Published online Nov 28, 2023. doi: 10.4329/wjr.v15.i11.324
Peer-review started: August 17, 2023
First decision: September 14, 2023
Revised: September 29, 2023
Accepted: October 23, 2023
Article in press: October 23, 2023
Published online: November 28, 2023
Processing time: 98 Days and 20.3 Hours
The prognostic value of late gadolinium enhancement (LGE) derived from cardiovascular magnetic resonance (CMR) is well studied, and several new metrics of LGE have emerged. However, some controversies remain; therefore, further discussion is needed, and more precise risk stratification should be explored.
To investigate the associations between the positivity, extent, location, and pattern of LGE and multiple outcomes in dilated cardiomyopathy (DCM).
PubMed, Ovid MEDLINE, and Cochrane Library were searched for studies that investigated the prognostic value of LGE in patients with DCM. Pooled hazard ratios (HRs) and 95% confidence intervals were calculated to assess the role of LGE in the risk stratification of DCM.
Nineteen studies involving 7330 patients with DCM were included in this meta-analysis and covered a wide spectrum of DCM, with a mean left ventricular ejection fraction between 21% and 50%. The meta-analysis revealed that the presence of LGE was associated with an increased risk of multiple adverse outcomes (all-cause mortality, HR: 2.14; arrhythmic events, HR: 5.12; and composite endpoints, HR: 2.38; all P < 0.001). Furthermore, every 1% increment in the extent of LGE was associated with an increased risk of all-cause mortality. Analysis of a subgroup revealed that the prognostic value varied based on different location and pattern of LGE. Additionally, we found that LGE was a stronger predictor of arrhythmic events in patients with greater left ventricular ejection fraction.
LGE by CMR in patients with DCM exhibited a substantial value in predicting adverse outcomes, and the extent, location, and pattern of LGE could provide additional information for risk stratification.
Core Tip: The prognostic value of late gadolinium enhancement (LGE) is well studied, and several new metrics of LGE have emerged. However, some controversies remain; therefore, further discussion is needed, and a more precise risk stratification should be explored.
- Citation: Feng XY, He WF, Zhang TY, Wang LL, Yang F, Feng YL, Li CP, Li R. Association between late gadolinium enhancement and outcome in dilated cardiomyopathy: A meta-analysis. World J Radiol 2023; 15(11): 324-337
- URL: https://www.wjgnet.com/1949-8470/full/v15/i11/324.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i11.324
Dilated cardiomyopathy (DCM) is a heterogeneous heart muscle disease with a prevalence of 1 in 2500 adults. Even with advances in therapy, the prognosis of patients with DCM remains poor (the 5-year mortality rate was as high as 20%) and varies considerably among individuals[1]. Therefore, prognostic stratification in DCM has become a demanding issue in clinical practice.
Currently, the left ventricular ejection fraction (LVEF) is a common applicable indicator for risk stratification in patients with DCM. However, the strategy of using the LVEF alone as a prognostic factor is inadequate in predicting adverse outcomes[2]. Occurring in approximately 30% of patients with DCM, myocardial scar is the most important underlying pathology of sudden cardiac death (SCD) events and the major substrate for ventricular arrhythmia. Late gadolinium enhancement (LGE) based on cardiovascular magnetic resonance (CMR) is currently the gold standard for evaluating localized myocardial fibrosis. Studies have confirmed that LGE is an independent and powerful predictor of adverse outcomes in DCM and could increase the prognostic value of LVEF[2-4].
However, most studies have focused on the presence of LGE and adverse outcomes. With the continuously developing research on LGE in patients with DCM, more studies have incorporated new indicators (the extent, location, and pattern of LGE) to assess the outcomes in patients with DCM, other than just the presence of LGE. Therefore, this meta-analysis was designed to evaluate the role of those novel indicators of LGE in the risk stratification of DCM.
The network meta-analysis extension of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) guidelines provided support in guiding this review[5].
The following databases were searched according to the methods recommended by the Cochrane Handbook: PubMed, Ovid MEDLINE, and Cochrane Library. Studies were searched using relevant controlled vocabulary thesauruses (Medical Subject Headings terms for PubMed) and synonyms for these terms: “dilated cardiomyopathy,” “cardiac magnetic resonance,” “late gadolinium enhancement,” and “prognosis.” The details of the search strategy adopted for PubMed, Ovid MEDLINE, and the Cochrane Library are shown in the Appendix. These searches were limited to cohort studies and were finalized in December 2021.
Observational cohort studies, both prospective and retrospective, were included in the meta-analysis if they reported the prognostic value of LGE in DCM and specified the exclusion of ischemic heart disease (i.e., any medical documentation (coronary angiography, myocardial perfusion imaging, or medical records) that indicated the presence of ischemic heart disease and significant coronary artery disease); moreover, studies with transmural LGE without any history of coronary artery disease or myocardial infarction were included if the imaging phenotype including LGE distribution was not coherent with an ischemic insult in a specific coronary artery territory. When the population of a study also included patients with cardiomyopathies other than DCM, only those with DCM were considered for the meta-analysis. Furthermore, only studies that quantified the extent of LGE as a percentage of left ventricular mass were included in the meta-analysis. The endpoints were classified into three subgroups: (1) All-cause mortality; (2) arrhythmic events, including SCD, cardiac arrest due to ventricular fibrillation (VF), sustained ventricular tachycardia (VT), or appropriate implantable cardioverter–defibrillator (ICD) intervention; and (3) composite endpoints, including all-cause mortality and cardiac events. Cardiac events, included cardiac death[2] (death after a period of clinical deterioration in the signs and symptoms of heart failure despite medical treatment), arrhythmic events, and heart failure. SCD was defined as an unexpected death either within 1 h of the onset of cardiac symptoms in the absence of progressive cardiac deterioration; during sleep; or within 24 h of last being seen alive[6]. VF was defined as irregular or regular tachycardia with regards to polarity, amplitude, morphology, and sequence of intracardiac electrograms, with a mean cycle length of £240 ms (i.e., ³250 beats/min). Sustained VT was defined as tachycardia originating in the ventricle with a rate >100 beats/min and lasting > 30 s or requiring an intervention for termination. Appropriate ICD intervention was defined as a device shock or antitachycardia overdrive pacing delivered in response to a ventricular tachyarrhythmia and documented by stored intracardiac electrocardiogram data[7].
Data were extracted and analyzed by two independent investigators and were reported on standardized forms; consensus was reached through a discussion in case of disagreements. Moreover, data on hazard ratios (HRs) and 95% confidence intervals (CIs), adjusted and unadjusted variables in the regression model, author names, year of publication, sample size, age, percentage of male patients, LGE status (its presence, extent, location, and pattern), follow-up duration, LVEF, and left ventricular end-diastolic volume index (LVEDVi) were recorded if available.
The Newcastle–Ottawa Quality Assessment Scale (NOS) was used for quality assessment, as shown in Table 1, and the quality of the selected studies was evaluated and determined based on the selection of the study groups and the outcomes of interest. The NOS scoring system is as follows. A study scored 1 star for each item within the selection and outcome categories, and a maximum of 2 stars was given for comparability. Nine NOS stars were used to assess the risk of bias. A study with nine stars (four for selection, two for comparability, and three for outcome) was considered to have a low risk of bias, and a study with ≥ 5 stars (two for selection, one for comparability, and two for outcome) was considered to have a medium risk of bias. A study with < 5 stars (0 stars for either of the three fields or 1 star for comparability or outcome) was considered to have a high risk of bias.
| Ref. | Selection | Comparability | Outcome | Total score | |||||
| Exposed cohort | Nonexposed cohort | Ascertainment of exposure | Outcome of interest | Assessment of outcome | Length of follow-up | Adequacy of follow-up | |||
| Halliday et al[6], 2019 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Gulati et al[2], 2013 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Behera et al[9], 2020 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Tateishi et al[10], 2015 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Lehrke et al[11], 2011 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Perazzolo et al[7], 2014 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Neilan et al[12], 2013 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Yamada et al[13], 2014 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Buss et al[14], 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Mikami et al[15], 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Puntmann et al[16], 2016 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 1 | 8 |
| Masci et al[17], 2012 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 7 |
| Šramko et al[8], 2013 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Amzulescu et al[20], 2015 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 5 |
| Alba et al[21], 2020 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 6 |
| Xu et al[18], 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Barison et al[19], 2020 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Halliday et al[4], 2017 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Di Marco et al[22], 2021 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 5 |
Pooled HR and 95%CIs were calculated using a fixed-effects model. The heterogeneity between studies was assessed using Q and I2 tests; heterogeneity was considered significant if I2 ≥ 50%, and the studies will be recalculated using a random-effects model. Furthermore, the meta-analysis was performed using the D–L method if heterogeneity existed, and sensitivity analysis was used to assess the stability of the results.
Publication bias was evaluated using Egger’s and Begg’s tests; trim and fill analysis was used if there was publication bias. Sensitivity analysis using the leave-one-out method was performed, which allowed the calculation of estimates by omitting one study at a time. All analyses were performed using STATA, version 16. P values of < 0.05 were used to denote statistical significance.
The study has been registered with PROSPERO (number: CRD42023382021).
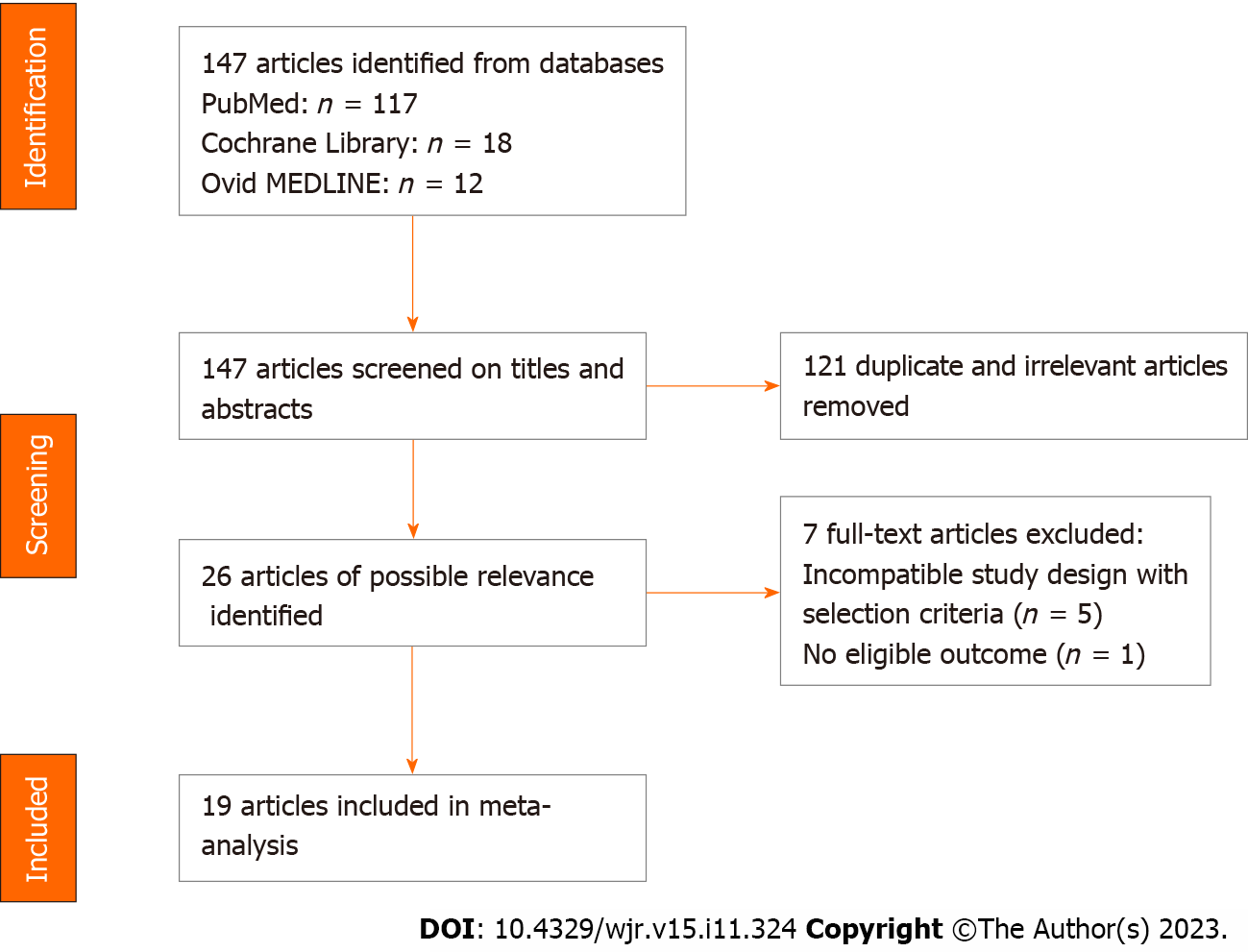
Figure 1 shows a flow chart for the search process and study selection. Overall, 147 citations were identified using PubMed (117 citations), Ovid MEDLINE (12 citations), and the Cochrane Library (18 citations). After screening the article titles and abstracts, 26 full-text articles of possible relevance were recruited, and 121 duplicate or irrelevant articles were excluded. Finally, 19 of the 26 relevant articles were included based on the inclusion criteria.
The quality of the included studies was assessed using the NOS, as shown in Table 1. None of the included studies had a high risk of bias; however, two studies had a low risk of bias[7,8]. For the study selection, 14 studies had a low risk of bias[6-19], and 5 had a medium risk of bias[3,5,20-22]. In terms of comparability, 8 studies had a low risk of bias[7-9,12,13,15,16,19], 7 studies had a medium risk of bias [3,11,14,17,18,21], and 5 studies had a high risk of bias[5,6,10,20,22]. Regarding outcomes, 8 studies had a low risk of bias [3,5,6,7,8,10,14,18] and 11 studies had a medium risk of bias[9,11-13,15-17,19-21,22].
This study analyzed data from 7330 individuals (age ranging from 33 to 76 years; 68% were men) from 19 full-text studies from Asia, Europe, and North America. Of the 7330 patients, 2856 (39.0%) had myocardial LGE, and the follow-up duration ranged from 1 to 132 mo.
Among the 19 selected studies, 4 studies were multicenter[12,16,21,22] and 3 studies were retrospective studies[9,21,22]. 14 studies reported the relationship between the presence of LGE and adverse outcomes[3,5,7,6-14,17,18,21,22], 4 studies that reported on the extent of LGE[3,12,16,20], 2 studies[6,15] that reported on the location of LGE and 1 study[6] that reported on the pattern of LGE were adjusted for age, sex, and LVEF, and a portion of those studies were modified for the New York Heart Association Functional Classification[5,6,10,11,21].
The detailed data on the author, year of publication, sample size, age, percentage of male patients, LGE status, follow-up duration, LVEF, and LVEDVi are shown in Table 2.
| Ref. | Type of study | Patients enrolled | Mean/median age (yr) | Male (%) | LGE present, n (%) | Mean/Median Follow-Up Time | Endpoint included in analysis | Mean/median LVEF (%) | Mean/median LVEDVi |
| Halliday et al[6], 2019 | Prospective | 874 | LGE negative: 51.0 ± 15.1; LGE extent (0.00%-2.55%): 52.8 ± 14.4; LGE extent (2.55%-5.10%): 53.7 ± 14.6; LGE extent (> 5.10%): 56.2 ± 14.6 | 588 (67.3) | 300 (34.3) | 4.9 yr (range, 3.5-7.0 yr) | All cause mortality; arrhythmic events: SCD and aborted SCD. | 39 (range, 29-50) | LGE negative: 126.3 ± 36.6; the extent of LGE (0.00-2.55%): 147.9 ± 46.1; the extent of LGE (2.55%-5.10%): 142.8 ± 49.8; the extent of LGE (> 5.10%): 135.5 ± 37.3 |
| Gulati et al[2], 2013 | Prospective | 472 | 51.1 ± 14.7 | 324 (68.6) | 142 (30.1) | 5.3 yr (range, 31 d-11.0 yr) | Arrhythmic events: SCD and aborted SCD; all cause mortality | 37±13 | 135.1 ± 44.3 |
| Behera et al[9], 2020 | Retrospective | 112 | LGE negative: 45.5 (range, 33.0-58.7); LGE positive: 40.0 (range, 24.5-54.5) | LGE negative: 42 (61.8); LGE positive: 30 (68.2) | 44 (39.3) | 745 ± 320 d | Composite endpoint: All-cause mortality, resuscitated cardiac arrest, sustained VT/appropriate ICD shock, HF hospitalization | LGE negative: 31.5 (range, 28.0-36.2); LGE positive: 32.5 (range, 27.0-41.0) | LGE negative: 104.0 (range, 77.0-125.0); LGE positive: 137.0 (range, 87.5-225.2) |
| Tateishi et al[10], 2015 | Prospective | 207 | 50 ± 16 | 165 (80) | 105 (50.7) | 44 mo (range, 23-62 mo) | Composite endpoint: Cardiac death, cardiac transplantation, LV assist device implantation, appropriate ICD discharge for VT or VF, and rehospitalisation for HF | 27 ± 11 | 143 ± 57 |
| Lehrke et al[11], 2011 | Prospective | 184 | 51.55 ± 1.1 | 138 (75) | 72 (39.1) | 685 ± 30 d | Composite endpoint: Cardiac death, hospitalisation for decompensated HF, or appropriate ICD discharge | LGE negative: 44 (range, 33.1-50.9); LGE positive: 31 (range, 20.9-42.2) | LGE negative: 109 (range, 92.7-137.6); LGE positive: 133 (range, 116-161) |
| Perazzolo et al[7], 2014 | Prospective | 137 | No arrhythmic events: 47 (range, 37-60); arrhythmic events: 59 (range, 43-70) | 108 (78.8) | 76 (55.5) | 3 yr (range, 31 d-9.6 yr) | Arrhythmic events: SCD, cardiac arrest due to VF, sustained VT, or appropriate ICD intervention. | No arrhythmic events:33 (range, 28-40); arrhythmic events: 30 (range, 29-40) | No arrhythmic events: 109 (range, 87-140); arrhythmic events: 123 (range, 105-143) |
| Neilan et al[12], 2013 | Prospective | 162 | 55 ± 14 | 106 (65) | 81 (50) | 29 ± 18 mo | Composite endpoint: Cardiovascular death and appropriate ICD therapy; arrhythmic events: ATP, ICD discharge, and non-heart failure cardiovascular death | 28 ± 9 | 140 ± 50 |
| Yamada et al[13], 2014 | Prospective | 57 | 55 ± 13 | 40 (70.2) | 25 (44) | 71 ± 32 mo | Composite endpoint: Cardiac death, hospitalization for decompensated HF, or documented lethal arrhythmia, including VT and VF | LGE negative: 36 ± 13; LGE positive: 30 ± 11 | LGE negative: 120 ± 39; LGE positive: 141 ± 47 |
| Buss et al[14], 2015 | Prospective | 210 | 52 ± 15 | 159 (76) | 79 (38) | 5.3 yr | Composite endpoint: Cardiac events together with the occurrence of hospitalization due to congestive HF | 36.1 ± 13.8 | 132 ± 48 |
| Mikami et al[15], 2016 | Prospective | 118 | 57 ± 14 | 68 (58) | 66 (56) | 2.1 ± 1.3 yr | Composite endpoint: Cardiac mortality or appropriate ICD therapy; arrhythmic events: Appropriate ICD therapy or SCD | 32 ± 12 | 119 ± 42 |
| Puntmann et al[16], 2016 | Prospective | 637 | 50 (range, 37-76 yr) | 395 (62) | 171 (27) | 22 mo (range, 19-25 mo) | All cause mortality | 47 (range, 29-50) | 109 (range, 89-132) |
| Masci et al[17], 2012 | Prospective | 125 | 59 ± 14 | 82 (65.6) | 50 (40) | 14.2 mo (range, 6.5–28.8 mo) | Composite endpoint: Cardiac death and HF hospitalization | 33 ± 10 | LGE negative: 124 ± 35; LGE positive: 140 ± 39 |
| Šramko et al[8], 2013 | Prospective | 42 | Idiopathic: 45 ± 12; inflammatory: 42 ± 8 | 30 (71.4) | 28 (66.7) | 25 ± 9 mo | Composite endpoint: Cardiac death, urgent heart transplantation, and hospitalization for worsening HF | Idiopathic DC: 22 ± 11; Inflammatory DC: 21 ± 9 | Idiopathic: 137 ± 39; Inflammatory: 148 ± 46 |
| Amzulescu et al[20], 2015 | Prospective | 162 | 55 ± 15 | 102 (63.0) | 63 (39) | 3.4 yr (range, 1.5-6.3 yr) | Composite endpoint: Cardiovascular death, heart transplantation, LV assist device implantation, resuscitated cardiac arrest, and appropriate device shocks | 24.6 ± 8.4 | 161.6 ± 52 |
| Alba et al[21], 2020 | Retrospective | 1672 | 56 ± 14 | 1185 (70.9) | 650 (39) | 2.3 yr (range, 1.0-4.3 yr) | Composite endpoint: All-cause mortality, heart transplantation, or left ventricular assist device implant; arrhythmic events: SCD or appropriate ICD shock | 33 ± 11 | 118 ± 27 |
| Xu et al[18], 2021 | Prospective | 412 | 48.0 ± 14.4 | 300 (72.8) | 201 (48.8) | 28.1 mo (range, 19.3-43.0 mo) | Composite endpoint: All-cause mortality and HF readmission; all-cause mortality | 23.7 ± 9.8 | 185.6 ± 58.9 |
| Barison et al[19], 2020 | Prospective | 183 | 66 (range, 56-73 yr) | 134 (73) | 116 (63) | 30 mo (range, 10-65 mo) | Composite endpoint: Appropriate ICD shock and cardiac death; arrhythmic events: appropriate ICD shock | 24 (range, 21-31) | 143 (range, 120-168) |
| Halliday et al[4], 2017 | Prospective | 399 | 49.9 ± 15.3 | 254 (63.7) | 101 (25.3) | 4.6 yr (range, 3.5-7.0 yr) | Arrhythmic events: SCD or Aborted SCD | 49.6 ± 4.9 | 111.1 ± 19.4 |
| Di Marco et al[22], 2021 | Retrospective | 1165 | 58 (range, 48-68) | 768 (65.9) | 486 (41.7) | 36 mo (range, 20-58 mo) | Arrhythmic events: Appropriate ICD therapies, sustained VT, resuscitated cardiac arrest, and sudden death | 39 (range, 30-46) | 118 (range, 99-142) |
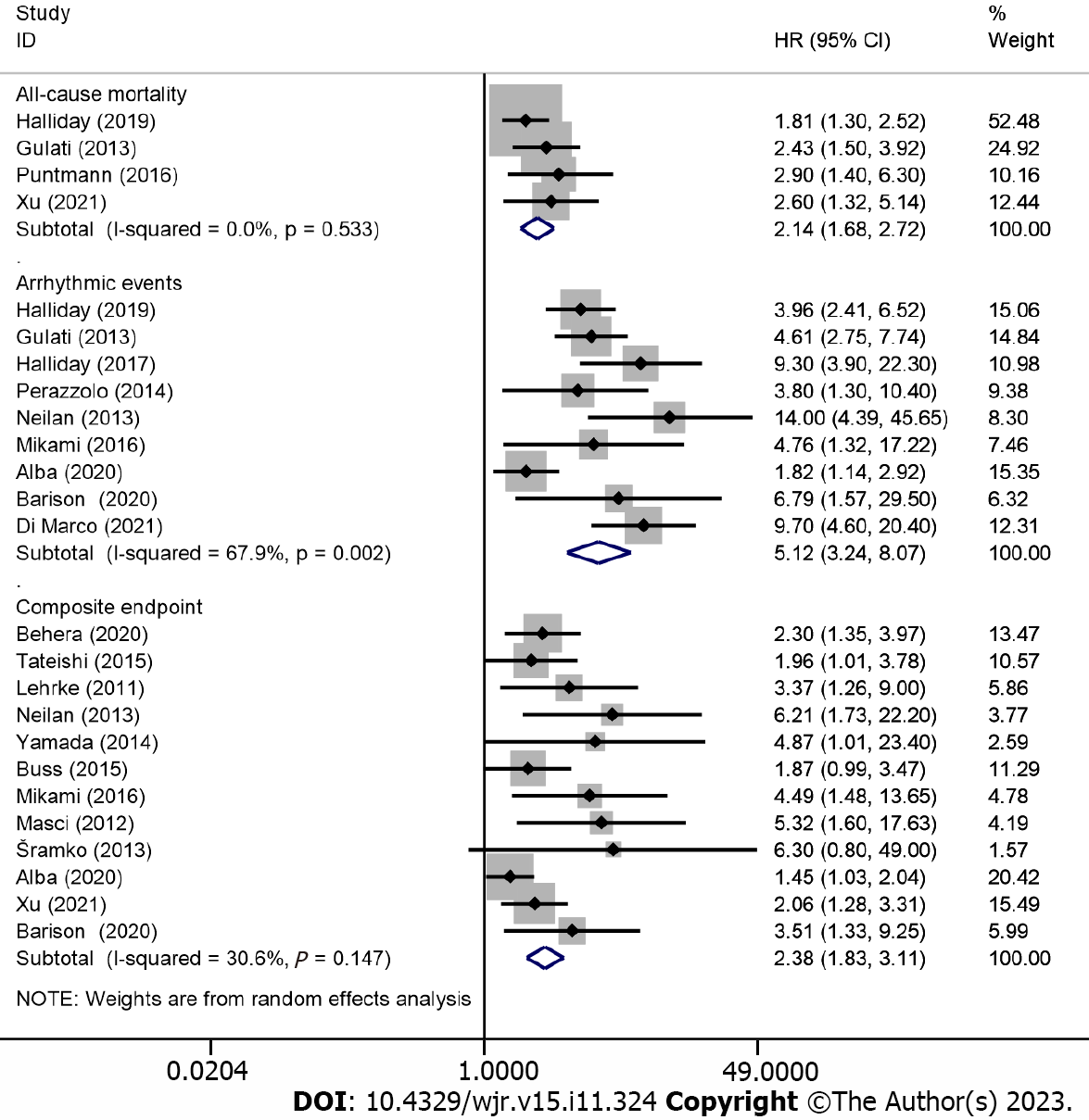
Positivity for LGE and adverse outcomes: Overall, 18 studies, involving 7168 patients (2793 had LGE), reported the association between the positivity of LGE and adverse outcomes. The included outcomes of the subgroup analysis were all-cause mortality (4 articles), arrhythmic events (9 articles), and composite endpoints (12 articles). All-cause mortality occurred in 313 patients (4.4% of patients of the 18 included studies), of who 172 had LGE (6.2% of LGE-positive patients) and 141 had no LGE (3.2% of LGE-negative patients), with a risk difference between LGE-positive and LGE-negative patients of 12.2% (95%CI: 9.1%–15.3%; P < 0.001). Besides, arrhythmic events and composite endpoints occurred in 256 (3.6%) and 603 (8.4%) patients, respectively.
In the pooled analysis, positivity for LGE was associated with an increased risk of all-cause mortality (HR: 2.14; 95%CI: 1.68–2.72; P < 0.001), arrhythmic events (HR: 5.12, 95%CI: 3.24–8.07, P < 0.001), and composite endpoints (HR: 2.38, 95%CI: 1.83–3.11, P < 0.001), as shown in Figure 2. A random-effects model was used for studies on arrhythmic events with significant heterogeneity (I2 = 67.9%; P = 0.002), and sensitivity analysis using the leave-one-out method showed that the study by Alba et al[21] may be the source of heterogeneity. However, Egger’s and Begg’s tests showed that there was publication bias in the presence of LGE and composite endpoints; after conducting a trim and fill analysis and adjusting for the effect size for funnel plot asymmetry, the P values before and after the trim and fill analysis were less than 0.001, indicating that no significant changes occurred and the results were reliable.
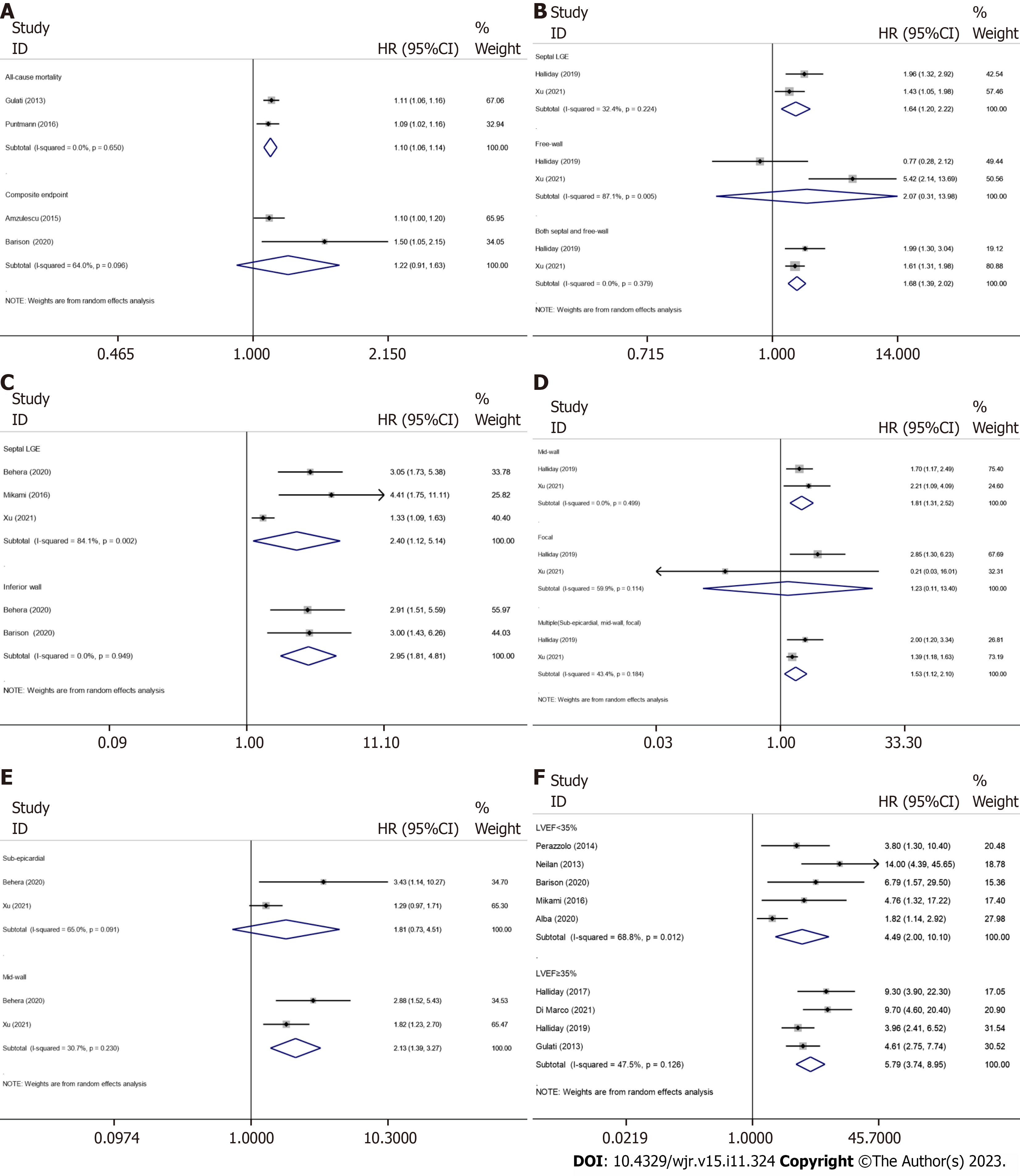
The extent of LGE and adverse outcomes: Two studies were included in the subgroup analysis of all-cause mortality, including 313 participants with LGE and 796 without LGE, with 101 all-cause mortality occurred during the follow-up period, and the pooled HR of all-cause mortality for every 1% increment in the extent of LGE was 1.10 (95%CI: 1.06–1.14; P < 0.001) (Figure 3A). In the analysis of the outcome of composite endpoints, two studies recruited 179 patients with LGE and 166 without LGE, with 79 composite endpoints occurred during the follow-up period, however, even random-effects model was used for studies on composite endpoints with significant heterogeneity (I2 = 64.0%; P = 0.096); the result showed that every 1% increment in the extent of LGE only has numerically increased the risk of composite endpoints without reaching statistical significance 1.22 (95%CI: 0.91–1.63; P < 0.001).
The LGE locations and adverse outcomes: Recently, several LGE metrics, including location and pattern, have emerged as new indicators for risk prediction. Subgroup analysis, which included two studies (LGE was present in 501 patients and absent in 785 patients; all-cause mortality occurred in 212 patients), showed that LGE located in the septum (pooled HR: 1.64; 95%CI: 1.20–2.22; P < 0.001) and in both the septum and free wall (pooled HR: 1.68; 95%CI: 1.39–2.02; P < 0.001) can predict all-cause mortality. However, LGE located in the free wall only has numerically increased the risk of all-cause mortality without reaching statistical significance (pooled HR: 2.07; 95%CI: 0.31–13.98; P = 0.457) (Figure 3B). A random-effects model was used for studies on free-wall LGE with significant heterogeneity (I2 = 87.1%; P = 0.005).
The subgroup analysis for predicting composite endpoints included five studies (Figure 3C), including 471 LGE-positive participants and 466 LGE-negative participants, and the number of composite endpoints was 297. The pooled HRs for septal and inferior LGE of composite endpoints were 2.40 (95%CI: 1.12–5.14, P = 0.025) and 2.95 (95%CI: 1.81–4.81, P < 0.001), respectively; a random-effects model was used to analyze the heterogeneity in the septal LGE group (I2 = 84.1%; P = 0.002), sensitivity analysis using the leave-one-out method showed that the study by Xu et al[18]. may be the source of heterogeneity, and the heterogeneity may result from the unadjusted HR.
The LGE patterns and adverse outcomes: In addition to the mid-wall pattern of LGE, recent studies have found that LGE has many other patterns, such as focal and sub-epicardial patterns, which can also provide stratification information for adverse outcomes. Subgroup analysis for all-cause mortality, which included 501 patients with myocardial LGE and 785 without LGE from two studies, was performed for the mid-wall, focal, and multiple (mid-wall, sub-epicardial, and focal) patterns of LGE; death occurred in 212 patients, and the pooled HRs were 1.81 (95%CI: 1.31–2.52; P < 0.001), 1.23 (95%CI: 0.11–13.40; P = 0.867; not statistical significance), and 1.53 (95%CI: 1.12–2.10; P < 0.001), respectively (Figure 3D).
The relationship between the patterns of LGE and composite endpoints was also analyzed; subgroup analysis was performed and included two studies involving 245 LGE-positive patients and 279 LGE-negative patients, with 195 composite endpoints during the follow-up period. Our analysis showed that mid-wall (pooled HR: 2.13; 95%CI: 1.39–3.27; P = 0.001) patterns of LGE can predict composite endpoints (Figure 3E), even random-effects model was used for studies on sub-epicardial LGE with significant heterogeneity (I2 = 65.0%; P = 0.091), these results showed that sub-epicardial LGE has numerically increased the risk of composite endpoints without reaching statistical significance (pooled HR: 1.81; 95%CI: 0.73–4.51; P = 0.202).
Positive effect of LGE and arrhythmic events on different ranges of LVEF: As the studies included in our meta-analysis all demonstrated indications of LVEF, we also performed a subgroup analysis to assess the impact of LGE on DCM for different ranges of LVEF by dividing those studies into two groups (LVEF < 35% and LVEF ≥ 35%). The analysis, which included nine studies (LGE was present in 2018 patients and absent in 3164 patients; arrhythmic events occurred in 256 patients), showed that LGE is a stronger predictor of arrhythmic events in patients with a greater LVEF, and the pooled HRs for positive LGE with LVEF < 35% and LVEF ≥ 35% were 4.49 (95%CI: 2.00–10.10; P < 0.001) and 5.79 (95%CI: 3.74–8.95; P < 0.001), respectively (Figure 3F). A random-effects model was used for studies on LVEF < 35% with significant heterogeneity (I2 = 68.8%; P = 0.012).
LGE detected by CMR represents a myocardial scar, which could provide a foundation for ventricular re-entrant arrhythmia and is the main cause of SCD in multiple myocardiopathies[23]. Although LGE is observed in approximately 30% of patients with DCM, several studies support the prognostic power of LGE[3,4]. This meta-analysis included 7330 patients with DCM, confirmed the prognostic value of LGE for multiple adverse endpoints, and investigated the association between the extent, location, and pattern of LGE and outcomes.
Prior meta-analyses have evaluated the prognostic value of LGE in DCM[24-26]. However, the sample size of our meta-analysis was larger, and given the increasing numbers of studies published in the past few years about the characteristics of LGE (extent, location, and pattern), we considered that focusing on novel indicators was appropriate. The relevant number of studies included allowed subgroup analyses between the extent, location, and pattern of LGE and outcomes.
Myocardial fibrosis is strongly associated with ventricular remodeling[11,27]. The prognostic value of LGE is inde
In our meta-analysis, LGE was present in approximately 40% of the patients with DCM under study and was strongly and significantly associated with multiple adverse outcomes. The relationship between LGE and the endpoints was particularly obvious for arrhythmic events (pooled HR: 5.12, 95%CI: 3.24–8.07, P < 0.001). This will be extremely meaningful for risk stratification mainly based on LVEF, as most SCD cases did not have severely reduced LVEF[23]. Moreover, the finding that the predictive power of LGE was better in patients with greater LVEF regarding the endpoint of arrhythmic events in our subgroup analysis confirmed the results of a previous study[22]. The severely reduced LVEF detected in patients with DCM represents an adversely remodeled left ventricle, which can lead to self-organized criticality and cascading mechanical collapse, and ultimately result in SCD[29]. However, in the absence of a severely remodeled left ventricle, LGE provides the foundation for ventricular re-entrant arrhythmia, which can be the major cause of adverse outcomes.
A study suggests that the susceptibility to re-entrant arrhythmias may be increased with the extent of LGE[19], indicating that the evaluation of the extent of LGE plays an important role in determining the prognosis of patients with DCM.
As a continuous variable, every 1% increment in LGE extent can predict all-cause mortality in our meta-analysis. However, even though only studies that quantified the extent of LGE as a percentage of the left ventricular mass were included in our meta-analysis, heterogeneity among studies was inevitable because of the differences in the measurement methods and parameters of LGE quantification used among the included studies. However, Halliday et al[6] and Behera et al[9] demonstrated that the correlation between the extent of LGE and outcomes seems to be nonlinear, as a small increase in the extent of LGE could significantly increase the risk of adverse outcomes.
One potential mechanism is that the induction of arrhythmia can be influenced by the texture and spatial distribution of fibrosis, the formation and dynamics of which are mostly determined by the maximal local fibrosis level; thus, the composition of myocardial fibrosis, rather than volume, may be the main determinant of arrhythmia[30]. Another mechanism that may explain these results is that ventricular arrhythmia most likely originates from the regions between myocardial fibrosis and healthy tissue with slow conduction[31,32]. Therefore, more precise identification of heterogeneous zones between healthy myocardium and myocardial fibrosis and the accurate discrimination of the texture and type of myocardial fibrosis may help better predict the prognosis of patients with DCM.
Studies mainly focused on septal LGE, which is the most common location of LGE. However, recent studies have shown that LGE can also occur in the free wall with a high incidence[6,18], suggesting that LGE located in this location is worth further exploration.
Our meta-analysis found that septal LGE is associated with all-cause mortality and composite endpoints, which is consistent with the findings of previous studies. Furthermore, combined septal and free-wall LGE was more closely associated with all-cause mortality, as multi-location LGE may result in a larger extent of myocardial fibrosis.
The location of LGE may be related to its underlying etiology. LGE of idiopathic DCM is frequently located in the septum for unclear reasons, whereas LGE of DCM caused by viral myocarditis is usually located in the free wall. This is because cardiotropic viruses that originate from the bloodstream can cause pericarditis; the free wall in direct contact with the pericardium is the prime location for migration of inflammatory cells. The scar microstructure may be varied for the underlying etiology and cause a different risk[33].
However, because the number of studies included in this study was small and whether free-wall LGE is a protective or adverse factor remains controversial[6,18], further investigation regarding the prognosis of the distribution of LGE is still required.
As the most typical LGE pattern, mid-wall LGE was associated with an increased risk of all-cause mortality and composite endpoints in this study. However, our subgroup analyses showed that sub-epicardial and focal pattern of LGE were not statistically significant in predicting adverse outcome; in this case, more studies are required to confirm their prognostic value.
The sub-endocardial and transmural patterns of LGE have been considered to be indicators of previous myocardial infarction[34,35]. However, several studies have investigated the role of transmural LGE, which is not coherent with coronary artery territory (indicating that this pattern is not of an ischemic origin), and sub-endocardial LGE in determining the prognosis of patients with DCM[21,22]. Di Marco et al[22] have found that transmural LGE was associated with a heightened risk of adverse outcomes. Through the aforementioned studies, it is suggested that more attention should be paid to those “ischemia LGE patterns” rather than simply seeing it as myocardial infarct in patients with DCM.
In addition to focusing on the pattern of LGE itself, the number of coexisting LGE patterns must also be considered. In our meta-analysis, patients with multiple patterns of LGE (sub-epicardial, mid-wall, and focal) were at a greater risk of all-cause mortality than those without LGE. One possibility is that more LGE patterns may represent a higher heterogeneity of fibrosis. However, the prognostic ability of multiple LGE patterns is not as good as that of one single pattern in some other studies[6,18]. The limited number of patients with multiple LGE patterns may be responsible for the controversial results. Therefore, more trials are needed to draw a more certain conclusion.
Furthermore, Di Marco et al[22] have found that the combination of LVEF strata and LGE status may help improve risk stratification in patients with DCM. Based on the results of this study, the next subdivision of various indicators (extent, location, and pattern) of LGE, particularly the free-wall LGE, and the integration of those indicators with varying clinical parameters may be more helpful in predicting the prognosis of patients with DCM.
The limitations of this study are that most included studies were observational; selection bias should be anticipated; and this meta-analysis can only detect associations but not causality. Additionally, the exclusion and inclusion criteria were different among the included studies, and there was a wide variety on the definitions of the endpoints. Finally, the data on the location and pattern of LGE were limited, which can explain the significant heterogeneity in some subgroup analyses; further studies enrolling more eligible patients are required.
CMR-LGE exhibited a substantial prognostic value in predicting all-cause mortality, arrhythmic events and composite endpoints in patients with DCM. The extent, location, and pattern of LGE could provide additional information for risk stratification.
LGE as a prognostic indicator of dilated cardiomyopathy (DCM) has been extensively studied, and several new metrics of late gadolinium enhancement (LGE), such as its extent, location, and pattern, have emerged. However, whether some indicators are protective or risk factors and whether the combination of left ventricular ejection fraction (LVEF) strata and LGE has a better predictive value remains controversial; therefore, further discussion is required, and more precise risk stratification should be explored.
This meta-analysis aimed to detect the predictive performance of the extent, location, and pattern of LGE, to compare and screen these new indicators of LGE, and provide novel concepts for improving the risk stratification algorithm for adverse outcomes of DCM.
The research objectives of this meta-analysis were to investigate the associations between the positivity and extent, location, and pattern of LGE derived from CMR and multiple outcomes. We found that the presence of LGE was associated with an increased risk of multiple adverse outcomes (all-cause mortality, arrhythmic events, and composite endpoints). Furthermore, an increase in the extent of LGE and a different location and pattern of LGE may impact the prognosis. Although the current studies and meta-analyses mainly focus on the relationship between the presence of LGE and prognosis, our study found that LGE is a stronger predictor of arrhythmic events in patients with greater LVEF, and that the different types of LGE were equally predictive, which suggested that these new indicators and their combinations may help improve the risk stratification.
We followed the guidelines of PRISMA-NMA, registered with PROSPERO, and extracted data from databases recommended by the Cochrane Handbook. After discussion, we reached a consensus and classified the endpoints into three; the pooled HRs and 95%CIs obtained by using STATA were applied to evaluate the effectiveness of the new metrics of LGE. The Newcastle–Ottawa Quality Assessment Scale was used for quality assessment. Publication bias was assessed using Egger’s and Begg’s tests, and a sensitivity analysis was performed using the leave-one-out method, to assess the stability of the results.
CMR-LGE is a strong prognostic marker for patients with DCM. The extent, location, and pattern of LGE provided additional information for risk stratification. Further studies are needed to determine whether free-wall LGE is a protective or risk factor, and whether the focal or sub-epicardial pattern of LGE has predictive value.
CMR-LGE is a strong prognostic marker for patients with DCM. The extent, location, and pattern of LGE provided additional information for risk stratification. Further studies are needed to determine whether free-wall LGE is a protective or risk factor, and whether the focal or sub-epicardial pattern of LGE has predictive value.
The prognostic value of different locations and patterns of LGE needs to be confirmed in future studies, and the combined predictive value of these predictors warrants further exploration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Barison A, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet. 2010;375:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 414] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 2. | Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O'Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 889] [Article Influence: 74.1] [Reference Citation Analysis (0)] |
| 3. | Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith RE, Prasad SK. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:1659-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 4. | Halliday BP, Gulati A, Ali A, Guha K, Newsome S, Arzanauskaite M, Vassiliou VS, Lota A, Izgi C, Tayal U, Khalique Z, Stirrat C, Auger D, Pareek N, Ismail TF, Rosen SD, Vazir A, Alpendurada F, Gregson J, Frenneaux MP, Cowie MR, Cleland JGF, Cook SA, Pennell DJ, Prasad SK. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation. 2017;135:2106-2115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 5. | Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catalá-López F, Gøtzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4165] [Cited by in RCA: 5298] [Article Influence: 529.8] [Reference Citation Analysis (1)] |
| 6. | Halliday BP, Baksi AJ, Gulati A, Ali A, Newsome S, Izgi C, Arzanauskaite M, Lota A, Tayal U, Vassiliou VS, Gregson J, Alpendurada F, Frenneaux MP, Cook SA, Cleland JGF, Pennell DJ, Prasad SK. Outcome in Dilated Cardiomyopathy Related to the Extent, Location, and Pattern of Late Gadolinium Enhancement. JACC Cardiovasc Imaging. 2019;12:1645-1655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 7. | Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, Vettor G, Tona F, Tarantini G, Cacciavillani L, Corbetti F, Giorgi B, Miotto D, Thiene G, Basso C, Iliceto S, Corrado D. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | Šramko M, Kubánek M, Tintěra J, Kautznerová D, Weichet J, Malušková J, Franeková J, Kautzner J. Utility of combination of cardiac magnetic resonance imaging and high-sensitivity cardiac troponin T assay in diagnosis of inflammatory cardiomyopathy. Am J Cardiol. 2013;111:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Behera DR, V K AK, K K NN, S S, Nair KKM, G S, T R K, Gopalakrishnan A, S H. Prognostic value of late gadolinium enhancement in cardiac MRI of non-ischemic dilated cardiomyopathy patients. Indian Heart J. 2020;72:362-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Tateishi E, Noguchi T, Goto Y, Morita Y, Ishibashi-Ueda H, Yamada N, Kanzaki H, Nishimura K, Miyamoto Y, Anzai T, Ogawa H, Yasuda S. Prognostic impact of blood pressure response plus gadolinium enhancement in dilated cardiomyopathy. Heart. 2015;101:774-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, Pribe R, Ehlermann P, Zugck C, Korosoglou G, Giannitsis E, Katus HA. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG, Jerosch-Herold M, Ghoshhajra BB, Kwong RY. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Yamada T, Hirashiki A, Okumura T, Adachi S, Shimazu S, Shimizu S, Morimoto R, Takeshita K, Naganawa S, Kondo T, Murohara T. Prognostic impact of combined late gadolinium enhancement on cardiovascular magnetic resonance and peak oxygen consumption in ambulatory patients with nonischemic dilated cardiomyopathy. J Card Fail. 2014;20:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T, Frankenstein L, Steen H, Meder B, Giannitsis E, Katus HA, Korosoglou G. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Mikami Y, Cornhill A, Heydari B, Joncas SX, Almehmadi F, Zahrani M, Bokhari M, Stirrat J, Yee R, Merchant N, Lydell CP, Howarth AG, White JA. Objective criteria for septal fibrosis in non-ischemic dilated cardiomyopathy: validation for the prediction of future cardiovascular events. J Cardiovasc Magn Reson. 2016;18:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Puntmann VO, Carr-White G, Jabbour A, Yu CY, Gebker R, Kelle S, Hinojar R, Doltra A, Varma N, Child N, Rogers T, Suna G, Arroyo Ucar E, Goodman B, Khan S, Dabir D, Herrmann E, Zeiher AM, Nagel E; International T1 Multicentre CMR Outcome Study. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc Imaging. 2016;9:40-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 363] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 17. | Masci PG, Barison A, Aquaro GD, Pingitore A, Mariotti R, Balbarini A, Passino C, Lombardi M, Emdin M. Myocardial delayed enhancement in paucisymptomatic nonischemic dilated cardiomyopathy. Int J Cardiol. 2012;157:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Xu Y, Lin J, Liang Y, Wan K, Li W, Wang J, Zhu Y, Mui D, Wang L, Li Y, Cheng W, Sun J, Zhang Q, Han Y, Chen Y. Prognostic value of left ventricular remodelling index in idiopathic dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2021;22:1197-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Barison A, Aimo A, Mirizzi G, Castiglione V, Ripoli A, Panchetti L, Rossi A, Giannoni A, Startari U, Aquaro GD, Emdin M, Piacenti M. The extent and location of late gadolinium enhancement predict defibrillator shock and cardiac mortality in patients with non-ischaemic dilated cardiomyopathy. Int J Cardiol. 2020;307:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Amzulescu MS, Rousseau MF, Ahn SA, Boileau L, de Meester de Ravenstein C, Vancraeynest D, Pasquet A, Vanoverschelde JL, Pouleur AC, Gerber BL. Prognostic Impact of Hypertrabeculation and Noncompaction Phenotype in Dilated Cardiomyopathy: A CMR Study. JACC Cardiovasc Imaging. 2015;8:934-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 21. | Alba AC, Gaztañaga J, Foroutan F, Thavendiranathan P, Merlo M, Alonso-Rodriguez D, Vallejo-García V, Vidal-Perez R, Corros-Vicente C, Barreiro-Pérez M, Pazos-López P, Perez-David E, Dykstra S, Flewitt J, Pérez-Rivera JÁ, Vazquez-Caamaño M, Katz SD, Sinagra G, Køber L, Poole J, Ross H, Farkouh ME, White JA. Prognostic Value of Late Gadolinium Enhancement for the Prediction of Cardiovascular Outcomes in Dilated Cardiomyopathy: An International, Multi-Institutional Study of the MINICOR Group. Circ Cardiovasc Imaging. 2020;13:e010105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 22. | Di Marco A, Brown PF, Bradley J, Nucifora G, Claver E, de Frutos F, Dallaglio PD, Comin-Colet J, Anguera I, Miller CA, Schmitt M. Improved Risk Stratification for Ventricular Arrhythmias and Sudden Death in Patients With Nonischemic Dilated Cardiomyopathy. J Am Coll Cardiol. 2021;77:2890-2905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 23. | Stecker EC, Vickers C, Waltz J, Socoteanu C, John BT, Mariani R, McAnulty JH, Gunson K, Jui J, Chugh SS. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47:1161-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 403] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 24. | Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The Prognostic Value of Late Gadolinium-Enhanced Cardiac Magnetic Resonance Imaging in Nonischemic Dilated Cardiomyopathy: A Review and Meta-Analysis. JACC Cardiovasc Imaging. 2018;11:1274-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 25. | Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA, Sramko M, Masci PG, Barison A, Mckenna P, Mordi I, Haugaa KH, Leyva F, Rodriguez Capitán J, Satoh H, Nabeta T, Dallaglio PD, Campbell NG, Sabaté X, Cequier Á. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017;5:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 26. | Duan X, Li J, Zhang Q, Zeng Z, Luo Y, Jiang J, Chen Y. Prognostic value of late gadolinium enhancement in dilated cardiomyopathy patients: a meta-analysis. Clin Radiol. 2015;70:999-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Wu KC, Weiss RG, Thiemann DR, Kitagawa K, Schmidt A, Dalal D, Lai S, Bluemke DA, Gerstenblith G, Marbán E, Tomaselli GF, Lima JA. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414-2421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 495] [Cited by in RCA: 458] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 28. | Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 856] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 29. | Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 30. | Kazbanov IV, ten Tusscher KH, Panfilov AV. Effects of Heterogeneous Diffuse Fibrosis on Arrhythmia Dynamics and Mechanism. Sci Rep. 2016;6:20835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Hsia HH, Marchlinski FE. Electrophysiology studies in patients with dilated cardiomyopathies. Card Electrophysiol Rev. 2002;6:472-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Estner HL, Zviman MM, Herzka D, Miller F, Castro V, Nazarian S, Ashikaga H, Dori Y, Berger RD, Calkins H, Lardo AC, Halperin HR. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging. Heart Rhythm. 2011;8:1942-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 33. | Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581-1590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 589] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 34. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 855] [Cited by in RCA: 813] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 35. | Zhao S. Letter to the editor: is it time for imaging to level with pathology? Int J Cardiovasc Imaging. 2020;36:2249-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |