papillary neoplasms. Semin Diagn Pathol. 2000;17:16-30. [PubMed] |
| 27. | Distler M, Kersting S, Niedergethmann M, Aust DE, Franz M, Rückert F, Ehehalt F, Pilarsky C, Post S, Saeger HD, Grützmann R. Pathohistological subtype predicts survival in patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg. 2013;258:324-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, Eshleman JR, Goggins MG, Wolfgang CL, Canto MI, Schulick RD, Edil BH, Choti MA, Adsay V, Klimstra DS, Offerhaus GJ, Klein AP, Kopelovich L, Carter H, Karchin R, Allen PJ, Schmidt CM, Naito Y, Diaz LA Jr, Kinzler KW, Papadopoulos N, Hruban RH, Vogelstein B. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011;108:21188-21193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 481] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 29. | Thompson LD, Becker RC, Przygodzki RM, Adair CF, Heffess CS. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol. 1999;23:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 220] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Ganeshan DM, Paulson E, Tamm EP, Taggart MW, Balachandran A, Bhosale P. Solid pseudo-papillary tumors of the pancreas: current update. Abdom Imaging. 2013;38:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Choi JY, Kim MJ, Lee JY, Lim JS, Chung JJ, Kim KW, Yoo HS. Typical and atypical manifestations of serous cystadenoma of the pancreas: imaging findings with pathologic correlation. AJR Am J Roentgenol. 2009;193:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Basturk O, Coban I, Adsay NV. Pancreatic cysts: pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 33. | Ishigami K, Nishie A, Asayama Y, Ushijima Y, Takayama Y, Fujita N, Takahata S, Ohtsuka T, Ito T, Igarashi H, Ikari S, Metz CM, Honda H. Imaging pitfalls of pancreatic serous cystic neoplasm and its potential mimickers. World J Radiol. 2014;6:36-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Jang KM, Kim SH, Song KD, Kim YK, Lee SJ, Choi D. Differentiation of solid-type serous cystic neoplasm from neuroendocrine tumour in the pancreas: value of abdominal MRI with diffusion-weighted imaging in comparison with MDCT. Clin Radiol. 2015;70:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Namba Y, Oshita A, Nishisaka T, Namba M, Sasaki T, Matsugu Y, Itamoto T. Lymphoepithelial cyst of the pancreas: A case report and summary of imaging features of pancreatic cysts. Int J Surg Case Rep. 2019;55:192-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Abdelkader A, Hunt B, Hartley CP, Panarelli NC, Giorgadze T. Cystic Lesions of the Pancreas: Differential Diagnosis and Cytologic-Histologic Correlation. Arch Pathol Lab Med. 2020;144:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 37. | Ciers P, Vanderhaeghe D, Vansteenkiste F, Moubax K, Vanooteghem S, Vanneste A, Van Moerkercke W. Lymphoepithelial cysts of the pancreas: case report and review of the literature. Acta Chir Belg. 2023;123:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 38. | Pereira da Costa A, Lopes de Castro G, Lourenço Lira D, Figueiredo HF, Castro Cláudio J, Alexandre MA. Lymphoepithelial cyst of uncinated process of the pancreas associated with chronic cholecystitis: Case report and review of literature. Ann Med Surg (Lond). 2022;80:104117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 39. | Satoh D, Sadamori H, Yagi T, Fujiwara T. Enlarging lymphoepithelial cyst of the pancreas during 12 months of observation: report of a case. Surg Today. 2015;45:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Adsay NV, Hasteh F, Cheng JD, Klimstra DS. Squamous-lined cysts of the pancreas: lymphoepithelial cysts, dermoid cysts (teratomas), and accessory-splenic epidermoid cysts. Semin Diagn Pathol. 2000;17:56-65. [PubMed] |
| 41. | Yokomizo H, Hifumi M, Yamane T, Hirata T, Terakura H, Murata K, Fujita H, Matsukane H. Epidermoid cyst of an accessory spleen at the pancreatic tail: diagnostic value of MRI. Abdom Imaging. 2002;27:557-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |