Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.600
Revised: August 20, 2024
Accepted: September 19, 2024
Published online: October 28, 2024
Processing time: 156 Days and 23.7 Hours
Post-transplant lymphoproliferative disorder (PTLD) is a rare but highly fatal complication occurring after allogeneic hematopoietic cell transplantation (allo-HCT) or solid organ transplantation (SOT). Unlike SOT, PTLD after allo-HCT usually originates from the donor and is rarely accompanied by a loss of donor chimerism.
We report a case of Epstein-Barr virus positive PTLD manifesting as diffuse large B-cell lymphoma (DLBCL) with significantly decreased T-cell chimerism early after allo-HCT. A 30-year-old patient with acute myeloid leukemia underwent unrelated allo-HCT after first complete remission. Nearly 3 mo after transplantation, the patient developed cervical lymph node enlargement and gastric lesions, both of which were pathologically suggestive of DLBCL. Meanwhile, the patient experienced a significant and persistent decrease in T-cell chimerism. A partial remission was achieved after chemotherapy with single agent rituximab and subsequent R-CHOP combined chemotherapy.
The loss of T-cell chimerism and the concomitant T-cell insufficiency may be the cause of PTLD in this patient.
Core Tip: In this paper, we report a case of post-transplant lymphoproliferative disorder (PTLD) after unrelated allogeneic hematopoietic cell transplantation in which the donor T-cell chimerism decreased significantly at the time of PTLD diagnosis, and the primary disease was still in remission. The decreased chimerism in the donor T-cell of this patient may lead to a decreased ability to control Epstein-Barr virus reactivation, which is directly related to the occurrence of PTLD.
- Citation: Guo QN, Liu HS, Li L, Jin DG, Shi JM, Lai XY, Liu LZ, Zhao YM, Yu J, Li YY, Yu FQ, Gao Z, Yan J, Huang H, Luo Y, Ye YS. Epstein-Barr virus positive post-transplant lymphoproliferative disorder with significantly decreased T-cell chimerism early after transplantation: A case report. World J Radiol 2024; 16(10): 600-607
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/600.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.600
Post-transplant lymphoproliferative disorder (PTLD) is a rare complication in patients with allogeneic hematopoietic cell transplantation (allo-HCT). However, it often has an acute onset and progresses rapidly. PTLD can progress to multiple organ failure in a short time with a high mortality. About 80% of PTLDs occur within 1 year after transplantation and are associated with Epstein-Barr virus (EBV) reactivation[1]. Patients are in an immunodeficient state early after trans
We report a case of PTLD after unrelated allo-HCT in which the donor T-cell chimerism decreased significantly at the time of PTLD diagnosis, and the primary disease was still in remission. The decreased chimerism in the donor T-cells of this patient may lead to a decreased ability to control EBV reactivation, which is directly related to the occurrence of PTLD.
A 30-year-old Chinese man was diagnosed with acute myeloid leukemia for 6 months, allo-HCT for 3 months, and cervical lymph node enlargement for 1 day.
Six months ago, the patient was diagnosed with acute myeloid leukemia (risk category was intermediate according to 2022 ELN risk classification). Venetoclax-homoharringtonine-acclarithromycin-cytarabine induction chemotherapy was given, and reexamination of bone marrow smear after hematopoietic recovery showed a complete remission (CR) and negative bone marrow minimal residual disease (MRD). Subsequently, two cycles of consolidation therapy with inter
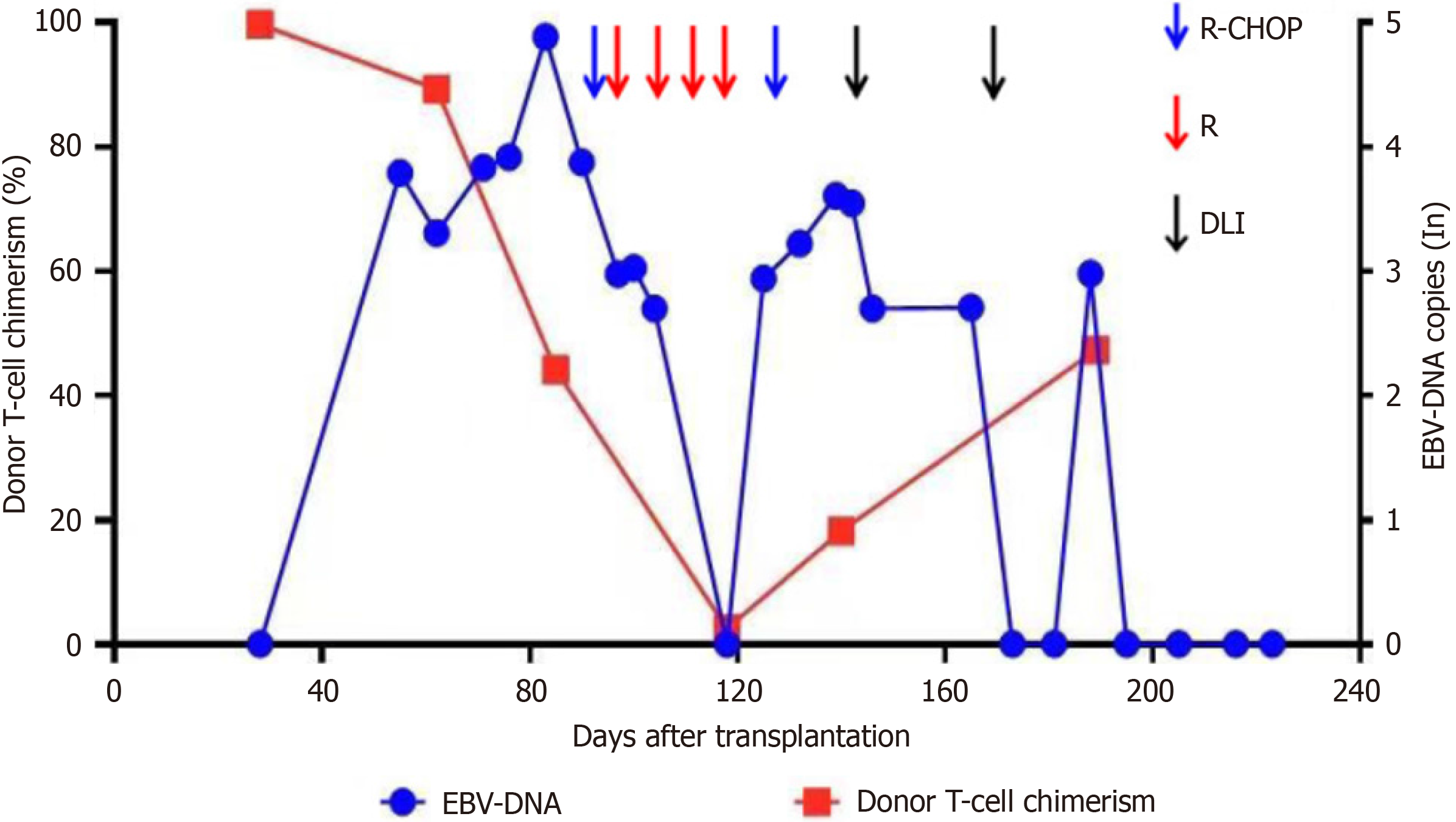
Three months ago, the patient underwent allo-HCT. Conditioning regimen with BUCY + MeCCNU + ATG was given on March 8, 2022, with Busulfan 0.8 mg/kg/d (days -7, -6, -5, and -4), cyclophosphamide 60 mg/kg/d (days -3 and -2), Semustine 250 mg/m2 (day -1), anti-human T-cell rabbit immunoglobulin 6 mg/kg/d (days -4, -3, -2, and -1). On March 15, 2022, the patient received peripheral blood hematopoietic stem cells from an unrelated donor (male, 45 years old, HLA match 9/10) from the China Marrow Donor Program with transfusion of mononucleated cells 13.49 × 108/kg and CD34+ cells 3.67 × 106/kg. The donor and recipient were positive for EBV-IgG and negative for EBV-IgM before transplantation. Cyclosporine A + Mycophenolatemofetil + methotrexate were given to prevent acute GVHD. The follow-up treatment is shown in Figure 1.
Reexamination of bone marrow smear on day +28 post-transplant showed CR and negative MRD. The bone marrow short tandem repeat sequence (STR) examination showed a full donor chimerism. On day +55, EBV-DNA was 6.17 × 103 copies/L. Then immunosuppressive agents were withdrawn and on day +62 when the STR report showed 89.35% for T-cell, 97.06% for B-cell and 97.73% for NK-cell chimerism, respectively. Meanwhile, the bone marrow smear showed continued CR and negative MRD. On day +83, EBV-DNA increased to 7.69 × 104 copies/L, and the patient presented with left cervical lymph node enlargement.
The patient had no history of past illness.
The patient denied any family history of malignancy.
Physical examination showed left cervical lymph node enlargement.
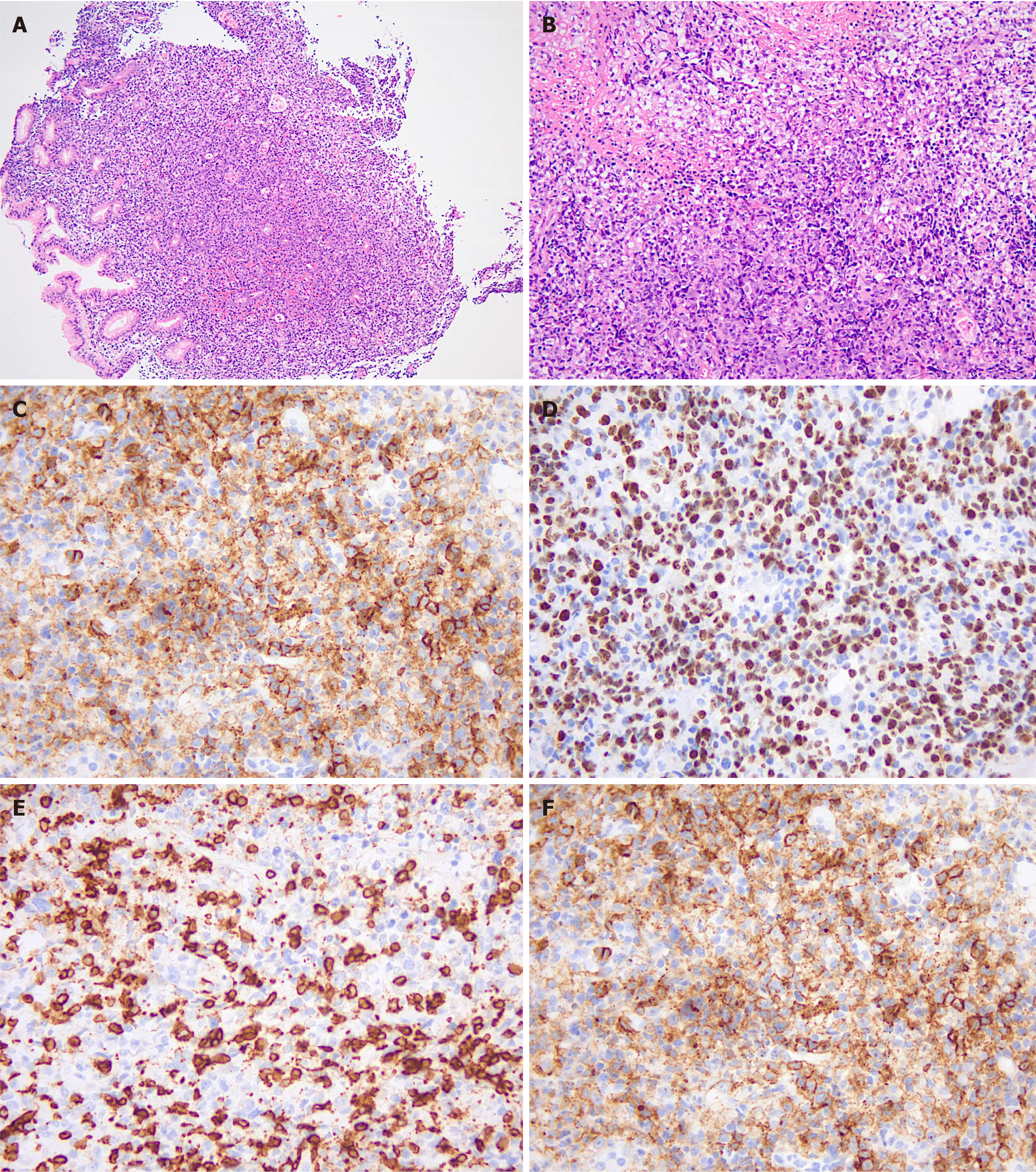
Pathology of the cervical lymph node showed dysplasia of lymphoid tissue with extensive necrosis, consistent with PTLD, B-cell, monomorphic [diffuse large B-cell lymphoma (DLBCL)], non-GCB, with EBV infection). Immunohistochemistry results were: CD20 (+), PAX-5 (+), CD10 (-), BCL-6 (partially +), MUM1 (+), CD5 (-), BCL-2 (+), C-Myc (-), CD30 (+20%), CD3 (-), CyclinD1 (-), CD21 (-), Ki-67 (+50%), PD-1 (+), EBER (+), ALK (-), CD4 (little +), CD8 (+), Lysozyme (-), MPO (-), CD34 (vascular +), CD117 (-), TDT (-), and CD56 (-) (Figure 2).
The bone marrow and chimerism were re-examined and the STR showed 44.12% for T-cells, 87.77% for B-cells, and 91.01% for NK cells, respectively. The bone marrow remained in CR and bone marrow biopsy showed no lymphoma involvement. Afterwards, the donor T-cell chimerism decreased dramatically and immunosuppressive therapy was discontinued on day +95. Seventeen milliliters of donor lymphocytes (CD3+ cells, 0.48 × 107/kg) were infused on day +91.
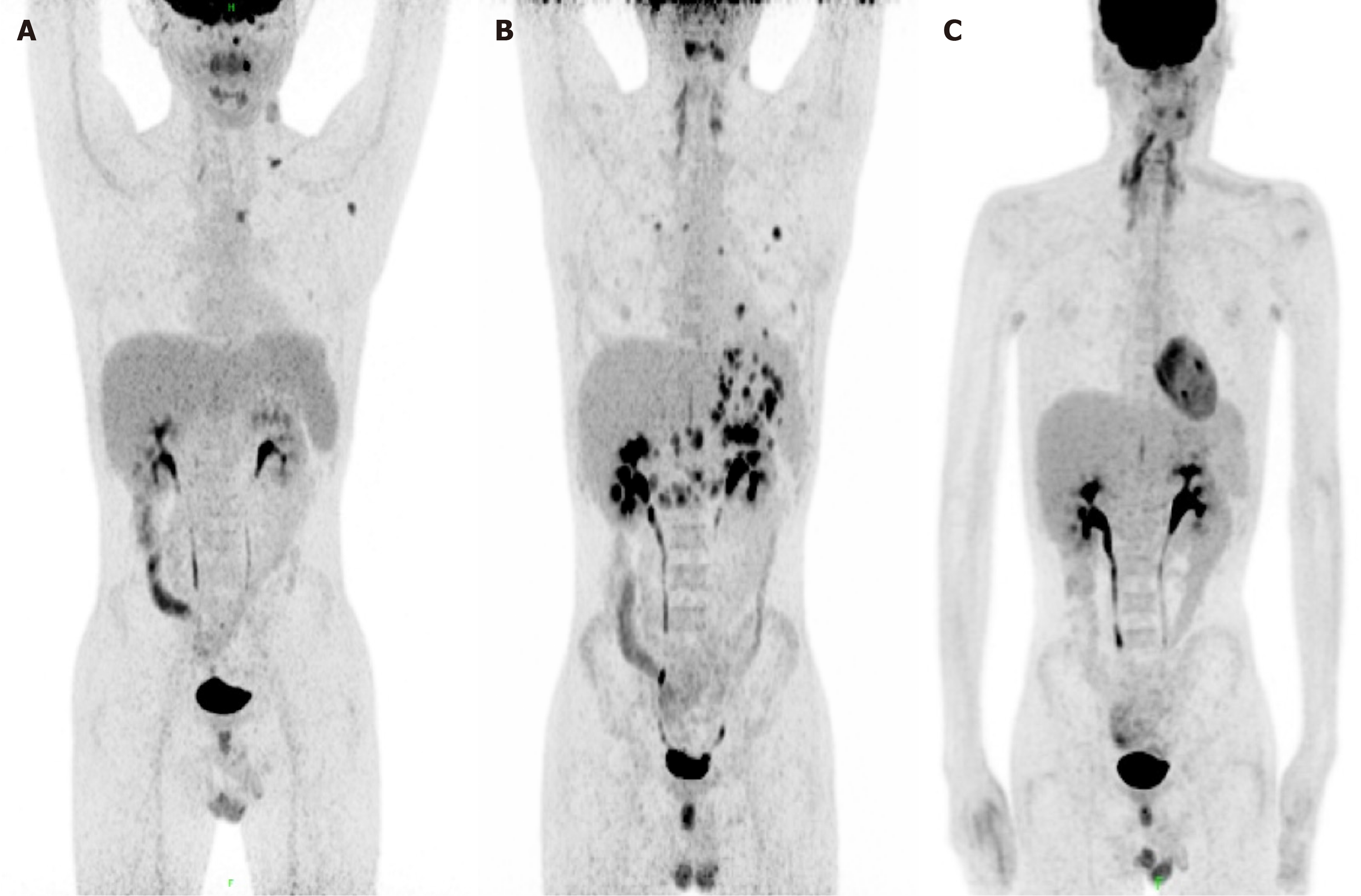
PET/CT on day +94 showed the left clavicular region and mediastinal region 3A, a few slightly enlarged lymph nodes in the left axilla, and increased FDG metabolism (SUVmax = 5.8) in the presacral region, suggestive of lymphoma infiltration; increased FDG metabolism in the left tonsil and nodule-like FDG in the left nasopharynx (SUVmax = 10.4), suggestive of lymphoma infiltration (mediastinal SUVmax = 1.9; liver SUVmax = 2.9 (Figure 3A).
EBV+ PTLD with significantly decreased T-cell chimerism early after transplantation.
Single agent rituximab (600 mg) was given on days +95, +104, +111, and +118, respectively. The enlarged lymph node subsided and ultrasound showed no abnormal enlarged lymph nodes. Reexamination of the STR test on day +118 showed that chimerism was 2.49% for T-cells, 37.33% for B-cells, and 32.75% for NK cells, respectively. On day +125, the patient presented with epigastric discomfort and PET/CT showed increased FDG metabolism in soft tissue density nodules in the right posterior parietal wall of the nasopharynx; multiple lymph nodes in the bilateral parotid area with increased FDG metabolism (SUVmax = 4.8); and slightly increased FDG metabolism (SUVmax = 7.8) in the left wall of the nasopharynx, suggestive of the possibility of lymphoma infiltration. Newly multiple irregular thickenings of the gastric wall and increased focal FDG metabolism (SUVmax = 8.9) were observed. Combined with the history, the possibility of lymphoma infiltration was considered. Multiple solid nodules were found scattered in both lungs with increased FDG metabolism (Figure 3B). Considering that the chimerism continued to decline, 34 mL of donor lymphocytes (CD3+ cells of 0.96× 107/kg) were reinfused on day +128. Gastroscopy showed multiple IIa + IIc mucosal bulges in the gastric fundus, body, angle, and sinus, about 0.5 cm-1.2 cm in size, with ulcers visible in the center of some bulges. Gastric tissue biopsy revealed diffuse infiltration of heterogeneous lymphocytes with diffuse EBV infection, consistent with PTLD manifesting as B-cell non-Hodgkin's lymphoma (diffuse large B-cell, non-GCB). Immunohistochemistry revealed: CD20 (mostly weakly +), CD19 (+), PAX-5 (partially +), CD10 (-), Bc1-6 (20% +), MUM1 (+), CD5 (-), Bc1-2 (90% +), c-Myc (20% +), CD30 (80% +), CD3 (-), Cyclin D1 (-), CD21 (-), Ki-67 (80% +), P53 (partially weakly +), PD-1 (40% +), EBER (+), CD23 (-), HE, ALK (-), CD34 (-), CD117 (-), and MPO (-) (Figure 4).
Fluorescence in situ hybridization revealed negativity for C-myc, BcL-2, and BcL-6 gene rearrangement. In order to better control disease, R-CHOP regimen (rituximab 600 mg d0, cyclophosphamide 1.15 g/d, doxorubicin liposome 46 mg d1, vincristine 4 mg d1, dexamethasone 15 mg/d1-5) was given on days +141 and +167, respectively.
PET/MRI on day +209 showed that compared with the original PET/MRI, the nasopharynx, uvula, bilateral parotid area, and hypermetabolic lesions in the gastric wall were not observed, suggesting that local tumor activity was significantly suppressed; the number of multiple lesions in the lung was significantly reduced, and partial remission was achieved; there was a diffuse increase in FDG metabolism in the bone marrow cavity of the scanned area, which was considered to be caused by granulocyte colony-stimulating factor in conjunction with the medical history. PET/MRI on day +322 showed that no significant abnormal foci of increased FDG metabolism (Figure 3C). Up until May 1, 2023 (day +412), the patient has no discomfort, with MRD negative CR and no sign of GVHD.
PTLD is one of the most serious complications associated with transplantation, which is rare but has a high mortality rate. A mortality rate of 84.6% was reported in early years. In an EBMT study, the overall incidence of PTLD after allo-HCT was 3.22%[4]. The incidence of PTLD is highest in 1 to 5 mo after transplantation, and the peak incidence occurred in the third month posttransplant. The clinical manifestations of PTLD are diverse, including fever, pharyngitis, lymphadenopathy, hepatosplenomegaly, central nervous system symptoms, and even multiple organ dysfunction, which can not be explained by other reasons such as infection or immunity. In this case, EBV viremia with decreased chimerism occurred nearly 2 mo after transplantation, which was followed by lymphadenopathy and a subsequent diagnosis of EBV-positive DLBCL. According to the ECIL-6 guidelines, the patient was in a high-risk group for developing PTLD[5]. The risk factors included mismatched unrelated donor, ATG-containing conditioning regimen, and increased EBV load after trans
EBV has been identified as the cause of several human cancers including PTLD. Allo-HCT associated PTLD is usually donor-derived and originates from EBV-infected B-lymphocytes[6]. EBV+ PTLD is classically associated with the most elaborate viral expression pattern latency III[7]. EBV is highly immunogenic, and during primary infection, healthy individuals mount a vigorous humoral and cellular immune response through the cellular components consisting of CD4+ and CD8+ T-cells, which controls both primary infection and the periodic reactivation that occurs in all EBV-sero
EBV latent infection latency III is directly associated with PTLD, although EBNA3, expressed only during latency III, is a target for cytotoxic T-cells[10]. The number and function of cytotoxic T-cells are reduced in immunocompromised patients, and EBV-specific cytotoxic T-cells play an important role in controlling the occurrence of PTLD. Decreased immune function along with decreased T-cell chimerism indicated that the number and function of T-cells were dimi
Donor chimerism decline or loss is rare in patients who develop PTLD. This case is unique in that the occurrence of PTLD was accompanied by a dramatic decline in chimerism, especially in T-cells. Meanwhile, the bone marrow exami
Recovery of T-cell counts in the first 6 mo after transplant is mainly dependent on the peripheral expansion of mature T-cells driven by cytokines [interleukin (IL)-15, IL-2, and IL-7]. These mature T-cells originate either from the donor in the case of a non T-cell depleted (TCD) bone marrow transplant or from host T-cells surviving the conditioning regimen in the case of a TCD transplant[11]. The decrease in T-cell chimerism in this patient was mainly considered to be related to the impaired or absent T-cell function of the donor itself. ATG has the function of removing T-lymphocytes from both donor and recipient, which is known as "in vivo T-lymphocytes depletion". In addition to the routine use of reduced-dose conditioning transplantation, ATG is also widely used in unrelated donor transplantation and HLA haploidentical donor transplantation. It has been shown that ATG can persist in vivo until +90 d after transplantation, and it is unclear whether the dramatic decrease in T-cell chimerism is related to the excessive immunosuppression caused by ATG. The first-line treatment for PTLD includes rituximab (375 mg/m2 once weekly), combination of rituximab with reduced dose of immunosuppressive agents, and EBV-specific cytotoxic T lymphocytes from donor or third-party sources. Unselected donor lymphocyte infusion from EBV-positive donors is used to restore T-cell reactivity, including EBV-specific re
The loss of T-cell chimerism and the concomitant T-cell insufficiency may be the cause of PTLD in this patient.
The authors are grateful to the patient for his enthusiastic collaboration and his informed consent to publish the present case report.
| 1. | Lowe T, Bhatia S, Somlo G. Second malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:1121-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | Zutter M, Martin P, Sale G, Shulman H, Fisher L, Thomas E, Durnam D. Epstein-Barr virus lymphoproliferation after bone marrow transplantation. Blood. 1988;72:520-529. [RCA] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 264] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 3. | Au WY, Lie AK, Lee CK, Ma SK, Wan TS, Shek TW, Liang R, Kwong YL. Late onset post-transplantation lymphoproliferative disease of recipient origin following cytogenetic relapse and occult autologous haematopoietic regeneration after allogeneic bone marrow transplantation for acute myeloid leukaemia. Bone Marrow Transplant. 2001;28:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, Omar H, Martino R, Halkes C, Faraci M, Theunissen K, Kalwak K, Hubacek P, Sica S, Nozzoli C, Fagioli F, Matthes S, Diaz MA, Migliavacca M, Balduzzi A, Tomaszewska A, Camara Rde L, van Biezen A, Hoek J, Iacobelli S, Einsele H, Cesaro S; Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, Ljungman P; Sixth European Conference on Infections in Leukemia, a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP), the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer (EORTC-IDG), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 6. | Dierickx D, Pociupany M, Natkunam Y. Epstein-Barr virus-associated posttransplant lymphoproliferative disorders: new insights in pathogenesis, classification and treatment. Curr Opin Oncol. 2022;34:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Morscio J, Tousseyn T. Recent insights in the pathogenesis of post-transplantation lymphoproliferative disorders. World J Transplant. 2016;6:505-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Heslop HE. How I treat EBV lymphoproliferation. Blood. 2009;114:4002-4008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | DeStefano CB, Desai SH, Shenoy AG, Catlett JP. Management of post-transplant lymphoproliferative disorders. Br J Haematol. 2018;182:330-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Fujimoto A, Suzuki R. Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disorders after Hematopoietic Stem Cell Transplantation: Pathogenesis, Risk Factors and Clinical Outcomes. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Toubert A, Glauzy S, Douay C, Clave E. Thymus and immune reconstitution after allogeneic hematopoietic stem cell transplantation in humans: never say never again. Tissue Antigens. 2012;79:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |