Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.545
Revised: September 14, 2024
Accepted: October 8, 2024
Published online: October 28, 2024
Processing time: 196 Days and 18.2 Hours
Exertional heat stroke (EHS) is a critical condition arising from prolonged physical exertion in high temperatures that typically presents with normal hemoglobin levels. However, atypical presentations can also occur, leading to significant complications such as hemolytic anemia and organ dysfunction.
This case report describes a male patient who experienced moderate-to-severe anemia that was difficult to correct, with a confirmed diagnosis of microangiopathic hemolytic anemia accompanying multiple organ dysfunction syndrome, indicative of critical EHS. Despite intensive resuscitation efforts, the patient’s condition deteriorated, necessitating admission to the intensive care unit for advanced management.
This case highlights the importance of recognizing atypical presentations of EHS, particularly that with significant hemolytic anemia and concurrent organ failure. Clinicians should maintain a high level of suspicion for these complications in patients displaying symptoms of heat-related illness, especially when caused by strenuous activity, as early diagnosis and intervention are crucial to improve patient outcomes.
Core Tip: This case report emphasizes the critical need for clinicians to recognize atypical presentations of exertional heat stroke, particularly when patients exhibit significant hemolytic anemia and multiple organ dysfunction. Early diagnosis and prompt intervention are essential to manage these complications to improve patient outcomes, especially after strenuous physical activity in high temperatures.
- Citation: Xiang CH, Zhang XM, Liu J, Xiang J, Li L, Song Q. Exertional heat stroke with pronounced presentation of microangiopathic hemolytic anemia: A case report. World J Radiol 2024; 16(10): 545-551
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/545.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.545
Exertional heat stroke (EHS), a type of heat stroke (HS), is a critical illness induced by heat injury, occurring when high-intensity physical activity causes an imbalance between heat production and heat dissipation in the body, leading to disease onset and a core body temperature exceeding 40 °C. This increase in body temperature may be accompanied by impaired consciousness and multiple organ dysfunction (MODS)[1], but is accompanied by a normal hemoglobin level[2].
The patient described in this report developed refractory moderate-to-severe anemia, with a peripheral blood smear showing a maximum ruptured red blood cell rate exceeding 15%, and a significantly decreased haptoglobin level. The patient was diagnosed with microangiopathic hemolytic anemia (MAHA) with concomitant MODS, indicating extremely severe EHS. In this report, we aimed to review the disease course, and the diagnosis and treatment protocols are analyzed to improve our understanding of EHS and concomitant MAHA. The diagnosis and treatment of this patient with extremely severe EHS are summarized to provide a reference for future diagnosis and treatment of similar critical cases.
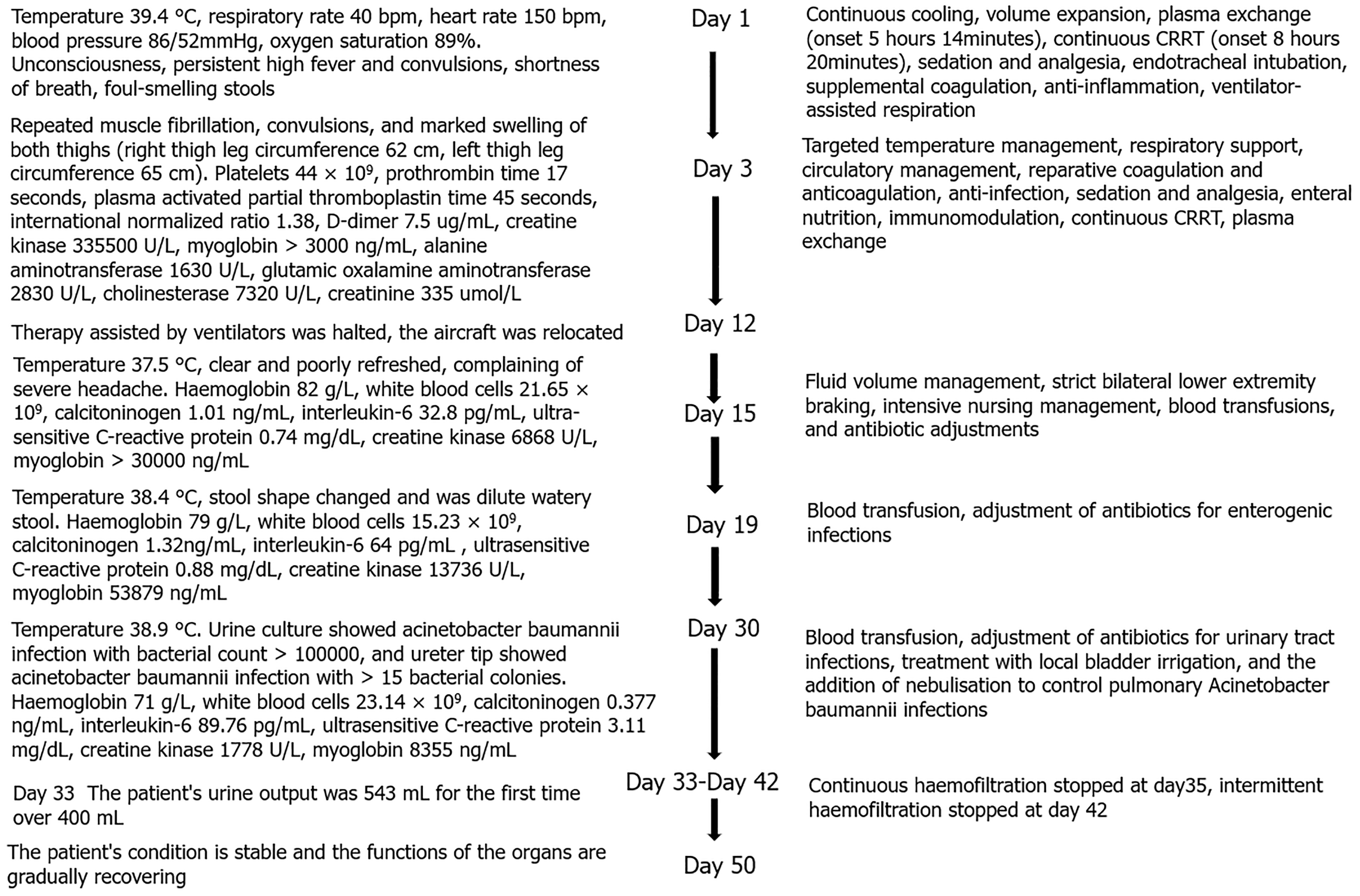
The patient was transferred to our department and admitted to our hospital due to high fever, loss of consciousness, and anuria for 13 days after an episode of exercise.
The patient was a 25-year-old male with a body mass index of 25.65, previously in good health and able to tolerate high-intensity physical activity. On August 4, 2023, he was admitted to the hospital after experiencing high fever, loss of consciousness, and anuria for 13 days following an episode of exercise.
On July 23, 2023, he participated in a 5 km training run in a 28 °C temperature and 66% humidity environment. He collapsed 200 m from the finish line after 54 minutes and became unresponsive. On-site medical staff recorded his highest body temperature at 40.3 °C, heart rate at 140 bpm, and blood pressure at 80/50 mmHg. The patient received fluid supplementation and cooling measures before being transferred to a local hospital, where he was diagnosed with EHS.
Blood gas analysis revealed severe acidosis and elevated lactic acid levels. He underwent endotracheal intubation and assisted ventilation due to respiratory distress. Despite treatment, including plasmapheresis, continuous renal repla
The patient had been in good health prior to this incident, with no known chronic illnesses or significant medical history. There were no previous episodes of exertional heat illness or other serious health conditions. He had not undergone any surgeries and did not have any known allergies. His physical activity level was high, as reported by his family and as evidenced by his participation in the training run.
Personal history: The patient was a 25-year-old male with no significant past medical history. He had not undergone any surgeries and had no known allergies. He was physically active, regularly participating in high-intensity workouts and training runs without prior incidents of heat-related illnesses.
Family history: The patient’s family history was unremarkable. There were no known hereditary diseases or conditions in the family.
General appearance: Acute illness presentation; cooperative during the examination.
Vital signs: Temperature, 36.8 °C; heart rate, 82 bpm; respiratory rate, 18 breaths/minute; blood pressure, 156 mmHg/78 mmHg.
Consciousness: Clear mental state.
Skin and eyes: Normal conjunctiva and skin; no jaundice or subcutaneous bleeding.
Abdomen: Liver and spleen not palpable; no tenderness noted.
Muscle strength: Bilateral upper limb muscle strength graded at 4+; bilateral lower limb muscle strength graded at 3+.
Upon admission, the patient’s laboratory results revealed the following: (1) White blood cell count: 10.16 × 109/L; (2) Hemoglobin: 87 g/L (post-transfusion of 1 U of red blood cells); (3) Platelet count: 407 × 109/L; (4) D-Dimer: 13273 ng/mL; (5) Alanine transaminase: 264.4 U/L; (6) Myoglobin: > 3000 ng/mL; (7) Troponin T: 1.1 ng/mL; (8) Creatinine: 307.2 μmol/L; (9) Creatine kinase (CK): 4717 U/L; (10) Lactate dehydrogenase (LDH): 265 U/L; (11) CK isoenzyme: 69.6 U/L; and (12) Pro B-type natriuretic peptide: 2937 pg/mL.
On day 21, the patient’s laboratory results were as follows: (1) Hemoglobin: 64 g/L; (2) Ruptured erythrocyte rate: 15%; (3) LDH: 1213 U/L; (4) Haptoglobin: < 6.63 mg/dL; (5) Serum transferrin: 134 mg/dL; (6) Serum ferritin: 1444 ng/mL; (7) Vitamin B12: 505 pg/mL; (8) Folic acid: 5.62 ng/mL; and (9) Coombs test: Negative.
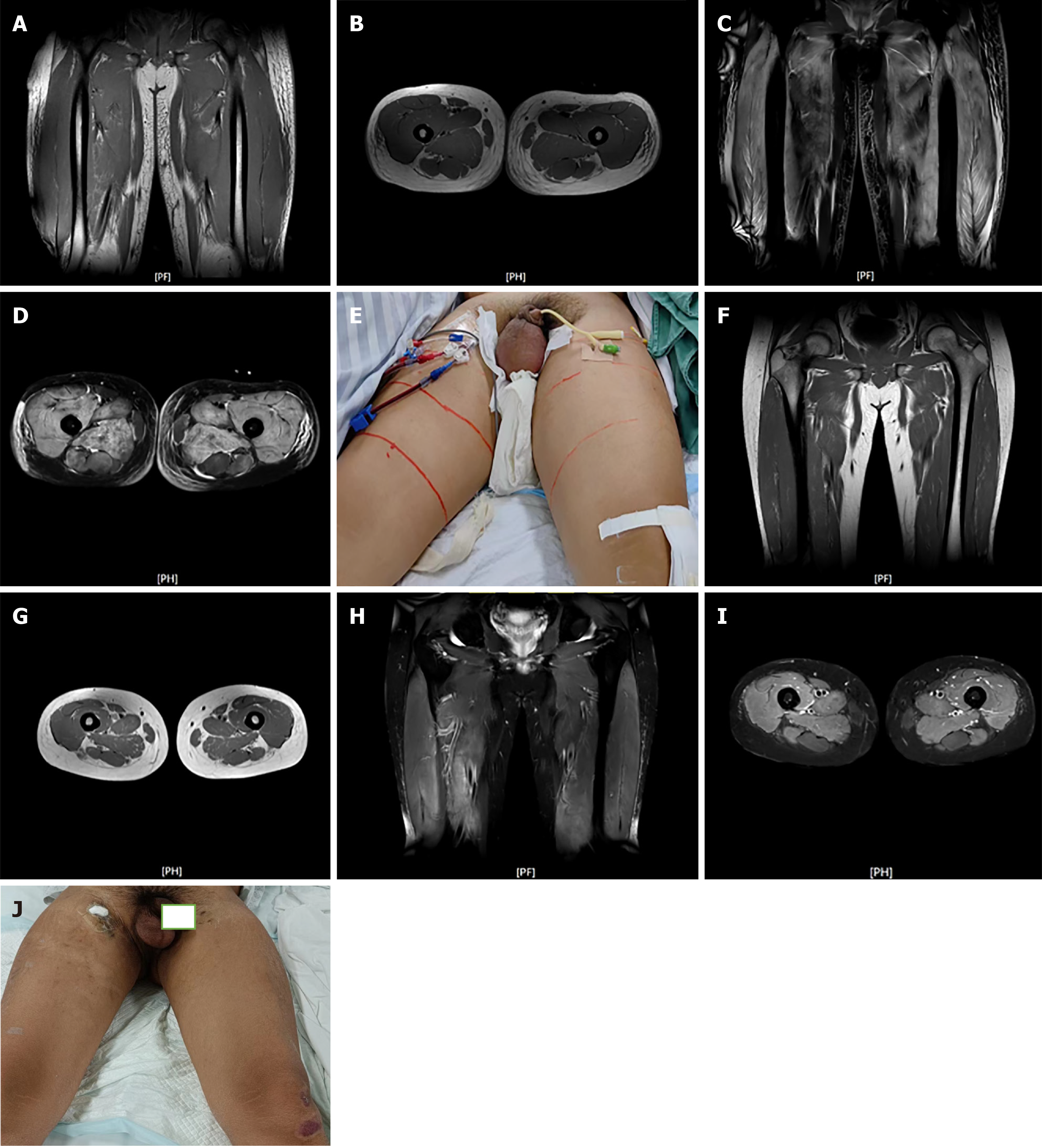
Computed tomography imaging revealed the following: (1) Bilateral lung inflammation with mild interstitial edema in the bilateral upper lung lobes; (2) Bilateral pleural effusion; (3) Increased gallbladder density, with cholestasis considered; and (4) Bilateral abdominal wall subcutaneous soft tissue swelling.
Magnetic resonance imaging on August 6 showed the following: (1) Multiple abnormal muscle group signals in the bilateral thighs; and (2) Extensive subcutaneous soft tissue swelling, with significant reduction by September 26 (Figure 2).
Ultrasound and magnetic resonance imaging of the kidneys demonstrated the following: Enhanced parenchymal echoes with unclear corticomedullary junction and intra-renal structures.
Given the complexity and critical nature of the patient’s condition, a multidisciplinary team (MDT) was convened both within and outside the hospital. Experts from various specialties, including critical care, nephrology, cardiology, hematology, and infectious diseases, participated in the consultation. The team conducted thorough reviews of the patient’s clinical progress, laboratory findings, and imaging results. The MDT’s primary focus was on stabilizing the patient’s vital functions, managing multi-organ failure, and addressing severe complications, including disseminated intravascular coagulation (DIC), refractory hemolytic anemia, acute renal failure, and rhabdomyolysis.
The treatment strategy was continuously adjusted based on the evolving clinical presentations. Interventions included administration of intravenous immunoglobulin sodium, dynamic adjustments in diuretic therapy, and implementation of CRRT. Emphasis was placed on minimizing invasive procedures and optimizing supportive care to facilitate gradual recovery. The MDT’s collaborative approach enabled timely modifications to the treatment plan in response to changes in the patient’s condition, contributing to the patient’s stabilization and gradual improvement.
The final diagnosis of the patient revealed the following: (1) EHS; (2) Acute renal injury; (3) Acute lung injury; (4) Acute respiratory distress syndrome (ARDS); (5) Coagulation abnormalities, including DIC; (6) Rhabdomyolysis; (7) Acute myocardial injury; (8) Moderate anemia; and (9) Lactic acidosis.
The patient’s treatment plan was comprehensive and included the following interventions: (1) Supportive care: immediate stabilization with intravenous fluids, electrolyte management, and continuous monitoring of vital signs; (2) Pharmacological therapy: administration of intravenous immunoglobulin and methylprednisolone sodium succinate to address inflammation and immune dysregulation; (3) Renal support: initiation of CRRT due to acute renal failure and persistent anuria; (4) Respiratory support: implementation of mechanical ventilation due to ARDS and acute lung injury; (5) Hematologic management: careful management of anemia and coagulation abnormalities, including transfusions and appropriate use of blood products; and (6) Other measures: use of diuretics to manage fluid overload, and analgesics and anti-inflammatory medications to alleviate symptoms and support the overall recovery.
The patient showed gradual improvement over the course of treatment. By day 75, the hemoglobin level had stabilized at 110 g/L, and other laboratory parameters, including creatinine and LDH, showed significant improvement. The patient was successfully weaned from mechanical ventilation and CRRT, and renal function began to recover.
Upon discharge on November 24, 2023, the patient exhibited stable vital signs and no significant sequelae. Follow-up care included regular outpatient visits to monitor renal function, respiratory status, and overall recovery. The patient was advised to adhere to a structured rehabilitation program to support full recovery and prevent complications.
Changes in routine blood parameters may be absent at the onset of HS[1], and anemia caused by EHS is seldom given sufficient attention in clinical practice. Our patient’s blood parameters had been normal in past tests. After disease onset, the blood concentration increased due to profuse sweating, the hemoglobin increased, but later continuously decreased, and the subsequent anemia persisted for an extended duration.
During HS onset, cell compliance decreases when erythrocytes are heated. When Abdullah et al[3] replicated the pathological damage caused by HS in humans within a baboon model, they observed erythrocyte plasma membrane fragmentation and rupture under heat stress conditions, showing that erythrocyte function is impaired under heat stress. Anemia in the early stages of patients with EHS is related to heat stroke-induced coagulopathy (HIC), particularly when cerebral hemorrhage, pulmonary hemorrhage, and gastrointestinal bleeding are present, which are usually fatal. The most severe form of coagulation disorder[4,5], DIC, can cause MAHA and adhesion of circulating erythrocytes when they pass through pores composed of fibrin filaments. These processes, combined with the force of blood flow, cause erythrocyte rupture[6]. Our experience has shown the importance of focusing on patients with early HIC, particularly those who later progress to the DIC stage of HS. In the initial stages of disease, routine blood and 6-item coagulation markers should be monitored once every 4 hours in patients with EHS in the ICU, and coagulation replenishment or anticoagulant therapy should be administered promptly based thereon. Aggressive blood transfusion should be performed when hemoglobin is below the tolerable limit, and surgery should be avoided during the coagulation disorder phase[7].
Patients with extremely severe EHS develop severe muscle injury after extremely strenuous exercise, when bioactive molecules secreted by muscles can significantly affect inflammatory responses and the recovery process. These processes lead to significant rhabdomyolysis, acute kidney injury, and lactic acidosis[3]. In a retrospective study of EHS, biomarkers of skeletal muscle injury, such as myoglobin and CK levels, were observed to have increased two- and 4.5-fold, respectively[8]. In our patient with extremely severe EHS, extreme elevations in myoglobin and CK levels and acute kidney injury occurred during the course of the disease.
Myoglobin causes nephrotoxicity through production of ferric oxide (Fe3+). In rhabdomyolysis, a myoglobin level over 8000 U/L is correlated with acute kidney injury[9]. A clinical study of 187 patients with EHS found that approximately 44% developed acute kidney injury, and that approximately 27% of those who develop acute kidney injury die within 90 days[10]. Our patient experienced continuous worsening of renal function during the course of disease, indicating that greater focus should be placed on assessing striated muscle injury in the early stages of the disease, and providing renal replacement therapy as soon as possible in patients with extremely severe EHS. After long-term renal replacement therapy, our patient’s renal function showed complete recovery.
Our study is the first to report MAHA caused by HS-related DIC. We focused on treating the patient’s anemia, a significant condition that affects EHS progression and whose pathogenesis is extremely important for our understanding of EHS. MODS caused by EHS should be treated differently from other critical illnesses, as patients with this condition are usually young, healthy people who can tolerate high-intensity physical activity[11]. There is a need to improve treatment confidence, establish and actively perform MDT, and adopt an evidence-based, multiprong treatment plan based on multidisciplinary recommendations.
We would like to express our sincere gratitude to all the healthcare professionals involved in the care of this patient, including the critical care team, nephrologists, pulmonologists, hematologists, and nursing staff. Their dedication, expertise, and coordinated efforts were instrumental in the successful recovery of the patient. We also thank the patient and their family for their trust and cooperation throughout the treatment process. We offer special thanks to the hospital administration for providing the necessary support and resources to facilitate this multidisciplinary approach.
| 1. | Liu SY, Wang Q, Lou YP, Gao Y, Ning B, Song Q, Li HL. Interpretations and comments for expert consensus on the diagnosis and treatment of heat stroke in China. Mil Med Res. 2020;7:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Yezli S, Yassin Y, Ghallab S, Abdullah M, Abuyassin B, Vishwakarma R, Bouchama A. Classic heat stroke in a desert climate: A systematic review of 2632 cases. J Intern Med. 2023;294:7-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Abdullah M, Ehaideb S, Roberts G, Bouchama A. Insights into pathophysiology and therapeutic strategies for heat stroke: Lessons from a baboon model. Exp Physiol. 2024;109:484-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Iba T, Connors JM, Levi M, Levy JH. Heatstroke-induced coagulopathy: Biomarkers, mechanistic insights, and patient management. EClinicalMedicine. 2022;44:101276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Song JC, Song Q, Zhang W, Liu SY, Li WQ, Zhou Z. [Expert consensus on the diagnosis and treatment of heatstroke-induced coagulopathy in China]. Jiefangjun Yixue Zazhi. 2023;48:1237-1247. [DOI] [Full Text] |
| 6. | George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 777] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 7. | Military Heat Stroke Prevention and Control Expert Group; Heat stroke Acute Diagnosis and Treatment Expert Group. [Acute diagnosis and treatment expert consensus for heat stroke (2021)]. Zhonghua Jizhen Yixue Zazhi. 2021;30:1290-1299. [DOI] [Full Text] |
| 8. | Laitano O, Oki K, Leon LR. The Role of Skeletal Muscles in Exertional Heat Stroke Pathophysiology. Int J Sports Med. 2021;42:673-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Candela N, Silva S, Georges B, Cartery C, Robert T, Moussi-Frances J, Rondeau E, Rebibou JM, Lavayssiere L, Belliere J, Krummel T, Lebas C, Cointault O, Sallee M, Faguer S; French Intensive Care Renal Network (F. I.R.N). Short- and long-term renal outcomes following severe rhabdomyolysis: a French multicenter retrospective study of 387 patients. Ann Intensive Care. 2020;10:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Wu M, Wang C, Liu Z, Zhong L, Yu B, Cheng B, Liu Z. Clinical Characteristics and Risk Factors Associated With Acute Kidney Injury Inpatient With Exertional Heatstroke: An Over 10-Year Intensive Care Survey. Front Med (Lausanne). 2021;8:678434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Bouchama A, Abuyassin B, Lehe C, Laitano O, Jay O, O'Connor FG, Leon LR. Classic and exertional heatstroke. Nat Rev Dis Primers. 2022;8:8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 214] [Article Influence: 71.3] [Reference Citation Analysis (0)] |