Published online Dec 28, 2023. doi: 10.4329/wjr.v15.i12.350
Peer-review started: August 26, 2023
First decision: October 9, 2023
Revised: October 26, 2023
Accepted: December 12, 2023
Article in press: December 12, 2023
Published online: December 28, 2023
Processing time: 121 Days and 12 Hours
Gastrointestinal stromal tumor (GIST) is a rare gastrointestinal mesenchymal tumor with potential malignancy. Once the tumor ruptures, regardless of tumor size and mitotic number, it can be identified into a high-risk group. It is of great significance for the diagnosis, treatment, and prognosis of GIST if non-invasive examination can be performed before surgery to accurately assess the risk of tumor.
To identify the factors associated with GIST rupture and pathological risk.
A cohort of 50 patients with GISTs, as confirmed by postoperative pathology, was selected from our hospital. Clinicopathological and computed tomography data of the patients were collected. Logistic regression analysis was used to evaluate factors associated with GIST rupture and pathological risk grade.
Pathological risk grade, tumor diameter, tumor morphology, internal necrosis, gas-liquid interface, and Ki-67 index exhibited significant associations with GIST rupture (P < 0.05). Gender, tumor diameter, tumor rupture, and Ki-67 index were found to be correlated with pathological risk grade of GIST (P < 0.05). Multifactorial logistic regression analysis revealed that male gender and tumor diameter ≥ 10 cm were independent predictors of a high pathological risk grade of GIST [odds ratio (OR) = 11.12, 95% confidence interval (95%CI): 1.81-68.52, P = 0.01; OR = 22.96, 95%CI: 2.19-240.93, P = 0.01]. Tumor diameter ≥ 10 cm, irregular shape, internal necrosis, gas-liquid interface, and Ki-67 index ≥ 10 were identified as independent predictors of a high risk of GIST rupture (OR = 9.67, 95%CI: 2.15-43.56, P = 0.01; OR = 35.44, 95%CI: 4.01-313.38, P < 0.01; OR = 18.75, 95%CI: 3.40-103.34, P < 0.01; OR = 27.00, 95%CI: 3.10-235.02, P < 0.01; OR = 4.43, 95%CI: 1.10-17.92, P = 0.04).
Tumor diameter, tumor morphology, internal necrosis, gas-liquid, and Ki-67 index are associated with GIST rupture, while gender and tumor diameter are linked to the pathological risk of GIST. These findings contribute to our understanding of GIST and may inform non-invasive examination strategies and risk assessment for this condition.
Core Tip: Gastrointestinal stromal tumor (GIST) biopsy is inconvenient, has a low yield, and easily leads to tumor metastasis. It is of great significance for the diagnosis, treatment, and prognosis of GIST if non-invasive examination can be performed before surgery to accurately assess the risk of tumor. The results of our study found that tumor diameter, tumor morphology, internal necrosis, and gas-liquid interface are related to the rupture of GIST, and sex and tumor diameter are related to the pathological risk of GIST. The results of this study provides ideas for non-invasive examination and risk assessment of GIST.
- Citation: Liu JZ, Jia ZW, Sun LL. Factors associated with gastrointestinal stromal tumor rupture and pathological risk: A single-center retrospective study. World J Radiol 2023; 15(12): 350-358
- URL: https://www.wjgnet.com/1949-8470/full/v15/i12/350.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i12.350
Gastrointestinal stromal tumor (GIST), a rare mesenchymal tumor of the gastrointestinal tract, presents a potential for malignancy and constitutes 1%-3% of gastrointestinal malignancies[1,2]. Immunohistochemical analysis of GIST typically reveals positive expression of CD117, CD34, or DOG-1[3,4]. Due to its invasive nature and propensity for recurrence and metastasis, the clinical assessment of prognosis following GIST surgery heavily relies on pathological evaluation. However, preoperative selection of appropriate treatment methods lacks a foundation based on pathological assessment. Notably, imaging characteristics of GIST have been observed, and significant disparities in postoperative pathological risk grades have been identified between GISTs exhibiting distinct computed tomography (CT) features prior to surgery, thereby highlighting the crucial role of CT in GIST diagnosis[5,6].
GISTs display unpredictable and variable biological behavior, rendering the distinction between benign and malignant tumors challenging[2,7]. In the early stages, GISTs were classified as either benign or malignant; however, clinical experience has revealed that tumors initially determined as "benign" by histopathology may later metastasize. Consequently, many pathologists advocate for grouping based on pathological risk grades[8,9]. Once the tumor ruptures, irrespective of size and mitotic count, it can be classified into a high-risk group.
GIST biopsy is inconvenient and has a limited yield, and open biopsies can potentially induce tumor metastasis, precluding risk assessment in such cases. Risk assessment cannot be performed for biopsied cases. Therefore, needle biopsy is not recommended prior to surgery for GISTs that can be completely resected[10]. Given the divergent treatment and prognosis of GISTs compared to non-epithelial tumors like lymphoma and schwannoma, preoperative imaging diagnosis and evaluation assume paramount importance. The ability to perform non-invasive examinations before surgery to accurately assess tumor risk would hold significant implications for GIST diagnosis, treatment, and prognosis. In light of this, we postulated that imaging findings possess clinical utility in predicting GIST rupture and pathological risk. Consequently, this study aimed to offer insights into non-invasive examination strategies and risk assessment for GISTs by examining the correlation between imaging findings and GIST rupture and pathological risk.
Fifty patients diagnosed with GISTs were included in this retrospective study, following confirmation of the diagnosis through postoperative pathology at our institution. The patients' clinicopathological and CT data were systematically collected. The study cohort consisted of individuals aged between 18 and 84 years, comprising 28 males and 22 females. In order to ensure the reliability and relevance of the data, specific inclusion and exclusion criteria were applied. The inclusion criteria encompassed patients who had undergone biopsy or surgery at our hospital, with complete and well-documented pathological data, clear risk grading, and comprehensive clinical and CT data available. Furthermore, only primary tumors were considered. Patients who had not undergone CT examination prior to surgery or whose CT image quality was deemed inadequate were excluded. Additionally, cases with uncertain tumor pathological risk grading or those involving tumor relapse were also excluded from the study cohort.
In this investigation, we meticulously gathered a comprehensive set of clinical and pathological data from a cohort of 50 patients diagnosed with GISTs. The dataset encompassed crucial patient demographics such as age and gender, as well as pivotal pathological indicators including risk grade, tumor diameter, morphology, necrosis, rupture status, gas-liquid interface, tumor location, mitotic figures, and Ki-67 index. The assessment of pathological risk was meticulously categorized into four distinct levels, namely, very low, low, moderate, and high, enabling a comprehensive evaluation of the disease severity[8,11,12] (Supplementary Table 1).
Contrast-enhanced CT scanning was performed using a 256-slice computed tomography scanner (Brilliance iCT, Philips) with the following scanning conditions: Peak kilovoltage of 120 and tube current (mA) ranging from 138 to 458. The following parameters were assessed: (1) Tumor diameter: The maximum diameter of the tumor was measured on the coronal image; (2) Tumor morphology: The shape of the tumor was evaluated to determine if it exhibited a regular shape. A tumor with an elliptical or round shape was considered regular; (3) Boundary: The boundary of the tumor was assessed based on the presence of a clear boundary or an unclear boundary. An unclear boundary indicated a potential for invasion; (4) Primary tumor site: The primary tumor site was determined based on the location of the initial lesion; (5) Necrosis: The presence of a necrotic area was determined based on the CT results; and (6) Gas-liquid interface: The presence of a gas-liquid interface was assessed based on the imaging results. These parameters were evaluated to assess the risk factors associated with GIST rupture and pathological risk.
The criteria for tumor rupture included: (1) Tumor rupture or overflow; (2) Presence of bloody ascites; (3) Gastrointestinal perforation at the tumor site; (4) Microscopic infiltration of adjacent organs; (5) Intra-lesional dissection or segmental resection; and (6) Incisional biopsy[12,13].
SPSS 26.0 (IBM Corp, Armonk, NY) software was used for statistical analyses. Enumeration data are expressed as frequencies, and statistical analysis was performed by the χ2 test. Pearson correlation was used to analyze the correlation between age, gender, pathological risk grade, tumor diameter, tumor morphology, internal necrosis, tumor rupture, gas-liquid interface, tumor site, mitotic figures, and Ki-67 index. P < 0.05 was considered statistically significant.
The results of the comparison of clinical data between the unruptured and ruptured GISTs are shown in Table 1. Statistical analysis showed that pathological risk, tumor diameter, tumor morphology, internal necrosis, and gas-liquid interface were associated with GIST rupture (P < 0.05). The differences in age, gender, primary site, mitotic count, and Ki-67 index of the ruptured group and the unruptured group were not statistically significant (P > 0.05). GISTs with a high pathological risk grade, large tumor diameter, irregular shape, internal tumor necrosis, and gas-fluid interface were prone to rupture.
| Unruptured group (n = 38) | Rupture group (n = 12) | Statistical value | P value | |
| Age (yr) | 64.79 ± 9.75 | 57.17 ± 20.61 | 1.24 | 0.24 |
| Gender | 2.31 | 0.13 | ||
| Male | 19 | 9 | ||
| Female | 19 | 3 | ||
| Pathological risk grade | 8.47 | 0.02 | ||
| Low risk | 21 | 3 | ||
| Intermediate risk | 6 | 0 | ||
| High risk | 11 | 9 | ||
| Tumor diameter (cm) | 3.25 (2, 5.25) | 9.0 (4.5, 13.63) | 2.60 | 0.01 |
| Tumor shape | 17.56 | < 0.01 | ||
| Irregular | 9 | 11 | ||
| Regular | 29 | 1 | ||
| Internal necrosis | 15.35 | < 0.01 | ||
| No | 30 | 2 | ||
| Yes | 8 | 10 | ||
| Gas-liquid interface | 23.68 | < 0.01 | ||
| Absence | 27 | 1 | ||
| Presence | 11 | 11 | ||
| Primary site | 0.23 | 0.63 | ||
| Gastric | 22 | 6 | ||
| Small bowel | 16 | 6 | ||
| Mitotic count (50/HPF) | 6.11 ± 2.60 | 6.17 ± 1.64 | 0.08 | 0.94 |
| Ki-67 index (%) | 5.50 (4.75, 8.00) | 7.50 (5.00, 14.25) | 1.15 | 0.25 |
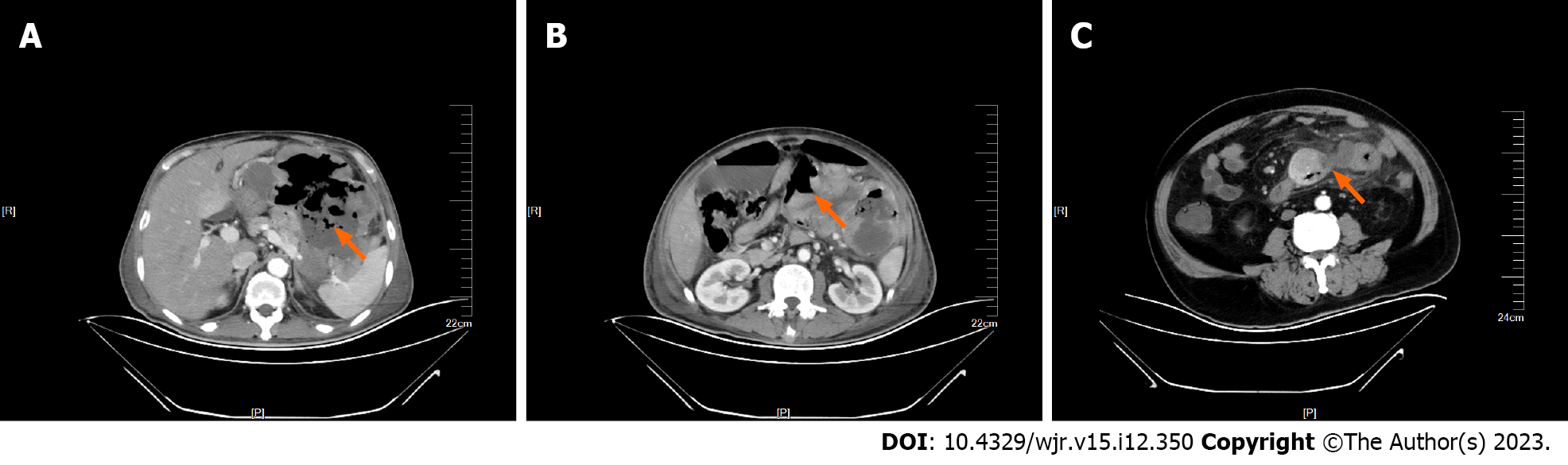
The pathological risk grade assessment of GISTs was carried out through various observation indicators of CT images. The results showed that there were 24 cases of low risk, 6 cases of intermediate risk, and 20 cases of high risk. The analysis results showed that gender, tumor diameter, tumor rupture, and Ki-67 index were associated with the pathological risk grade of GISTs (P < 0.05) (Table 2). We found that male GIST patients had a higher pathological risk grade, and the longer the tumor diameter, the higher the pathological risk of GISTs. GIST patients whose tumors were prone to rupture had a higher pathological risk grade, multiple gas shadows were common in the central necrotic area of ruptured tumors (Figure 1A), gas-liquid interface (Figure 1B) was visible in the tumor, and pus coating was formed next to the tumor (Figure 1C).
| Low risk (n = 24) | Intermediate risk (n = 6) | High risk (n = 20) | χ2 value | P value | |
| Age (yr) | 1.94 | 0.38 | |||
| < 60 | 5 | 2 | 8 | ||
| ≥ 60 | 19 | 4 | 12 | ||
| Gender | 8.10 | 0.02 | |||
| Male | 9 | 3 | 16 | ||
| Female | 15 | 3 | 4 | ||
| Tumor diameter (cm) | 10.47 | 0.01 | |||
| < 10 | 22 | 6 | 11 | ||
| ≥ 10 | 2 | 0 | 9 | ||
| Tumor shape | 2.26 | 0.32 | |||
| Irregular | 9 | 1 | 10 | ||
| Regular | 15 | 5 | 10 | ||
| Internal necrosis | 5.37 | 0.07 | |||
| No | 18 | 5 | 9 | ||
| Yes | 6 | 1 | 11 | ||
| Tumor rupture | 8.47 | 0.02 | |||
| No | 21 | 6 | 11 | ||
| Yes | 3 | 0 | 9 | ||
| Gas-liquid interface | 0.79 | 0.67 | |||
| Absence | 15 | 3 | 10 | ||
| Presence | 9 | 3 | 10 | ||
| Primary site | 3.46 | 0.18 | |||
| Gastric | 16 | 4 | 8 | ||
| Small bowel | 8 | 2 | 12 | ||
| Mitotic count (50/HPF) | 4.49 | 0.11 | |||
| < 5 | 8 | 3 | 3 | ||
| ≥ 5 | 16 | 2 | 17 | ||
| Ki-67 index (%) | 6.30 | 0.04 | |||
| < 10 | 21 | 5 | 11 | ||
| ≥ 10 | 3 | 1 | 9 |
The results of the logistic regression analysis of the factors associated with the pathological risk grade of GISTs are shown in Table 3. Multifactorial logistic regression analysis showed that male gender and tumor diameter ≥ 10 cm were independently correlated with a high pathological risk grade of GISTs [odds ratio (OR) = 11.12, 95% confidence interval (95%CI): 1.81-68.52, P = 0.01; OR = 22.96, 95%CI: 2.19-240.93, P = 0.01].
| B | S.E. | Wald | Sig. | Exp (B) | 95%CI for Exp (B) | ||
| Lower | Upper | ||||||
| Age ≥ 60 yr | 0.44 | 0.90 | 0.24 | 0.63 | 1.55 | 0.27 | 9.07 |
| Male gender | 2.41 | 0.93 | 6.75 | 0.01 | 11.12 | 1.81 | 68.52 |
| Tumor diameter ≥ 10 cm | 3.13 | 1.20 | 6.83 | 0.01 | 22.96 | 2.19 | 240.93 |
| Irregular shape | 0.50 | 1.12 | 0.20 | 0.65 | 1.65 | 0.18 | 14.92 |
| Internal necrosis | -1.40 | 1.21 | 1.35 | 0.25 | 0.25 | 0.02 | 2.62 |
| Gas-liquid interface | 0.50 | 1.26 | 0.16 | 0.69 | 1.64 | 0.14 | 19.48 |
| Small bowel tumor | -0.15 | 0.87 | 0.03 | 0.87 | 0.87 | 0.16 | 4.76 |
| 50/HPF ≥ 5 | -0.33 | 0.87 | 0.14 | 0.71 | 0.72 | 0.13 | 3.96 |
| Ki-67 index ≥ 10 | 2.18 | 1.15 | 3.56 | 0.06 | 8.82 | 0.92 | 84.49 |
The results of the logistic regression analysis of the factors associated with the tumor rupture of GIST are shown in Table 4. Multifactorial logistic regression analysis showed that tumor diameter ≥ 10 cm, irregular shape, internal necrosis, gas-liquid interface, and Ki-67 index ≥ 10 were independently correlated with a high risk of tumor rupture of GISTs (OR = 9.67, 95%CI: 2.15-43.56, P = 0.01; OR = 35.44, 95%CI: 4.01-313.38, P < 0.01; OR = 18.75, 95%CI: 3.40-103.34, P < 0.01; OR = 27.00, 95%CI: 3.10-235.02, P < 0.01; OR = 4.43, 95%CI: 1.10-17.92, P = 0.04).
| B | S.E. | Wald | Sig. | Exp (B) | 95%CI for Exp (B) | ||
| Lower | Upper | ||||||
| Age ≥ 60 yr | 0.21 | 0.71 | 0.08 | 0.77 | 1.23 | 0.31 | 4.93 |
| Male gender | -1.10 | 0.74 | 2.20 | 0.14 | 0.33 | 0.08 | 1.43 |
| Tumor diameter ≥ 10 cm | 2.27 | 0.77 | 8.72 | < 0.01 | 9.67 | 2.15 | 43.56 |
| Irregular shape | 3.57 | 1.11 | 10.30 | < 0.01 | 35.44 | 4.01 | 313.38 |
| Internal necrosis | 2.93 | 0.87 | 11.33 | < 0.01 | 18.75 | 3.40 | 103.34 |
| Gas-liquid interface | 3.30 | 1.10 | 8.91 | < 0.01 | 27.00 | 3.10 | 235.02 |
| Small bowel tumor | -0.32 | 0.66 | 0.23 | 0.63 | 0.73 | 0.20 | 2.67 |
| 50/HPF ≥ 5 | -0.84 | 0.85 | 0.97 | 0.33 | 0.43 | 0.08 | 2.29 |
| Ki-67 index ≥ 10 | 1.49 | 0.71 | 4.36 | 0.04 | 4.43 | 1.10 | 17.92 |
| High pathological risk grade | -1.31 | 0.74 | 3.12 | 0.08 | 0.27 | 0.06 | 1.16 |
In this study, our findings indicated that certain factors are associated with the rupture of GISTs in the patients that we screened. These factors include tumor diameter, tumor shape, internal necrosis, and gas-liquid interface. Additionally, we found that being male and having a tumor diameter ≥ 10 cm are independent correlates of a high pathological risk grade of GISTs.
GIST is a gastrointestinal tumor that has seen a significant increase in the incidence and diagnosis rate in recent years. Rupture and bleeding of GISTs are considered to be serious and dangerous complications that require urgent attention[2,14]. The clinical manifestations of spontaneous tumor rupture and hemorrhage are atypical, characterized by a rapid onset. Many patients are admitted to the hospital with acute abdomen, resulting in delayed surgery. Therefore, timely diagnosis and treatment are crucial for improving patient prognosis[15,16].
Tumor rupture is an important risk factor for recurrence after GIST resection and is also an indicator for adjuvant imatinib therapy[13]. Numerous studies have confirmed that tumor rupture is associated with an increased risk of recurrence. For example, Yanagimoto et al[17] identified that tumor size, mitotic count, tumor location, and tumor rupture were important prognostic factors for GIST. Hølmebakk et al[18] and Nishida et al[19] found that tumor rupture was an independent prognostic factor for recurrence-free survival. These findings highlight the significance of tumor rupture in evaluating the prognosis of GIST patients and its association with the poor outcomes.
Furthermore, approximately half of GIST ruptures are spontaneous and cannot be prevented. Therefore, there is growing interest in studying factors related to tumor rupture[19-22]. Our study identified tumor diameter, tumor shape, internal necrosis, and the presence of gas-liquid interface as factors associated with GIST rupture. Previous research has also reported that larger tumor diameters are associated with a higher risk of rupture[19], and that larger tumors are more likely to experience necrosis in the central region[23]. Positive resection margins have also been strongly linked to tumor rupture[24]. Moreover, the clinical presentation of GISTs, such as an unclear tumor boundary, irregular tumor shape, and the presence of a gas-liquid interface in imaging scans, can indicate aggressive behavior and malignancy. Gas-liquid interface detection in GISTs is currently uncommon, but researchers believe that it predicts severe disease in GIST patients[25-27]. Our study found that necrosis and rupture were more likely to occur when an air-liquid interface was present, and these factors were important indicators of poor prognosis in GIST patients. However, it is important to note that the definition of tumor rupture remains controversial, and consistent standards have yet to be established[18]. Some researchers consider macroscopic damage of tumor pseudocapsule as tumor rupture[19].
In cases of GISTs with high pathological risk grades, CT signs of malignancy include invasive tumor growth, large size with uneven density and unclear boundaries, hemorrhage, liquefaction, necrosis or cystic degeneration, inhomogeneous enhancement on CT enhancement, and the presence of thick tumor blood vessels around the tumor in the arterial phase. Additionally, GISTs metastasizing to other organs and extra-GISTs located outside the gastrointestinal tract are prone to malignancy. Our study found that gender, tumor diameter, rupture, and Ki-67 index were closely associated with pathological risk grades. Lower pathological risk grades of GIST are characterized by slow tumor growth, smaller tumor diameters (usually less than 5.0 cm), round or oval shapes, uniform enhancement on scans, no invasion of surrounding tissues, and no distant organ metastasis. Conversely, higher pathological risk grades indicate worse growth and larger tumor diameters. These tumors are more likely to experience liquefaction and necrosis due to a relative lack of blood supply. Our study suggests that combining CT examination with tumor diameter, morphology, internal necrosis, gas-liquid interface, and Ki-67 index can facilitate early non-invasive assessment of GIST tumor rupture risk, providing valuable information for clinical decision-making. Additionally, clinical diagnostic information can be used to predict the pathological risk grades of GISTs, aiding in further clinical diagnosis and treatment.
There are some limitations to this study that should be acknowledged. First, the small number of GIST samples included warrants further studies with larger sample sizes. Second, the study primarily focused on GIST cases occurring in the gastric and small bowel, which may not fully reflect the relationship between tumor location and tumor rupture and pathological risk grade. Therefore, it is necessary to include more GIST cases in uncommon sites. Lastly, the study lacks information on treatment modalities and the presence of metastases.
In summary, our study has substantiated the association between tumor diameter, tumor shape, internal necrosis, and gas-liquid interface with the occurrence of GIST rupture. Furthermore, we have identified gender and tumor diameter as independent factors influencing the pathological risk grade of GISTs. By leveraging the power of CT detection and integrating the aforementioned factors, we have successfully demonstrated the potential of non-invasive early assessment for GIST rupture and pathological risk grade. These findings hold significant promise in enhancing the clinical decision-making process by providing valuable insights.
Gastrointestinal stromal tumor (GIST) is a rare gastrointestinal mesenchymal tumor. It is of great significance for the diagnosis, treatment, and prognosis of GIST if non-invasive examination can be performed before surgery to accurately assess the risk of tumor.
If accurate assessment of GIST tumor risk through non-invasive examination is the focus of this study, it can provide valuable insights into non-invasive examination strategies and risk assessment of GISTs.
To investigate the factors associated with GIST rupture and pathological risk, and provide insights into non-invasive examination techniques and risk assessment for GISTs.
A cohort of 50 GIST patients was selected from our hospital. Clinicopathological and CT data of the patients were collected. Logistic regression analysis was used to evaluate factors associated with GIST rupture and pathological risk grade.
Male gender and tumor diameter ≥ 10 cm were independent predictors of a high pathological risk grade of GISTs [odds ratio (OR) = 11.12, 95% confidence interval (95%CI): 1.81-68.52, P = 0.01; OR = 22.96, 95%CI: 2.19-240.93, P = 0.01]. Tumor diameter ≥ 10 cm, irregular shape, internal necrosis, gas-liquid interface, and Ki-67 index ≥ 10 were identified as independent predictors of a high risk of GIST rupture (OR = 9.67, 95%CI: 2.15-43.56, P = 0.01; OR = 35.44, 95%CI: 4.01-313.38, P < 0.01; OR = 18.75, 95%CI: 3.40-103.34, P < 0.01; OR = 27.00, 95%CI: 3.10-235.02, P < 0.01; OR = 4.43, 95%CI: 1.10-17.92, P = 0.04).
Tumor diameter, tumor morphology, internal necrosis, gas-liquid interface, and Ki-67 index are associated with GIST rupture, while gender and tumor diameter are linked to the pathological risk of GISTs. These findings contribute to our understanding of GISTs and may inform non-invasive examination strategies and risk assessment for this condition.
In later studies, we can further verify our conclusions in large-sample clinical studies to better guide clinical non-invasive examination and risk assessment of GISTs.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Dimofte GM, Romania; Nishida T, Japan S-Editor: Lin C L-Editor: Wang TQ P-Editor: Lin C
| 1. | Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:21. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Ho MY, Blanke CD. Gastrointestinal stromal tumors: disease and treatment update. Gastroenterology. 2011;140:1372-6.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A, Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, Nishida T, Shen L, Chen LT, Kang YK. Asian Consensus Guidelines for the Diagnosis and Management of Gastrointestinal Stromal Tumor. Cancer Res Treat. 2016;48:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 4. | Qi ZP, Shi Q, Liu JZ, Yao LQ, Xu MD, Cai SL, Li B, Take I, Zhang YQ, Chen WF, Zhong YS, Zhou PH. Efficacy and safety of endoscopic submucosal dissection for submucosal tumors of the colon and rectum. Gastrointest Endosc. 2018;87:540-548.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Choi YR, Kim SH, Kim SA, Shin CI, Kim HJ, Han JK, Choi BI. Differentiation of large (≥ 5 cm) gastrointestinal stromal tumors from benign subepithelial tumors in the stomach: radiologists' performance using CT. Eur J Radiol. 2014;83:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Teoh WC, Teo SY, Ong CL. Gastrointestinal stromal tumors presenting as gynecological masses: usefulness of multidetector computed tomography. Ultrasound Obstet Gynecol. 2011;37:107-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Ibrahim A, Chopra S. Succinate Dehydrogenase-Deficient Gastrointestinal Stromal Tumors. Arch Pathol Lab Med. 2020;144:655-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Raut CP, Espat NJ, Maki RG, Araujo DM, Trent J, Williams TF, Purkayastha DD, DeMatteo RP. Efficacy and Tolerability of 5-Year Adjuvant Imatinib Treatment for Patients With Resected Intermediate- or High-Risk Primary Gastrointestinal Stromal Tumor: The PERSIST-5 Clinical Trial. JAMA Oncol. 2018;4:e184060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Chu H, Pang P, He J, Zhang D, Zhang M, Qiu Y, Li X, Lei P, Fan B, Xu R. Value of radiomics model based on enhanced computed tomography in risk grade prediction of gastrointestinal stromal tumors. Sci Rep. 2021;11:12009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Wei ZG, Han C, Li JD. Treatment Experts Group for Gastrointestinal Stromal. Shijie Huaren Xiaohua Zazhi. 2010;18:65-69. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 11. | Wei SC, Xu L, Li WH, Li Y, Guo SF, Sun XR, Li WW. Risk stratification in GIST: shape quantification with CT is a predictive factor. Eur Radiol. 2020;30:1856-1865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP, Shmookler B, Sobin LH, Weiss SW. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2231] [Cited by in RCA: 2149] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 13. | Nishida T, Hølmebakk T, Raut CP, Rutkowski P. Defining Tumor Rupture in Gastrointestinal Stromal Tumor. Ann Surg Oncol. 2019;26:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 14. | Faigel DO, Abulhawa S. Gastrointestinal stromal tumors: the role of the gastroenterologist in diagnosis and risk stratification. J Clin Gastroenterol. 2012;46:629-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 249] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 16. | Fan X, Han H, Sun Z, Zhang L, Chen G, Mzee SAS, Yang H, Chen J. Prognostic Value of Bleeding in Gastrointestinal Stromal Tumors: A Meta-Analysis. Technol Cancer Res Treat. 2021;20:15330338211034259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Yanagimoto Y, Takahashi T, Muguruma K, Toyokawa T, Kusanagi H, Omori T, Masuzawa T, Tanaka K, Hirota S, Nishida T. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer. 2015;18:426-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Hølmebakk T, Hompland I, Bjerkehagen B, Stoldt S, Bruland ØS, Hall KS, Boye K. Recurrence-Free Survival After Resection of Gastric Gastrointestinal Stromal Tumors Classified According to a Strict Definition of Tumor Rupture: A Population-Based Study. Ann Surg Oncol. 2018;25:1133-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Nishida T, Cho H, Hirota S, Masuzawa T, Chiguchi G, Tsujinaka T; Kinki GIST Study Group. Clinicopathological Features and Prognosis of Primary GISTs with Tumor Rupture in the Real World. Ann Surg Oncol. 2018;25:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Hacking S, Jackson K, Johnston R, Grogan E, Walmsley R, Armstrong L, Coleman J, Grieve J, Robinson S, Frew K, Aujayeb A. GIST-related malignant ascites with large-volume paracentesis complicated by myocardial infarction and tumour rupture. BMJ Support Palliat Care. 2023;13:e93-e95. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Misawa S, Takeda M, Sakamoto H, Kirii Y, Ota H, Takagi H. Spontaneous rupture of a giant gastrointestinal stromal tumor of the jejunum: a case report and literature review. World J Surg Oncol. 2014;12:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Santoyo-Villalba J, Triguero-Cabrera J, García-Jiménez A. Acute abdomen secondary to massive bleeding due to rupture of liver metastases of a gastrointestinal stroma tumor. Cir Cir. 2021;89:93-96. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1304] [Article Influence: 72.4] [Reference Citation Analysis (33)] |
| 24. | Hølmebakk T, Bjerkehagen B, Hompland I, Stoldt S, Boye K. Relationship between R1 resection, tumour rupture and recurrence in resected gastrointestinal stromal tumour. Br J Surg. 2019;106:419-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 25. | Sureka B, Bansal K, Arora A. Torricelli-Bernoulli Sign in Gastrointestinal Stromal Tumor. AJR Am J Roentgenol. 2015;205:W468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Fortman BJ. Torricelli-Bernoulli sign in an ulcerating gastric leiomyosarcoma. AJR Am J Roentgenol. 1999;173:199-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Liu T, Lin G, Peng H, Huang L, Jiang X, Li H, Cai K, Jiang J, Guo L, Du X, Tang J, Zhang W, Chen J, Ye Y. Clinicopathological characteristics and prognosis of gastrointestinal stromal tumors containing air-fluid levels. PLoS One. 2021;16:e0261566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |