Published online Oct 28, 2023. doi: 10.4329/wjr.v15.i10.293
Peer-review started: September 6, 2023
First decision: September 19, 2023
Revised: September 20, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 28, 2023
Processing time: 47 Days and 18.3 Hours
Hepatic steatosis is a very common problem worldwide.
To assess the performance of two- and six-point Dixon magnetic resonance (MR) techniques in the detection, quantification and grading of hepatic steatosis.
A single-center retrospective study was performed in 62 patients with suspected parenchymal liver disease. MR sequences included two-point Dixon, six-point Dixon, MR spectroscopy (MRS) and MR elastography. Fat fraction (FF) estimates on the Dixon techniques were compared to the MRS-proton density FF (PDFF). Statistical tests used included Pearson’s correlation and receiver operating characteristic.
FF estimates on the Dixon techniques showed excellent correlation (≥ 0.95) with MRS-PDFF, and excellent accuracy [area under the receiver operating characteristic (AUROC) ≥ 0.95] in: (1) Detecting steatosis; and (2) Grading severe steatosis, (P < 0.001). In iron overload, two-point Dixon was not evaluable due to confounding T2* effects. FF estimates on six-point Dixon vs MRS-PDFF showed a moderate correlation (0.82) in iron overload vs an excellent correlation (0.97) without iron overload, (P < 0.03). The accuracy of six-point Dixon in grading mild steatosis improved (AUROC: 0.59 to 0.99) when iron overload cases were excluded. The excellent correlation (> 0.9) between the Dixon techniques vs MRS-PDFF did not change in the presence of liver fibrosis (P < 0.01).
Dixon techniques performed satisfactorily for the evaluation of hepatic steatosis but with exceptions.
Core Tip: Fat fraction (FF) estimates on the Dixon techniques (two-point Dixon and six-point Dixon) have excellent correlation with magnetic resonance spectroscopy-proton density FF (MRS-PDFF), and excellent accuracy in detecting steatosis, and grading severe steatosis. However, in iron overload, two-point Dixon was not evaluable due to confounding effect on liver signal from T2* decay. The excellent correlation between the Dixon techniques vs MRS-PDFF was not affected by co-existing liver fibrosis.
- Citation: Elfaal M, Supersad A, Ferguson C, Locas S, Manolea F, Wilson MP, Sam M, Tu W, Low G. Two-point Dixon and six-point Dixon magnetic resonance techniques in the detection, quantification and grading of hepatic steatosis. World J Radiol 2023; 15(10): 293-303
- URL: https://www.wjgnet.com/1949-8470/full/v15/i10/293.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i10.293
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease worldwide with a global prevalence of 25% (estimated at 1 billion people)[1-5]. Roughly 20% have non-alcoholic steatohepatitis (NASH), characterized by hepatocyte injury and inflammation. These patients are at risk for progression to liver fibrosis and cirrhosis[6]. Consequently, steatosis and fibrosis may frequently co-exist in the liver. NAFLD is also associated with metabolic syndrome, type 2 diabetes and cardiovascular disease[6]. Accurate detection, quantification and grading of steatosis are necessary for risk stratification, to monitor disease progression and to assess the treatment response.
Liver biopsy is the reference standard for liver fat evaluation but is invasive and can be associated with pain, bleeding and death (1 in 10000)[7]. It has poor patient acceptability and is not suitable as a screening tool or for longitudinal patient monitoring. Biopsy is associated with high sampling variability as it only evaluates 1:50000th of the liver[8]. As steatosis often affects the liver non-uniformly, biopsy may be poorly representative. Finally, the histopathology assessment is a qualitative process prone to intra- and inter-reader variability[7].
B-mode ultrasound (US) is the first-line imaging test for investigating patients with suspected parenchymal liver disease. It is non-invasive, easy to perform, inexpensive and widely available. However, it has questionable utility as a screening tool due to poor sensitivity in mild steatosis[1,6,9]. Magnetic resonance spectroscopy (MRS) is widely regarded as the non-invasive reference standard for liver fat quantification. It is strongly correlated with histopathology and has been touted as a replacement for biopsy[10-12]. It can directly measure the chemical composition of liver fat and produces a proton density fat fraction (PDFF) according to the formula: PDFF = F/(F + W), where F and W are the unconfounded signals of protons within mobile fat and mobile water molecules, respectively. MRS has drawbacks including a limited spatial coverage and is technically demanding requiring specialized software and expertise so is generally only available in large academic centers.
Chemical shift encoded fat and water separation MR techniques are based on the principle that fat and water protons oscillate at different resonant frequencies. The fat-water resonance frequency differences (225 Hz at 1.5 T and 450 Hz at 3 T) create phase shifts that can be used to separate fat and water signals. The first chemical shift fat and water separation method, termed ‘two-point Dixon’, was described in 1984[13]. This technique involves the dual-echo acquisition of T1 weighted in-phase (IP) and opposed-phased (OP) images that can be used to generate separated fat-only and water-only images. This allows the hepatic FF to be calculated as follows[6]: FF% = (IP - OP)/(2 × IP). Two-point Dixon sequences are part of the routine toolkit of MR liver imaging worldwide, and have been performed in many clinical applications over the years. These are familiar sequences to practicing body MR radiologists, easy to perform, can sample large areas of the liver, and do not require sophisticated software. However, 1st generation fat quantification on two-point Dixon is prone to underestimation due to confounders such as B0 inhomogeneity, T1 bias, T2* effects, noise bias, spectral complexity of fat, eddy currents and J-coupling[6]. Fat quantification is also adversely affected by liver iron overload due to T2* decay which has opposing effects to fat on liver signal leading to inaccuracies. Additionally, the two-point Dixon technique has a narrower dynamic range of 0%-50% liver FF although this approximates the normal biologic range in humans so is not generally a practical limitation[1,14]. Recent advances have led to the development of modern Dixon sequences that are corrected for confounders leading to more accurate liver fat quantification[1,14,15]. These complex multi-echo chemical shift encoded techniques utilize both the phase and magnitude of the MR signal and can provide fat quantification [MR imaging (MRI)-PDFF] over the entire physical range (0%-100%). The most common method involves the acquisition of 6 echos and is termed ‘six-point Dixon’. By performing automatic segmentectomy, multi-echo Dixon techniques can generate whole liver fat quantification. Confounder corrected R2* maps, acquired simultaneously, provide fat-corrected liver iron quantification. Unlike two-point Dixon which is performed ubiquitously in clinical liver imaging worldwide, six-point Dixon requires dedicated software that has to be purchased separately, so access to this technique is limited clinically.
On this background, the primary aim of this study was to assess the diagnostic performance of 1st generation two-point Dixon (widely available clinically) and modern six-point Dixon MR techniques (limited access clinically) in the detection, quantification and grading of hepatic steatosis, using MRS as the reference standard. The secondary aims were: (1) To assess the confounding effects of iron overload on the Dixon techniques, a subgroup analysis was performed in patients with and without iron overload; (2) As steatosis and fibrosis are often found together in NAFLD patients, a subgroup analysis was performed to determine if liver fibrosis had an influence on FF estimates; and (3) To assess the accuracy of US for detecting and grading hepatic steatosis, a subgroup analysis was performed in patients with an US examination within 6 mo of the MR.
This was a retrospective single-center cross-sectional study. Institutional ethics and review board approval was granted and the requirement for informed patient consent was waived. From July 2021 to February 2022 (8 mo), all consecutive adult patients (≥ 18 years old) that underwent a per-protocol clinical MR examination for the combined assessment of liver fat, iron and fibrosis for suspected diffuse parenchymal liver disease and without known focal liver lesions, were entered into the study. Three patients were excluded from the study due to technically poor-quality MR examinations.
All examinations were performed on a 1.5-T MR system (Magnetom Aera, Siemens Healthcare, Erlangen, Germany) using an 18-channel body matrix array coil. The technical MR parameters are included in Table 1. Additionally, an MR elastography (MRE) examination was also performed using a commercially available clinical system (Resoundant, MN, United States).
| Two-point Dixon | Six-point Dixon | MRS | |
| TR (ms) | 6.68 | 15.6 | 3000 |
| TE (ms) | 2.39, 4.77 | 2.38, 4.76, 7.14, 9.52, 11.90, 14.28 | 12, 24, 36, 48, 72 |
| Flip angle | 10° | 4° | 90° |
| Matrix size | 288 mm × 176 mm | 160 mm × 97 mm | N/A1 |
| FOV (mm) | 400 | 450 | N/A1 |
| Bandwidth (kHz/pixel) | 470 | 1080 | 1200 |
| Slice thickness (mm) | 3 | 3.5 | N/A1 |
| NSA | 1 | 1 | 1 |
| Parallel imaging, acceleration factor | CAIPIRINHA 2 | CAIPIRINHA 4 | - |
For the MR examination, the proprietary liver fat and iron quantification software (LiverLab, Siemen Healthcare) automatically generates the following information on picture archiving and communication system (PACS): (1) A report of the mean MRI-PDFF (%) and mean R2* (s-1 ) of the entire liver (segmentectomy) and of a selected region of interest (ROI); and (2) A spectroscopy report of the mean MRS-PDFF (%) of a 27 cm3 voxel. The MRI-PDFF (segmentectomy) and MRS-PDFF data as well as liver stiffness data acquired on MRE were imported into an Excel spreadsheet. Only the primary investigative team (Mohamed Elfaal and Alanna Supersad) had access to this data.
After removing patient identifying information, the following anonymized datasets were entered into 1 of 2 research folders on PACS. Cases were randomized within each folder and allocated unique research identification numbers. Folder 1 contained the T1 IP and OP MR images (two-point Dixon) of all patients. Folder 2 contained the static and cine US images of patients with an US within 6 mo of the MR. Three fellowship trained board-certified radiologists (with 2 years, 2 years, and 5 years of clinical experience, respectively) independently analyzed cases in folder 1. The readers were blinded to the clinical data. Using a well-established and validated technique, each reader manually placed 4 circular ROIs over the liver (2 in each hepatic lobe) on both the T1 IP and OP MR images[16]. The ROI size was set to a minimum threshold of 4 cm in diameter/12.6 cm2 area and ROIs were placed taking care to avoid large vessels, bile ducts and the liver margin. The hepatic FF on two-point Dixon was calculated using the formula[6], FF = (IP - OP)/(2 × IP).
Three other fellowship trained board-certified radiologists (with 3 years, 4 years, and 2 years of clinical experience, respectively) independently analyzed cases in folders 2. The readers were blinded to the background clinical data. For folder 2, the readers were required to assess if hepatic steatosis was present or absent on the US images, and determine the grade of steatosis. The following US criteria were used[17,18]: (1) Mild steatosis: Liver echogenicity exceeds that of the renal cortex; (2) Moderate steatosis: Poor delineation of the echogenic walls of the intrahepatic portal vein branches; and (3) Severe steatosis: Loss of definition of the posterior hepatic margin or diaphragm outline.
Reader data on folders 1 and 2 were submitted to the primary investigative team for export into the excel spreadsheet. For folder 1, a group averaged FF on two-point Dixon was calculated for each case. For folder 2, the group decision on whether steatosis was present or absent on each case was determined by the majority rule. Cases that did not meet agreement by at least 2 of the 3 readers were not included in the final analysis.
An a-prior power analysis was performed on G* Power. Assuming a mild effect size of 0.4, alpha of 0.05 and beta of 0.2, a minimum sample size of 52 was needed to achieve a power of 0.8.
The following statistical tests were used: (1) Intraclass correlation coefficient (ICC) to calculate the inter-reader agreement for FF estimates on two-point Dixon; (2) Pearson’s correlation and Bland Altman to assess the inter-test correlation and variability, respectively, for FF estimates on two-point Dixon vs MRS, and six-point Dixon vs MRS; (3) Linear regression to model the relationship between two-point Dixon vs MRS, and six-point Dixon vs MRS for fat quantification; (4) Receiver operating characteristic (ROC) analysis to determine the accuracy of two-point Dixon, six-point Dixon and US for the detection and grading of steatosis; and (5) Fleiss kappa to calculate the inter-reader agreement for detecting steatosis on US. The kappa (k) value interpretation as suggested by Cohen was used: k < 0.20 (poor agreement), 0.21-0.40 (fair agreement), 0.41-0.60 (moderate agreement), 0.61-0.80 (good agreement), and 0.81-1.00 (excellent agreement)[19].
Statistical analysis was performed on IBM SPSS (version 26) and MedCalc (version 19.6.1). A P value of < 0.05 was considered statistically significant. Statistical review was performed by a study author with training in medical statistics.
There were 62 patients including 33 males (53.2%) and 29 females (46.8%). The mean age was 54.4 ± 13.2 years and ranged from 18 to 80 years. Past medical history included obesity in 23 (37.1%), diabetes in 16 (25.8%), dyslipidemia in 15 (24.2%), hypertension in 14 (22.6%), NASH in 3 (4.8%), NAFLD in 2 (3.2%), hepatitis B in 1 (1.6%), hepatitis C in 1 (1.6%) and hypothyroidism in 1 (1.6%). The body mass index (BMI) was available in 33 patients (53.2%) with a mean BMI of 34.7 ± 7.8 kg/m2 (range 19.8-60.4 kg/m2). Accordingly, 25 (40.3%) were obese (BMI ≥ 30 kg/m2) and 6 (9.7%) were overweight (BMI 25-29.9 kg/m2).
The mean MRS-PDFF was 13.6% ± 8.9% (range 2.6%-34.3%). As proposed by Starekova et al[6], PDFF thresholds for mild, moderate and severe hepatic steatosis were set as 5%, 15% and 25%, respectively. Using MRS-PDFF as the reference standard, mild steatosis was found in 23 (37.1%), moderate steatosis in 15 (24.2%), severe steatosis in 9 (14.5%), and a non-steatotic liver in 15 (24.2%).
Seven patients (11.3%) had hepatic iron overload - all were of mild grade. This was associated with a mean R2* of 88.2 ± 21.7 s-1 (range 70.4-133.3 s-1) and a mean liver iron concentration of 2.8 ± 2.8 mg/g Fedw (range 2.2-4.4 mg/g Fedw). The FF was not evaluable on two-point Dixon in any of these patients as T2* effects from iron led to net signal loss on the IP images relative to the OP images resulting in erroneously negative calculations using the FF formula. The 7 cases were removed from the two-point Dixon dataset so the resulting dataset (n = 55) consisted of only cases with a normal liver iron concentration (< 2 mg/g Fedw). In contrast, six-point Dixon was not failed by iron overload and this dataset (n = 62) included a mixed cohort of cases with and without iron overload.
The liver stiffness was acquired in 60 patients and not evaluable in two others due to technical failure of MRE. A liver stiffness cut-off of ≥ 2.9 Ka was used to indicate the presence of liver fibrosis (F1 or higher)[20]. Accordingly, there were 24 patients (36.9%) with liver fibrosis and 36 patients (55.4%) without liver fibrosis.
ICC showed an excellent inter-reader agreement of 0.99 [95% confidence interval (CI): 0.99-1, P < 0.001] for calculating the FF estimates on two-point Dixon. Pearson showed an excellent inter-test correlation of 0.95 and 0.96, respectively, for FF estimates on two-point Dixon vs MRS-PDFF and six-point Dixon vs MRS-PDFF, P < 0.001. On six-point Dixon vs MRS-PDFF, a moderate correlation (0.82) was noted in iron overload vs an excellent correlation (0.97) without iron overload, P < 0.03. In comparison, liver fibrosis did not affect the correlation between FF estimates and the MRS-PDFF. Excellent correlations of 0.98 (fibrosis) vs 0.98 (no fibrosis) on two-point Dixon and 0.92 (fibrosis) vs 0.94 (no fibrosis) on six-point Dixon were noted, P < 0.01.
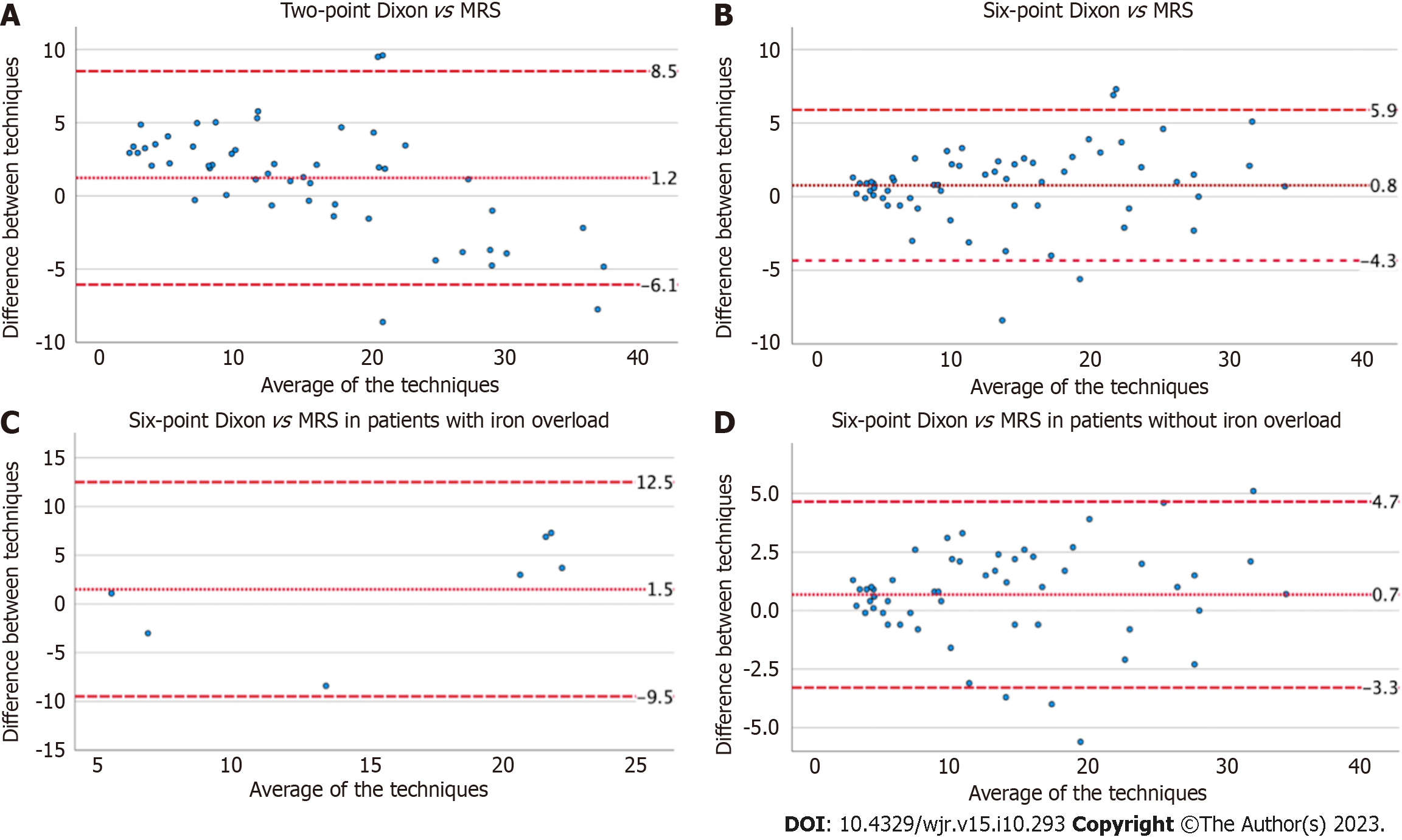
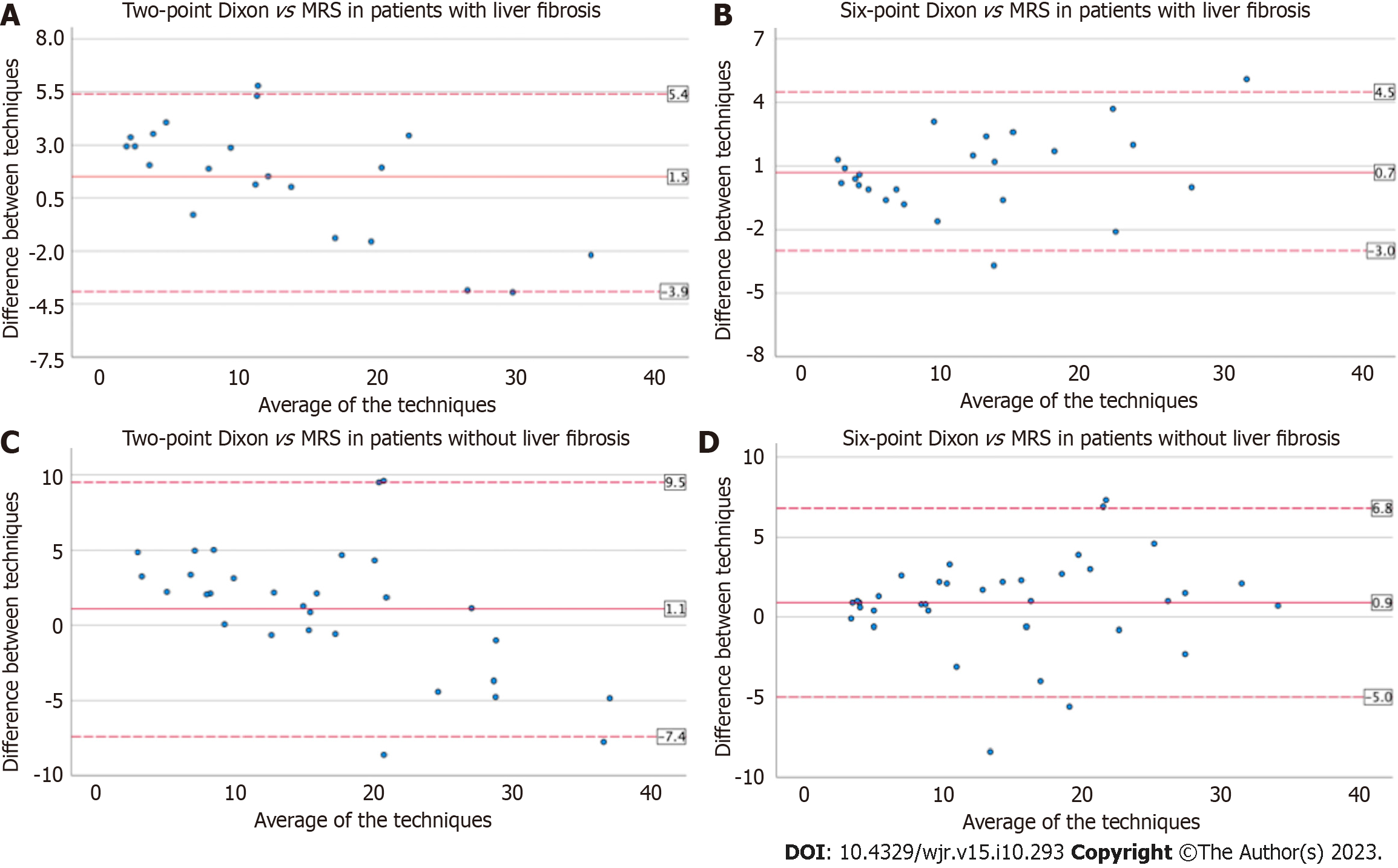
Bland Altman was used to assess the inter-test variability of FF estimates on the Dixon techniques vs MRS-PDFF (Figures 1 and 2). The mean difference between tests and 95% lower and upper limits of agreement (LOA) were: (1) 1.2% and -6.1%, 8.5% on two-point Dixon vs 0.8% and -4.3%, 5.9% on six-point Dixon; (2) 1.5% and -9.5%, 12.5% (iron overload) vs 0.7% and -3.3%, 4.7% (no iron overload), on six-point Dixon; (3) 1.5% and -3.9%, 5.4% (liver fibrosis) vs 1.1% and -7.4%, 9.5% (no liver fibrosis), on two-point Dixon; and (4) 0.7% and -3%, 4.5% (liver fibrosis) vs 0.9% and -5%, 6.8% (no liver fibrosis), on six-point Dixon.
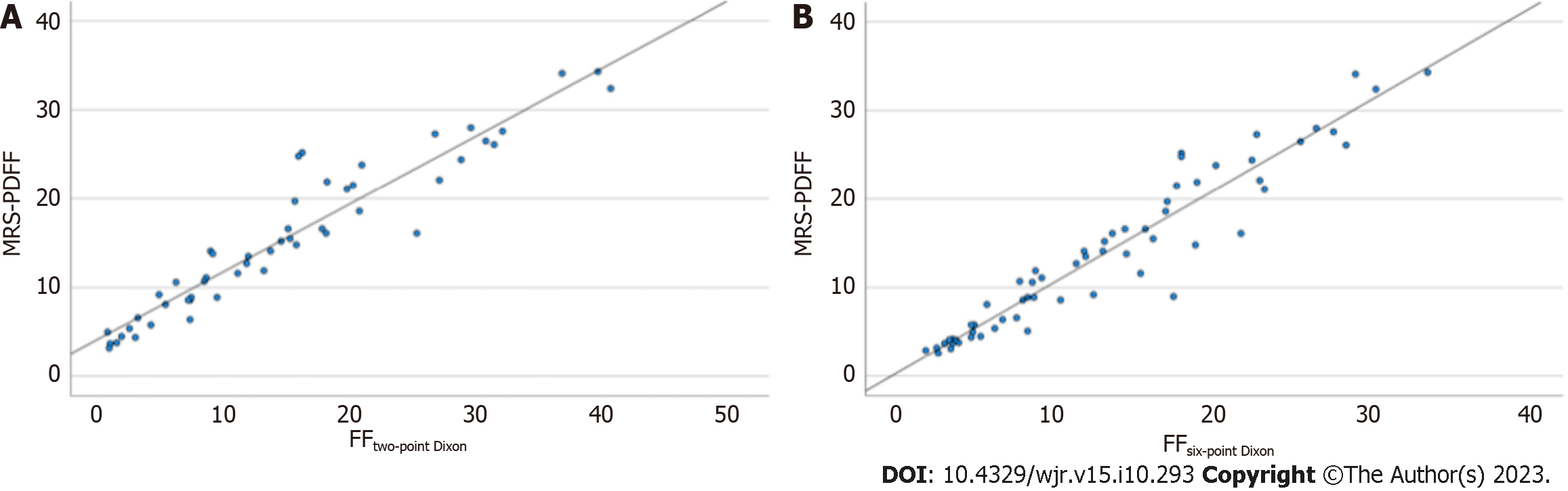
Linear regression showed a highly significant linear relationship between the Dixon techniques and MRS for liver fat quantification (P < 0.001) (Figure 3). For two-point Dixon, the regression equation was: MRS-PDFF (%) = 4.67 + 0.76 (FFtwo-point Dixon). The R2 was 0.90 signifying that 90% of the variance in MRS-PDFF was predictable from the FF estimates on two-point Dixon. For six-point Dixon, the regression equation was: MRS-PDFF (%) = 0.59 + 1.02 (FFsix-point Dixon). The R2 was 0.92 signifying that 92% of the variance in MRS-PDFF was predictable from the FF estimates on six-point Dixon.
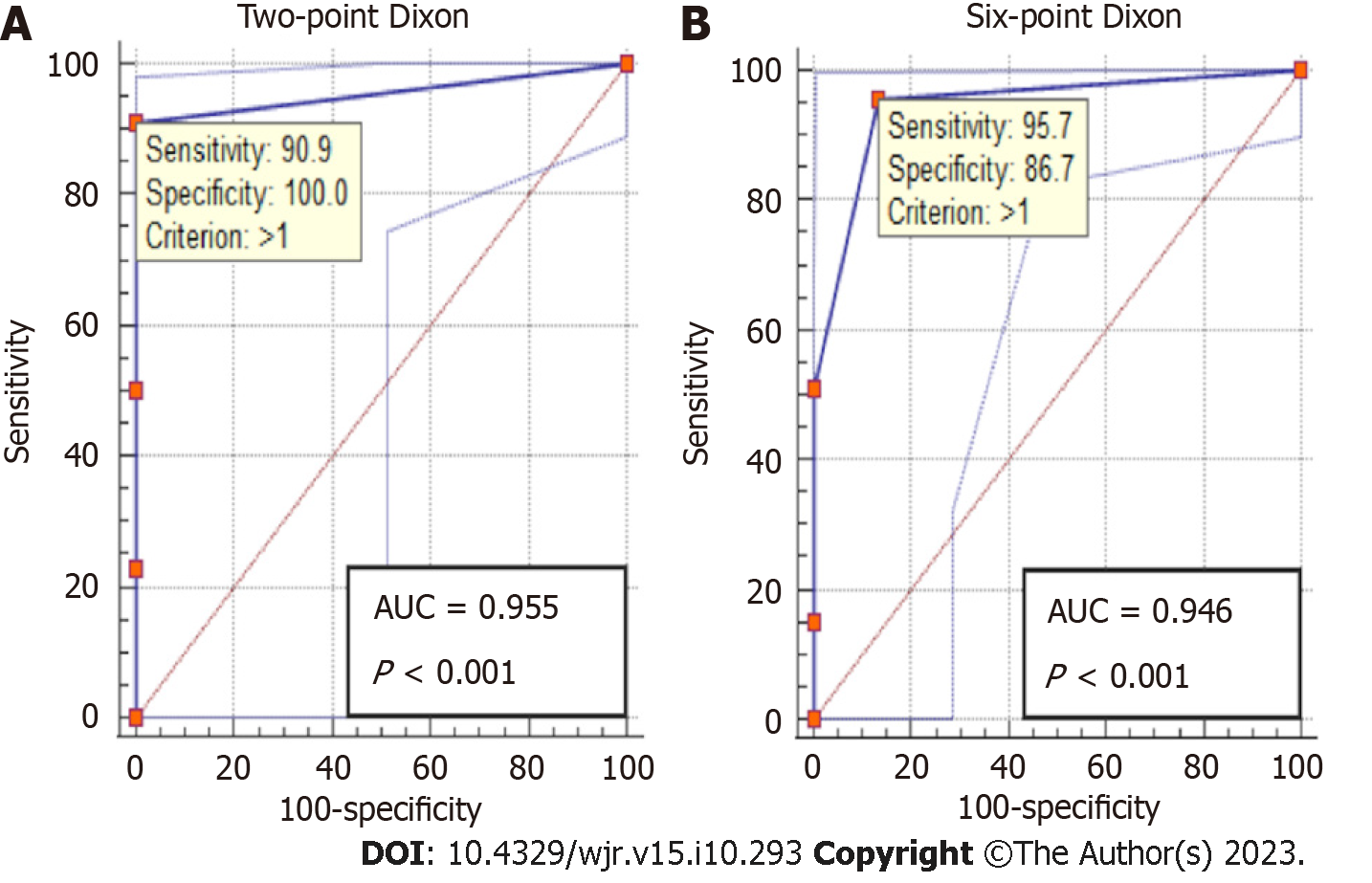
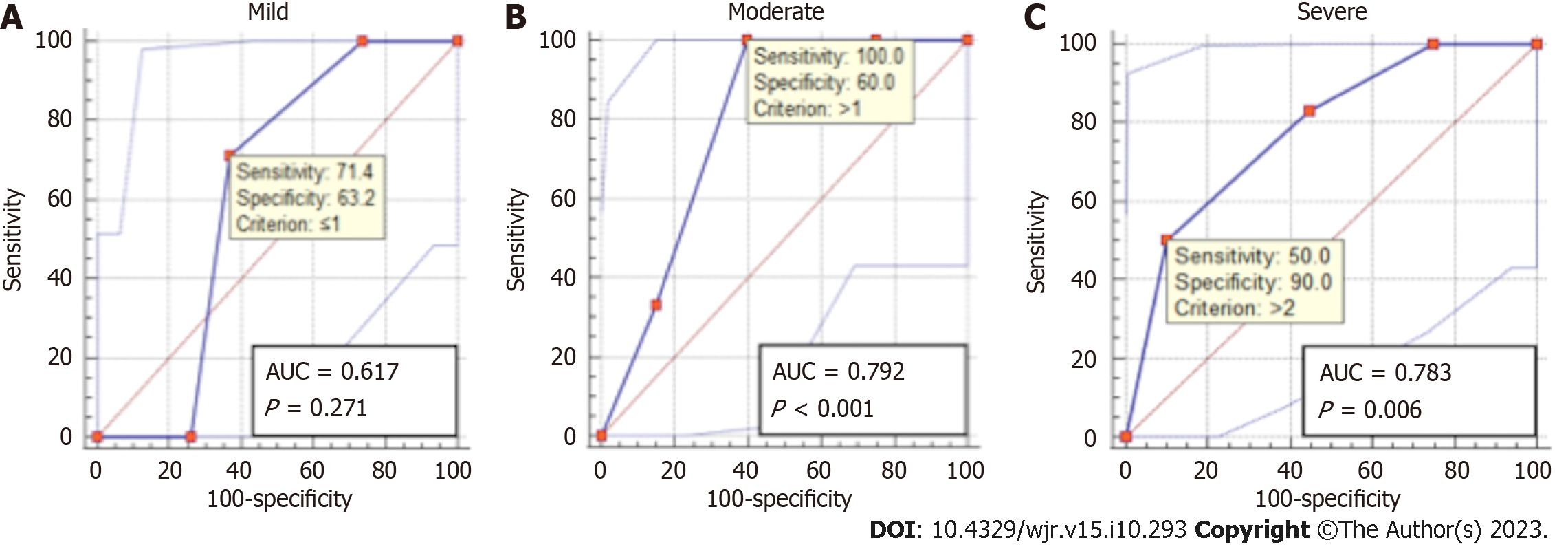
ROC analysis showed an excellent accuracy for detecting steatosis on both two-point Dixon [area under the ROC (AUROC) = 0.96, 95%CI: 0.86-0.99, P < 0.001] and six-point Dixon (AUROC = 0.95, 95%CI: 0.86-0.99, P < 0.001) (Figure 4). Similarly, an excellent accuracy was found for grading severe steatosis on both two-point Dixon (AUROC = 0.96, 95%CI: 0.87-1, P < 0.001) and six-point Dixon (AUROC = 0.97, 95%CI: 0.89-1, P < 0.001) (Figure 5). For grading moderate steatosis, there was fair accuracy on both two-point Dixon (AUROC = 0.72, 95%CI: 0.58-0.84, P = 0.001) and six-point Dixon (AUROC = 0.75, 95%CI: 0.62-0.85, P < 0.001). For grading mild steatosis, the accuracy was fair on two-point Dixon (AUROC = 0.75, 95%CI: 0.61-0.86, P < 0.01) but poor on six-point Dixon (AUROC = 0.59, 95%CI: 0.46-0.71, P = 0.21). When patients with iron overload were removed from the analysis, the accuracy for grading mild steatosis increased to excellent on six-point Dixon (AUROC = 0.99, 95%CI: 0.91-1.00, P < 0.001) (Figure 6A).
There were 29 patients that had an US examination within 6 mo of the MR. Fleiss kappa found a moderate inter-reader agreement (k = 0.45, 95%CI: 0.33-0.58, P < 0.001) for detecting steatosis on US. For grading steatosis, the inter-reader agreement was moderate for both mild steatosis (k = 0.44, 95%CI: 0.23-0.65, P < 0.001) and severe steatosis (k = 0.42, 95%CI: 0.21-0.63, P < 0.001), and fair for moderate steatosis (k = 0.28, 95%CI: 0.10-0.49, P = 0.01). Conversely, the inter-reader agreement for detecting a non-steatotic liver on US was excellent (k = 0.82, 95%CI: 0.61-1, P < 0.001). ROC analysis demonstrated that US had a good accuracy for detecting steatosis (AUROC = 0.86, 95%CI: 0.67-0.96, P < 0.001) but was inferior in grading steatosis. US showed poor accuracy in grading mild steatosis (AUROC = 0.62, 95%CI: 0.41-0.80, P = 0.27) and only fair accuracy in grading both moderate steatosis (AUROC = 0.79, 95%CI: 0.59-0.93, P < 0.001) and severe steatosis (AUROC = 0.78, 95%CI: 0.58-0.92, P < 0.01) (Figures 6B and 7).
MRS-PDFF is widely recognized as the most accurate non-invasive biomarker of hepatic steatosis[10-12,21-24]. In our study, we found an excellent correlation (≥ 0.95) between FF estimates on two- and six-point Dixon vs MRS-PDFF (P < 0.001). The findings are concordant with the correlations (0.8-0.99) reported in other studies[25-28]. We also demonstrated a strong linear relationship between the Dixon techniques and MRS for liver fat quantification as defined by the equations MRS-PDFF (%) = 4.67 + 0.76 (FFtwo-point Dixon) and MRS-PDFF (%) = 0.59 + 1.02 (FFsix-point Dixon). Findings on ROC analysis demonstrate that the Dixon techniques have excellent accuracy for detecting hepatic steatosis (AUROC ≥ 0.95, P < 0.001) and in grading severe steatosis (AUROC ≥ 0.96, P < 0.001) as well as fair accuracy in grading moderate steatosis (AUROC ≥ 0.72, P = 0.001). Two-point Dixon also showed fair accuracy for grading mild steatosis (AUROC = 0.75, P < 0.01). However, the main limitation of two-point Dixon is that it cannot be used to estimate the FF in iron overload. Despite this, two-point Dixon, a ubiquitous abdominal MR sequence, has the notable practical advantage of being widely available clinically worldwide unlike six-point Dixon or MRS, where clinical access is limited to a number of large specialized centres. In many centers that do not have access to the latter, two-point Dixon may be used as a pragmatic option (‘’a poor man’s fat quantification’’) in the absence of iron overload. Moreover, FF estimates on two-point Dixon are technically simple to perform manually and have excellent inter-reader agreement (0.99, P < 0.001). In comparison, six-point Dixon can be used in iron overload although subgroup analysis suggests that this test performs better in patients without iron overload. Comparing FF estimates vs MRS-PDFF, inter-test correlation was moderate (0.82) in patients with iron overload vs excellent (0.97) in patients without iron overload, P < 0.001. Bland Altman showed a slightly wider inter-test disparity between FF estimates vs MRS-PDFF in patients with iron overload (mean difference = 1.5%, LOA of -9.5%, 12.5%) compared to patients without iron overload (mean difference = 0.7%, LOA of -3.3%, 4.7%). Iron overload also negatively affected the accuracy of six-point Dixon in grading mild steatosis. When patients with iron overload were removed from the analysis, the AUROC increased from 0.59 to 0.99 (P < 0.001). Unlike iron overload, liver fibrosis did not affect the correlation between FF estimates on the Dixon techniques and MRS-PDFF. Correlation remained excellent (> 0.9) whether fibrosis was present or absent, P < 0.01. Inter-test variability on Bland Altman was similar in patients with or without fibrosis.
US had a poorer diagnostic performance in evaluating hepatic steatosis compared to MRI. The inter-reader agreement was moderate on US vs excellent on the Dixon techniques. The accuracy for detecting steatosis was also lower on US (AUROC = 0.86, P < 0.001) compared to the Dixon techniques (AUROC ≥ 0.95, P < 0.001). Similarly, US had a lower accuracy (AUROC = 0.78, P < 0.01) than the Dixon techniques (AUROC ≥ 0.96, P < 0.001) in grading severe steatosis.
The study had several limitations. This was a single-center retrospective analysis performed on a relatively modest number of patients. Notwithstanding, the study was adequately powered to detect statistically significant differences. Future multi-institution studies are necessary to determine the generalizability of our findings. Our study cohort was drawn from patients with suspected diffuse parenchymal liver disease, of which 75.8% had hepatic steatosis on MRS-PDFF. While the high prevalence of steatosis in the study provided a valuable source of data for analysis, we acknowledge that our study is skewed by selection bias and does not reflect the true prevalence of steatosis in the general population. Finally, our study was performed exclusively on a single vendor 1.5-T MR platform using clinically available proprietary software. We did not evaluate the confounding effects of higher magnet strengths (e.g., 3-T), different manufacturer platforms, software or protocols as it was not within the scope of our study. An ex-vivo phantom study reported that multi-echo Dixon techniques were accurate and reliable across 1.5-T and 3-T, different clinical platforms and multiple institutions[29]. However, a similar study in a large patient population with various grades of hepatic steatosis would be helpful to establish the clinical translatability of the findings.
In conclusion, our study findings suggest that in general the Dixon techniques perform satisfactorily for the detection, quantification and grading of hepatic steatosis but with exceptions. Two-point Dixon is a clinically widely available 1st generation technique while six-point Dixon is a modern technique that is not routinely available clinically in many centers. The main weakness of two-point Dixon is that it cannot be used in iron overload due to unmitigated signal decay from T2* effects[30]. In comparison, six-point Dixon can be used in iron overload, although its ability to grade mild steatosis may be compromised suggesting imperfect confounder correction. Additionally, liver fibrosis did not appear to have an impact on FF estimates on the Dixon techniques - a finding that to our knowledge has not been previously reported. Lastly, both Dixon techniques showed superior inter-reader agreement and accuracy for detecting steatosis compared to US. Ability to grade severe steatosis was also better on MR.
Hepatic steatosis is a global healthcare concern.
There is a clinical need to determine how good existing magnetic resonance (MR) techniques are in evaluating hepatic steatosis.
The primary objective was to test the diagnostic performance of two- and six-point Dixon MR techniques in evaluating hepatic steatosis.
A retrospective single center study was performed in patients with suspected diffuse parenchymal liver disease. All patients underwent MR imaging assessment with two-point Dixon, six-point Dixon and MR spectroscopy. Findings on two-point Dixon and six-point Dixon were compared against the reference standard, MR spectroscopy.
We found an excellent correlation (≥ 0.95) between fat fraction estimates on two- and six-point Dixon when compared against magnetic resonance spectroscopy (P < 0.001).
Two- and six-point Dixon are useful MR techniques for evaluating patients with hepatic steatosis.
MR techniques can provide accurate non-invasive assessment of hepatic steatosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Radiological Society of North America, 00413675.
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji G, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhao S
| 1. | Starekova J, Reeder SB. Liver fat quantification: where do we stand? Abdom Radiol (NY). 2020;45:3386-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62:S47-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1516] [Cited by in RCA: 2140] [Article Influence: 214.0] [Reference Citation Analysis (0)] |
| 3. | Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1703] [Article Influence: 243.3] [Reference Citation Analysis (0)] |
| 4. | Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23:8263-8276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 543] [Cited by in RCA: 516] [Article Influence: 64.5] [Reference Citation Analysis (6)] |
| 5. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7533] [Article Influence: 837.0] [Reference Citation Analysis (0)] |
| 6. | Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology. 2021;301:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 7. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1736] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 8. | Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T; LIDO Study Group. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898-1906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1376] [Cited by in RCA: 1549] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 9. | Cleveland E, Bandy A, VanWagner LB. Diagnostic challenges of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Clin Liver Dis (Hoboken). 2018;11:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Ríos C, Méndez-Sánchez N. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: Correlation with biochemical method and morphometry. J Hepatol. 2010;53:732-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | van Werven JR, Schreuder TC, Aarts EO, Nederveen AJ, Meijer JW, Berends FJ, Janssen IM, Mulder CJ, Jansen PL, Stoker J. Hepatic steatosis in morbidly obese patients undergoing gastric bypass surgery: assessment with open-system 1H-MR spectroscopy. AJR Am J Roentgenol. 2011;196:W736-W742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | McPherson S, Jonsson JR, Cowin GJ, O'Rourke P, Clouston AD, Volp A, Horsfall L, Jothimani D, Fawcett J, Galloway GJ, Benson M, Powell EE. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 13. | Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1697] [Cited by in RCA: 1640] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 14. | Dyke JP. Quantitative MRI Proton Density Fat Fraction: A Coming of Age. Radiology. 2021;298:652-653. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 15. | Reeder SB, Sirlin CB. Quantification of liver fat with magnetic resonance imaging. Magn Reson Imaging Clin N Am. 2010;18:337-357, ix. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 16. | Hong CW, Cui JY, Batakis D, Xu Y, Wolfson T, Gamst AC, Schlein AN, Negrete LM, Middleton MS, Hamilton G, Loomba R, Schwimmer JB, Fowler KJ, Sirlin CB. Repeatability and accuracy of various region-of-interest sampling strategies for hepatic MRI proton density fat fraction quantification. Abdom Radiol (NY). 2021;46:3105-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1565] [Cited by in RCA: 1447] [Article Influence: 62.9] [Reference Citation Analysis (0)] |
| 18. | Rodge GA, Goenka MK, Goenka U, Afzalpurkar S, Shah BB. Quantification of Liver Fat by MRI-PDFF Imaging in Patients with Suspected Non-alcoholic Fatty Liver Disease and Its Correlation with Metabolic Syndrome, Liver Function Test and Ultrasonography. J Clin Exp Hepatol. 2021;11:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [PubMed] |
| 20. | Guglielmo FF, Venkatesh SK, Mitchell DG. Liver MR Elastography Technique and Image Interpretation: Pearls and Pitfalls. Radiographics. 2019;39:1983-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, Xin Y. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29:3564-3573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 180] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 22. | Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34:729-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 23. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1184] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 24. | Ajmera V, Loomba R. Imaging biomarkers of NAFLD, NASH, and fibrosis. Mol Metab. 2021;50:101167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 134] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 25. | Kramer H, Pickhardt PJ, Kliewer MA, Hernando D, Chen GH, Zagzebski JA, Reeder SB. Accuracy of Liver Fat Quantification With Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison With MR Spectroscopy. AJR Am J Roentgenol. 2017;208:92-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 26. | Yurdaisik I, Nurili F. Accuracy of Multi-echo Dixon Sequence in Quantification of Hepatic Steatosis. Cureus. 2020;12:e7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Hayashi T, Saitoh S, Takahashi J, Tsuji Y, Ikeda K, Kobayashi M, Kawamura Y, Fujii T, Inoue M, Miyati T, Kumada H. Hepatic fat quantification using the two-point Dixon method and fat color maps based on non-alcoholic fatty liver disease activity score. Hepatol Res. 2017;47:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Idilman IS, Keskin O, Celik A, Savas B, Elhan AH, Idilman R, Karcaaltincaba M. A comparison of liver fat content as determined by magnetic resonance imaging-proton density fat fraction and MRS versus liver histology in non-alcoholic fatty liver disease. Acta Radiol. 2016;57:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 29. | Hu HH, Yokoo T, Bashir MR, Sirlin CB, Hernando D, Malyarenko D, Chenevert TL, Smith MA, Serai SD, Middleton MS, Henderson WC, Hamilton G, Shaffer J, Shu Y, Tkach JA, Trout AT, Obuchowski N, Brittain JH, Jackson EF, Reeder SB; RSNA Quantitative Imaging Biomarkers Alliance PDFF Biomarker Committee. Linearity and Bias of Proton Density Fat Fraction as a Quantitative Imaging Biomarker: A Multicenter, Multiplatform, Multivendor Phantom Study. Radiology. 2021;298:640-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 30. | Colgan TJ, Zhao R, Roberts NT, Hernando D, Reeder SB. Limits of Fat Quantification in the Presence of Iron Overload. J Magn Reson Imaging. 2021;54:1166-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |