Published online Jan 28, 2024. doi: 10.4329/wjr.v16.i1.20
Peer-review started: October 16, 2023
First decision: November 9, 2023
Revised: December 6, 2023
Accepted: December 25, 2023
Article in press: December 25, 2023
Published online: January 28, 2024
Processing time: 98 Days and 15.7 Hours
After approval for clinical use in 2017 early investigations of ultra-high-field abdominal magnetic resonance imaging (MRI) have demonstrated the feasibility as well as diagnostic capabilities of liver, kidney, and prostate MRI at 7-Tesla. However, the elevation of the field strength to 7-Tesla not only brought advan
To offer a comprehensive overview of current literature on clinical abdominal 7T MRI that emphasizes current trends, details relevant challenges, and provides a concise set of potential solutions.
This systematic review adheres to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. A PubMed search, utilizing Medical Subject Headings terms such as "7-Tesla" and organ-specific terms, was conducted for articles published between January 1, 1985, and July 25, 2023. Eligibility criteria included studies exploring 7T MRI for imaging human abdominal organs, encompassing various study types (in-vivo/ex-vivo, method development, reviews/meta-analyses). Exclusion criteria involved animal studies and those lacking extractable data. Study selection involved initial identification via title/abstract, followed by a full-text review by two researchers, with discrepancies resolved through discussion. Data extraction covered publication details, study design, population, sample size, 7T MRI protocol, image characteristics, endpoints, and conclusions.
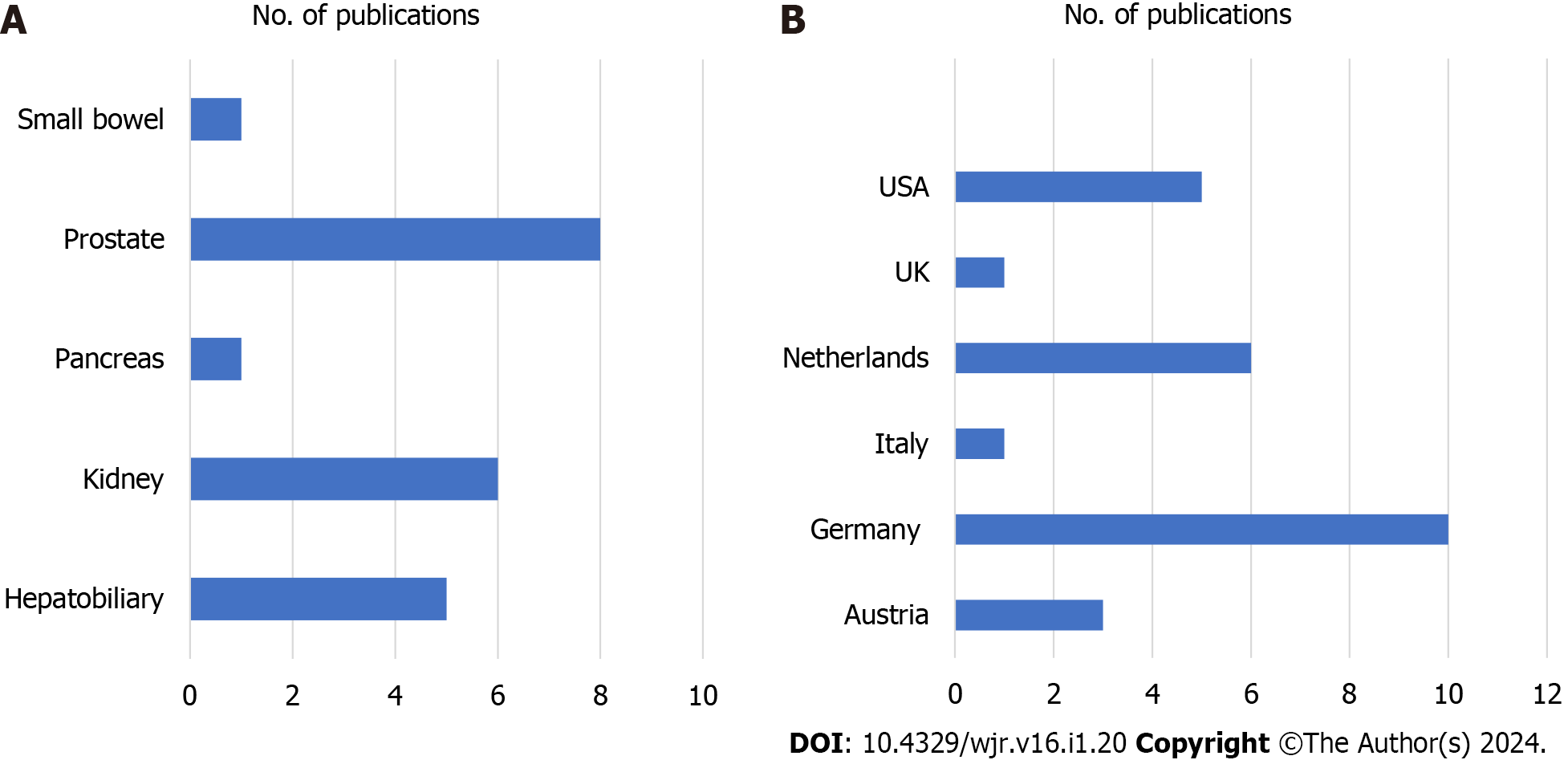
The systematic review included a total of 21 studies. The distribution of clinical 7T abdominal imaging studies revealed a predominant focus on the prostate (n = 8), followed by the kidney (n = 6) and the hepatobiliary system
Further increase of abdominal clinical MRI field strength to 7T demonstrated high imaging potential, yet also limitations mainly due to the inhomogeneous radiofrequency (RF) excitation field relative to lower field strengths. Hence, further optimization of dedicated RF coil elements and pulse sequences are expected to better optimize clinical imaging at high magnetic field strength.
Core Tip: At 7T non-enhanced T1w imaging, especially time-of-flight magnetic resonance angiography, excels in liver vessel assessment, outperforming both steady-state free precession and T2-weighted TSE techniques. Additionally, 7T magnetic resonance spectroscopy (MRS), particularly 31P-MRS, provides valuable insights into hepatic energy metabolism in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis. In pancreatic evaluation, 7T magnetic resonance imaging (MRI) holds promise for tumor characterization. Renal 7T MRI demonstrates potential for reducing contrast use, and prostate imaging explores metabolomic profiles and multi-voxel MRS for cancer detection. Imaging the small bowel at 7T currently offers no significant advantages. Despite challenges 7T MRI holds promise for advancing abdominal diagnostics.
- Citation: Perera Molligoda Arachchige AS, Teixeira de Castro Gonçalves Ortega AC, Catapano F, Politi LS, Hoff MN. From strength to precision: A systematic review exploring the clinical utility of 7-Tesla magnetic resonance imaging in abdominal imaging. World J Radiol 2024; 16(1): 20-31
- URL: https://www.wjgnet.com/1949-8470/full/v16/i1/20.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i1.20
Magnetic resonance imaging (MRI) of the abdomen is a proven and useful tool for the evaluation, assessment of severity, and follow-up of diseases of the abdomen as summarized by the American College of Radiology[1]. It is an evolving technology involving a variety of pulse sequences and protocols that are continuously being modified and improved.
It is well-known that unlike traditional X-rays, standard clinical MRI relies on the behaviour of hydrogen protons in response to these magnetic fields. Higher MRI magnetic field strengths ensure that more protons align with the field, generating more usable tissue magnetization. This enhances image signal relative to background noise. Initially, scientists believed 0.5T would be the maximum clinicaI magnet strength due to concerns about radiofrequency penetration in live tissue[2]. However, 1.5T clinical scanners started becoming available in the 1980s, and by 2002, 3T scanners gained approval. Although the first 7T research scanners emerged in 1999, they were limited to neuroimaging and some extremity applications. The first body images showcasing potential for prostate imaging at 7T were presented in 2007. Motivated by the desire to enhance resolution and contrast, 7T body MRI expanded, with the first abdominal images published in 2009. Today, 7T MRI is even employed in imaging challenging anatomical regions such as cardiac tissue[3]. Although multiple groups have worked on developing 7T methods and overcoming associated challenges, it wasn't until 2017 that 7T scanners received United States Food and Drug Administration and European Medicines Agency approval for clinical use[4]. Since then, there has been growth of in vivo body applications of 7T MRI in humans. Initial efforts involved acquiring landscaping image sets to establish the overall feasibility and safety of 7T whole body, breast, cardiac and metabolic magnetic resonance (MR) imaging. Subsequent publications have shifted focus towards implementing standardized imaging protocols to explore the diagnostic capacity of 7T abdominal MRI in dedicated examinations of non-enhanced and contrast-enhanced liver and kidney imaging, as well as renal MR angiographic applications[5-9]. This systematic review aims to provide an overview of the work currently published on clinical abdominal 7T MRI, where challenges to its application will be detailed along with a short overview of possible solutions.
The systematic review follows the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. We used the PubMed database to perform the literature search using the following MeSH terms: “7-Tesla” AND “abdominal” OR “renal” OR “kidney” OR “pancreas” OR “pancreatitis” OR “pancreatic” OR “liver” OR “hepatic” OR “hepatobiliary” OR “spleen” OR “intestine” OR “colon” OR “bladder” OR “uterus” OR “ovaries” OR “prostate”. Articles published between January 1, 1985 to July 25, 2023 were searched.
Studies were included that investigated the use of 7T MRI for any human abdominal organ and their associated disorders. In particular, studies were included if they were: (1) Human studies; (2) in-vivo/post-mortem/histological/ex-vivo studies; (3) related to MRI sequence/method development; and/or (4) reviews/case series or meta-analyses. Studies were excluded if they were: (1) Animal studies; (2) simulations; or (3) yielded no extractable data.
Studies were first identified by a single researcher through review of titles/abstracts. Any study that used 7T MRI to investigate the abdominal organs and disorders was moved to the next stage of screening. The second round of screening was conducted by two researchers based on the full text, following the eligibility criteria mentioned above. Any discrepancies in judgment were resolved through discussion.
The following data were extracted: publication characteristics (first author and year of publication), study design, study population, sample size, 7T MRI protocol, imaging protocol/characteristics, endpoints, and conclusions.
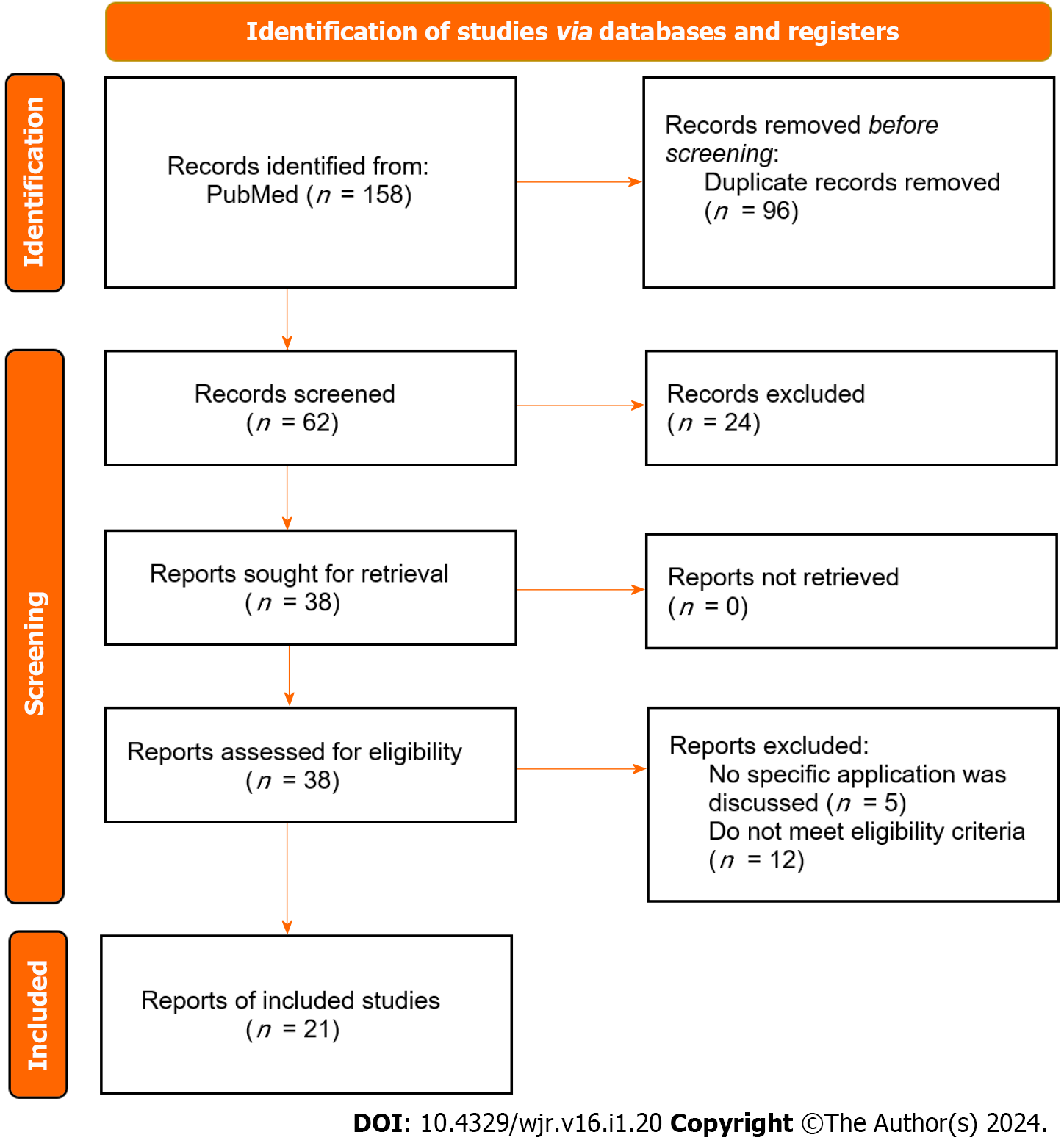
The search strategy produced 158 results, amongst which 62 studies were screened by title/abstract to yield 38 studies for further full-text review. Finally, after filtering by eligibility criteria, data extraction for qualitative synthesis was conducted on 21 studies via manual citation review (Figure 1)[10].
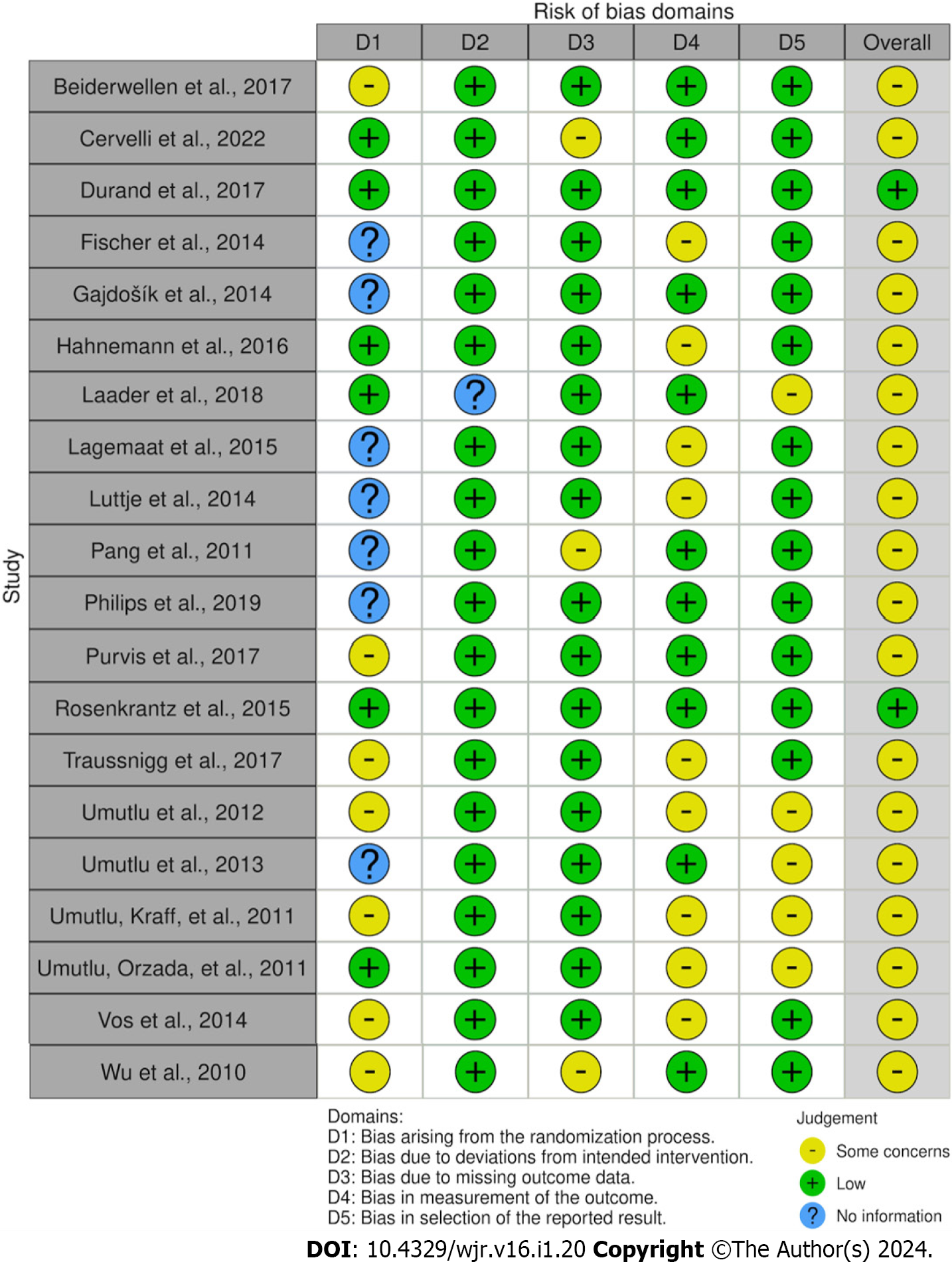
We used Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2.0) to obtain the Traffic-Light plot for the risk of bias assessment for all original investigative studies[11]. The risk of bias was initially evaluated by one researcher, everything was reviewed by the second researcher, and any disagreements were discussed before arriving at a conclusion. Risk of bias was assessed across five domains D1-D5, with D1 being the bias due to randomization, D2 being bias due to deviation from intended intervention, D3 being bias due to missing outcome data, D4 being bias in the measurement of outcome, and D5 being bias during the selection of the reported result, see Figure 2.
The focus on the studies were predominantly on the prostate (n = 8), followed by the kidney (n = 6) and the hepatobiliary system (n = 5). While the studies on the prostate, kidney, hepatobiliary system, and pancreas demonstrated clear advantages at 7T, small bowel studies showed no significant improvements relative to traditional MRI (1.5 T). The largest number of studies are reported from Germany (n = 10) followed by the Netherlands (n = 5), the United States (n = 5), Austria (n = 2), United Kingdom (n = 1), Italy (n = 1), see Figure 3.
Overall, 22 studies have been summarized in Table 1 with details on study design, imaging protocols, study sample, and endpoints/applications. Note that the study by Tenbergen et al[12] is not included in the table since we only included original investigations.
| Ref. | Study Design | MRI model, RF Coil & Imaging Parameters | No. of patients | Endpoints/Applications |
| Fischer et al[17], 2014 | Prospective study | Siemens Terra: 8-channel transmit/receive body coil; T1w 2D FLASH TOF MRA; transversal orientation; TR = 17ms; TE = 4.7 ms; TA = 0:33 s | 12 | For non-enhanced MR imaging of the arterial, venous and portal liver vessels in candidates for hepatectomy or liver transplantation with acute or chronic renal insufficiency |
| Traussnigg et al[20], 2017 | Prospective Non-randomized feasibility study | Siemens Terra; surface coil (1H/31P, 10 cm diameter, Rapid Biomedical GmbH), 2D 31P-MR CSI sequence, TA = approximately 10 min | 30 | As a non-invasive tool for obtaining pathomechanistic insights to improve risk stratification using changes in energy metabolism including dynamic ATP flux in inflammation and fibrosis in non-alcoholic fatty liver disease (NAFLD) and steatohepatitis (NASH) |
| Beiderwellen et al[28], 2017 | Prospective study | Siemens Terra; 8-channel transmit/receive body coil; T1w 3D spoiled gradient-echo VIBE sequence; coronal; post-contrast; TR = 2.90 ms; TE = 1.09 ms; TA = 27s; Resolution = 1.25 mm × 1.25 mm × 1.6 mm | 10 | For Gadobutrol dose reduction (to 0.025 mmol/kg of body weight) while maintaining diagnostic image quality for the diagnosis of renal artery stenosis in patients with renal impairment |
| Purvis et al[21], 2017 | Prospective study | Siemens Terra; 16-channel 31P RF array heart/liver butterfly-loop coil pair; 3D UTE CSI sequence; TA = approximately 28 min | 26 | For studying metabolism in liver disease |
| Wu et al[30], 2010 | Pilot study (ex-vivo) | Siemens Terra; T2w TSE and PRESS with CHESS water suppression; TE/TR = 30/1700 ms; resolution = 3 mm3 isotropic; TA = approximately 23 min; MRS data processed using NMR Software Nuts (Acorn NMR Inc., Livermore, CA, United States) | N/A | To construct a malignancy index based on prostate cancer metabolomic profiles |
| Rosenkrantz et al[33], 2015 | Feasibility study | Siemens Terra: Axial TSE T2w sequence; TR = 11670 ms; TE = 80 ms; FA = 160; Resolution = 0.75 mm × 0.75 mm × 3 mm; BW 254 Hz/voxel; NEX = 1 | 3 | For tumor localization using T2w MRI at 7T before prostatectomy |
| Luttje et al[32], 2014 | Feasibility study | Siemens Terra: 2D 1H MRSI grid positioned in the tumor location as seen, 3D 31P MRSI grid positioned over whole prostate; TE/TR = 56/2000 ms; resolution = 5 mm × 5 mm × 5 mm; TA = 448s; 3D 31P MRSI TE/TR = 0.42/200 ms; FA = 20°, resolution = 12 mm × 12 mm × 12 mm, TA = 610s | 5 | To acquire both 1H and 31P MRS within the same scan session in patients with prostate cancer |
| Lagemaat et al[35], 2015 | Prospective study | B0 and B1+ field-mapping to optimize B0 and B1 field homogeneity in the prostate; T2wTE = 71 ms; TR = 3000–3640 ms; resolution 0.75 mm × 0.75 mm × 3 mm with overlaid 2D 31P T1 3D 31P MRSI Nuclear Overhauser Effect (NOE) measurements | 12 | Optimization of phosphorus (31P) MRSI of the human prostate at 7 T by the evaluation of T1 relaxation times and the NOE of phosphorus-containing metabolites |
| Vos et al[31], 2014 | Prospective study | Siemens Terra; 8-channel TxRx body array coil + an endorectal coil tuned to the P31 frequency; Axial/sagittal T2w FSE TR = 3000/3640 ms; TA = 90/113s | 17 | Prostate cancer is visualized on T2-weighted MRI with periprostatic lipids appearing hypo-intense compared to healthy peripheral zone tissue |
| Durand et al[34], 2017 | Feasibility study (ex-vivo) | N/A | To determine high-resolution ex-vivo MRI protocol parameters for characterization of prostate tissue at histological length scales | |
| Philips et al[36], 2019 | Pilot study | Siemens Terra; Combined 31P Tx/Rx and 1H Rx endorectal coil (31P/ 1H ERC), 8-channel external multitransmit 1H array; T2w TSE, resolution 0.43 mm × 0.43 mm × 3 mm; TA = 119s; DWI EPI (RESOLVE) b0, b100, b400, and b800, resolution 1.75 mm × 1.75 mm × 3 mm, TA = 274s; 31P 3D MRSI with non-selective BIR-4 excitation, TA = 789s; 1H MRSI PRESS TA = 421s | 4 | To perform high resolution multiparametric MR imaging and 1H and 31P spectroscopy of the prostate using a 31P Tx/Rx 1H Rx endorectal coil in combination with an external multitransmit 1H body array |
| Hahnemann et al[37], 2016 | Prospective comparative study | Siemens Terra; custom-built 8-channel Tx/Rx RF body coil with B1+ shimming; coronal bSSFP resolution 0.8mm × 0.8 mm × 2.0 mm, TA = 26s; axial bSSFP, resolution 0.7 mm × 0.7 mm × 2.0 mm, TA = 20s; coronal T2w HASTE, resolution 1.0 mm × 1.0 mm × 5.0 mm, TA = 25s | 12 | To perform non-enhanced high quality T2w MRI of the small bowel |
| Gajdošík et al[22], 2014 | Prospective study | Siemens Terra; 10 cm diameter Tx/Rx surface coil (Rapid Biomedical GmbH, Rimpar, Germany); STEAM sequence; resolution 30 mm × 30 mm × 30 mm | 11 | To assess the proton T1 and T2 relaxation of in vivo hepatic water, choline and lipid metabolites with possible J-coupling behavior of lipids in healthy volunteers |
| Pang et al[15], 2011 | Pilot study | bisected microstrip transceiver surface coil; SSFP sequence; TA < 20s | - | For liver imaging |
| Cervelli et al[23], 2022 | Feasibility study (ex-vivo) | GE Signa; 8-channel Tx/Rx knee RF coil; morphologic sequences: 3D-T2w-CUBE, IDEAL T1w and T2w, 2D- and 3D-MRCP; Quantitative sequences: MP2-RAGE, MSE, IDEAL, 2D-MRF | N/A | For differentiating between tumour and non-target pancreatic tissue using conventional T1w-, T2w- sequences and MRF-derived relaxometry. The MRF sequence obtained reliable relaxation time data |
| Umutlu et al[24], 2011 | Prospective study | Siemens Terra; a custom-built 8-channel Tx/Rx RF body coil; 2D FLASH, resolution = 0.8 mm × 0.8 mm × 2.0 mm, TA = 31s; 3D FLASH, resolution 1.3 mm × 1.3 mm × 1.6 mm, TA = 27s; IP and O/P GRE, resolution 1.1 mm × 1.1 mm × 3 .0 mm, TA = 20s; bSSFP, resolution 1.3 mm × 1.6 mm × 4.0 mm, TA = 19s; T2w TSE, resolution 1.4 mm × 1.4 mm × 5.5 mm, TA = 34s | 8 | For renal imaging |
| Umutlu et al[25], 2011 | Prospective study | Siemens Terra; custom-built body Tx/Rx RF coil; T2w BSSFP, resolution 1.3 mm × 1.6 mm × 4.0 mm; T2w TSE; I/P and O/PGRE; T1w 2D FLASH, resolution 0.8 mm × 0.8 mm × 2.0 mm | 10 | To assess the feasibility of dynamic CE renal imaging |
| Umutlu et al[26], 2012 | Prospective study | Siemens Terra; custom-built Tx/Rx RF body array coil; Coronal Fat-Saturated 2D FLASH , TA = 31s; Coronal Fat-Saturated 3D FLASH TA = 27s; Axial Fat-Saturated 2D TOF MRA TA = 33s | 12 | To investigate the feasibility of 7T nonenhanced high-field MRA of the renal vasculature and to evaluate the diagnostic potential of various non-enhanced T1-weighted spoiled gradient-echo sequences |
| Laader et al[29], 2018 | Prospective study | Siemens Terra; custom-built eight-channel Tx/Rx RF body coil; axial 2D T1w TOF MRA TA = 33s; Coronal fat-saturated 3D low-dose CE T1w FLASH VIBE, TA = 27s | 10 | To evaluate the performance of time-of-flight MRA versus low-dose CE renal MRA at 7T in order to reduce and/or completely omit contrast agent use for renal MRA at 7T |
| Umutlu et al[27], 2013 | Prospective study | Siemens Terra; custom-built Tx/Rx RF coil; coronal T1w3D FLASH sequence | 8 | To assess the feasibility of first-pass CE renal MRA at 7T |
The advancements in shimming of the B1 field generated by the 7T multi-channel transmit/receive radiofrequency (RF) body coil has created a foundation for effectively applying 7T MRI in abdominal imaging. Numerous studies have reviewed the progress in high field imaging ranging from non-enhanced MR angiography (MRA) studies to the use of magnetic resonance spectroscopy (MRS) for the detection of metabolic alterations associated with liver diseases including non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and cirrhosis, emphasizing the inherent ability of 7T to improve the signal-to-noise ratio (SNR) in various anatomical regions[13,14]. Liver imaging at 7T is particularly interesting due to the expected improvements in SNR, and short echo times can enable the acquisition of high-resolution images (in particular in T1w sequences) with reduced motion artifacts. The susceptibility effect is also more prominent at higher field strengths, where 7T susceptibility-weighted imaging has demonstrated enhanced detection and quantification of hepatic iron stores[15].
Despite these advantages, there exist technical challenges in transferring liver imaging protocols to high magnetic field scanners, specifically related to RF coil and sequence design. For example, a finite-difference time-domain method has been employed to analyse RF field penetration behaviour at 7T, revealing limitations in imaging coverage and RF penetration in human liver imaging at 7T[15]. Elliptically-shaped tissues like the brain may experience a dielectric effect where the short RF wavelength at 7T couples with tissue and forms standing waves, yielding increased B1 penetration and inhomogeneity due to enhanced constructive and destructive interference respectively[16]. The irregular geometry of the liver may limit this effect, leading to reduced B1 penetration and image coverage. To overcome this limitation, a large-sized microstrip transceiver coil for deeper penetration in liver imaging was proposed. On the other hand, a benefit of lessened dielectric effect in the liver is lessened dielectric shading artifacts.
Non-enhanced T1w imaging, [especially time-of-flight magnetic resonance angiography (TOF-MRA)] holds promise for high-quality liver vessel assessment at 7T, offering sharply defined vessel signals and effective background signal suppression with successful visualization of intracranial, renal, and lower extremity vessels. Despite the longer examination time for TOF-MRA, Fischer et al[17] highlighted its advantages in delineating three types of liver vessels: the right portal vein, the inferior vena cava and the middle hepatic vein. It was shown that compared to non-enhanced T1w 2D FLASH and T1w 3D FLASH sequences, TOF-MRA has high overall image quality and vessel delineation showing significantly higher contrast ratios for all liver vessels, with the least impairment by B1 inhomogeneity and susceptibility artifacts.
Both steady-state free precession (SSFP), the most common technique for non-enhanced MR angiography, and T2-weighted (T2W) turbo spin echo (TSE) imaging techniques face limitations in non-contrast-enhanced liver vessel imaging at 7T. SSFP imaging is susceptible to signal heterogeneity in the static magnetic field B0, leading to signal loss artifacts. Additionally, SSFP sequences at 7T encounter challenges related to specific absorption rate (SAR) limitations, with SAR increasing with the square of the magnetic field and the flip angle, potentially constraining usable flip angles such that image signal is inadequate. T2W TSE imaging is also challenging at higher field strengths, since B1 field inhomogeneity and constraints in available RF power for generating accurate refocusing pulses and high flip angles can limit the efficacy of this sequence at higher magnetic field strengths. Consequently, exploiting the inherently hyperintense vessel signal in T1w MRI could be a more promising approach for non-contrast-enhanced liver vessel imaging at 7T[17].
MRS at 7T has been noted for its potential clinical applicability stemming from the high spectral resolution and SNR achieved in a tertiary (research) setting. Studies have highlighted the limitations of liver biopsy as the reference standard for diagnosing NASH, and there has been a focus on the need for non-invasive tools to monitor the severity and progression of NAFLD[18,19]. As a result, proton (1H)- and phosphorus (31P)-MRS have been considered potential candidates for real-time detection of hepatic fat, cell membrane, and energy metabolism. While 3T 1H-MRS is effective for quantifying liver steatosis, a study by Traussnigg et al[20] additionally studied the clinical potential of 7T 31P-MRS in NAFLD patients[8]. Their study sought to obtain novel mechanistic insights into lipid, cell membrane, and energy homeostasis in NAFLD by leveraging the increased SNR and spectral resolution of 7T 31P-MRS. The study performed a retrospective analysis of spectra acquired from young healthy volunteers, which indicated a statistical increase in phosphocreatine (PCr) to total phosphorus signal from the liver of patients with advanced fibrosis. The presence of PCr signals in NAFLD or diabetes patients has not been shown in previous studies[20]. The study acknowledged some technical limitations, such as the limited transmit bandwidth restricting effective excitation of β-ATP signal at 7T. Nevertheless, 7T 31P-MRS could be considered a highly accurate and fast non-invasive method for generating comprehensive in vivo profiles of lipid, cell membrane, and energy metabolism in the human liver. The findings indicate significant differences in hepatic energy metabolism between NAFL and NASH, providing valuable insights for further mechanistic and therapeutic studies of NAFLD/NASH[20].
Similarly, in the same year another study explored the utility of 7T 31P spectroscopy for investigating liver metabolism, providing novel insights by measuring T1 values for key metabolites like phosphatidylcholine (PtdC), phosphoenolpyruvate (PEP), nicotinamide adenine dinucleotide (NAD+), and uridine diphosphoglucose (UDPG). Chemical Shift Imaging (CSI) was employed with a receive array that achieved approximately 46% coverage of the liver to localize spectra from a specific volume[21]. The study validated the protocol's accuracy and repeatability in both healthy volunteers and cirrhosis patients, demonstrating significant differences in metabolites such as inorganic phosphate (Pi), PEP/PtdC, and glycerophosphoetanolamine. Notably, the study reported a 12% reduction in Pi in cirrhosis patients, indicating the potential of 7T 31P spectroscopy to detect metabolic alterations associated with liver diseases. They concluded that 7T 31P spectroscopy is a powerful and reliable tool for in-depth investigations into liver metabolism, offering valuable insights into T1 relaxation times and metabolic changes associated with cirrhosis[21].
Lastly, Gajdošík et al[22] delved into the 1H magnetic resonance relaxation behaviour within hepatic tissue at 7T. In vivo T1 and T2 relaxation times for water, methyl groups, and various lipids were quantified. The study successfully resolved key metabolites and maintained a well-tolerated protocol within a 60-min timeframe for volunteers. The findings highlight the potential for future applications in characterizing diverse liver pathologies.
For what concerns the pancreas, the aim of 7T MRI so far has been to identify useful imaging biomarkers by evaluating the radiological-histopathological correlation of pancreatic lesions. A recent study[23] evaluated the correlation between 7T MRI findings and histological features in Pancreatic Ductal Adenocarcinoma (PDAC) lesions, with a secondary endpoint of identifying useful MR quantitative sequences for defining an optimal acquisition protocol. MRI findings were compared with the histological composition of ex-vivo specimens from lesions preoperatively diagnosed as PDAC via computed tomography scan, where ex-vivo samples were chosen to overcome limitations of the study[23]. Despite careful coregistration of 7T MRI data with macroscopic and microscopic histological evaluations of pancreaticobiliary lesions, this feat was challenged by a lack of sample uniformity in tumour histology. The results demonstrated the utility of Magnetization Prepared 2 Rapid Acquisition Gradient Echo (MP2RAGE), multiple spin-echo (MSE), and magnetic resonance fingerprinting (MRF)-derived relaxometry in distinguishing tumoral regions from unaffected pancreatic tissue, with MRF demonstrating the capacity for obtaining reliable relaxation time data within a breath hold. Yet, further exploration is needed to understand MRF's role in differentiating the pancreaticobiliary lesion, peritumoral stroma, and residual pancreatic gland. The study concluded that with further validation MRF could play a valuable role in the imaging evaluation of pancreatic tumours[23].
Some studies conducted a series of renal imaging studies at 7T. Initial studies were feasibility studies while subsequent studies compared different imaging sequences for their ability to obtain high quality images by reducing or avoiding the use of contrast[24-27].
Initially, they successfully performed renal 7T MRI in eight subjects, with the T1w GRE sequence delivering high anatomical detail and excellent visibility of non-enhanced vasculature, and B1 shimming able to reduce signal voids in most sequence types. Nevertheless, the study suffered from B0 and B1 inhomogeneities (especially in T2w TSE sequences), chemical shift artifacts, RF wavelength effects, and specific absorption rate (SAR) limitations. Despite these difficulties, all 7T examinations were well-tolerated by subjects with an average examination time of 25 min[24]. In the same year, the same group assessed the feasibility of intravenous injection of Gadobutrol for dynamic contrast-enhanced renal MRI at 7T in ten healthy subjects. Among the sequences tested, T1w 2D FLASH yielded the best overall image quality, while T2w TSE had the poorest image quality. Additionally, quantitative analysis highlighted the advantages of arterial phase 3D FLASH imaging in terms of contrast to noise (CNR) between cortex and medulla[25].
In the following year, the same researchers explored the feasibility of non-enhanced 7T MRA for assessing renal perfusion and to evaluate renal vasculature using various T1w sequences. The study also addressed safety concerns related to Gadolinium-based contrast agents and their link to nephrogenic systemic fibrosis. Initial results demonstrated the feasibility and challenges of non-enhanced 7T renal MRA, ultimately emphasizing the superiority of TOF imaging in the tested sequences[26]. Shortly thereafter, they evaluated first-pass contrast-enhanced renal 7T MRA. Eight healthy subjects underwent dynamic imaging with contrast, resulting in high definition of the arterial vasculature and increased SNR and CNR upon administration of contrast. The study concluded that first-pass contrast-enhanced renal 7T MRA is technically feasible and provides superior vessel assessment compared to non-enhanced MRA[27]. Finally, they explored the viability of reducing 7T MRA renal Gadobutrol dosage in ten healthy volunteers, and showed that a reduced dose of 0.025 mmol/kg body weight can maintain sufficient image quality[28].
Another study comparing non-enhanced TOF MRA and low-dose contrast-enhanced renal MRA at 7T showed that both methods exhibited moderate overall image quality, with TOF MRA demonstrating less artifacts and better delineation of segmental branches within the renal parenchyma[29]. This indicates the feasibility of reducing or eliminating contrast agents for renal 7T MRA, with a call for further research to validate these findings.
Overall, despite the demonstrated feasibility of various 7T MRI sequences for kidney imaging, several challenges persist. SAR limitations may require sacrifices that include long imaging times and spurious artifacts, especially in T2w sequences; these may be partially addressed with parallel imaging and high amplitude stimulated echoes, which unfortunately may compromise SNR. RF inhomogeneity may be managed through RF shimming, an area of ongoing advan
In the context of prostate imaging, 7T MRI has been investigated for the possibility of identifying imaging biomarkers of prostate cancer using different enhanced and non-enhanced sequences. In addition, MRS has been extensively investigated for its accuracy in evaluating metabolomic profiles in prostate cancer, and feasibility studies have been conducted for combined 1H and 31P MRS and related endorectal coils. One of the first reported prostate studies employing 7T MRI evaluated the feasibility of applying metabolomic profiles obtained from intact-tissue 14T MRS to the imaging of whole prostates using multi-voxel 7T MRS. Cancerous samples could be differentiated from histologically benign ones using the metabolic profile with an overall accuracy of 93%, demonstrating that metabolomic profiles at ultra-high fields could be translated for use in real-world clinical settings[30].
A prostate feasibility study in 2014 by Vos et al[31] demonstrated that 7T T2w imaging could employ dedicated multi-transmit RF coils with shimming and SAR-monitoring to generate good image quality. All 17 patients completed the protocol without adverse incident to yield image quality rated as satisfactory by one reader (R1) and good by the other two readers (R2 and R3), with moderate agreement (α = 0.44) amongst them. Lesions were successfully visualized in all 7 patients with confirmed prostate cancer. Moderate motion artifacts were observed, and six lesions with low Gleason scores or small volume were not recognized, highlighting the need for future investigations into the impact of the absence of lipid signal at 7T on the detection of extracapsular extension of prostate cancer into periprostatic tissue[31].
That year another study by Luttje et al[32] demonstrated the feasibility of combining 7T 1H MRSI and 31P MRSI in the human prostate. The researchers used a customized endorectal coil (ERC) matched and tuned to both 1H and 31P without significant efficiency losses or sensitivity discrepancies between channels. 1H MRSI revealed distinct resonances of choline, polyamines, creatine, and citrate, offering the potential for individual fitting and increased sensitivity in detecting elevated choline levels. They proposed the use of adiabatic RF pulses and coil hardware combinations to combat residual B1+ nonuniformity. In vivo 31P MRSI measurements identified signals of phospholipid metabolites, and contrary to previous findings indicated lower levels of phosphoethanolamine in tumor areas compared to healthy tissue. Combining 1H and 31P MRSI in the same scan session potentially improved diagnostic sensitivity by correlating individual compounds of the choline pool with tumor aggressiveness[32]. Another similar study evaluated safety concerns of a similar 31P/1H ERC in terms of its proximity to tissue. Electromagnetic field simulations and phantom experiments indicated an absence of significant coupling between the 1H and 31P ERC channels and the external body array, with SAR and temperature simulations/measurements confirming procedural safety. Successful application of the 31P/1H ERC in volunteer and patient measurements demonstrated the feasibility and safety for clinical use of 7T prostate imaging and spectroscopy[33].
Other 7T MRSI work in the prostate has been performed, including a study by Lagemaat et al[34] aimed at characterization of vivo 31P MR spectra. They assessed T1 relaxation times and the nuclear overhauser effect (NOE) of phosphorus-containing metabolites, and determined that the NOE enhanced fitting accuracy with some variability, and that reducing flip angle (≤ 45°) enabled optimal acquisition in 15 min. Another study used MRSI to discern and localize prostate cancer through distinct choline and citrate signal patterns. Tailored coil designs to address B1+ inhomogeneities and chemical shift artifacts permitted 31P 7T MRSI to enable identification of phosphorus-containing metabolites. Overall insights into prostate tissue biochemistry could be gleaned clinically by leveraging the high 7T SNR to execute 3D 31P MRSI within a clinically acceptable timeframe[12].
Other studies included a two patient 7T T2w TSE MRI analysis using a two-loop transmit system that revealed a focal region of decreased T2 signal in the peripheral zone suspicious for tumors in both patients[35]. Post-prostatectomy analysis of the specimens confirmed good correspondence between these findings and histopathologic assessment of the dominant peripheral zone tumor[35]. Another study by Durand et al[36] sought to balance 7T resolution and scan time to image the prostate gland. High resolution 100 μm × 107 μm × 750 μm (11 min) and 60 μm × 60 μm × 90 μm (3 h) protocols allowed visualization of prostatic microanatomy, aiding in distinguishing benign and malignant tissues, including adenocarcinomas with Gleason scores of 7, 8, and 9. The study suggests that these images could serve as a diagnostic non-invasive alternative to biopsy, and improve treatment planning precision through accurate tumor volume estimation[36].
Finally, there have been indications of other potential clinical applications of 7T MRI in prostate imaging. Dynamic contrast-enhanced MRI (DCE MRI) at 7T is a promising method due to the possibility of enhanced spatial and temporal resolution, vital for precise pharmacokinetic modeling and qualitative assessment in prostate cancer detection. The R2* relaxation rate using Gadolinium-based contrast agents purportedly quadruples at 7T relative to 3T, significantly impacting DCE-MRI signal, especially in blood[12]. 7T MRI also holds promise for detecting lymph node metastases in prostate cancer patients, offering enhanced spatial resolution for visualizing nodes smaller than 5 mm. Functional MRI contrast agents such as ferumoxtran-10 may aid in distinguishing between normal and metastatic lymph nodes, although efforts in this capacity have thus far been limited to clinical trials[12].
As of the writing of this article, there does not seem to be any significant advantage to imaging the small bowel at 7T relative to traditional MRI field strengths. A study by Hahnemann et al assessed the feasibility and efficacy of non-enhanced small bowel MRI at both 1.5 T and 7T using fast imaging with balanced steady-state free precession (bSSFP) and half-Fourier acquisition single-shot turbo spin-echo sequences[37]. The comparison revealed that mesentery contrast and tissue detail delineation were generally superior and occasionally similar at 1.5T relative to 7T across all subjects. Contrast of the jejunum and ileum were mostly equivalent at 1.5T and 7T, with one subject exhibiting superior contrast of the ileum at 1.5T[37]. Quantitative analysis demonstrated no statistically significant differences in tissue contrast between the bowel wall and lumen for 1.5T and 7T in coronal bSSFP, although some axial images revealed significantly higher contrast at 1.5T. Overwhelming B1 inhomogeneity and susceptibility artifact in 7T bSSFP imaging led the study to conclude that although small bowel MRI at 7T is technically feasible, tissue contrast and image fidelity are at best comparable to that achieved at 1.5 T MRI[37].
It is essential to recognize several limitations of our systematic review. First, our search of records could have missed some publications indexed in other databases like EMBASE, Scopus, Google scholar, etc. However, PubMed is known for its appropriate and high-quality content from peer-reviewed medical journals, which indicates its applicability for conducting systematic reviews in medicine. Systematic reviews involve a trade-off between comprehensiveness and feasibility, and including too many databases can make processing time unwieldy. Note that a manual search in EMBASE using the same search criteria showed very few missed studies, suggesting that the sacrifice of avoiding its use was relatively small. Second, some areas of study were not adequately represented in the literature, such as imaging of the small bowel and pancreas at 7T. Lastly, a meta-analysis of diagnostic accuracy could not be conducted due to the lack of adequate studies with similar objectives.
While 7T MRI demonstrates remarkable imaging potential, the limitations stemming from inhomogeneous excitation fields call for ongoing efforts in optimizing dedicated RF coil and pulse design. Further research and technological advancements are crucial to harness the benefits of high magnetic field strengths while mitigating the challenges associated with these advancements in MRI technology.
Clinical 7-Tesla HASTE was approved for clinical use in 2017. Since then, it has been used widely in specialized research centers mainly for neuroimaging studies. However, it has been also used in the imaging of abdominal organs even though the studies are few.
To summarize all the current evidence concerning the utilization of 7-Tesla MRI in clinical abdominal imaging since to our knowledge there has been no review paper discussing this before.
To offer a comprehensive overview of current literature on clinical abdominal 7T MRI that emphasizes current trends, to summarize the current imaging sequences/parameters used, to describe relevant challenges, and to provide a concise set of potential solutions.
This systematic review adheres to PRISMA guidelines. A PubMed search, utilizing Medical Subject Headings terms such as "7-Tesla" and organ-specific terms, was conducted for articles published between January 1, 1985, and July 25, 2023. Eligibility criteria included studies exploring 7T MRI for imaging human abdominal organs, encompassing various study types (in-vivo/ex-vivo, method development, reviews/meta-analyses). Exclusion criteria involved animal studies and those lacking extractable data. Study selection involved initial identification via title/abstract, followed by a full-text review by two researchers, with discrepancies resolved through discussion. Data extraction covered publication details, study design, population, sample size, 7T MRI protocol, image characteristics, endpoints, and conclusions.
The systematic review encompassed a total of 21 studies. Analysis of the distribution of clinical 7T abdominal imaging studies indicated a predominant emphasis on the prostate (n = 8), followed by the kidney (n = 6), and the hepatobiliary system (n = 5). Research on these organs, as well as the pancreas, demonstrated evident advantages at 7T. Conversely, studies on the small bowel did not reveal significant enhancements compared to traditional MRI at 1.5T. The majority of the evaluated studies originated from Germany (n = 10), followed by the Netherlands (n = 5), the United States (n = 5), Austria (n = 2), the United Kingdom (n = 1), and Italy (n = 1).
7T MRI showcases remarkable imaging potential, however, the limitations arising from inhomogeneous excitation fields underscore the need for ongoing efforts in optimizing dedicated RF coil and pulse design. Continued research and technological advancements are imperative to fully harness the advantages of high magnetic field strengths while addressing the challenges associated with advancements in MRI technology.
More studies are necessary to elucidate the full potential of 7-Tesla MRI in abdominal imaging especially when it comes to the imaging of the pancreas, and the intestines which had very few investigative studies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alzerwi NAN, Saudi Arabia S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Caraiani C, Yi D, Petresc B, Dietrich C. Indications for abdominal imaging: When and what to choose? J Ultrason. 2020;20:e43-e54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Harmonay V. What is the Strongest MRI Machine Today? Atlantis WORLDWIDE. 04th Dec 2023. Available from: https://info.atlantisworldwide.com/blog/what-is-the-strongest-mri-machine. |
| 3. | Nowogrodzki A. The world's strongest MRI machines are pushing human imaging to new limits. Nature. 2018;563:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 4. | Arachchige ASPM. 7-Tesla PET/MRI: A promising tool for multimodal brain imaging? AIMS Neurosci. 2022;9:516-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Hoff MN, McKinney A 4th, Shellock FG, Rassner U, Gilk T, Watson RE Jr, Greenberg TD, Froelich J, Kanal E. Safety Considerations of 7-T MRI in Clinical Practice. Radiology. 2019;292:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Reiter T, Lohr D, Hock M, Ankenbrand MJ, Stefanescu MR, Kosmala A, Kaspar M, Juchem C, Terekhov M, Schreiber LM. On the way to routine cardiac MRI at 7 Tesla - a pilot study on consecutive 84 examinations. PLoS One. 2021;16:e0252797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Snyder CJ, DelaBarre L, Metzger GJ, van de Moortele PF, Akgun C, Ugurbil K, Vaughan JT. Initial results of cardiac imaging at 7 Tesla. Magn Reson Med. 2009;61:517-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Korteweg MA, Veldhuis WB, Visser F, Luijten PR, Mali WP, van Diest PJ, van den Bosch MA, Klomp DJ. Feasibility of 7 Tesla breast magnetic resonance imaging determination of intrinsic sensitivity and high-resolution magnetic resonance imaging, diffusion-weighted imaging, and (1)H-magnetic resonance spectroscopy of breast cancer patients receiving neoadjuvant therapy. Invest Radiol. 2011;46:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Vaughan JT, Snyder CJ, DelaBarre LJ, Bolan PJ, Tian J, Bolinger L, Adriany G, Andersen P, Strupp J, Ugurbil K. Whole-body imaging at 7T: preliminary results. Magn Reson Med. 2009;61:244-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 40131] [Article Influence: 10032.8] [Reference Citation Analysis (2)] |
| 11. | Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, Emberson JR, Hernán MA, Hopewell S, Hróbjartsson A, Junqueira DR, Jüni P, Kirkham JJ, Lasserson T, Li T, McAleenan A, Reeves BC, Shepperd S, Shrier I, Stewart LA, Tilling K, White IR, Whiting PF, Higgins JPT. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6581] [Cited by in RCA: 15131] [Article Influence: 2521.8] [Reference Citation Analysis (0)] |
| 12. | Tenbergen CJA, Metzger GJ, Scheenen TWJ. Ultra-high-field MR in Prostate cancer: Feasibility and Potential. MAGMA. 2022;35:631-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Verma Y, Ramesh S, Perera Molligoda Arachchige AS. 7 T Versus 3 T in the Diagnosis of Small Unruptured Intracranial Aneurysms: Reply to Radojewski et al. Clin Neuroradiol. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Trattnig S, Springer E, Bogner W, Hangel G, Strasser B, Dymerska B, Cardoso PL, Robinson SD. Key clinical benefits of neuroimaging at 7T. Neuroimage. 2018;168:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Pang Y, Wu B, Wang C, Vigneron DB, Zhang X. Numerical Analysis of Human Sample Effect on RF Penetration and Liver MR Imaging at Ultrahigh Field. Concepts Magn Reson Part B Magn Reson Eng. 2011;39B:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Ibrahim TS, Lee R, Abduljalil AM, Baertlein BA, Robitaille PM. Dielectric resonances and B(1) field inhomogeneity in UHFMRI: computational analysis and experimental findings. Magn Reson Imaging. 2001;19:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Fischer A, Kraff O, Maderwald S, Beiderwellen K, Ladd ME, Forsting M, Lauenstein TC, Umutlu L. Non-enhanced T1-weighted liver vessel imaging at 7 Tesla. PLoS One. 2014;9:e97465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475-485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 370] [Cited by in RCA: 469] [Article Influence: 42.6] [Reference Citation Analysis (2)] |
| 19. | Tincopa MA, Loomba R. Non-invasive diagnosis and monitoring of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Lancet Gastroenterol Hepatol. 2023;8:660-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 20. | Traussnigg S, Kienbacher C, Gajdošík M, Valkovič L, Halilbasic E, Stift J, Rechling C, Hofer H, Steindl-Munda P, Ferenci P, Wrba F, Trattnig S, Krššák M, Trauner M. Ultra-high-field magnetic resonance spectroscopy in non-alcoholic fatty liver disease: Novel mechanistic and diagnostic insights of energy metabolism in non-alcoholic steatohepatitis and advanced fibrosis. Liver Int. 2017;37:1544-1553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Purvis LAB, Clarke WT, Valkovič L, Levick C, Pavlides M, Barnes E, Cobbold JF, Robson MD, Rodgers CT. Phosphodiester content measured in human liver by in vivo (31) P MR spectroscopy at 7 tesla. Magn Reson Med. 2017;78:2095-2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Gajdošík M, Chmelík M, Just-Kukurová I, Bogner W, Valkovič L, Trattnig S, Krššák M. In vivo relaxation behavior of liver compounds at 7 Tesla, measured by single-voxel proton MR spectroscopy. J Magn Reson Imaging. 2014;40:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Cervelli R, Cencini M, Cacciato Insilla A, Aringhieri G, Boggi U, Campani D, Tosetti M, Crocetti L. Ex-vivo human pancreatic specimen evaluation by 7 Tesla MRI: a prospective radiological-pathological correlation study. Radiol Med. 2022;127:950-959. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Umutlu L, Orzada S, Kinner S, Maderwald S, Brote I, Bitz AK, Kraff O, Ladd SC, Antoch G, Ladd ME, Quick HH, Lauenstein TC. Renal imaging at 7 Tesla: preliminary results. Eur Radiol. 2011;21:841-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Umutlu L, Kraff O, Orzada S, Fischer A, Kinner S, Maderwald S, Antoch G, Quick HH, Forsting M, Ladd ME, Lauenstein TC. Dynamic contrast-enhanced renal MRI at 7 Tesla: preliminary results. Invest Radiol. 2011;46:425-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Umutlu L, Maderwald S, Kraff O, Kinner S, Schaefer LC, Wrede K, Antoch G, Forsting M, Ladd ME, Lauenstein TC, Quick HH. New look at renal vasculature: 7 tesla nonenhanced T1-weighted FLASH imaging. J Magn Reson Imaging. 2012;36:714-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Umutlu L, Maderwald S, Kinner S, Kraff O, Bitz AK, Orzada S, Johst S, Wrede K, Forsting M, Ladd ME, Lauenstein TC, Quick HH. First-pass contrast-enhanced renal MRA at 7 Tesla: initial results. Eur Radiol. 2013;23:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Beiderwellen K, Kraff O, Laader A, Maderwald S, Orzada S, Ladd ME, Forsting M, Lauenstein TC, Umutlu L. Contrast enhanced renal MR angiography at 7 Tesla: How much gadolinium do we need? Eur J Radiol. 2017;86:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Laader A, Beiderwellen K, Kraff O, Maderwald S, Ladd ME, Forsting M, Umutlu L. Non-enhanced versus low-dose contrast-enhanced renal magnetic resonance angiography at 7 T: a feasibility study. Acta Radiol. 2018;59:296-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Wu CL, Jordan KW, Ratai EM, Sheng J, Adkins CB, Defeo EM, Jenkins BG, Ying L, McDougal WS, Cheng LL. Metabolomic imaging for human prostate cancer detection. Sci Transl Med. 2010;2:16ra8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Vos EK, Lagemaat MW, Barentsz JO, Fütterer JJ, Zámecnik P, Roozen H, Orzada S, Bitz AK, Maas MC, Scheenen TW. Image quality and cancer visibility of T2-weighted magnetic resonance imaging of the prostate at 7 Tesla. Eur Radiol. 2014;24:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Luttje MP, Italiaander MG, Arteaga de Castro CS, van der Kemp WJ, Luijten PR, van Vulpen M, van der Heide UA, Klomp DW. (31) P MR spectroscopic imaging combined with (1) H MR spectroscopic imaging in the human prostate using a double tuned endorectal coil at 7T. Magn Reson Med. 2014;72:1516-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Philips BWJ, van Uden MJ, Rietsch SHG, Orzada S, Scheenen TWJ. A multitransmit external body array combined with a (1) H and (31) P endorectal coil to enable a multiparametric and multimetabolic MRI examination of the prostate at 7T. Med Phys 2019; 46: 3893-3905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Lagemaat MW, Maas MC, Vos EK, Bitz AK, Orzada S, Weiland E, van Uden MJ, Kobus T, Heerschap A, Scheenen TW. (31) P MR spectroscopic imaging of the human prostate at 7 T: T1 relaxation times, Nuclear Overhauser Effect, and spectral characterization. Magn Reson Med 2015; 73: 909-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Rosenkrantz AB, Zhang B, Ben-Eliezer N, Le Nobin J, Melamed J, Deng FM, Taneja SS, Wiggins GC. T2-weighted prostate MRI at 7 Tesla using a simplified external transmit-receive coil array: correlation with radical prostatectomy findings in two prostate cancer patients. J Magn Reson Imaging 2015; 41: 226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Durand M, Jain M, Robinson B, Aronowitz E, El Douahy Y, Leung R, Scherr DS, Ng A, Donzeau D, Amiel J, Spincemaille P, Villers A, Ballon DJ. Magnetic resonance microscopy may enable distinction between normal histomorphological features and prostate cancer in the resected prostate gland. BJU Int 2017; 119: 414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Hahnemann ML, Kraff O, Maderwald S, Johst S, Orzada S, Umutlu L, Ladd ME, Quick HH, Lauenstein TC. Non-enhanced magnetic resonance imaging of the small bowel at 7 Tesla in comparison to 1.5 Tesla: First steps towards clinical application. Magn Reson Imaging. 2016;34:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |