Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.579
Revised: September 5, 2024
Accepted: September 24, 2024
Published online: October 28, 2024
Processing time: 165 Days and 4.1 Hours
Macrophage activation syndrome (MAS), a sub-type of hemophagocytic lymphohistiocytosis (HLH) secondary to autoimmune rheumatic diseases, is a critical and potentially fatal condition characterized by an excessive inflammatory response. Despite the established efficacy of the HLH-2004 guideline in diagnosing and tre
In this case study, we propose a potentially effective strategy for managing a pa
Low dose etoposide proves to be an effective approach in alleviating HLH while mitigating the risk of infection.
Core Tip: Etoposide has been utilized in severe and refractory cases of macrophage activation syndrome (MAS). However, in cases where severe infections and autoimmune disorders are present concurrently, the use of etoposide carries the risk of bone marrow suppression and exacerbation of infections. In this case report, we successfully avoided bone marrow suppression and controlled both MAS and severe infection in patients in such condition by using a modified etoposide regimen.
- Citation: Gao SP, Luo XF, Kosari M, Li WJ, Yang L, Tu W, Zhong JX. Successful management of infection and macrophage activation syndrome patient using low-dose etoposide: A case report. World J Radiol 2024; 16(10): 579-585
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/579.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.579
Hemophagocytic lymphohistiocytosis (HLH) encompasses a range of hematological conditions with potentially life-threatening conditions, characterized by hyperactivation of the immune system, especially macrophages, cytotoxic T-cells, and NK cells. There are two distinct types of HLH, the primary (genetic) HLH, which is commonly seen in the pe
The overall mortality rate of MAS is reported to be around 12%[5], and notably exceeds 42% in SLE patients[6]. Etoposide (VP-16), an antineoplastic agent that exerts its effects through the inhibition of topoisomerase II, has been approved by FDA for the treatment of small cell lung cancer. However, due to its excellent efficacy in managing MAS/HLH, it was recommended for the treatment of HLH in HLH-2004 guideline[7]. By inducing DNA damage and sub
Her major complaint upon arriving at our hospital was an unremitting high fever of unknown origin that had lasted for over a week, peaking at 39.1 °C. The patient also reported proximal muscle weakness in the upper extremities and a dry mouth.
In February 2023, a 40-year-old Chinese female was referred to our hospital shortly after receiving diagnoses of SLE and pulmonary infection at a local medical facility. She provided a verbal informed consent for the documentation of her case. Other investigations were unremarkable. Prior to admission to Tongji Hospital, she had been taking oral prednisone 5 mg once daily.
Additionally, she was infected with COVID-19 two months prior to her admission and continued to experience intermittent episodes of coughing with white frothy sputum.
Apart from a cesarean-section surgery in 2007, her medical history held no significant events.
During our examination, we observed a non-pruritic butterfly-shaped rash and facial swelling. On auscultation, bilateral moist rales were detected.
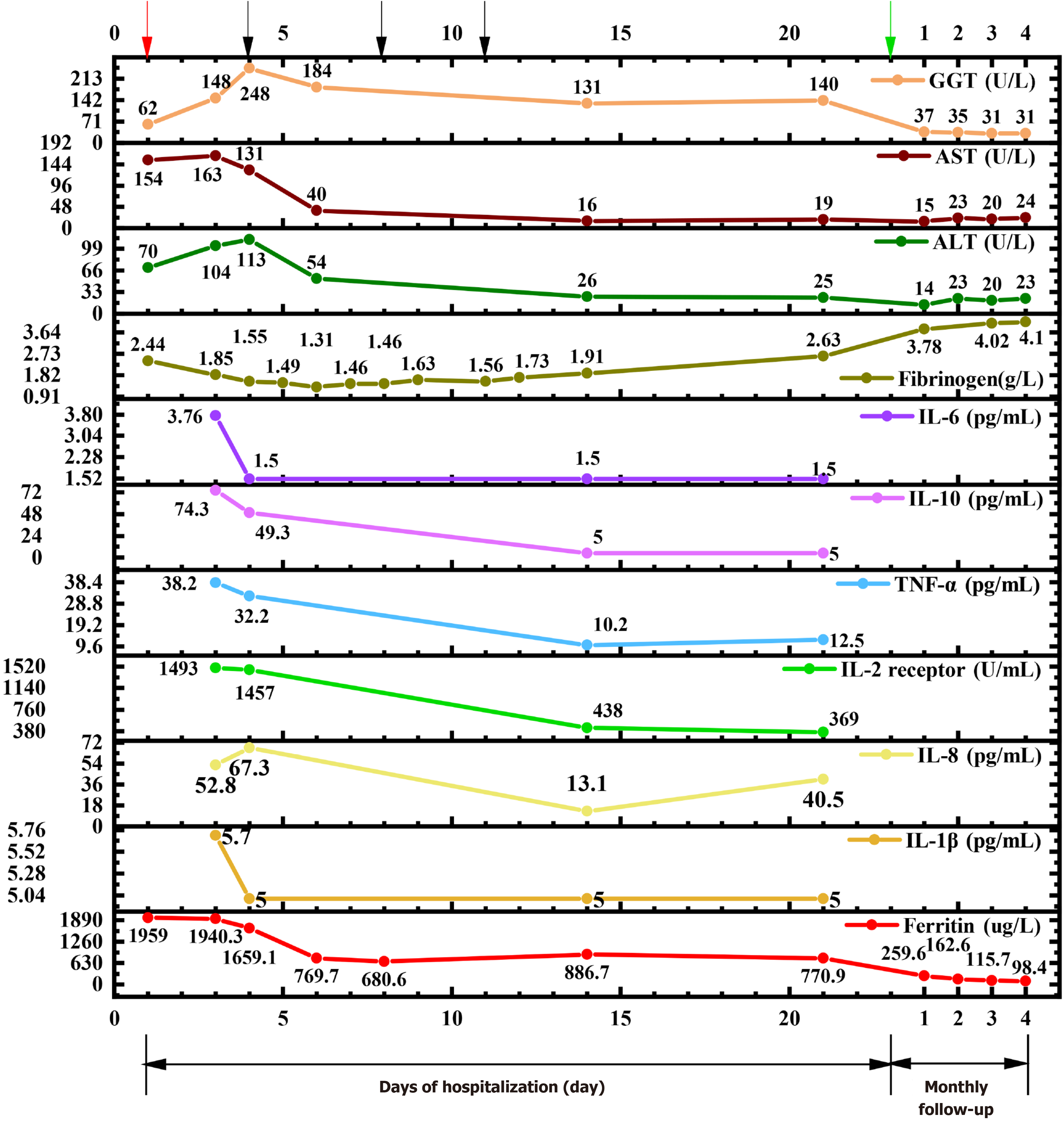
Upon admission, we conducted a thorough investigation, affirming our diagnosis of MAS based on her prior medical history involving EBV, CMV, COVID-19 infection plus SLE. Notably, we observed significant elevations in ferritin, aspartate transaminase (AST), alanine transaminase (ALT), gamma-glutamic transaminase (GGT), and IL-2R (sCD25), while fibrinogen levels were found to be diminished (Figure 1). The patient had fever over a week, peaking at 39.1 before admission to our hospital, hypertriglyceridemia (3.51 mmol/L), high ferritin (1959 μg/L), low number of NK cells [NK cells count: 75/μL (Reference range: 150-1100/μL)]; high sCD25 (IL-2R) level [1493 U/mL (reference range: 223-710 U/mL)], supporting the diagnosis of HLH according to the HLH 2004 criteria. However, hemophagocytosis was not evident in the bone marrow biopsy, and no hepatosplenomegaly was observed in the ultrasound. The rheumatological blood panel disclosed several abnormal markers consistent with an SLE diagnosis. Specifically, we detected elevations in ANA, anti-dsDNA, anti-Smith antibody, anti-u1-nRNP, anti-histone antibody, anti-nucleosome antibody, and anti-RO52, while complement 3 and complement 4 were low. Although both CMV and EBV nucleic acid tests were negative, serological markers indicated a pattern of past infection. Urinalysis showed a total urine protein of 84 mg/L, with a total trace protein of 14.4 mg/L. A 24-hour urine collection showed a total urine protein of 243.6 mg/24 hours, and a total trace protein of 41.8 mg/24 hours, indicating damage to kidney due to SLE.
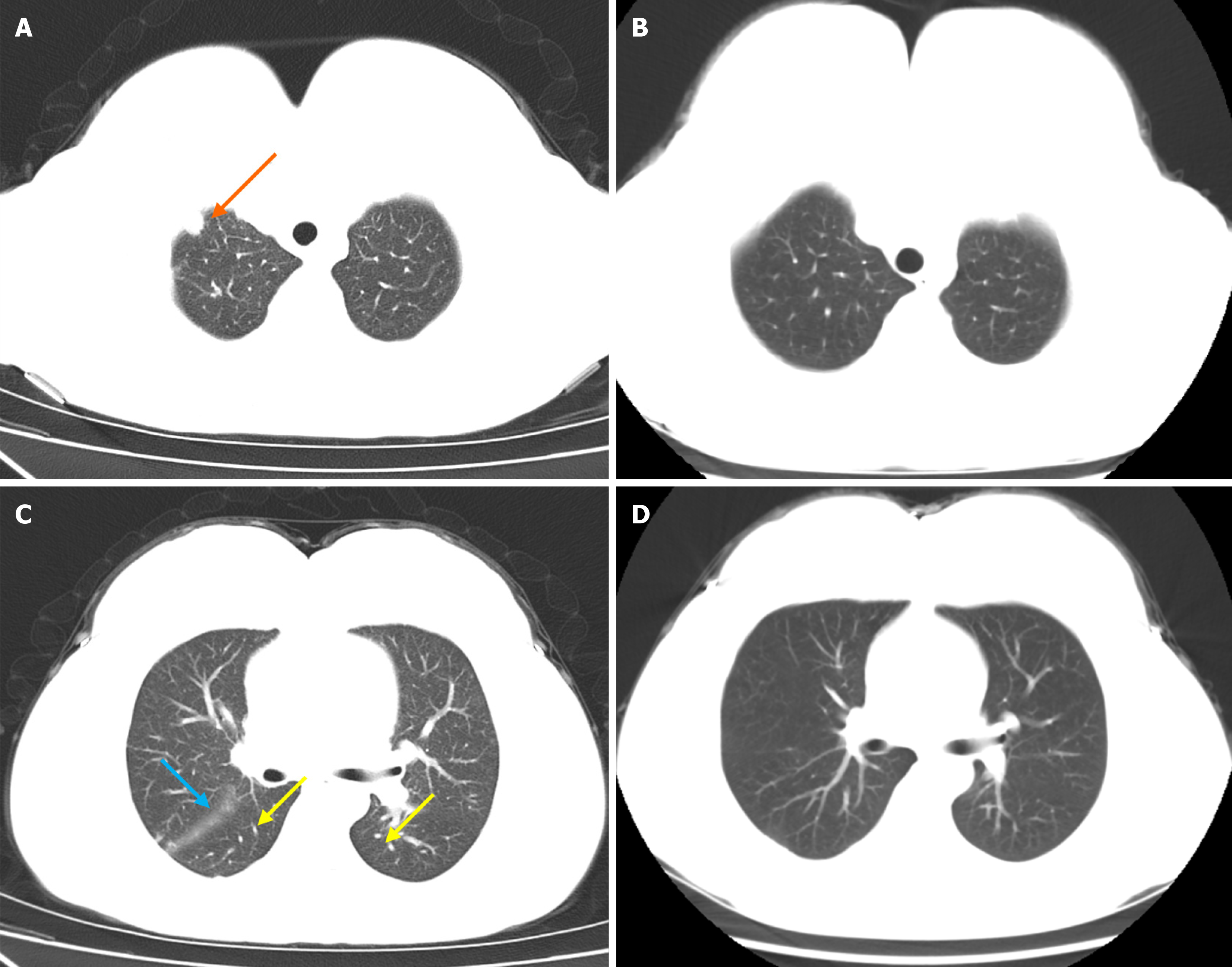
Chest computed tomography (CT) revealed several key features: Multiple nodules in both lungs, notably a larger nodule at the right lung apex; thickening of interlobular septa in both lungs, indicative of interstitial pulmonary edema; a small volume of pericardial effusion; a slight bilateral pleural effusion; and a slight bilateral interlobular effusion.
The patient was diagnosed with MAS and SLE with pulmonary infection.
The patient was given methylprednisolone succinate 40 mg IV (daily), cyclosporine A, cyclophosphamide, and a limited course of etoposide 100 mg twice weekly for 3 sessions upon admission. Prior to initiating etoposide treatment, the patient developed hypoxemia. A chest CT angiography ruled out embolism, prompting consideration of hypoxemia origins: Pulmonary inflammatory exudation or microthrombosis linked to hemophagocytic syndrome. As precautionary measure, prophylactic heparin was prescribed. Following the application of etoposide, various indicators exhibited significant improvement. Initial laboratory results before etoposide treatment revealed a hyper-inflammatory state, characterized by elevated levels of ferritin (1659.1 ug/L), lactate dehydrogenase (325 U/L), IL-2 receptor (1457 U/mL), IL-10 (49.3 pg/mL), and TNF-α (32.2 pg/mL). Despite several days of treatment with methylprednisolone succinate and cyclophosphamide, no favorable changes were observed. Due to diagnosis of hemophagocytic syndrome and pulmonary infection, a low-dose etoposide regimen (100mg IV) was administered on the 4th, 8th, and 11th days of hospitalization, totaling 300 mg. Interestingly, after the first etoposide administration, a remarkable decrease in ferritin level (from 1659.1 to 769.7 ug/L) was seen. Furthermore, a notable decrease of at least two-fold was observed in IL-2 receptor, IL-10, and TNF-α. Fibrinogen level dropped below 2 g/L and remained low for 10 days (Figure 1). Hence, a single dose of human fibrinogen 500 mg IV was infused, resulting in a gradual and sustained increase in fibrinogen levels, which was normalized by the 21st day of hospitalization. Fibrinogen infusion and the third dose of etoposide were administered on the 11th day of hospitalization. Because both treatments were given on the same day, it's possible that the improvement was a result of both primary and supportive treatments. In conjunction with etoposide, other treatments were introduced, including “IVIG” 5 g/day for five consecutive days (totaling 25 g), as well as cefoperazone + sulbactam 3 g IV daily, ganciclovir 250 mg IV daily, fluconazole 200mg PO daily to prevent further pulmonary complications. Cefoperazone and sulbactam were used on the second day of hospitalization. On the first day, white blood counts (WBC) count was 2.45 × 109/L (reference range: 3.5-9.5 × 109/L), neutrophils count was 1.61 × 109/L (reference range: 1.8-6.3 × 109/L), lym
Monthly appointments were arranged until 4 months after discharge, revealing evident improvement in lung lesions. Anti-dsDNA testing yielded negative results, indicating remission of SLE. No coagulopathy was present. Ferritin levels remained within the desirable range. Chest CT result showed the presence of micronodules in the upper lobe of the right lung and lower lobe of the left lung. A few cord-like foci were observed in the lingular segment of the upper lobe of the left lung and the lower lobe of the right lung. Some nodules had disappeared compared with the previous CT scan (Figure 2). There was no evidence of malignancy 4 months after discharge.
Etoposide is typically considered for refractory cases of HLH or when the first-line treatment (high-dose corticosteroid and cyclosporine) fails to yield desirable outcomes. In this case report, given the undesirable responses to treatments with methylprednisolone succinate and cyclophosphamide, we decided to introduce etoposide. However, administering high doses of etoposide may potentially lead to bone marrow suppression, which posed a concern given the patient’s con
Cytotoxic T cells (CTLs), also known as killer T cells or CD8+ T cells, are a type of T cells that are able to eliminate cancer cells, virus-infected cells, as well as damaged cells. Low-dose etoposide is believed to restore immune homeostasis by clearing activated immune cells including CTLs and macrophages, along with restraining their production of inflammatory cytokines. This action not only diminishes macrophage activity, but also prompts newly activated CTLs to clear macrophages and virus-infected cells[15].
In conclusion, employing a low dose and short course of etoposide proves to be an effective approach in alleviating MAS while mitigating the risk of infection. This approach is particularly suitable for MAS patients with concurrent infection. By reducing the cumulative etoposide dosage and optimizing the dosages of other medications to their minimal effective levels can minimize the risk of potential adverse effects. However, it’s worth noting that the available data are limited, thus further research is necessary to fully explore the potential benefit of low dose etoposide.
| 1. | Hadchouel M, Prieur AM, Griscelli C. Acute hemorrhagic, hepatic, and neurologic manifestations in juvenile rheumatoid arthritis: possible relationship to drugs or infection. J Pediatr. 1985;106:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 152] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Rouphael NG, Talati NJ, Vaughan C, Cunningham K, Moreira R, Gould C. Infections associated with haemophagocytic syndrome. Lancet Infect Dis. 2007;7:814-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 3. | Gao ZW, Wang X, Lin F, Dong K. The correlation between SARS-CoV-2 infection and rheumatic disease. Autoimmun Rev. 2020;19:102557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, Requena-Caballero L, Jordan MB, Abdel-Wahab O, Allen CE, Charlotte F, Diamond EL, Egeler RM, Fischer A, Herrera JG, Henter JI, Janku F, Merad M, Picarsic J, Rodriguez-Galindo C, Rollins BJ, Tazi A, Vassallo R, Weiss LM; Histiocyte Society. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 973] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 5. | He L, Yao S, Zhang R, Liu M, Hua Z, Zou H, Wang Z, Wang Y. Macrophage activation syndrome in adults: Characteristics, outcomes, and therapeutic effectiveness of etoposide-based regimen. Front Immunol. 2022;13:955523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 6. | Nam SH, Ahn SM, Oh JS, Hong S, Lee CK, Yoo B, Kim YG. Macrophage activation syndrome in rheumatic disease: Clinical characteristics and prognosis of 20 adult patients. PLoS One. 2022;17:e0267715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 7. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3599] [Article Influence: 199.9] [Reference Citation Analysis (1)] |
| 8. | Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Koyama M, Sawada A, Yasui M, Inoue M, Kawa K. Encouraging results of low-dose etoposide in the treatment of early-onset hemophagocytic syndrome following allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2007;86:466-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Horne A, von Bahr Greenwood T, Chiang SCC, Meeths M, Björklund C, Ekelund M, Erensjö P, Berg S, Hagelberg S, Bryceson YT, Andersson U, Henter JI. Efficacy of Moderately Dosed Etoposide in Macrophage Activation Syndrome-Hemophagocytic Lymphohistiocytosis. J Rheumatol. 2021;48:1596-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 11. | Wang R, Li T, Ye S, Lv L, Chen S, Wang X, Bao CD, Fu Q. Short-term, low-dose etoposide in refractory adult-onset Still's disease-associated macrophage activation syndrome. Clin Rheumatol. 2022;41:2817-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Tokushige J, Ueki T, Sato K, Fujikawa Y, Shimizu I, Akahane D, Sumi M, Ueno M, Ichikawa N, Kobayashi H. [Successful treatment with low-dose etoposide for hemophagocytic syndrome following reduced-intensity conditioning for cord blood transplantation in a patient with acute myelgenous leukemia]. Rinsho Ketsueki. 2012;53:535-539. [PubMed] |
| 13. | Seo YI, Park R, Choi TY, Shin JW, Won JH, Park HS, Lee NS, Cho D. [A case of therapy-related acute monocytic leukemia following low-dose of etoposide treatment for hemophagocytic lymphohistiocytosis]. Korean J Lab Med. 2007;27:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Wang HT, Li T, Ye S, Wang XD. [Clinical analysis of low-dose etoposide in the treatment of refractory adult Still's disease]. Zhonghua Fengshibing Zazhi. 2018;22:5. |
| 15. | Takami A. Possible role of low-dose etoposide therapy for hemophagocytic lymphohistiocytosis by COVID-19. Int J Hematol. 2020;112:122-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |