Published online Feb 28, 2023. doi: 10.4329/wjr.v15.i2.42
Peer-review started: September 28, 2022
First decision: October 17, 2022
Revised: October 24, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: February 28, 2023
Processing time: 147 Days and 17.9 Hours
Paraduodenal pancreatitis (PP) represents a diagnostic challenge, especially in non-referral centers, given its potential imaging overlap with pancreatic cancer. There are two main histological variants of PP, the cystic and the solid, with slightly different imaging appearances. Moreover, imaging findings in PP may change over time because of disease progression and/or as an effect of its risk factors exposition, namely alcohol intake and smoking.
To describe multimodality imaging findings in patients affected by PP to help clinicians in the differential diagnosis with pancreatic cancer.
The systematic review was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analyses 2009 guidelines. A Literature search was performed on PubMed, Embase and Cochrane Library using (groove pancreatitis [Title/Abstract]) OR (PP [Title/Abstract]) as key words. A total of 593 articles were considered for inclusion. After eliminating duplicates, and title and abstract screening, 53 full-text articles were assessed for eligibility. Eligibility criteria were: Original studies including 8 or more patients, fully written in English, describing imaging findings in PP, with pathological confirmation or clinical-radiological follow-up as the gold standard. Finally, 14 studies were included in our systematic review.
Computed tomography (CT) findings were described in 292 patients, magnetic resonance imaging (MRI) findings in 231 and endoscopic ultrasound (EUS) findings in 115. Duodenal wall thickening was observed in 88.8% of the cases: Detection rate was 96.5% at EUS, 91.0% at MRI and 84.1% at CT. Second duodenal portion increased enhancement was recognizable in 76.3% of the cases: Detection rate was 84.4% at MRI and 72.1% at CT. Cysts within the duodenal wall were detected in 82.6% of the cases: Detection rate was 94.4% at EUS, 81.9% at MRI and 75.7% at CT. A solid mass in the groove region was described in 40.9% of the cases; in 78.3% of the cases, it showed patchy enhancement in the portal venous phase, and in 100% appeared iso/hyperintense during delayed phase imaging. Only 3.6% of the lesions showed restricted diffusion. The prevalence of radio
PP has peculiar imaging findings. MRI is the best radiological imaging modality for diagnosing PP, but EUS is more accurate than MRI in depicting duodenal wall alterations.
Core Tip: Paraduodenal pancreatitis (PP) represents a diagnostic challenge, especially in non-referral centers, given its potential imaging overlap with neoplastic processes, namely pancreatic and duodenal carcinoma. Numerous articles show imaging features of PP, but most of them are represented by case reports or reviews with poor scientific background. This systematic review describes the multimodality imaging features (computed tomography, magnetic resonance imaging and endoscopic ultrasound) of PP according to original research articles with pathologic samples and or clinical-radiological follow-up as the gold standard.
- Citation: Bonatti M, De Pretis N, Zamboni GA, Brillo A, Crinò SF, Valletta R, Lombardo F, Mansueto G, Frulloni L. Imaging of paraduodenal pancreatitis: A systematic review. World J Radiol 2023; 15(2): 42-55
- URL: https://www.wjgnet.com/1949-8470/full/v15/i2/42.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i2.42
Paraduodenal pancreatitis (PP), also known as groove pancreatitis, is a peculiar form of chronic pancreatitis characterized by an inflammatory mass-forming involvement of the duodenal wall in the so-called groove area, located between the head of the pancreas, the duodenum, and the common bile duct[1]. The inflammatory process may lead to a solid thickening of the duodenal wall and/or to the development of cystic changes centered in the groove area. PP has been subdivided into cystic or solid type, based on the presence or absence of cysts in the groove area at imaging or pathology. According to a large Italian study, two thirds of patients present the cystic type of PP and one third the solid one[2]; similar data were reported on a more limited series from India[3]. The inflammatory process, arising from the groove area, might also extend to the whole pancreas secondary to the compression and obstruction of the main pancreatic duct by the inflamed and thickened groove area, leading to obstructive chronic pancreatitis. No definitive epidemiological data have been published, but PP is a rare disease considering that in an observational study including 893 patients with chronic pancreatitis, PP prevalence was 6%[4]. On the other hand, a German study published in 2014 reported 3.5% of PP on 373 consecutive pancreatic resections in a single center[5].
Adsay et al[1] described the typical histological features of PP, namely dilated ducts in the duodenal wall with pseudocystic changes and granulation tissue, Brunner’s gland hyperplasia, dense myoid stromal proliferation and fibrosis of the pancreas and of the surrounding soft tissue of the groove area[1].
As reported by many previously published papers, patients suffering from PP are typically middle-aged men, heavy smokers, and drinkers[2-4,6-14]. Acute pancreatitis and abdominal pain have been described as the most frequent presenting symptoms, followed by symptoms related to duodenal obstruction (vomiting and weight loss) and to common bile duct obstruction (jaundice)[2,9-11]. Symptoms related to pancreatic insufficiency (diabetes and steatorrhea) are less frequent and generally reported in patients with advanced disease.
PP diagnosis may be challenging since patients often present with symptoms mimicking pancreatic cancer, such as abdominal pain, vomiting, weight loss or jaundice, and, especially in the solid type, also at imaging the differential diagnosis with pancreatic cancer can be extremely difficult. Therefore, a significant proportion of patients (reported between 5% and 21%, even in referral centers) undergo demolitive pancreatic surgery because of misdiagnosis or malignancy suspicion[2,6,15,16].
Many different therapeutic strategies have been proposed for symptoms’ management in PP and, nowadays, no definitive data have been published about the best choice between medical treatment and endoscopic or surgical interventions. A step-up approach should probably be considered, starting with medical treatment based on pain control, alcohol consumption cessation, and smoke cessation. Endoscopic treatment might be considered in the case of bile duct stenosis and surgery should be reserved for patients with intractable pain, duodenal obstruction, or recurrent bile duct obstruction and cholangitis.
Despite the rarity of the disease, a precise radiological and clinical diagnosis is crucial for patients’ management and a multidisciplinary approach is needed to reduce the risk of misdiagnosis and of inappropriate surgical resections. Therefore, the aim of our study was to conduct a systematic literature review to show the multimodality imaging appearance of PP and to assess imaging performance in the differential diagnosis between PP and pancreatic cancer.
The study was conducted according to the Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) guidelines. We performed a database search on PubMed, Embase and Cochrane Library, looking for articles published from January 1990 to July 2022. The following string was used: (Groove pancreatitis [Title/Abstract]) OR (PP [Title/Abstract]). A total of 593 papers were identified and considered for inclusion. After eliminating duplicates, and title and abstract screening, 53 full-text articles were assessed for eligibility by two radiologists independently. Discrepancies were solved by consensus, which was necessary in 2 cases. Eligibility criteria were original studies including 8 or more patients, written in English, describing imaging findings in PP, with pathological confirmation or clinical-radiological follow-up as the gold standard (Figure 1). Finally, 14 studies were included in our systematic review[2,3,7,9,11,12,15-22].
Study characteristics, including publication date, journal type, inclusion period, aim of the study, study design, characteristics of the patients considered for inclusion, number of patients with PP included, and study limitations were extracted from the included studies (Table 1). The presence of potential bias was evaluated by two Authors in consensus using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies (https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp) (Table 2). The maximum number of points given to each item was 4, 2 and 3, with a total maximum number of 9 points.
| Ref. | Year | Journal type | Aim | Inclusion period | Study design | Patients considered for inclusion | Paraduodenal pancreatitis patients included | Limitations |
| Ishigami et al[17] | 2010 | Radiological | Differential diagnosis cancer vs paraduodenal pancreatitis | 2001-2008 | Retrospective, single center | Institutional database search using “groove pancreatitis or groove pancreatic carcinoma” (n = 22) | 15 | Small population, no clear distinction between computed tomography and magnetic resonance imaging findings |
| Kalb et al[18] | 2013 | Radiological | Differential diagnosis cancer vs paraduodenal pancreatitis | 2007-2010 | Retrospective, single center | Institutional database search using “Whipple and/or pancreatectomy” and diagnosis of cancer or paraduodenal pancreatitis (n = 47) | 17 | Surgically resected patients only, small population |
| Zaheer et al[19] | 2014 | Radiological | Findings description | 2002-2013 | Retrospective, single center | Patients undergoing pancreaticoduodenectomy and histological paraduodenal pancreatitis diagnosis (n = 12) | 12 | Surgically resected patients only, small population |
| Arvanitakis et al[11] | 2014 | Gastroenterological | Endoscopic and medical management | 1995-2010 | Retrospective, single center | Institutional database search using “paraduodenal pancreatitis” (n = 51) | 51 | Poor imaging findings description based on radiological reports |
| Wagner et al[20] | 2015 | Radiological | Findings description | "14 yr" | Retrospective, single center | Patients with cystic dystrophy in heterotopic pancreas diagnosis at endoscopic ultrasound (n = 138) | 76 | Only cystic variant of paraduodenal pancreatitis included |
| Arora et al[3] | 2015 | Radiological | Findings description | 2010-2014 | Retrospective, single center | Patients treated for paraduodenal pancreatitis at gastroenterology or surgical units (n = 33) | 33 | Poor imaging findings description based on radiological reports, no clear distinction between computed tomography and magnetic resonance imaging findings |
| Shin et al[21] | 2016 | Radiological | Differential diagnosis cancer vs paraduodenal pancreatitis | 2005-2011 | Retrospective, 2 centers | Multidetector computed tomography for pancreas protocols (n = 2561) with groove mass | 8 | Surgically resected patients only, small population |
| Boninsegna et al[22] | 2017 | Radiological | Differential diagnosis cancer vs paraduodenal pancreatitis | 2012-2015 | Retrospective, single center | Abdominal Magnetic Resonance Imaging with groove mass | 28 | None |
| de Pretis et al[2] | 2017 | Multidisciplinary | Clinical and morphological features | 1994-2012 | Retrospective, single center | Patients with diagnosis of paraduodenal pancreatitis (n = 120) | 120 | Poor imaging findings description based on radiological reports, no clear distinction between computed tomography and magnetic resonance imaging findings |
| Muraki et al[9] | 2017 | Surgical | Imaging and pathologic correlation | 2004-2015 | Retrospective, single center | All pancreatic resections | 47 | Surgically resected patients only, poor imaging findings description, no clear distinction between computed tomography and magnetic resonance imaging findings |
| Tarvainen et al[16] | 2021 | Multidisciplinary | Diagnosis, natural course and treatment | 2005-2015 | Retrospective, multicentric | Institutional database search using “groove and/or paraduodenal” (n = 192) | 33 | Poor imaging findings description, no clear distinction between computed tomography and magnetic resonance imaging findings |
| Ooka et al[7] | 2021 | Gastroenterological | Clinical management | 2000-2014 | Retrospective, single center | Institutional database search using “groove pancreatitis and/or paraduodenal pancreatitis” (n = 211) | 48 | No clear distinction between computed tomography and magnetic resonance imaging findings |
| Değer et al[15] | 2022 | Surgical | Clinical features and outcome | 2013-2019 | Retrospective, single center | Institutional database search using “groove and/or paraduodenal” (n = 28) | 25 | Poor imaging findings description based on radiological reports, no clear distinction between computed tomography and magnetic resonance imaging findings |
| Kulkarni et al[12] | 2022 | Radiological | Findings description | 2007-2020 | Retrospective, single center | Patients with pancreatitis (n = 2120) | 30 | None |
| Ref. | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Outcome of interest was not present at start of study | Comparability of cohorts | Assessment of outcome | Follow-up long enough | Adequacy of follow up | Total |
| Ishigami et al[17] | ± | - | - | - | - | - | NA | NA | 6 |
| Kalb et al[18] | ± | - | - | - | - | - | NA | NA | 6 |
| Zaheer et al[19] | ± | NA | - | + | NA | - | NA | NA | 2 |
| Arvanitakis et al[11] | ± | NA | ± | + | NA | - | - | - | 3 |
| Wagner et al[20] | + | NA | ± | - | NA | + | NA | NA | 1 |
| Arora et al[3] | ± | NA | ± | + | NA | - | - | - | 3 |
| Shin et al[21] | ± | - | - | - | ± | - | NA | NA | 4 |
| Boninsegna et al[22] | - | ± | - | - | ± | - | NA | NA | 4 |
| de Pretis et al[2] | - | NA | - | - | NA | - | - | - | 6 |
| Muraki et al[9] | - | - | - | - | - | - | NA | NA | 7 |
| Tarvainen et al[16] | ± | ± | - | - | - | - | - | - | 6 |
| Ooka et al[7] | - | - | - | - | - | - | - | - | 9 |
| Değer et al[15] | - | NA | - | - | NA | - | - | - | 6 |
| Kulkarni et al[12] | - | NA | - | - | NA | - | NA | NA | 4 |
The following data were extracted from the included studies: Number of patients examined with the different imaging modalities [computed tomography (CT), magnetic resonance imaging (MRI), ultrasound (US), and endoscopic ultrasound (EUS)], PP variant (cystic/solid), lesions’ size (mean maximum and minimum diameter), presence of duodenal wall thickening (yes/no), duodenal wall thickening distribution (eccentric/circumferential), presence of second duodenal portion increased wall enhancement (yes/no), presence (yes/no) number (single/multiple) and size (mm) of duodenal wall cysts, presence of a discrete pancreatic mass (yes/no), lesion’s signal intensity on T2-weighted images, on T1-weighted images, on high b value diffusion-weighted images and on apparent diffusion coefficient (ADC) map (hypo-/iso-/hyper-intense in comparison to “normal” pancreas), enhancement on arterial, portal venous and delayed phase images (hypo-/iso-/hyper-intense/dense in comparison to “normal” pancreas), enhancement pattern in portal venous phase (hypo/patchy/rim), presence of pancreatic cysts (yes/no), presence of main pancreatic duct dilatation (yes/no), presence of pancreatic calcifications (yes/no), presence of biliary duct dilatation (yes/no), presence of portal vein stenosis (yes/no), presence of gastroduodenal artery displacement (yes/no), presence of peripancreatic fat stranding (yes/no), presence of peripancreatic enlarged lymph nodes (yes/no). The above-mentioned variables were not considered in every study (Tables 3 and 4). The absolute number of patients for which the variable was evaluated is reported in the text as (n = #).
| Ref. | Duodenal wall thickening | Thickening distribution | Duodenal wall enhancement | Duodenal wall cysts | Cysts number | Cysts size | Pancreatic mass | Signal intensity on T2-weighted images | Signal intensity on T1-weighted images, diffusion-weighted images, apparent diffusion coefficient map | Arterial phase enhancement | Portal venous phase enhancement |
| Ishigami et al[17] | + | + | |||||||||
| Kalb et al[18] | + | + | + | 1 | |||||||
| Zaheer et al[19] | 1, 2 | 1 | 1 | 1, 2 | 1 | 1 | |||||
| Arvanitakis et al[11] | 2 | +, 2 | +, 2 | ||||||||
| Wagner et al[20] | 1, 2 | +, 1, 2 | 1 | 1 | |||||||
| Arora et al[3] | +, 1 | +, 1 | +, 1 | ||||||||
| Shin et al[21] | 1 | 1 | |||||||||
| Boninsegna et al[22] | + | + | + | + | + | ||||||
| de Pretis et al[2] | |||||||||||
| Muraki et al[9] | + | ||||||||||
| Tarvainen et al[16] | |||||||||||
| Ooka et al[7] | 1 | 1 | |||||||||
| Değer et al[15] | +, 1 | +, 1 | 1 | ||||||||
| Kulkarni et al[12] | 1 | 1 | 1 |
| Ref. | Delayed enhancement | Enhancement pattern | Pancreatic cysts | Main pancreatic duct dilatation | Pancreatic calcifications | Biliary duct dilatation | Portal vein stenosis | Gastroduodenal artery displacement | Peripancreatic fat stranding | Peripancreatic lymph nodes |
| Ishigami et al[17] | + | + | 1 | + | ||||||
| Kalb et al[18] | + | + | + | + | ||||||
| Zaheer et al[19] | 2 | 1 | 1 | 1 | 1 | 1 | ||||
| Arvanitakis et al[11] | +, 1 | 1 | +, 1 | |||||||
| Wagner et al[20] | +, 1 | 1 | +, 1 | 1 | ||||||
| Arora et al[3] | +, 1 | +, 1 | 1 | +, 1 | +, 1 | |||||
| Shin et al[21] | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
| Boninsegna et al[22] | + | + | + | + | ||||||
| de Pretis et al[2] | 1 | |||||||||
| Muraki et al[9] | + | + | +, 1 | |||||||
| Tarvainen et al[16] | +, 1 | +, 1 | 1 | +, 1 | ||||||
| Ooka et al[7] | 1 | 1 | 1 | 1 | ||||||
| Değer et al[15] | +, 1 | |||||||||
| Kulkarni et al[12] | 1 | 1 | 1 | 1 | 1 |
Diagnostic performance of imaging studies in the differential diagnosis between PP and pancreatic cancer was also assessed.
Absolute numbers and percentages were used to describe quantitative variables. For continuous data, mean values were calculated. Sensitivity, specificity, negative predictive value and positive predictive value in differentiating between PP and pancreatic cancer were reported, when available. P values <0.05 were considered statistically significant.
All the included studies had a retrospective design and encompassed a total of 543 patients, 489 (90%) males and 54 (10%) females, with a mean age of 48 years. History of chronic alcohol abuse was reported in 87% of the cases (n = 524) and 78% of the patients were heavy smokers (n = 334). The included studies were published on radiological journals in 8/14 cases (n = 219), on multidisciplinary journals in 2/14 (n = 153), on gastroenterological journals in 2/14 (n = 99), and on surgical journals in 2/14 (n = 72).
Pathology was the gold standard in 9/14 studies (n = 261), pathology or clinical-radiological follow up in 3/14 (n = 183), follow-up alone in 2/14 (n = 99). Cross-sectional images were reviewed by one or two Radiologists in 10/14 studies (n = 314), whereas in 4/14 studies (n = 229) the described CT and MRI imaging findings were based on the original radiological reports.
Nine out of the 14 evaluated studies included imaging findings obtained from 2 or more imaging modalities, whereas 4 studies were based on CT images only and 3 on MRI only. In 7 of the included studies, it was not always possible to clearly understand if the described findings were derived from CT or MRI images. Therefore, CT findings were described for 292 patients, MRI findings for 231 and EUS findings for 115; US findings were not described in any of the included studies.
Duodenal wall thickening was described in 88.8% of the cases (n = 420); at EUS, duodenal thickening was recognizable in 96.5% of the cases (n = 115), at MRI in 91.0% (n = 78) and at CT in 84.1% (n = 227). The cutoff value for the duodenal wall thickening definition was reported in three studies[18,21,22] (n = 53) and was 3 mm in all of them. Mean maximum duodenal wall thickness was assessed in two studies[19,20] and was 19 mm (n = 88). Wall thickening distribution was evaluated in one study only[19] and was eccentric, involving the second duodenal portion medial wall, in 81.8% of the cases and concentric in 18.2% (n = 11). The second duodenal portion showed an increased enhancement in comparison to the adjacent intestinal walls in 76.3% of the cases (n = 93); second duodenal portion increased enhancement was recognizable in 84.4% of the cases at MRI (n = 32) and in 72.1% at CT (n = 61).
Cysts within the duodenal wall were detected in 82.6% of the cases (n = 419); duodenal wall cysts were recognizable in 94.4% of the cases at EUS (n = 108), in 81.9% of the cases at MRI (n = 138) and in 75.7% of the cases at CT (n = 173). Duodenal wall cysts were single in 65.8% of the cases and multiple in 34.2% (n = 149). Cyst size was evaluated in three studies[9,18,20]. Muraki et al[9] and Wagner et al[20] reported a mean maximum size of the cystic component of 13 mm (n = 123), whereas Kalb et al[18] reported cystic components diameters ranging from 6 to 27 mm (n = 17).
The cystic variant of PP was depicted in 72.0% of the cases and the solid variant in 28.0% (n = 543).
A solid mass in the groove region was described in 40.9% of the cases (n = 88). Mean maximum diameter of the lesion was 38 mm (n = 75), whereas mean minimum diameter was 16 mm (n = 27). Lesions’ signal intensity on T2-weighted images was evaluated in two articles[17,22] (n = 43): The solid lesion was iso-intense to “normal” pancreatic parenchyma in 48.8% of the cases, hyperintense in 30.2% and hypointense in 21.0%. Lesions’ signal intensity on other imaging sequences was assessed only by Boninsegna et al[22] (n = 28): On T1-weighted images the lesion was hypointense in 64.3% of the cases and isointense in 35.7%, on high b-value diffusion-weighted images it was isointense in 71.4% of the cases and hypointense in 28.6%, whereas on ADC maps it was isointense in 71.4% of the cases, hyperintense in 25.0% and hypointense in 3.6%. During the arterial phase of the dynamic study, the lesion appeared hypovascular in 82.4% of the cases and isovascular in 17.6% (n = 34). During the portal venous phase, the lesion appeared isovascular in 47.6% of the cases, hypovascular in 42.9% and hypervascular in 9.5% (n = 42). Enhancement pattern during the portal venous phase was described as “patchy” in 78.3% of the cases, whereas no cases of ring enhancement were detected (n = 23). During the delayed phase, the lesion appeared hyperintense in 53.6% of the cases and isointense in 46.4% (n = 28).
Main pancreatic duct dilatation was present in 56.5% of the cases (n = 499); in the single included studies, prevalence of main pancreatic duct dilatation ranged from 28.9%[16] to 95.5%[20]. Pancreatic cysts were detected in 64.5% of the cases (n = 269); pancreatic cysts detection rate was 80.3% at MRI (n = 122), 52.4% at CT (n = 147) and 42.9% (n = 7) at EUS. Pancreatic calcifications were present in 48.3% of the cases (n = 383); in the single included studies, prevalence of pancreatic calcifications ranged from 20%[7] to 100%[11]. Calcifications in the region of the minor papilla were recognizable in 43.4% of the cases (n = 76).
Biliary duct dilatation was observed in 41.2% of the cases (n = 417), portal vein stenosis in 47.1% (n = 17) and gastroduodenal artery displacement in 64.3% (n = 84). Peripancreatic fat stranding was described in 88.1% of the cases (n = 134) and enlarged peripancreatic lymph nodes were appreciable in 65.0% (n = 20).
Four articles[17,18,21,22] explored imaging accuracy in the differential diagnosis between PP and pancreatic cancer, including a total of 68 patients with PP and 73 with pancreatic adenocarcinoma. Shin et al[21] showed that, at CT, absence of the malignant appearance of biliary duct stenosis (i.e. abrupt duct cutoff or shouldering), presence of duodenal wall thickening and presence of cysts in the groove region are significantly associated with PP (P = 0.002, 0.026 and 0.001, respectively). Ishigami et al[17] found that a patchy enhancement pattern in the portal venous phase at CT and/or MRI is significantly associated with PP (P < 0.0001). Kalb et al[18] showed that poorly experienced radiologists can correctly diagnose PP at MRI with an accuracy of 87.2% (88.2% sensitivity, 86.7% specificity, 78.9% PPV, 92.9% NPV) by looking for the presence of 3 key imaging findings: Focal thickening (> 3 mm) of the second portion of the duodenum, increased enhancement of the second portion of the duodenum and cysts in the groove region. Boninsegna et al[22] observed that, at MRI, iso-/hypo-intensity on high b-value diffusion weighted imaging (DWI), iso-/hyper-intensity on ADC maps and delayed phase iso-/hyper-intensity are significantly associated with PP (P = 0.004, 0.005 and 0.003, respectively), as well as focal thickening of the second portion of the duodenum, presence of cysts in the groove area and absence of main pancreatic duct dilatation (P = 0.001, 0.001 and 0.005, respectively). Moreover, mean maximum diameter was significantly larger in PP than in adenocarcinoma (P = 0.0003).
Our systematic review included 14 original articles showing multimodality imaging findings in PP. Imaging was the main topic in eight of the included articles, whereas it was ancillary in six of them; in these latter articles, imaging findings were not always extensively and accurately described. A total of 22 different imaging features were considered by the Authors in the included articles, with a mean of 4,4 imaging features per article. Surprisingly, the most frequently described imaging features were not directly correlated with PP appearance and were in the presence of main pancreatic duct dilatation (reported in 13 studies), presence of biliary duct dilatation (11 studies) and presence of pancreatic calcifications (10 studies). Presence of duodenal wall thickening and of duodenal walls cysts were also frequently assessed in the included studies (10 and 8 studies, respectively).
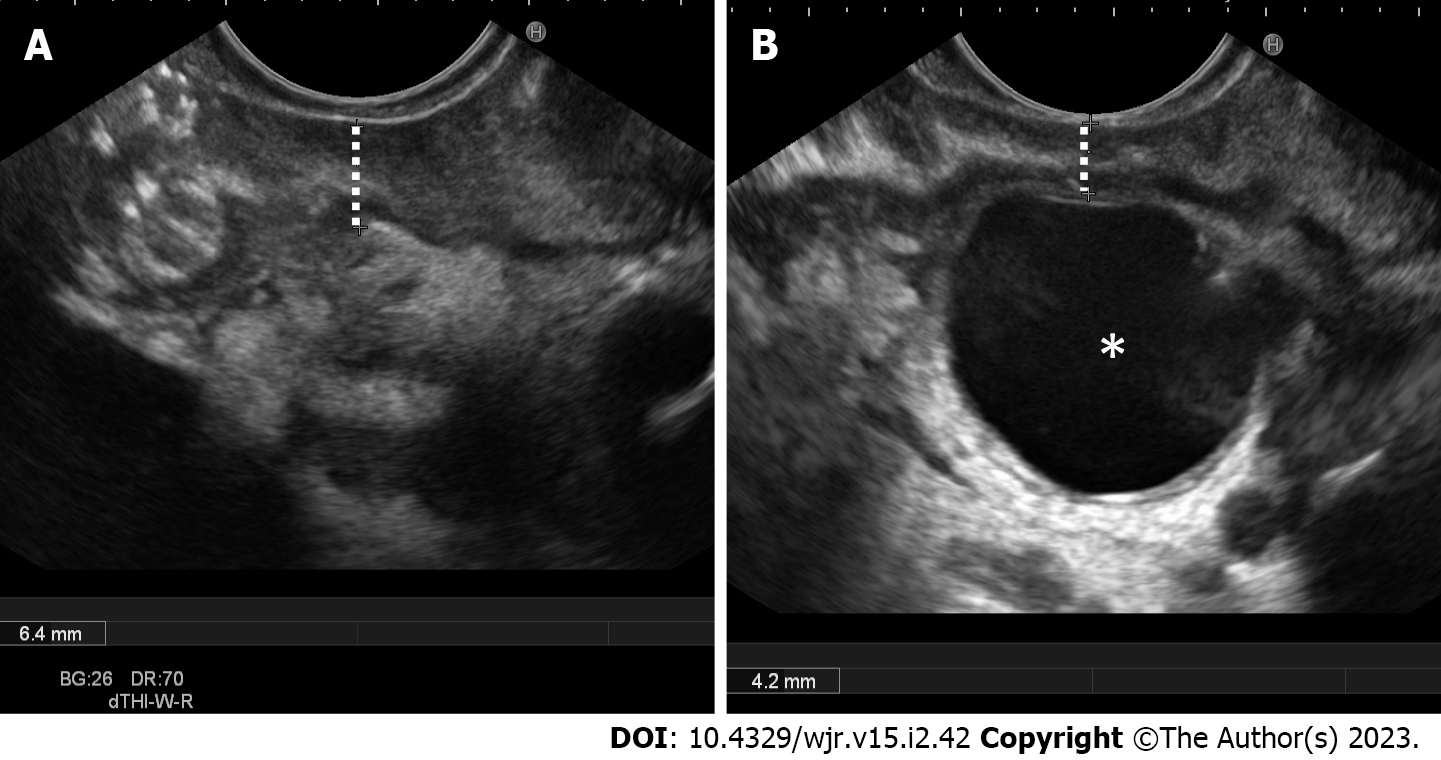
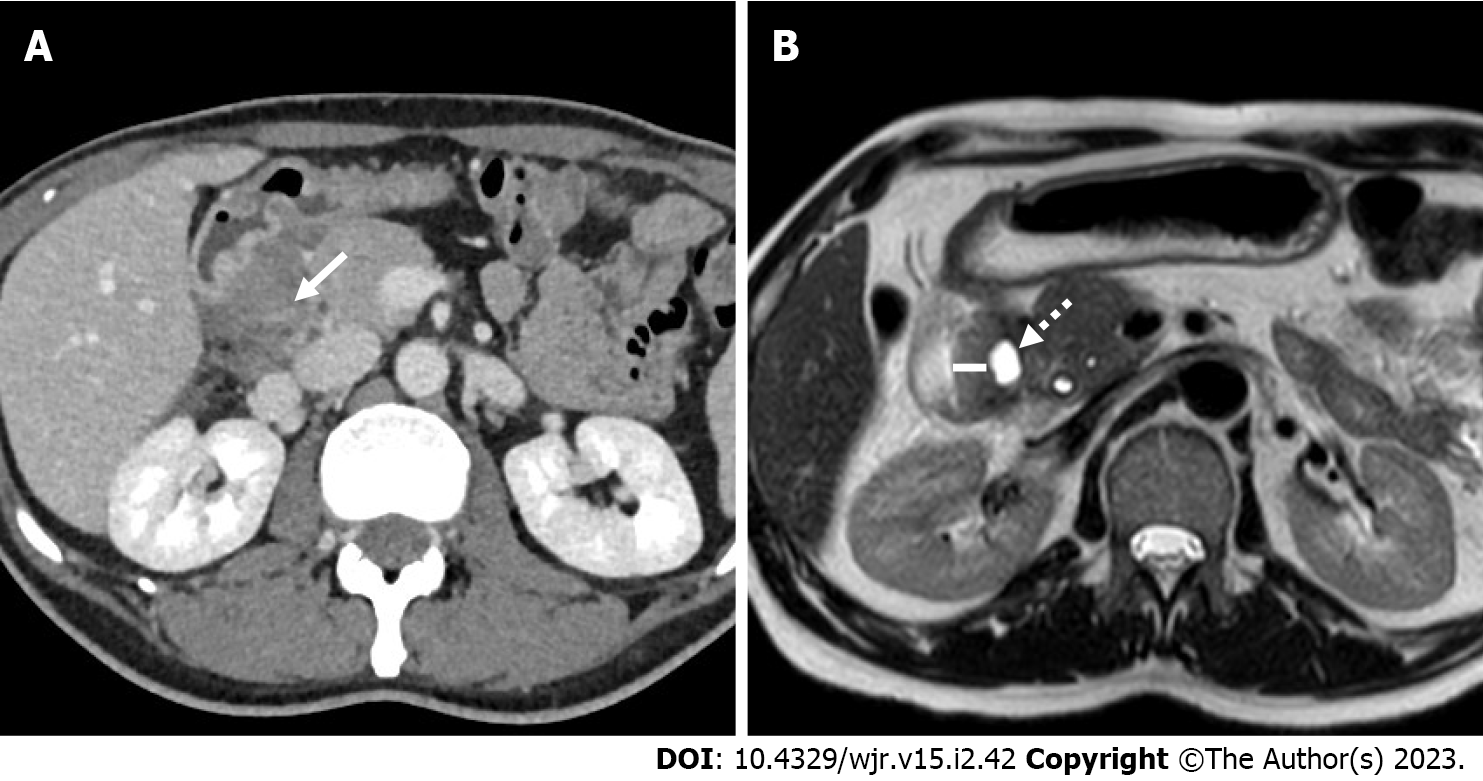
Typical imaging findings in PP are second duodenal portion wall thickening (88.8% of the cases), which is usually eccentric (81.8%), associated with the presence of duodenal wall cysts (82.6%) and second duodenal portion increased wall enhancement (76.3%). Duodenal wall cysts were more frequently single (65.8%) and showed a mean maximum diameter of 13 mm. The above-described imaging findings detection rates varied largely according to the adopted imaging modality. For example, duodenal wall thickening prevalence was 96.5% at EUS (Figure 2A), 91.0% at MRI and 84.1% at CT, and, similarly, duodenal wall cysts prevalence was 94.4% at EUS, 81.9% at MRI and 75.7% at CT. These differences are probably the consequence of the increased tissue contrast resolution of EUS over MRI and of MRI over CT (Figures 2B and 3). Consequently, the prevalence of cystic and solid subtypes of PP can be extremely variable and depends on the patients’ population characteristics (solid subtype prevalence increases in the surgical series, given to the difficulty in differential diagnosis with pancreatic cancer, and decreases in the gastroenterological series) and from the adopted imaging modality (cystic subtype prevalence is higher in MRI and EUS series in comparison to CT series).
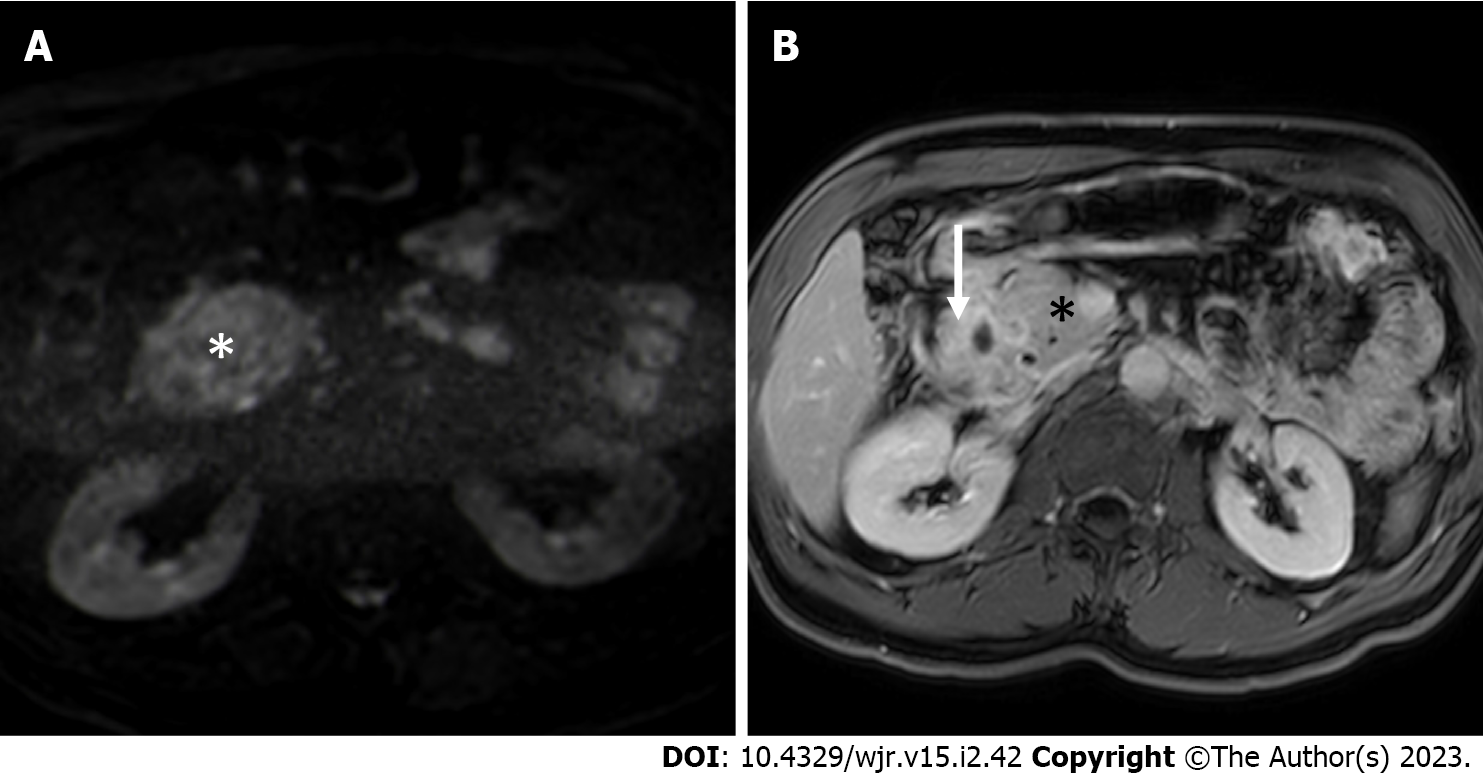
A solid mass in the groove region was described in less than a half (40.9%) of patients with PP. At MRI, lesion signal intensity was quite variable on T1- and T2-weighted images. On the other hand, the included lesions were hypo- to iso-intense in comparison to a normal pancreas on high b-value DWI in 100% of the cases (Figure 4A) and were iso- to hyper-intense on the ADC map in 96.4%. Therefore, the presence of increased diffusivity restriction (i.e. hyperintensity on high b-value DWI and hypointensity on the ADC map) has high negative predictive value for the diagnosis of PP. The solid components typically (82.4%) appeared hypovascular in the arterial phase of the dynamic study and showed a progressive enhancement during the portal venous (57.1% iso- to hyper-intense/attenuating) and the delayed (100% iso- to hyper-intense/attenuating) phases (Figure 4B). The enhancement pattern during the portal venous phase was mainly described as “patchy” (78.3% of the cases). Both patchy enhan
Presence of radiological signs of obstructive chronic pancreatitis were reported with extremely variable prevalence in the included studies. For example, prevalence of main pancreatic duct dilatation ranged from 28.9% to 95.5%, prevalence of pancreatic calcifications from 20.0% to 100%, and prevalence of pancreatic cysts from 35.1% to 94.1%. The rationale of these wide differences is clearly explained in the work of de Pretis et al[2], which demonstrated that the prevalence of both pancreatic calcifications and main pancreatic duct dilatation significantly increases during the course of the disease. Therefore, despite the results reported by Boninsegna et al[22], signs of obstructive chronic pancreatitis should not be used for a differential diagnosis between PP and pancreatic cancer.
Given its expansile inflammatory nature, PP determines reactive alterations in the adjacent structures. The most frequently encountered finding was peripancreatic fat stranding, which was appreciable in 88.1% of the cases, often associated with enlarged reactive peripancreatic lymph nodes (65%). Gastroduodenal artery displacement, without infiltration or occlusion, must also be considered a common finding in PP (64.3%).
Given the central role of duodenal wall changes depiction in the differential diagnosis between PP and pancreatic cancer[21,22], MRI is mandatory if CT is inconclusive, and EUS must be performed if doubts remain even after MRI[23]. Moreover, EUS-guided fine needle aspiration/biopsy should be performed in inconclusive cases, warranting diagnostic sensitivity, specificity, positive predictive value, negative predictive value, and accuracy in differentiating PP from pancreatic cancer of 90%, 100%, 100%, 93%, and 96%, respectively[13].
The main strength of our study is that it is the first systematic literature review of imaging findings in PP. By systematically reviewing 14 different original articles dealing with imaging findings in PP, we have been able to bring together a total of 543 patients affected by PP. The article has also some weaknesses, mainly due to selection bias in the included articles and to the extreme variability of the evaluated and described imaging findings. Moreover, the differential diagnosis between PP and pancreatic cancer, which represents the main criticality, was only addressed in 4 Papers.
PP has peculiar imaging findings that enable differential diagnosis with pancreatic cancer, namely second duodenal portion eccentric wall thickening, increased enhancement, and cystic changes. Absence of increased diffusivity restriction in the groove area, patchy enhancement during the portal venous phase and delayed phase enhancement are also imaging features strongly correlated with PP. Signs of obstructive chronic pancreatitis and biliary obstruction are often present in advanced disease and must not be considered worrisome features.
CT can be considered the first line imaging modality in pancreatic pathologies and enables clinicians to perform a differential diagnosis between PP and pancreatic cancer in most of the cases. Given its higher tissue contrast resolution, MRI represents the second level imaging modality of choice in the case of inconclusive CT findings. EUS has higher accuracy than CT and MRI in depicting duodenal wall changes, offers the possibility of obtaining cyto-histologic samples, but is more invasive and less tolerated; therefore, EUS must be considered a problem-solving technique in difficult cases.
Paraduodenal pancreatitis (PP) is a relatively rare benign inflammatory pathology that can create differential diagnosis dilemmas with pancreatic cancer. Many articles deal with imaging findings in PP, but most of them are represented by case reports, short series, or reviews.
The aim of our work was to perform a systematic literature review of imaging findings in PP considering only original research articles with pathology and/or clinical-radiological follow-up as the reference standard.
To critically describe multimodality imaging findings in PP to help clinicians in the differential diagnosis with pancreatic cancer.
Systematic review of original articles describing imaging findings in 8 or more patients affected by PP with pathological confirmation or clinical-radiological follow-up as the gold standard.
14 articles including 543 patients were included. Computed tomography, magnetic resonance imaging (MRI) and Endoscopic ultrasound (EUS) findings were described.
PP has typical findings at imaging. MRI is the most accurate radiological imaging modality, but EUS has higher sensitivity in depicting duodenal wall alterations.
Radiomics features extraction may be an option in order to further increase imaging accuracy in the differential diagnosis between PP and pancreatic cancer.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Kaneko J, Japan; Tellez-Avila F, United States S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Adsay NV, Zamboni G. Paraduodenal pancreatitis: a clinico-pathologically distinct entity unifying "cystic dystrophy of heterotopic pancreas", "para-duodenal wall cyst", and "groove pancreatitis". Semin Diagn Pathol. 2004;21:247-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | de Pretis N, Capuano F, Amodio A, Pellicciari M, Casetti L, Manfredi R, Zamboni G, Capelli P, Negrelli R, Campagnola P, Fuini A, Gabbrielli A, Bassi C, Frulloni L. Clinical and Morphological Features of Paraduodenal Pancreatitis: An Italian Experience With 120 Patients. Pancreas. 2017;46:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Arora A, Rajesh S, Mukund A, Patidar Y, Thapar S, Arora A, Bhatia V. Clinicoradiological appraisal of 'paraduodenal pancreatitis': Pancreatitis outside the pancreas! Indian J Radiol Imaging. 2015;25:303-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Frulloni L, Gabbrielli A, Pezzilli R, Zerbi A, Cavestro GM, Marotta F, Falconi M, Gaia E, Uomo G, Maringhini A, Mutignani M, Maisonneuve P, Di Carlo V, Cavallini G; PanCroInfAISP Study Group. Chronic pancreatitis: report from a multicenter Italian survey (PanCroInfAISP) on 893 patients. Dig Liver Dis. 2009;41:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Vitali F, Hansen T, Kiesslich R, Heinrich S, Kumar A, Mildenberger P, Amodio A, Benini L, Vantini I, Frulloni L. Frequency and characterization of benign lesions in patients undergoing surgery for the suspicion of solid pancreatic neoplasm. Pancreas. 2014;43:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Balduzzi A, Marchegiani G, Andrianello S, Romeo F, Amodio A, De Pretis N, Zamboni G, Malleo G, Frulloni L, Salvia R, Bassi C. Pancreaticoduodenectomy for paraduodenal pancreatitis is associated with a higher incidence of diabetes but a similar quality of life and pain control when compared to medical treatment. Pancreatology. 2020;20:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Ooka K, Singh H, Warndorf MG, Saul M, Althouse AD, Dasyam AK, Paragomi P, Phillips AE, Zureikat AH, Lee KK, Slivka A, Papachristou GI, Yadav D. Groove pancreatitis has a spectrum of severity and can be managed conservatively. Pancreatology. 2021;21:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Casetti L, Bassi C, Salvia R, Butturini G, Graziani R, Falconi M, Frulloni L, Crippa S, Zamboni G, Pederzoli P. "Paraduodenal" pancreatitis: results of surgery on 58 consecutives patients from a single institution. World J Surg. 2009;33:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Muraki T, Kim GE, Reid MD, Mittal P, Bedolla G, Memis B, Pehlivanoglu B, Freedman A, Erbarut Seven I, Choi H, Kooby D, Maithel SK, Sarmiento JM, Krasinskas A, Adsay V. Paraduodenal Pancreatitis: Imaging and Pathologic Correlation of 47 Cases Elucidates Distinct Subtypes and the Factors Involved in its Etiopathogenesis. Am J Surg Pathol. 2017;41:1347-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Vujasinovic M, Pozzi Mucelli R, Grigoriadis A, Palmér I, Asplund E, Rutkowski W, Baldaque-Silva F, Waldthaler A, Ghorbani P, Verbeke CS, Löhr JM. Paraduodenal pancreatitis - problem in the groove. Scand J Gastroenterol. 2022;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Arvanitakis M, Rigaux J, Toussaint E, Eisendrath P, Bali MA, Matos C, Demetter P, Loi P, Closset J, Deviere J, Delhaye M. Endotherapy for paraduodenal pancreatitis: a large retrospective case series. Endoscopy. 2014;46:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Kulkarni CB, Moorthy S, Pullara SK, Prabhu NK. CT imaging patterns of paraduodenal pancreatitis: a unique clinicoradiological entity. Clin Radiol. 2022;77:e613-e619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Jun JH, Lee SK, Kim SY, Cho DH, Song TJ, Park DH, Lee SS, Seo DW, Kim MH. Comparison between groove carcinoma and groove pancreatitis. Pancreatology. 2018;18:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Egorov VI, Vankovich AN, Petrov RV, Starostina NS, Butkevich ATs, Sazhin AV, Stepanova EA. Pancreas-preserving approach to "paraduodenal pancreatitis" treatment: why, when, and how? Biomed Res Int. 2014;2014:185265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Değer KC, Köker İH, Destek S, Toprak H, Yapalak Y, Gönültaş C, Şentürk H. The clinical feature and outcome of groove pancreatitis in a cohort: A single center experience with review of the literature. Ulus Travma Acil Cerrahi Derg. 2022;28:1186-1192. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Tarvainen T, Nykänen T, Parviainen H, Kuronen J, Kylänpää L, Sirén J, Kokkola A, Sallinen V. Diagnosis, natural course and treatment outcomes of groove pancreatitis. HPB (Oxford). 2021;23:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Ishigami K, Tajima T, Nishie A, Kakihara D, Fujita N, Asayama Y, Ushijima Y, Irie H, Nakamura M, Takahata S, Ito T, Honda H. Differential diagnosis of groove pancreatic carcinomas vs. groove pancreatitis: usefulness of the portal venous phase. Eur J Radiol. 2010;74:e95-e100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Kalb B, Martin DR, Sarmiento JM, Erickson SH, Gober D, Tapper EB, Chen Z, Adsay NV. Paraduodenal pancreatitis: clinical performance of MR imaging in distinguishing from carcinoma. Radiology. 2013;269:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (1)] |
| 19. | Zaheer A, Haider M, Kawamoto S, Hruban RH, Fishman EK. Dual-phase CT findings of groove pancreatitis. Eur J Radiol. 2014;83:1337-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Wagner M, Vullierme MP, Rebours V, Ronot M, Ruszniewski P, Vilgrain V. Cystic form of paraduodenal pancreatitis (cystic dystrophy in heterotopic pancreas (CDHP)): a potential link with minor papilla abnormalities? Eur Radiol. 2016;26:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Shin LK, Jeffrey RB, Pai RK, Raman SP, Fishman EK, Olcott EW. Multidetector CT imaging of the pancreatic groove: differentiating carcinomas from paraduodenal pancreatitis. Clin Imaging. 2016;40:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Boninsegna E, Negrelli R, Zamboni GA, Tedesco G, Manfredi R, Pozzi Mucelli R. Paraduodenal pancreatitis as a mimicker of pancreatic adenocarcinoma: MRI evaluation. Eur J Radiol. 2017;95:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Oza VM, Skeans JM, Muscarella P, Walker JP, Sklaw BC, Cronley KM, El-Dika S, Swanson B, Hinton A, Conwell DL, Krishna SG. Groove Pancreatitis, a Masquerading Yet Distinct Clinicopathological Entity: Analysis of Risk Factors and Differentiation. Pancreas. 2015;44:901-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |