Published online Dec 28, 2023. doi: 10.4329/wjr.v15.i12.338
Peer-review started: October 22, 2023
First decision: November 2, 2023
Revised: November 12, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: December 28, 2023
Processing time: 64 Days and 3.8 Hours
It has been reported that deep learning-based reconstruction (DLR) can reduce image noise and artifacts, thereby improving the signal-to-noise ratio and image sharpness. However, no previous studies have evaluated the efficacy of DLR in improving image quality in reduced-field-of-view (reduced-FOV) diffusion-weighted imaging (DWI) [field-of-view optimized and constrained undistorted single-shot (FOCUS)] of the pancreas. We hypothesized that a combination of these techniques would improve DWI image quality without prolonging the scan time but would influence the apparent diffusion coefficient calculation.
To evaluate the efficacy of DLR for image quality improvement of FOCUS of the pancreas.
This was a retrospective study evaluated 37 patients with pancreatic cystic lesions who underwent magnetic resonance imaging between August 2021 and October 2021. We evaluated three types of FOCUS examinations: FOCUS with DLR (FOCUS-DLR+), FOCUS without DLR (FOCUS-DLR−), and conventional FOCUS (FOCUS-conv). The three types of FOCUS and their apparent diffusion coefficient (ADC) maps were compared qualitatively and quantitatively.
FOCUS-DLR+ (3.62, average score of two radiologists) showed significantly better qualitative scores for image noise than FOCUS-DLR− (2.62) and FOCUS-conv (2.88) (P < 0.05). Furthermore, FOCUS-DLR+ showed the highest contrast ratio (CR) between the pancreatic parenchyma and adjacent fat tissue for b-values of 0 and 600 s/mm2 (0.72 ± 0.08 and 0.68 ± 0.08) and FOCUS-DLR− showed the highest CR between cystic lesions and the pancreatic parenchyma for the b-values of 0 and 600 s/mm2 (0.62 ± 0.21 and 0.62 ± 0.21) (P < 0.05), respectively. FOCUS-DLR+ provided significantly higher ADCs of the pancreas and lesion (1.44 ± 0.24 and 3.00 ± 0.66) compared to FOCUS-DLR− (1.39 ± 0.22 and 2.86 ± 0.61) and significantly lower ADCs compared to FOCUS-conv (1.84 ± 0.45 and 3.32 ± 0.70) (P < 0.05), respectively.
This study evaluated the efficacy of DLR for image quality improvement in reduced-FOV DWI of the pancreas. DLR can significantly denoise images without prolonging the scan time or decreasing the spatial resolution. The denoising level of DWI can be controlled to make the images appear more natural to the human eye. However, this study revealed that DLR did not ameliorate pancreatic distortion. Additionally, physicians should pay attention to the interpretation of ADCs after DLR application because ADCs are significantly changed by DLR.
Core Tip: This study evaluated the efficacy of deep learning-based reconstruction (DLR) for image quality improvement in reduced-field-of-view diffusion-weighted imaging (DWI) of the pancreas. DLR can significantly denoise images without prolonging the scan time or decreasing the spatial resolution. The denoising level of DWI can be controlled to make the images appear more natural to the human eye. However, this study revealed that DLR did not ameliorate pancreatic distortion. Additionally, physicians should pay attention to the interpretation of apparent diffusion coefficients (ADCs) after DLR application because ADCs are significantly changed by DLR.
- Citation: Takayama Y, Sato K, Tanaka S, Murayama R, Goto N, Yoshimitsu K. Deep learning-based magnetic resonance imaging reconstruction for improving the image quality of reduced-field-of-view diffusion-weighted imaging of the pancreas. World J Radiol 2023; 15(12): 338-349
- URL: https://www.wjgnet.com/1949-8470/full/v15/i12/338.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i12.338
Diffusion-weighted imaging (DWI) is a widely adopted magnetic resonance imaging (MRI) technique in clinical practice[1-3]. DWI is useful for detecting and characterizing malignant and non-malignant tumors[2,4]. The detection of pancreatic cancer using DWI has been reported to be equivalent to that using dynamic contrast-enhanced computed tomography when DWI is added to magnetic resonance cholangiopancreatography (MRCP)[5,6]. DWI can be used to predict the histological grade of pancreatic neuroendocrine tumors and differentiate malignant from benign intraductal papillary neoplasms (IPMNs)[7-9].
The diagnosis of abdominal lesions based on DWI can be difficult due to artifacts such as motion, ghosting, and distortion; the pancreas is especially susceptible to these artifacts because it exists deep in the abdomen. Reduced-field-of-view (reduced-FOV) DWI is one solution to reduce artifacts in DWI[8,10-13]. In particular, imaging of the pancreas has been shown to improve image quality, such as visualization of anatomical structures, contrast-to-noise ratio (CNR), and lesion conspicuity, and reduce artifacts, such as ghosting, susceptibility, motion, and aliasing artifacts, compared to full-FOV DWI[8,10,14]. Further improvements in the image quality of pancreatic DWI would allow radiologists to detect pancreatic tumors earlier, especially small pancreatic lesions, and help predict tumor malignancy or aggressiveness.
Recently, deep learning (DL) has been applied to radiology for the detection of lesions, evaluation, and image segmentation[9,15,16]. DL is a subcategory of machine learning; therefore, a subset of artificial intelligence[17,18]. DL-based reconstruction (DLR) can reduce image noise and truncation artifacts, improving the signal-to-noise ratio (SNR) and the sharpness of anatomical structures and lesions[9,15,16]. We hypothesized that a combination of reduced-FOV DWI and DLR would improve the DWI image quality of the pancreas without prolonging scan time. To the best of our knowledge, no previous studies have evaluated this hypothesis. This study aimed to evaluate the efficacy of DLR in improving the image quality in reduced-FOV DWI of the pancreas.
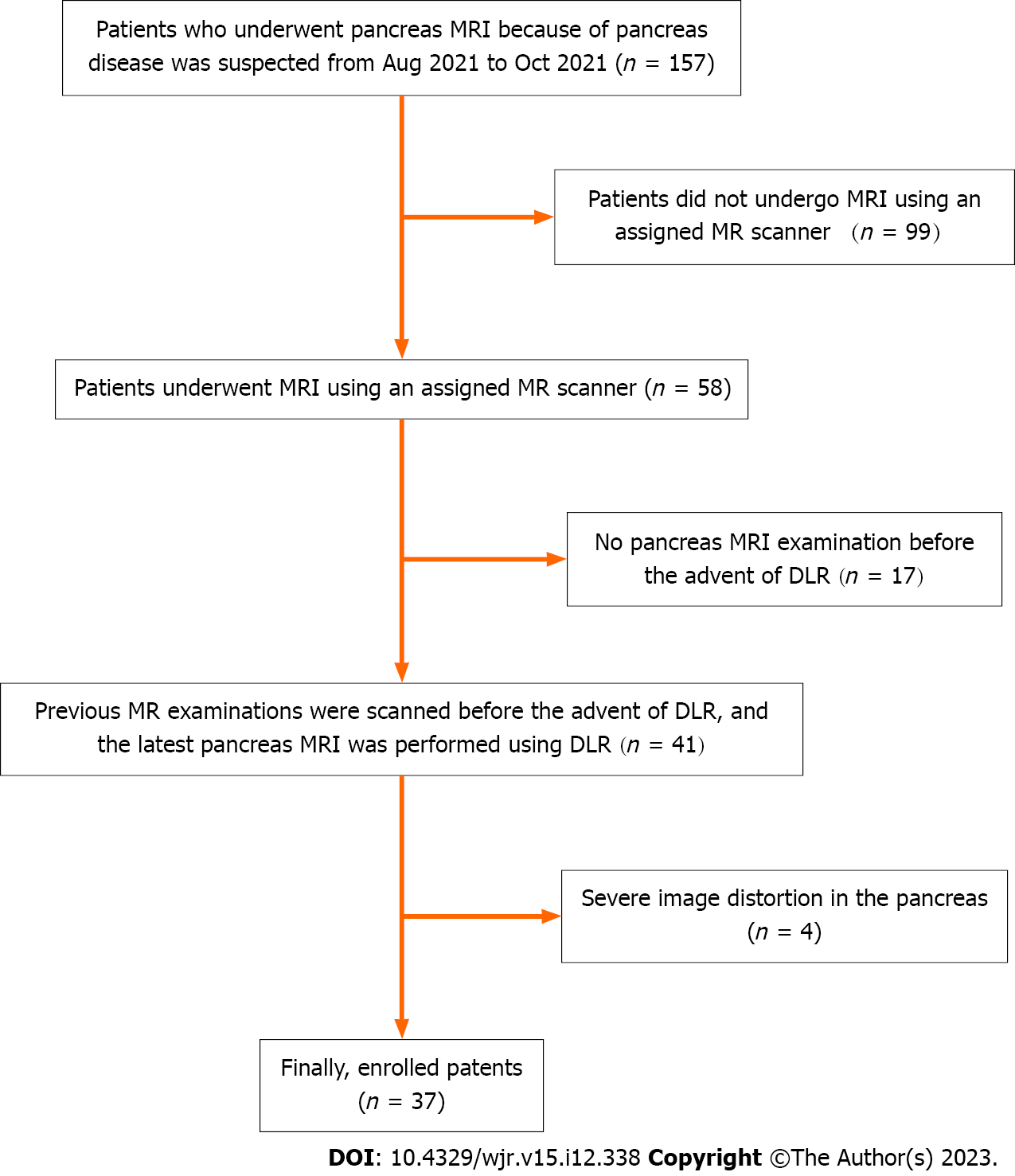
This study was approved by the Institutional Review Board of our hospital. The requirement of written informed consent was waived because this was a retrospective analysis of image post-processing of clinical magnetic resonance (MR) data. Between August 2021 and October 2021, 157 consecutive patients who underwent pancreatic MRI at our institute were investigated. The inclusion criteria were as follows: (1) Patients undergoing annual MRI studies for follow-up of pancreatic cystic lesions; (2) whose MR examinations were performed using an assigned MR scanner; and (3) previous pancreatic MR images were scanned before the advent of DLR, but the latest pancreatic MR images were performed using DLR. Patients were excluded if the pancreas could not be evaluated due to severe distortion. Ultimately, 37 patients [15 females, 22 males; median age (range), 66 years (41–85 years)] were enrolled. Figure 1 shows a flow chart of the patient selection process. Among the 37 patients, 21 were suspected to have IPMN on endoscopic retrograde cholangiopancreatography and/or MRI. The other 16 patients were diagnosed with unspecified pancreatic cystic lesions, such as IPMN and lymphoepithelial cysts, but their diagnosis was not confirmed.
All examinations were performed using a clinical 3.0-Tesla MR system (Discovery MR750w 3.0T; GE Healthcare, Waukesha, WI, United States). In addition to routine MRI, such as T1-weighted imaging, T2-weighted imaging, full-FOV DWI, and MRCP, each patient underwent reduced-FOV DWI with field-of-view optimized and constrained undistorted single-shot (FOCUS).
The DLR, i.e., AIR™ Recon DL (GE Healthcare), is a vendor-supplied MRI reconstruction algorithm based on a deep convolutional network trained on a database of more than 10000 pairs of artifact-free, high-SNR, high-spatial-resolution image, plus the corresponding low SNR, low-spatial-resolution images[19]. It converts truncation artifacts into improved image sharpness while simultaneously denoising the images[19]. The AIR™ Recon DL was already trained before being installed on the assigned MRI machine, so it was ready to integrate into our MRI reconstruction pipeline. Our motivation for introducing DLR was to improve the image quality of FOCUS of the pancreas, because it suffers from a low SNR and the limitation of not providing good results at higher b-value settings.
In this study, three types of FOCUS were evaluated. First, two types of FOCUS images are generated from a single set of raw FOCUS scanning data. One type of FOCUS was reconstructed with the use of DLR; it is referred to hereafter as “FOCUS-DLR+.” The other type of FOCUS was reconstructed without DLR (“FOCUS-DLR−”). Furthermore, all enrolled patients had undergone a previous MR examination that included a conventional FOCUS (“FOCUS-conv”), which was widely used in clinical practice before the advent of DLR, and before other improvements that are currently standard on the MR scanner. The average difference (range) in the length of time between FOCUS-DLR+/− and FOCUS-conv was 842.8 (181–2007) d.
The details of the imaging parameters of FOCUS-DLR+/− and FOCUS-conv are shown in Table 1. A two-dimensional (2D) spatially selective echoplanar radiofrequency (RF) excitation pulse was used for FOCUS. This reduces the excitation volume in the phase-encoding and slice-selective directions[11]. In a 2D RF pulse, the displacement between fat and water is designed such that the excited fat profile is completely outside the excited water profile; therefore, a fat-suppression technique is unnecessary[11]. A b-value of 600 s/mm2 was used as the maximum b-value in this study, because FOCUS-conv with a b-value of 600 s/mm2 provided acceptable image quality to visualize the pancreatic parenchyma. We also obtained an apparent diffusion coefficient (ADC) map for each type of FOCUS based on the signal intensity (SI) decay of each pixel on DWI with b-values of 0 and 600 s/mm2.
| Parameters | FOCUS-DLR+/– | FOCUS-conv |
| Repetition time, ms | 3000–10000 | 3500–15000 |
| Echo time, ms | 60 | 60 |
| Flip angle, degree | 90 | 90 |
| FOV, mm2 | 220 × 110 | 220 × 110 |
| Matrix | 120 × 64 | 130 × 40 |
| FOV reduction | Anterior-posterior | Anterior-posterior |
| Slice thickness, mm | 3 | 4 |
| Slice gap, mm | 3 | 5 |
| Number of slices | 20–30 | 15–20 |
| Number of excitations | 4 | 8 |
| b-values, s/mm2 | 0 and 600 | 0 and 600 |
| Band width, Hz/pixel | 1950 | 1300 |
| Respiratory compensation | Respiratory-triggered with navigator echo | Respiratory-triggered with or without navigator echo |
| Deep learning reconstruction factor | Moderate | N/A |
| Scan time, min | 2–5 | 3–10 |
We conducted qualitative and quantitative comparisons among the three FOCUS types and their ADC maps. The comparison between FOCUS-DLR+ and FOCUS-DLR− was aimed at evaluating DLR by comparing the efficacy of DLR to improve DWI image quality and assessing ADC maps. We also conducted a comparison between FOCUS-DLR+ and FOCUS-conv and between FOCUS-DLR− and FOCUS-conv. This was because the differences between FOCUS-DLR+/− and FOCUS-conv included not only the use of DLR but also updates to the MR scanner, including the update of the MR console to include the AIR™ Recon software.
A study coordinator (Takayama Y, with 23 years of experience in interpreting abdominal MRI) searched and displayed the patients’ MRI datasets using a picture archiving and communication system (PACS) (Rapideye, Canon Medical Systems, Tokyo). For qualitative comparison: (1) Sharpness of the pancreatic contour; (2) image noise; (3) distortion of the pancreas; (4) visualization of pancreatic cystic lesions; and (5) visualization of the main pancreatic duct (MPD) were independently evaluated by two radiologists (R1: Tanaka S and R2: Sato K, with 7 and 6 years of experience in interpreting abdominal MRI, respectively), who were blinded to imaging information and the patient’s clinical data. (1), (2), (3), and (4) were evaluated using each type of FOCUS with a b-value of 600 s/mm2, and (5) was evaluated using ADC maps. Qualitative assessments were performed using a 4-point scoring system. The image-quality scores are listed in Table 2.
| Qualitative assessment | 1 | 2 | 3 | 4 |
| Sharpness of the pancreas contour | Entire pancreas contour is unclear | < 50% of the pancreas contour is clear | ≥ 50% of the pancreas contour is clear | Entire pancreas contour is clear |
| Image noise | Severe noise; no visualization of any organs | Moderate noise; compromised diagnostic capability of FOCUS is more than ≥ 50% of the image | Mild noise; compromised the diagnostic capability of FOCUS is < 50% of the image | No or slight noise on the image |
| Distortion of the pancreas | Severe distortion; no visualization of the whole pancreas | Moderate distortion; no visualization is ≥ 50% of the pancreas | Mild distortion; no visualization is < 50% of the pancreas | No distortion of the pancreas |
| Visualization of pancreas cystic lesion | No visualization of pancreas cystic lesion | Pancreas cystic lesion is visible, but its SI is low | Pancreas cystic lesion is visible with high SI, but its contour is unclear | Pancreas cystic lesion is clearly visible with high SI and clear contour |
| Visualization of MPD on ADC map | No visualization of MPD | Visible MPD is < 50% of the pancreas | Visible MPD is ≥ 50% of the pancreas | Whole MPD is visible |
For quantitative comparison, we calculated the following: (1) Contrast ratios (CRs) between the pancreatic parenchyma and adjacent fat tissue, hereafter referred to as “CRpancreas-fat”; (2) CRs between pancreatic cystic lesion and pancreatic parenchyma (“CRlesion-pancreas”); (3) the ADC of pancreatic parenchyma (“ADCpancreas”); and (4) the ADC of pancreatic cystic lesions (“ADClesion”) for the three types of FOCUS and their ADC maps after drawing polygonal regions of interest (ROIs) on the pancreatic parenchyma, adjacent fat tissue and pancreatic cystic lesion. CRs were calculated for each type of FOCUS, with b-values of 0 and 600 s/mm2. CRs and ADCs were evaluated by the same two radiologists (R1 and R2) using the same PACS after completion of the qualitative assessments. CRpancreas-fat and CRlesion-pancreas were calculated instead of SNR or CNR because: (1) FOCUS does not include background air within the imaging area; and (2) a parallel imaging technique was used for the scan; thus, it was impossible to measure background air noise. CRs were calculated using the following formula:
CRpancreas-fat = (SIpancreas – SIfat)/ (SIpanceras + SIfat)
CRlesion-pancreas = (SIlesion – SIpancreas)/ (SIlesion + SIpancreas)
SIpancreas is the SI of the pancreatic parenchyma, SIfat is the SI of adjacent fat tissue, and SIlesion is the SI of the pancreatic cystic lesion. In this study, all CRs are presented as absolute values.
Regarding the calculation of CRs and ADCs, the routine MRI findings of the patients were used for the localization of MPD and pancreatic cystic lesions. To calculate CRpancreas-fat and ADCpancreas, three as-large-as-possible polygonal ROIs were drawn for each patient on the head, body, and tail of the pancreas to avoid MPD, lesions, and artifacts on the FOCUS images using b-values of 0 and 600 s/mm2. Three additional large-as-possible polygonal ROIs were drawn near the head, body, and tail of the pancreas to avoid vessels, lesions, air, and artifacts for each patient. For CRlesion-pancreas and ADClesion, as-large-as-possible polygonal ROIs were drawn within the pancreatic cystic lesion and the adjacent pancreatic parenchyma on the same axial slice where the lesions showed the maximum diameter. If there were several lesions in the pancreas, the largest lesion was selected for the calculation.
The same ROIs were duplicated for the FOCUS-DLR+, FOCUS-DLR−, and ADC maps. ROIs of similar size for FOCUS-conv and its ADC map were drawn as those of FOCUS-DLR+/−. In addition to qualitative and quantitative comparisons, we compared the scan time between FOCUS-DLR+/− and FOCUS-conv.
To compare image-quality scores, CRs, and ADCs among the three types of FOCUS, the Friedman test was performed. When the Friedman test showed a significant result, the Bonferroni post-hoc test was performed for pairwise comparisons among the three types of FOCUS.
The inter-reader agreement between the image-quality scores of the two radiologists was analyzed using weighted kappa statistics. The kappa values are interpreted as follows: < 0: No agreement; 0–0.20: Slight agreement; 0.21–0.40: Fair agreement; 0.41–0.60: Moderate agreement; 0.61–0.80: Substantial agreement; and 0.81–1.00: Almost perfect agreement.
Comparisons of CRs and ADCs among the three types of FOCUS were analyzed after the measurement results of the two radiologists were combined because it was difficult for them to draw the same ROIs at the same locations of the pancreatic parenchyma, adjacent fat tissue, and pancreatic cystic lesions. Finally, the paired t-test was performed for the comparison of scan time between FOCUS-DLR+/− and FOCUS-conv. All statistical analyses were performed with IBM SPSS Statistics 25.0 (IBM Japan, Tokyo). For the Friedman test and paired t-test, P values <0.05 were considered significant and P < 0.0167 (0.05/3) for the Bonferroni post-hoc test was considered significant.
The detailed results of the qualitative assessments by the two radiologists are shown in Table 3. Radiologists obtained similar results. The Friedman test showed significant differences between the three types of FOCUS in image-quality scores for pancreatic contour sharpness, image noise, visualization of pancreatic cystic lesions, and visualization of MPD on the ADC map (P < 0.05). There were no significant differences in the image-quality scores for pancreatic distortion among the three types of FOCUS (P > 0.05).
| Qualitative assessment | Reader | Mean image-quality score | P value | |||||
| Friedman test | Bonferroni post-hoc test | |||||||
| FOCUS-DLR+ | FOCUS-DLR- | FOCUS-conv | FOCUS-DLR+ vs FOCUS-DLR- | FOCUS-DLR+ vs FOCUS-conv | FOCUS-DLR- vs FOCUS-conv | |||
| Sharpness of pancreas contour | R1 | 3.32 | 3 | 2.24 | < 0.0011 | 0.31 | < 0.0011 | 0.0011 |
| R2 | 3.32 | 3.03 | 2.05 | < 0.0011 | 0.49 | < 0.0011 | < 0.0011 | |
| Image noise | R1 | 3.65 | 2.7 | 2.86 | < 0.00 11 | < 0.0011 | < 0.0011 | 1 |
| R2 | 3.59 | 2.54 | 2.7 | < 0.0011 | < 0.0011 | < 0.0011 | 0.97 | |
| Distortion of pancreas | R1 | 3.16 | 3.11 | 3.05 | 0.05 | N/A | N/A | N/A |
| R2 | 3.11 | 3.18 | 3.05 | 0.73 | N/A | N/A | N/A | |
| Visualization of pancreas cystic lesion | R1 | 3.7 | 3.49 | 2.89 | < 0.0011 | 0.67 | 0.0161 | 0.35 |
| R2 | 3.62 | 3.32 | 2.7 | < 0.0011 | 0.24 | 0.0011 | 0.17 | |
| Visualization of MPD on ADC map | R1 | 2.97 | 2.54 | 2.19 | < 0.0011 | 0.17 | 0.0031 | 0.49 |
| R2 | 2.73 | 2.24 | 1.99 | < 0.0011 | 0.11 | 0.0011 | 0.35 | |
The Bonferroni post-hoc test revealed that FOCUS-DLR+ and FOCUS-DLR− showed significantly higher image-quality scores for the sharpness of the pancreas contour than FOCUS-conv (P < 0.0167), but there were no significant differences between FOCUS-DLR+ and FOCUS-DLR− (P > 0.0167). Regarding image-quality scores of the image noise, FOCUS-DLR+ showed significantly higher scores than FOCUS-DLR− and FOCUS-conv (P < 0.0167), but there were no significant differences in scores between FOCUS-DLR− and FOCUS-conv (P > 0.0167). FOCUS-DLR+ showed significantly higher image-quality scores for visualization of the pancreatic cystic lesion and visualization of MPD on the ADC map compared to FOCUS-conv (P < 0.0167), but there were no significant differences between FOCUS-DLR+ and FOCUS-DLR− or between FOCUS-DLR+ and FOCUS-conv (P > 0.0167).
Table 4 provides the results of the inter-reader agreement between the two radiologists. All qualitative assessments showed significant agreement (P < 0.001).
| Qualitative assessment | Imaging | κ value (95%CI) | P value |
| Sharpness of pancreas contour | FOCUS-DLR+ | 0.73 (0.52–0.95) | < 0.0011 |
| FOCUS-DLR- | 0.69 (0.45–0.93) | < 0.0011 | |
| FOCUS-conv | 0.66 (0.43–0.89) | < 0.0011 | |
| Image noise | FOCUS-DLR+ | 0.69 (0.46–0.92) | < 0.0011 |
| FOCUS-DLR- | 0.70 (0.50–0.90) | < 0.0011 | |
| FOCUS-conv | 0.64 (0.42–0.87) | < 0.0011 | |
| Distortion of pancreas | FOCUS-DLR+ | 0.61 (0.40–0.82) | < 0.0011 |
| FOCUS-DLR- | 0.71 (0.53–0.89) | < 0.0011 | |
| FOCUS-conv | 0.61 (0.41–0.82) | < 0.0011 | |
| Visualization of pancreas cystic lesion | FOCUS-DLR+ | 0.80 (0.56–1.03) | < 0.0011 |
| FOCUS-DLR- | 0.76 (0.60–0.93) | < 0.0011 | |
| FOCUS-conv | 0.71 (0.51–0.91) | < 0.0011 | |
| Visualization of MPD on ADC map | FOCUS-DLR+ | 0.67 (0.51–0.83) | < 0.0011 |
| FOCUS-DLR- | 0.75 (0.60–0.90) | < 0.0011 | |
| FOCUS-conv | 0.74 (0.54–0.94) | < 0.0011 |
The average (range) of the ROIs of the pancreatic parenchyma, the adjacent fat tissue, and the cystic lesion of the pancreas drawn by the two radiologists were the following: R1, 170.9 mm2 (57.3–325.0 mm2), 235.3 mm2 (50.2–791.75 mm2) and 92.7 mm2 (22.2–457.6 mm2); R2, 236.3 mm2 (72.4–676.53 mm2), 151.5 ± 38.2 mm2 (70.9–253.66 mm2) and 104.6 mm2 (22.2–551.0 mm2), respectively.
The detailed results of the quantitative assessment are presented in Table 5. The Friedman test showed significant differences between the three types of FOCUS regarding CRpancreas-fat using b-values of 0 and 600 s/mm2, CRlesion-pancreas using b-values of 0 and 600 s/mm2, ADCpancreas and ADClesion (P < 0.05).
| Quantitative assessments | mean ± SD | P values | |||||
| Friedman test | Bonferroni post-hoc test | ||||||
| FOCUS-DLR+ | FOCUS-DLR- | FOCUS-conv | FOCUS-DLR+ vs FOCUS-DLR- | FOCUS-DLR+ vs FOCUS-conv | FOCUS-DLR- vs FOCUS-conv | ||
| CRpancreas-fat on FOCUS with b value of 600 s/mm2 | 0.68 ± 0.08 | 0.49 ± 0.10 | 0.27 ± 0.11 | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
| CRpancreas-fat on FOCUS with b value of 0 s/mm2 | 0.72 ± 0.08 | 0.65 ± 0.08 | 0.40 ± 0.11 | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
| CRlesion-pancreas on FOCUS with b value of 600 s/mm2 | 0.51 ± 0.26 | 0.62 ± 0.21 | 0.01 ± 0.26 | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
| CRlesion-pancreas on FOCUS with b value of 0 s/mm2 | 0.53 ± 0.21 | 0.62 ± 0.21 | 0.17 ± 0.19 | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
| ADCpancreas+ (× 10-3 mm2/s) | 1.44 ± 0.24 | 1.39 ± 0.22 | 1.84 ± 0.45 | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
| ADClesion (× 10-3 mm2/s) | 3.00 ± 0.66 | 2.86 ± 0.61 | 3.32 ± 0.70 | < 0.0011 | < 0.0011 | < 0.0011 | < 0.0011 |
The Bonferroni post-hoc test revealed that FOCUS-DLR+ showed significantly higher CRpancreas-fat using b-values of 0 and 600 s/mm2 compared to FOCUS-DLR− and FOCUS-conv, and FOCUS-DLR− showed significantly higher CRpancreas-fat than FOCUS-conv (P < 0.0167).
FOCUS- DLR− showed a significantly higher CRlesion-pancreas using b-values of 0 and 600 s/mm2 compared to FOCUS-DLR+ and FOCUS-conv, and FOCUS-DLR+ showed significantly higher CRlesion-pancreas than FOCUS-conv (P < 0.0167).
FOCUS-conv showed significantly higher ADCpancreas and ADClesion compared to FOCUS-DLR+ and FOCUS-DLR−, and FOCUS-DLR+ showed significantly higher ADCpancreas and ADClesion compared to FOCUS-DLR− (P < 0.0167).
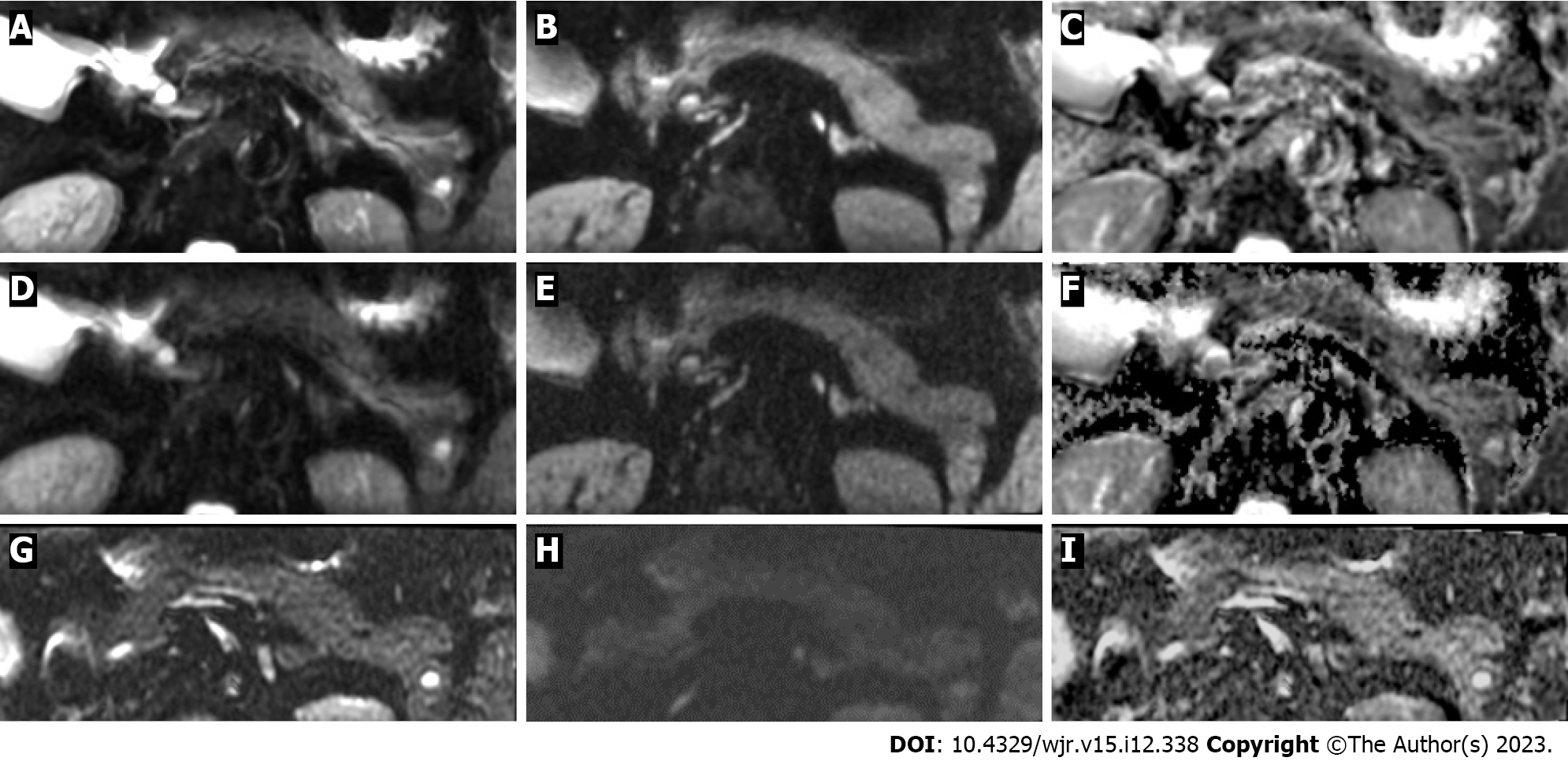
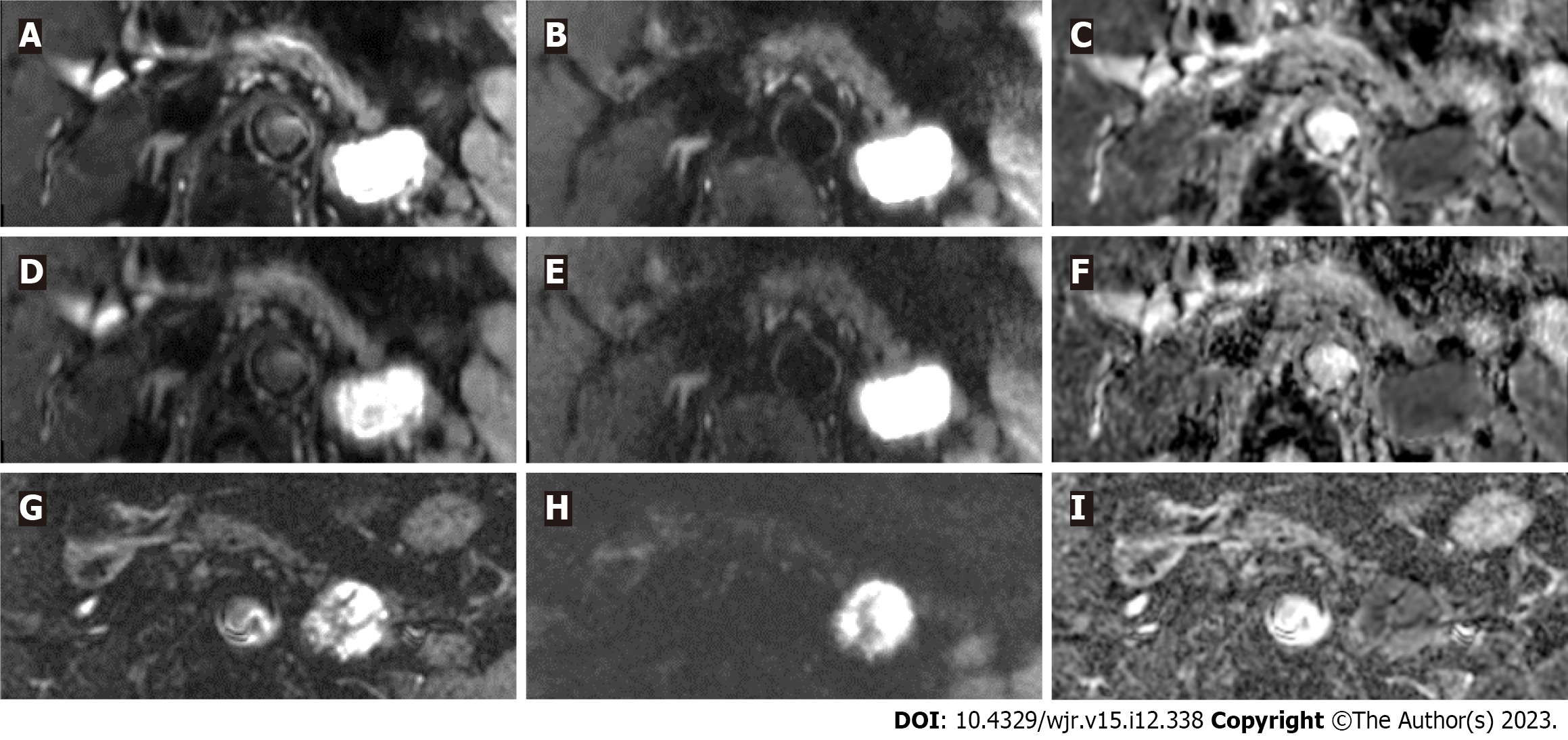
The average scan time of FOCUS-DLR+/− (3 min 27 s) was significantly shorter than that of FOCUS-conv (6 min 28 S) (P < 0.001). Figures 2 and 3 show representative images of FOCUS-DLR+, FOCUS-DLR−, and FOCUS-conv.
Our findings showed that FOCUS-DLR+ can significantly denoise images without prolonging the scan time or decreasing the spatial resolution compared to FOCUS-DLR− and FOCUS-conv. This result is consistent with studies that analyzed the effectiveness of DLR in brain, musculoskeletal, and prostate MRI examinations[8,16,20]. DLR has demonstrated superiority over other denoising methods. Filter-based noise reduction is commonly applied to data reconstruction pipelines to mitigate image noise[21]. However, this method removes image noise and degrades SIs of structural details, resulting in blurred images[9]. On average, an increased number of signals is also effective in obtaining higher-SNR images; however, this method requires longer scan times[4]. A decrease in spatial resolution can reduce image noise because the image SNR is proportional to the voxel size[11]. However, the decrease in spatial resolution is a disadvantage for diagnosis, especially for the depiction of small lesions.
Another benefit of DLR for denoising is that it can control the level of denoising of DWI to make the images appear more natural to the human eye. DLR can improve CRpancreas-fat on FOCUS using a b-value of 600 s/mm2, and CRpancreas-fat and CRlesion-pancreas on FOCUS using a b-value of 0 s/mm2. We speculated that a higher CR would clarify the pancreatic parenchyma and lesions. In fact, FOCUS-DLR− showed higher CRlesion-pancreas than FOCUS-DLR+ with a b-value of 600 s/mm2. This result could be related to an increased in SIs of the pancreatic parenchyma on FOCUS-DLR+ compared to that on FOCUS-DLR−. However, the results of CRlesion-pancreas on FOCUS with a b-value of 600 s/mm2 did not indicate that the detection of pancreatic cystic lesions would be affected by the use of DLR. Instead, DLR is helpful to determine whether there is a lesion inside or outside the tissue.
FOCUS-DLR+ showed a higher image-quality score for the sharpness of the pancreas contour compared to FOCUS-conv, but no significant differences were observed between FOCUS-DLR+ and FOCUS-DLR−. We suggest that FOCUS-DLR+ may be effective in visualizing anatomical structures and lesions in the pancreas. DLR has been reported to be useful in improving image sharpness because it can effectively eliminate truncation artifacts, while denoising is controlled independently[9,16]. Truncation artifacts are caused by incomplete sampling of high spatial frequencies in the Fourier domain (k-space), creating edge ringing in the final reconstructed image, which can be mitigated by increasing the spatial resolution[11,16]. Reduced-FOV DWI can provide a higher spatial resolution than full-FOV DWI[11]. Thus, reduced-FOV DWI may decrease truncation artifacts, regardless of the application of DLR. We speculated that the improvement in the sharpness of the pancreatic contour could be so subtle that it could be difficult for the human eye to recognize.
Our results also revealed that DLR did not ameliorate pancreatic distortion. No previous study has concluded that DLR could be effective in improving image distortion; therefore, our current findings seem reasonable. We used the single-shot echoplanar imaging sequence, which is occasionally disturbed by distortion artifacts in the phase-encoding direction[8,10,14]. In the present study, the pancreas of some patients was distorted due to adjacent air in the gastrointestinal tract. We concluded that DLR could not modify the severe distortions of pancreatic images in the post-processing pipeline after the scan. To reduce image distortion, air must be removed within the scan area or the parameter settings must be modified.
Regarding the comparison of ADCs, FOCUS-DLR+ showed higher image-quality scores for the visualization of MPD on an ADC map compared to FOCUS-conv, but no significant differences were observed between FOCUS-DLR+ and FOCUS-DLR− or between FOCUS- DLR− and FOCUS-conv. This result may be related to the differences in image noise and CRs among the three types of FOCUS. Generally, MPD shows a higher ADC than the pancreatic parenchyma. The high ADC of the MPD is easy for the human eye to recognize on the ADC map; therefore, the DLR might not influence the qualitative assessment of the visualization of MPD on ADC maps.
ADCpancreas and ADClesion acquired from FOCUS-DLR+ were significantly higher than those of FOCUS-DLR− and significantly lower than those of FOCUS-conv. ADCs can vary depending on the MRI apparatus, selection of b-values, and the existence of artifacts[13,22]. Image noise on DWI may also affect the calculation of ADC[23]. The ADC metrics derived from reduced-FOV DWI are controversial; both increased and decreased ADCs of reduced-FOV DWI have been reported compared to full-FOV DWI[13]. Our results indicated that ADCs could vary with the use of DLR due to differences in the SIs of the pancreatic parenchyma and pancreatic cystic lesions and in the level of image noise between the three types of FOCUS. Although ADC measurements may be helpful in differentiating malignancy from non-malignancy as a supplement to other imaging modalities, the interpretation of ADCs after DLR requires further study. One limitation of this study is that we were unable to evaluate ADCpancreas or ADClesion by referring to standard references of pathological findings or larger patient populations. In summary, we could not estimate how DLR affected the calculation results of ADCs and lesion characterization.
There are some limitations to this study. First, we analyzed a small number of patients from a single center. It may be difficult to avoid bias in our results and speculations. A large-scale, multicenter study would be necessary to validate our results. The retrospective design of this study is also a potential source of bias. Second, none of the patients enrolled had solid pancreatic tumors, such as pancreatic carcinoma or neuroendocrine tumors. The diagnosis of such lesions on the FOCUS and ADC maps is of great interest to radiologists. Unfortunately, it was impossible to evaluate these lesions by comparing FOCUS-DLR+/− with FOCUS-conv simply because no patients with such lesions presented for annual follow-up MR examinations. The mechanism by which DLR affects lesion detectability, especially in small pancreatic carcinomas, remains unknown. Third, we used the vendor-supplied DLR that was already trained before being installed on MRI machine. On the other hand, the machine learning model is widely regarded as a black box. It meant that we could not know detailed processes of DLR to improve the image quality of FOCUS-DLR+. Although we evaluated our data using common analysis methods, it might be necessary to prove whether or not our methodology was appropriate to evaluate the effectiveness of DLR. Finally, the b-values used in the analyses of the reduced-FOV were 0 and 600 s/mm2 for the aforementioned reasons. These b-values make it impossible to compare our findings with those of previous studies.
The use of DLR improved the image noise and CRs on FOCUS without prolonging the scan time. However, the interpretation of ADCs on FOCUS, with or without DLR, requires further study.
A combination of these techniques would improve diffusion-weighted imaging (DWI) image quality without prolonging the scan time but would influence the apparent diffusion coefficient calculation.
The image quality of reduced-field-of-view DWI [field-of-view optimized and constrained undistorted single-shot (FOCUS)] of the pancreas suffers from a low signal-to-noise ratio and the limitation of not providing good results at higher b-value settings.
This study aimed to evaluate the efficacy of deep learning-based reconstruction (DLR) for image quality improvement of FOCUS of the pancreas.
This was a retrospective study evaluated 37 patients with pancreatic cystic lesions who underwent magnetic resonance imaging between August 2021 and October 2021. We evaluated three types of FOCUS examinations: FOCUS with DLR (FOCUS-DLR+), FOCUS without DLR (FOCUS-DLR−), and conventional FOCUS (FOCUS-conv). The three types of FOCUS and their apparent diffusion coefficient (ADC) maps were compared qualitatively and quantitatively.
FOCUS-DLR+ (3.62, average score of two radiologists) showed significantly better qualitative scores for image noise than FOCUS-DLR− (2.62) and FOCUS-conv (2.88) (P < 0.05). Furthermore, FOCUS-DLR+ showed the highest contrast ratios (CRs) between the pancreatic parenchyma and adjacent fat tissue for b-values of 0 and 600 s/mm2 (0.72 ± 0.08 and 0.68 ± 0.08) and FOCUS-DLR− showed the highest CR between cystic lesions and the pancreatic parenchyma for the b-values of 0 and 600 s/mm2 (0.62 ± 0.21, and 0.62 ± 0.21) (P < 0.05), respectively. FOCUS-DLR+ provided significantly higher ADCs of the pancreas and lesion (1.44 ± 0.24 and 3.00 ± 0.66) compared to FOCUS-DLR− (1.39 ± 0.22 and 2.86 ± 0.61) and significantly lower ADCs compared to FOCUS-conv (1.84 ± 0.45 and 3.32 ± 0.70) (P < 0.05), respectively.
DLR improved image noise and CRs on FOCUS without prolonging the scan time. However, caution should be exercised when interpreting the ADCs after DLR.
This study revealed that DLR can significantly denoise images without prolonging the scan time or decreasing the spatial resolution. However, DLR did not ameliorate pancreatic distortion and physicians should pay attention to the interpretation of ADCs after DLR application.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Zhao CF, China; Xiang-Ke Niu, China S-Editor: Lin C L-Editor: A P-Editor: Lin C
| 1. | Kwee TC, Takahara T, Ochiai R, Nievelstein RA, Luijten PR. Diffusion-weighted whole-body imaging with background body signal suppression (DWIBS): features and potential applications in oncology. Eur Radiol. 2008;18:1937-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 305] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 2. | Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 3. | Takahara T, Imai Y, Yamashita T, Yasuda S, Nasu S, Van Cauteren M. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat Med. 2004;22:275-282. [PubMed] |
| 4. | Takayama Y, Nishie A, Asayama Y, Ishigami K, Kakihara D, Ushijima Y, Fujita N, Shirabe K, Takemura A, Honda H. Image quality and diagnostic performance of free-breathing diffusion-weighted imaging for hepatocellular carcinoma. World J Hepatol. 2017;9:657-666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Park MJ, Kim YK, Choi SY, Rhim H, Lee WJ, Choi D. Preoperative detection of small pancreatic carcinoma: value of adding diffusion-weighted imaging to conventional MR imaging for improving confidence level. Radiology. 2014;273:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Takakura K, Sumiyama K, Munakata K, Ashida H, Arihiro S, Kakutani H, Tajiri H. Clinical usefulness of diffusion-weighted MR imaging for detection of pancreatic cancer: comparison with enhanced multidetector-row CT. Abdom Imaging. 2011;36:457-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Jiang D, Dou W, Vosters L, Xu X, Sun Y, Tan T. Denoising of 3D magnetic resonance images with multi-channel residual learning of convolutional neural network. Jpn J Radiol. 2018;36:566-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 8. | Kim H, Lee JM, Yoon JH, Jang JY, Kim SW, Ryu JK, Kannengiesser S, Han JK, Choi BI. Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging of the Pancreas: Comparison with Conventional Single-Shot Echo-Planar Imaging. Korean J Radiol. 2015;16:1216-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Ogawa R, Kido T, Nakamura M, Nozaki A, Lebel RM, Mochizuki T. Reconstruction of cardiovascular black-blood T2-weighted image by deep learning algorithm: A comparison with intensity filter. Acta Radiol Open. 2021;10:20584601211044779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Tanabe M, Higashi M, Benkert T, Imai H, Miyoshi K, Kameda F, Ariyoshi S, Ihara K, Ito K. Reduced Field-of-View Diffusion-Weighted Magnetic Resonance Imaging of the Pancreas With Tilted Excitation Plane: A Preliminary Study. J Magn Reson Imaging. 2021;54:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Saritas EU, Cunningham CH, Lee JH, Han ET, Nishimura DG. DWI of the spinal cord with reduced FOV single-shot EPI. Magn Reson Med. 2008;60:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 214] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Mannelli L, Monti S, Corrias G, Fung MM, Nyman C, Golia Pernicka JS, Do RKG. Comparison of Navigator Triggering Reduced Field of View and Large Field of View Diffusion-Weighted Imaging of the Pancreas. J Comput Assist Tomogr. 2019;43:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Warndahl BA, Borisch EA, Kawashima A, Riederer SJ, Froemming AT. Conventional vs. reduced field of view diffusion weighted imaging of the prostate: Comparison of image quality, correlation with histology, and inter-reader agreement. Magn Reson Imaging. 2018;47:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Donati F, Casini C, Cervelli R, Morganti R, Boraschi P. Diffusion-weighted MRI of solid pancreatic lesions: Comparison between reduced field-of-view and large field-of-view sequences. Eur J Radiol. 2021;143:109936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 15. | Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O. Deep learning with convolutional neural network in radiology. Jpn J Radiol. 2018;36:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 16. | Wang X, Ma J, Bhosale P, Ibarra Rovira JJ, Qayyum A, Sun J, Bayram E, Szklaruk J. Novel deep learning-based noise reduction technique for prostate magnetic resonance imaging. Abdom Radiol (NY). 2021;46:3378-3386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 17. | Baştanlar Y, Ozuysal M. Introduction to machine learning. Methods Mol Biol. 2014;1107:105-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Nilsson TH, Nelson TM. Delayed monochromatic hue matches indicate characteristics of visual memory. J Exp Psychol Hum Percept Perform. 1981;7:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Kim M, Kim HS, Kim HJ, Park JE, Park SY, Kim YH, Kim SJ, Lee J, Lebel MR. Thin-Slice Pituitary MRI with Deep Learning-based Reconstruction: Diagnostic Performance in a Postoperative Setting. Radiology. 2021;298:114-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 20. | Kidoh M, Shinoda K, Kitajima M, Isogawa K, Nambu M, Uetani H, Morita K, Nakaura T, Tateishi M, Yamashita Y. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn Reson Med Sci. 2020;19:195-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 154] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 21. | Bouhrara M, Maring MC, Spencer RG. A simple and fast adaptive nonlocal multispectral filtering algorithm for efficient noise reduction in magnetic resonance imaging. Magn Reson Imaging. 2019;55:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Zhu J, Zhang J, Gao JY, Li JN, Yang DW, Chen M, Zhou C, Yang ZH. Apparent diffusion coefficient normalization of normal liver: Will it improve the reproducibility of diffusion-weighted imaging at different MR scanners as a new biomarker? Medicine (Baltimore). 2017;96:e5910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Jones DK, Basser PJ. "Squashing peanuts and smashing pumpkins": how noise distorts diffusion-weighted MR data. Magn Reson Med. 2004;52:979-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 436] [Article Influence: 21.8] [Reference Citation Analysis (0)] |