Published online Sep 28, 2022. doi: 10.4329/wjr.v14.i9.342
Peer-review started: December 14, 2021
First decision: March 7, 2022
Revised: March 26, 2022
Accepted: August 21, 2022
Article in press: August 21, 2022
Published online: September 28, 2022
Processing time: 282 Days and 2.8 Hours
We suggest an augmentation of the excellent comprehensive review article titled “Comprehensive literature review on the radiographic findings, imaging modalities, and the role of radiology in the coronavirus disease 2019 (COVID-19) pandemic” under the following categories: (1) “Inclusion of additional radiological features, related to pulmonary infarcts and to COVID-19 pneumonia”; (2) “Amplified discussion of cardiovascular COVID-19 manifestations and the role of cardiac magnetic resonance imaging in monitoring and prognosis”; (3) “Imaging findings related to fluorodeoxyglucose positron emission tomography, optical, thermal and other imaging modalities/devices, including ‘intelligent edge’ and other remote monitoring devices”; (4) “Artificial intelligence in COVID-19 imaging”; (5) “Additional annotations to the radiological images in the manuscript to illustrate the additional signs discussed”; and (6) “A minor correction to a passage on pulmonary destruction”.
Core Tip: Utility of classical radiographic findings suggestive of coronavirus disease 2019 (COVID-19) mediated pulmonary infarction (Hampton’s hump, Westermark sign, subpleural sparing and reversed halo sign) should improve the diagnostic accuracy of identification of COVID-19 pulmonary complications. This gain in accuracy would apply whether these findings are seen on plain chest X-ray or computed tomography. The former is important in financially constrained locales with limited medical technology infrastructure. Distinctive COVID-19-associated coagulopathy is more frequent with worsening disease severity in COVID-19. Cardiac magnetic resonance imaging can play an important role in monitoring and prognosis. “Artificial intelligence in COVID-19” and “‘Intelligent edge’ and other remote monitoring devices” are also discussed.
- Citation: Merchant SA, Nadkarni P, Shaikh MJS. Augmentation of literature review of COVID-19 radiology. World J Radiol 2022; 14(9): 342-351
- URL: https://www.wjgnet.com/1949-8470/full/v14/i9/342.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i9.342
We compliment Pal et al[1] for their excellent review. It is a comprehensive review indeed. An excellent effort with great details, including in depth pathophysiology, detailed illustrations, etc. Their coverage of imaging modalities is quite extensive too and includes a detailed look into the role of ultrasound in coronavirus disease 2019 (COVID-19), including point-of-care ultrasound, an invaluable addition. For the benefit of your readers, we wish to augment their excellent work and submit the following suggestions for the benefit of your readers.
We are involved in an ongoing multicentric international study on COVID-19 chest imaging and developing artificial intelligence (AI) algorithms for diagnosis, risk stratification, monitoring, prognostication, etc. Our 2020 publication has described additional important and distinctive COVID-19 chest-imaging features[2]. These include the following, seen on both plain chest radiographs and computed tomography (CT).
Hampton’s hump: Triangular/wedge shaped opacities with their bases towards the periphery of the lung/lobe/lobule. This sign has sensitivity and specificity of 22% and 82%, respectively[3,4].
Westermark sign: Oligemia, a rarefied area due to blood vessel collapse, distal to the site of occlusion by a pulmonary embolus. This sign has sensitivity and specificity of 14% and 92%, respectively[3,5].
Palla’s sign: An enlarged right pulmonary artery, suggesting embolism of segmental/subsegmental pulmonary arteries when seen together with Westermark sign. Sensitivity is reported to be “low” and specificity unknown. These findings are likely due to the microvascular thrombosis propensity in COVID-19[6-8], as discussed below, leading to a relatively increased incidence of pulmonary thromboembolism in COVID-19 pneumonia patients[9].
It is time to revisit these time-tested radiological signs for pulmonary infarcts[2]. Utilizing classic signs of infarcts and pneumonia will increase diagnostic accuracy and help raise awareness about the utility of chest radiographs, even in the current era; especially in cost-constrained locales lacking sophisticated infrastructure. It will also help develop more accurate AI algorithms for dia
Reported in 23% of COVID-19 cases in an Iranian study[10], subpleural sparing is commonly associated with nonspecific interstitial pneumonia and is described with lung contusions, pulmonary alveolar proteinosis, severe acute respiratory syndrome (SARS) and pneumocystis jirovecii infection[11]. The specificity of this finding depends on the prior probability of COVID-19 based on molecular detection via polymerase chain reaction (PCR).
The reversed halo sign is a focal ring-shaped area of ground-glass opacity within a peripheral rim of consolidation, suggesting an organizing/healing pneumonia[12]. It offers prognostic potential in COVID-19[13,14]. Data on sensitivity/specificity are not currently available. Utilizing classic signs of infarcts and pneumonia will increase diagnostic accuracy, and also help raise awareness about chest radiographs’ utility, even in the current era, especially in cost-constrained locales lacking sophisticated infrastructure. It will also help develop more accurate AI algorithms for diagnosis/prognosis of COVID-19. Co-occurrences of these signs are uncommon across COVID-19 patients: When seen in tandem, however, they may constitute a highly specific diagnostic signature. This speculation, of course, needs validation by larger studies.
The paper’s images[1] show the following (currently unannotated) features: Subpleural sparing, figures 4B just under arrow marked as ground glass opacities, 7C and 7F; Hampton’s humps, figures 2E, 2F, 4B (marked as consolidation), 4C and 7A (larger, but fewer, in the right lung than left lung); Westermark sign, figure 2F; and pericardial air, figure 2C.
While correctly noting the ability of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, to invade cells by binding with high affinity to angiotensin-converting enzyme 2 and transmembrane protease serine 2 receptors, the authors have not discussed the cardiovascular system, where COVID-19’s impact has been reviewed widely[6,15-17]. The angiotensin-converting enzyme 2 receptor is also expressed in the cardiovascular system in the endothelium of coronary arteries, cardiomyocytes, cardiac fibroblasts, epicardial adipocytes, vascular endothelial and smooth muscle cells[18-20].
Binding of SARS-CoV-2 to the endothelium predisposes to microthrombosis via endothelial inflammation, complement activation, thrombin generation, platelet and leukocyte recruitment and initiation of innate and adaptive immune responses with complications such as deep vein thrombosis, pulmonary embolism, cortical venous thrombosis, stroke, cardiac inflammation and injury, arrhythmias, blood clots[18] and acute/chronic myocardial injury[21]. An assay of the fibrin degradation product D-dimer (a thrombosis marker) on admission for prognostication of in-hospital mortality is now mandated in most clinical protocols to differentiate mild from severe COVID-19[7,22], especially when coupled with thrombocytopenia[8]. In infants and children reports of coronary artery aneurysms (CAA), including giant CAAs are gathering momentum as a part of multisystem inflammatory syndrome in post COVID-19 children[23-26].
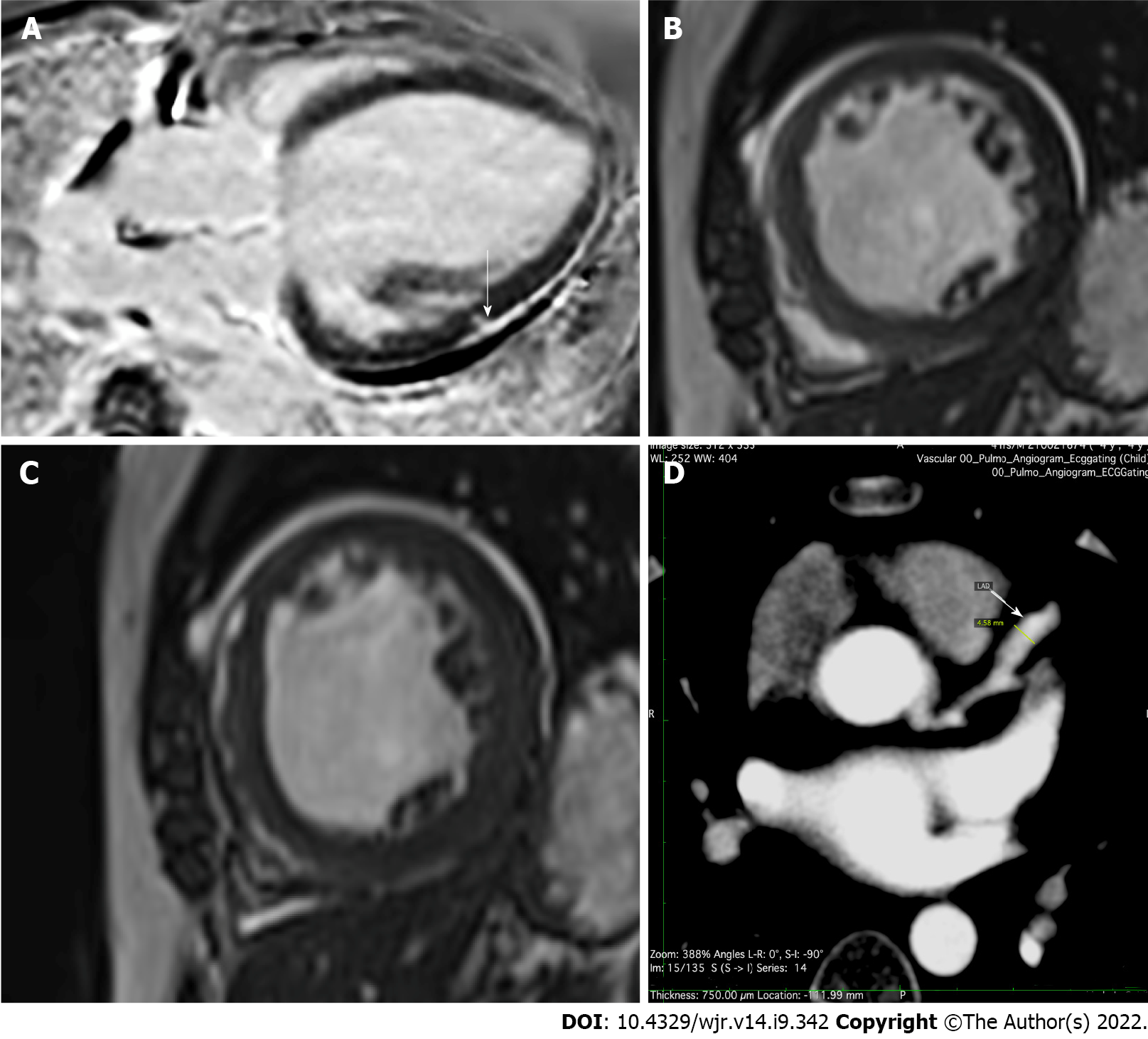
While the authors correctly note that cardiac magnetic resonance imaging (MRI) may be useful in the future to detect complications in patients with abnormal echocardiography, this is a current need too. Up to 60% of hospitalized COVID-19 patients have been reported to have evidence of myocardial injury[21] (Figure 1A). Among post-discharge patients, approximately 10% complain of palpitations, with half of these having ongoing chest pain 6 mo after discharge[15]. Dilated cardiomyopathy is a known complication of COVID-19 cardiac injury[27] (Figures 1B and C). In post-COVID-vaccination patients, distinct self-limited myocarditis and pericarditis have appeared. While myocarditis developed rapidly in younger patients, mostly after the second vaccination, pericarditis affected older patients later, after either the first or second dose[28].
A recent report implicates the booster dose of the COVID-19 vaccine for acute myocarditis too[29]. In infants and children with COVID-19 reports of CAAs, including giant CAAs are gathering momentum[23-26], and cardiac MRI/CT can be an invaluable in diagnosing these too. This is particularly important as these aneurysms (and their catastrophic consequences) are potentially regressible with ‘steroid therapy’. In addition these aneurysms would need to be monitored and managed, including for their potential to develop thrombosis[24]. Management includes cardiac support, immunomodulatory agents and anticoagulation[26]. Richardson et al[24] stated that in infants rapidly progressing CAAs are noted post COVID-19 infection. They also stated that as opposed to published reports these may be seen even in the absence of hemodynamic instability, ventricular dysfunction, myocardial ischemia or myopericarditis. In view of the risk of progression of cardiac signs and symptoms, Sperotto et al[26] recommended long-term follow-up of these patients. Coronary arteries should therefore be thoroughly assessed in patients presenting with multisystem inflammatory syndrome in children symptoms[25]. For its non-ionizing radiation nature MRI would be the first choice in children. However, CT on account of its speed (and current low radiation protocols) can be utilized effectively too (Figure 1D).
In their Radiology 2021 editorial, Lima et al[30] stated that prolonged symptoms due to “long-haul” COVID-19 portend the potential for chronic cardiac sequelae, whose duration and severity remain unknown. They introduced the work of Kravchenko et al[31], which demonstrated the value of cardiac MRI in identifying inflammation, adverse patterns of hypertrophy, fibrosis and myocardial injury due to myocarditis, pericarditis, cardiomyopathy and healing.
Although thoracic CT is widely used for imaging of COVID-19 infection, thoracic MRI can also be used as an alternative diagnostic tool because of its advantages[32]. This is particularly important in patients requiring avoidance of exposure to ionizing radiation, e.g., in children and during pregnancy where pulmonary MRI may be preferred over pulmonary CT[33]. Pulmonary abnormalities caused by COVID-19 pneumonia can be detected on True FISP MRI sequences and correspond to the patterns known from CT. Spiro et al[34] made a useful suggestion for the current pandemic: Following MRI of the abdomen or heart, there should be careful evaluation of the visualized parts of the lungs for COVID-19 findings. This would enable the identification and isolation of undetected cases of COVID-19.
Necker et al[35] reported a cinematic rendering of SARS-CoV-2 pneumonia. Cinematic rendering is a digital three-dimensional visualization technique that converts grayscale slices from CT or MRI into colored three-dimensional volumes via transfer functions illuminating the reconstruction with physical light simulation. They have stated that this type of rendering produces a natural, photorealistic image that is intuitively understandable and can be well applied for clinical purposes. Cinematic rendering of CT images is a new way to show the three dimensionality of the various densities contained in volumetric CT/MRI data. We agree with them and feel that such cinematic rendering can make complicated volume rendered CT/MRI images easy to understand for other clinicians, administrators, policy makers as well as patients alike.
The authors’ suggestion of using fluorodeoxyglucose-positron emission tomography (PET) in the future for prognosis and monitoring is wonderful. We wish to add that the “rim sign”, a slight and continuous fluorodeoxyglucose uptake at the border of a peripheral lung consolidation[36], is easily recognizable on fluorodeoxyglucose PET/CT (though data on sensitivity/specificity are not available). When present, it strongly suggests pulmonary infarction and is observable even without suggestive finding of pulmonary infarction. The reverse halo sign would also be seen. Though highly sensitive, use of PET/CT for primary detection of COVID-19 is constrained by poor specificity as well as considerations of cost, radiation burden and prolonged exposure times for imaging staff. However, in patients who may require nuclear medicine studies for other clinical indications, PET imaging may yield the earliest detection of nascent infection in otherwise asymptomatic individuals. This may be extremely vital for immunocompromised patients, including those with coexistent malignancies, where the early diagnosis of infection and subsequent initiation of care needed will contribute vitally to improving outcomes and reducing morbidity and mortality[33].
Lukose et al[37] stated that the currently popular method of collecting samples using the nasopharyngeal swab and subsequent detection of RNA using real-time PCR has false-positive results and a longer diagnostic time frame. Various optical techniques such as optical sensing, spectroscopy and imaging show great promise in virus detection, and the progress in the field of optical techniques for virus detection unambiguously show great promise in the development of rapid photonics-based devices for COVID-19 detection. They also provided a comprehensive review of the various photonics technologies employed for virus detection, especially the SARS-CoV family, such as near-infrared spectroscopy, fourier transform infrared spectroscopy, raman spectroscopy, fluorescence-based techniques, super-resolution microscopy and surface plasmon resonance-based detection.
Gomez-Gonzalez et al[38] reported a proof of concept of optical imaging spectroscopy for rapid, primary screening of SARS-CoV-2. A study by Shah et al[39] found that home pulse oximetry monitoring identified the need for hospitalization in initially non-severe COVID-19 patients when a cutoff SpO2 of 92% was used and that home SpO2 monitoring also reduced unnecessary emergency department revisits. McKay et al[40] stated that due to its portability, affordability and potential to serve as a screening tool for a conventionally lab-based invasive test, the mobile phone capillaroscope could serve as an important point-of-care tool and that the simplicity and portability of their technique may enable the development of an effective non-invasive tool for white blood cell screening in point-of-care and global health settings. This would be extremely useful in the COVID-19 pandemic scenario as white blood cell monitoring forms an essential part of COVID-19 management and follow-up[41,42].
Infrared thermography has been considered a gold standard method for screening febrile individuals during pandemics since the SARS outbreak in 2003. Khaksari et al[43] showed that in addition to an elevated body temperature a patient with COVID-19 will exhibit changes in other parameters such as oxygenation of tissues and cardiovascular and respiratory system functions. They also promulgated a compelling need to develop a new technique that would have the ability to screen all these signals and utilize the same for early detection of viral infections. In their opinion, keeping the advent of wireless technologies in mind, the development of such sensors that have point-of-care home-accessible capabilities will go a long way in better managing the increasing numbers of patients with COVID-19 who are opting for home quarantine and that this will eventually reduce the burden on the healthcare system.
The COVID-19 pandemic is changing the landscape of healthcare delivery worldwide. There is a discernible shift toward remote patient monitoring. It is pertinent to note that a large number of remote patient monitoring platforms are already utilizing optical technologies[44]. This area of research has great potential for growth, and the biomedical optics community has great prospects in the development, testing and commodification of new wearable remote patient monitoring technologies to add to the available healthcare armamentarium and contribute to the rapidly changing healthcare and research environment, not just for the COVID-19 era but far beyond[44].
Various other ingenious methods/modalities have been used for early detection/screening for COVID-19. These include smartwatches[45], smart phones and other intelligent edge devices. Mishra et al[45] developed a method utilizing data from smartwatches to detect the onset of COVID-19 infection in real-time that detected 67% of infection cases at or before symptom onset. They stated that their study provided a roadmap to a rapid and universal diagnostic method for the large-scale detection of respiratory viral infections in advance of symptoms, highlighting a useful approach for managing epidemics using digital tracking and health monitoring. Seshadri et al[46] stated that when used in conjunction with predictive platforms, wearable device users could receive alerts when changes in their metrics match those related to COVID-19 and that such anonymous data localized to regions such as neighborhoods or zip codes could provide public health officials and researchers a valuable tool to track and mitigate the spread of the virus. Their manuscript describes clinically relevant physiological metrics that can be measured from commercial devices today and highlights their role in tracking the health, stability, and recovery of COVID-19 + individuals and front-line workers.
Schuller et al[47] in their paper tilted ‘COVID-19 and Computer Audition: An Overview on What Speech & Sound Analysis Could Contribute in the SARS-CoV-2 Corona Crisis’ provided an overview on the potential for computer audition, i.e., the usage of speech and sound analysis by AI, to help in the COVID-19 pandemic scenario and concluded that computer audition appears ready for implementation of (pre-)diagnosis and monitoring tools and more generally provides rich and significant, yet so far untapped, potential in the fight against COVID-19 spread.
AI in COVID-19 imaging. Telemedicine has advanced by leaps and bounds. AI algorithms enable faster diagnosis (including remote diagnosis), with a fair degree of accuracy[48]. While the application of AI to medical imaging of cancers and other diseases is being developed over the past decades, the recent COVID-19 pandemic hastened the: (1) Need; (2) Development; (3) Training; and (4) Testing of AI algorithms, within a relatively shorter time-span of less than 2 years[49]. This was extremely beneficial for radiologists and other physicians involved in performing rapid diagnosis, keeping in mind this was a time when there was immense overloading of the healthcare system[50]. The benefits including for management were obvious. However limitations such as: (1) Limited datasets; (2) Inaccurate execution of training and testing procedures; and (3) Use of incorrect performance criteria needed to be dealt with. The above limitations can be overcome by the utilization of federated learning[48,51,52].
The technique of federated learning was originally pioneered by Google[53] as an application of their well-known MapReduce algorithm[54] and allows for iteratively training a machine learning model across geographically separated hardware, including mobile devices. The machine learning algorithm is distributed, while data remains local. It can be employed for both statistical and deep learning. Despite its drawbacks, specifically wide-area network bandwidth limits computation speed, federated learning appears to be a great way forward, especially for multicenter collaborations, getting around the ‘tricky’ data privacy issue and enabling algorithms/outcomes with much more accuracy than otherwise possible[51].
If AI is to make an even greater impact, Merchant et al[48] suggested getting down to the basics and incorporating time tested key medical ‘teaching’ and/or key ‘clinical’ parameters, including prognostic indicators, for more effective AI algorithms and their better clinical utility. They also stated that “Artificial Intelligence needs real Intelligence to guide it!”. Combining the wisdom gained over the years with the immense versatility of AI algorithms will maximize the accuracy and utility of AI applications in medical diagnosis and treatment modalities. We have gained wisdom regarding COVID-19 imaging over the past few years and should utilize the same for creation of better algorithms for screening/detection/prognostication and management.
El Naqa et al[55], as part of a Medical Imaging Data and Resource Center initiative, noted that the pandemic has led to the coupling of interdisciplinary experts that include: (1) Clinicians; (2) Medical physicists; (3) Imaging scientists; (4) Computer scientists; and (5) Informatics experts, all of whom are working towards solving the challenges of the COVID-19 pandemic, specifically AI methods applied to medical imaging. They stated that the lessons learned during the transitioning to AI in the medical imaging of COVID-19 can inform and enhance future AI applications, making the entire transition more than every discipline combined to respond to emergencies like the COVID-19 pandemic. AI has been used in multiple imaging fields for COVID-19 imaging.
The model by Manokaran et al[56] could achieve an accuracy of 94.00% in detecting COVID-19 and an overall accuracy of 92.19%, which was based on DenseNet-201. The model can achieve an area under receiver operating characteristic curve of 0.99 for COVID-19, 0.97 for normal and 0.97 for pneumonia. Their automated diagnostic model yielded an accuracy of 94.00% in the initial screening of COVID-19 patients and an overall accuracy of 92.19% using chest X-ray images.
Kusakunniran et al[57] proposed a solution to automatically classify COVID-19 cases in chest X-ray images using the ResNet-101 architecture, which was adopted as the main network with over 44 million parameters. A heatmap was constructed under the region of interest of the lung segment to visualize and emphasize signals of COVID-19. Their method achieved a sensitivity, specificity and accuracy of 97%, 98% and 98%, respectively. Rao et al[58] stated that separable SVRNet and separable SVDNet models greatly reduced the number of parameters while improving the accuracy and increasing the operating speed.
Yi et al[50] utilized a large CT database (1112 patients) provided by the China Consortium of Chest CT Image Investigation and investigated multiple solutions in detecting COVID-19 and distinguishing it from other common pneumonia and normal controls. They compared the performance of different models for complete and segmented CT slices, in particular studying the effects of CT-superimposition depths into volumes, on the performance of their models and showed that an optimal model could identify COVID-19 slices with 99.76% accuracy (99.96% recall, 99.35% precision and 99.65% F1-score).
Chaddad et al[59] investigated the potential of deep transfer learning to predict COVID-19 infection using chest CT and X-ray images. They opined that combining chest CT and X-ray images with DarkNet architecture achieved the highest accuracy of 99.09% and area under receiver operating characteristic curve of 99.89% in classifying COVID-19 from non-COVID-19 and that their results confirmed the ability of deep convolutional neural networks with transfer learning to predict COVID-19 in both chest CT and X-ray images. They concluded that this approach could help radiologists improve the accuracy of their diagnosis and improve overall efficiency of COVID-19 management.
Cho et al[60] performed quantitative CT analysis on chest CT images using supervised machine learning to measure regional ground glass opacities and inspiratory and expiratory image matching to measure regional air trapping in survivors of COVID-19. They summarized that quantitative analysis of expiratory chest CT images demonstrated that small airway disease with the presence of air trapping is a long-lasting sequelae of SARS-CoV-2 infection.
Fuhrman et al[61] developed a cascaded transfer learning approach to extract quantitative features from thoracic CT sections using a fine-tuned VGG19 network where a CT-scan-level representation of thoracic characteristics and a support vector machine was trained to distinguish between patients who required steroid administration and those who did not. They demonstrated significant differences between patients who received steroids and those who did not and concluded that their ‘cascade deep learning method’ has great potential in clinical decision-making and for monitoring patient treatment.
Quantum computers and quantum microscopes, new quantum repeaters enabling a scalable super secure quantum internet (distance will no longer be a hindrance, not just internet of things but ‘intelligent edge’ devices commonplace[62]) will give a quantum boost to COVID-19 and other health care algorithms/strategies, including in other related fields, improving healthcare in ways beyond the realm of dreams[51]. Cloud computing could be complemented by edge computing, taking advantage of the burgeoning intelligent edge devices (smartphones are commonplace in the remotest of locations). Besides latency, edge computing is preferred over cloud computing in remote locations, where there is limited or no connectivity to a centralized location (a requirement of cloud computing), which requires local storage, similar to a mini data center at their location[63]. Medical imaging including COVID-19/other pandemic imaging and AI will never be the same again, in the era of quantum computing and quantum AI imaging and health care will reach stratospheric levels and beyond[47].
Correction of “pulmonary destruction”. The author’s state: “The migration of fluid into the alveolar sacs is governed by the imbalance in Starling forces. The diffuse alveolar damage caused by the viral particles results in an increased capillary wall permeability (high k value), thereby increasing the force at which fluid migrates from the capillaries to the alveolar space.” emphasis added. Surely the authors mean “rate” instead of “force”. Permeability is the inverse of resistance. By analogy with Ohm’s Law for electricity (current = voltage/resistance) or its equivalent for blood pressure (cardiac output = blood pressure/peripheral resistance), capillary outflow will increase under fixed/constant pressure if permeability increases.
We hope that this augmentation of the excellent review by Pal et al[1] will enhance your readers’ ability to evaluate COVID-19 patients on imaging. COVID-19 is here to stay. Each effort at adding to the information available in the literature will go a long way in improving patient care overall.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Konovalov AB, Russia; Schoenhagen P, United States; Taydas O, Turkey; Zhu JB, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Pal A, Ali A, Young TR, Oostenbrink J, Prabhakar A, Deacon N, Arnold A, Eltayeb A, Yap C, Young DM, Tang A, Lakshmanan S, Lim YY, Pokarowski M, Kakodkar P. Comprehensive literature review on the radiographic findings, imaging modalities, and the role of radiology in the COVID-19 pandemic. World J Radiol. 2021;13:258-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (5)] |
| 2. | Merchant SA, Ansari SMS, Merchant N. Additional Chest Imaging Signs That Have the Potential of Being COVID-19 Imaging Markers. AJR Am J Roentgenol. 2020;215:W57-W58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Worsley DF, Alavi A, Aronchick JM, Chen JT, Greenspan RH, Ravin CE. Chest radiographic findings in patients with acute pulmonary embolism: observations from the PIOPED Study. Radiology. 1993;189:133-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 163] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Han D, Lee KS, Franquet T, Müller NL, Kim TS, Kim H, Kwon OJ, Byun HS. Thrombotic and nonthrombotic pulmonary arterial embolism: spectrum of imaging findings. Radiographics. 2003;23:1521-1539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Stein PD, Beemath A, Matta F, Weg JG, Yusen RD, Hales CA, Hull RD, Leeper KV Jr, Sostman HD, Tapson VF, Buckley JD, Gottschalk A, Goodman LR, Wakefied TW, Woodard PK. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med. 2007;120:871-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1095] [Cited by in RCA: 1249] [Article Influence: 249.8] [Reference Citation Analysis (2)] |
| 7. | Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 837] [Cited by in RCA: 836] [Article Influence: 167.2] [Reference Citation Analysis (0)] |
| 8. | McFadyen JD, Stevens H, Peter K. The Emerging Threat of (Micro)Thrombosis in COVID-19 and Its Therapeutic Implications. Circ Res. 2020;127:571-587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 9. | Léonard-Lorant I, Delabranche X, Séverac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Molière S, Leyendecker P, Roy C, Ohana M. Acute Pulmonary Embolism in Patients with COVID-19 at CT Angiography and Relationship to d-Dimer Levels. Radiology. 2020;296:E189-E191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 385] [Cited by in RCA: 443] [Article Influence: 88.6] [Reference Citation Analysis (0)] |
| 10. | Tabatabaei SMH, Talari H, Moghaddas F, Rajebi H. CT Features and Short-term Prognosis of COVID-19 Pneumonia: A Single-Center Study from Kashan, Iran. Radiol Cardiothorac Imaging. 2020;2:e200130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 11. | Chong WH, Saha BK, Austin A, Chopra A. The Significance of Subpleural Sparing in CT Chest: A State-of-the-Art Review. Am J Med Sci. 2021;361:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Maturu VN, Agarwal R. Reversed halo sign: a systematic review. Respir Care. 2014;59:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Sales AR, Casagrande EM, Hochhegger B, Zanetti G, Marchiori E. The Reversed Halo Sign and COVID-19: Possible Histopathological Mechanisms Related to the Appearance of This Imaging Finding. Arch Bronconeumol. 2021;57:73-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Marchiori E, Nobre LF, Hochhegger B, Zanetti G. CT characteristics of COVID-19: reversed halo sign or target sign? Diagn Interv Radiol. 2021;27:306-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3187] [Cited by in RCA: 2887] [Article Influence: 721.8] [Reference Citation Analysis (1)] |
| 16. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 17. | Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2333] [Cited by in RCA: 2077] [Article Influence: 415.4] [Reference Citation Analysis (0)] |
| 18. | Salamanna F, Maglio M, Landini MP, Fini M. Body Localization of ACE-2: On the Trail of the Keyhole of SARS-CoV-2. Front Med (Lausanne). 2020;7:594495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 19. | Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Response by Gheblawi et al to Letter Regarding Article, "Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2". Circ Res. 2020;127:e46-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 621] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 21. | Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, Danilov T, Kukar N, Shaban N, Kini A, Camaj A, Bienstock SW, Rashed ER, Rahman K, Oates CP, Buckley S, Elbaum LS, Arkonac D, Fiter R, Singh R, Li E, Razuk V, Robinson SE, Miller M, Bier B, Donghi V, Pisaniello M, Mantovani R, Pinto G, Rota I, Baggio S, Chiarito M, Fazzari F, Cusmano I, Curzi M, Ro R, Malick W, Kamran M, Kohli-Seth R, Bassily-Marcus AM, Neibart E, Serrao G, Perk G, Mancini D, Reddy VY, Pinney SP, Dangas G, Blasi F, Sharma SK, Mehran R, Condorelli G, Stone GW, Fuster V, Lerakis S, Goldman ME. Characterization of Myocardial Injury in Patients With COVID-19. J Am Coll Cardiol. 2020;76:2043-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 22. | Velavan TP, Meyer CG. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 337] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 23. | Navaeifar MR, Shahbaznejad L, Sadeghi Lotfabadi A, Rezai MS. COVID-19-Associated Multisystem Inflammatory Syndrome Complicated with Giant Coronary Artery Aneurysm. Case Rep Pediatr. 2021;2021:8836403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Richardson KL, Jain A, Evans J, Uzun O. Giant coronary artery aneurysm as a feature of coronavirus-related inflammatory syndrome. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Pick JM, Wang S, Wagner-Lees S, Badran S, Szmuszkovicz JR, Wong P, Votava-Smith J. Abstract 17092: Coronary Artery Aneurysms Are More Common in Post-COVID-19 Multisystem Inflammatory Syndrome in Children (MIS-C) Than Pre-Pandemic Kawasaki Disease. Circulation. 2020;142:A17092. [DOI] [Full Text] |
| 26. | Sperotto F, Friedman KG, Son MBF, VanderPluym CJ, Newburger JW, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180:307-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 214] [Cited by in RCA: 248] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 27. | Omidi F, Hajikhani B, Kazemi SN, Tajbakhsh A, Riazi S, Mirsaeidi M, Ansari A, Ghanbari Boroujeni M, Khalili F, Hadadi S, Nasiri MJ. COVID-19 and Cardiomyopathy: A Systematic Review. Front Cardiovasc Med. 2021;8:695206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A. Myocarditis and Pericarditis After Vaccination for COVID-19. JAMA. 2021;326:1210-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 291] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 29. | Sanchez Tijmes F, Zamorano A, Thavendiranathan P, Hanneman K. Imaging of Myocarditis Following mRNA COVID-19 Booster Vaccination. Radiol Cardiothorac Imaging. 2022;4:e220019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Lima JAC, Bluemke DA. Myocardial Scar in COVID-19: Innocent Marker versus Harbinger of Clinical Disease. Radiology. 2021;301:E434-E435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kravchenko D, Isaak A, Zimmer S, Mesropyan N, Reinert M, Faron A, Pieper CC, Heine A, Velten M, Nattermann J, Kuetting D, Duerr GD, Attenberger UI, Luetkens JA. Cardiac MRI in Patients with Prolonged Cardiorespiratory Symptoms after Mild to Moderate COVID-19. Radiology. 2021;301:E419-E425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Ates OF, Taydas O, Dheir H. Thorax Magnetic Resonance Imaging Findings in Patients with Coronavirus Disease (COVID-19). Acad Radiol. 2020;27:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Fields BKK, Demirjian NL, Dadgar H, Gholamrezanezhad A. Imaging of COVID-19: CT, MRI, and PET. Semin Nucl Med. 2021;51:312-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Spiro JE, Curta A, Mansournia S, Marschner CA, Maurus S, Weckbach LT, Hedderich DM, Dinkel J. Appearance of COVID-19 pneumonia on 1.5 T TrueFISP MRI. Radiol Bras. 2021;54:211-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Necker FN, Scholz M. Chest CT Cinematic Rendering of SARS-CoV-2 Pneumonia. Radiology. 2022;303:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Soussan M, Rust E, Pop G, Morère JF, Brillet PY, Eder V. The rim sign: FDG-PET/CT pattern of pulmonary infarction. Insights Imaging. 2012;3:629-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Lukose J, Chidangil S, George SD. Optical technologies for the detection of viruses like COVID-19: Progress and prospects. Biosens Bioelectron. 2021;178:113004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 38. | Gomez-Gonzalez E, Barriga-Rivera A, Fernandez-Muñoz B, Navas-Garcia JM, Fernandez-Lizaranzu I, Munoz-Gonzalez FJ, Parrilla-Giraldez R, Requena-Lancharro D, Gil-Gamboa P, Rosell-Valle C, Gomez-Gonzalez C, Mayorga-Buiza MJ, Martin-Lopez M, Muñoz O, Gomez-Martin JC, Relimpio-Lopez MI, Aceituno-Castro J, Perales-Esteve MA, Puppo-Moreno A, Garcia-Cozar FJ, Olvera-Collantes L, Gomez-Diaz R, de Los Santos-Trigo S, Huguet-Carrasco M, Rey M, Gomez E, Sanchez-Pernaute R, Padillo-Ruiz J, Marquez-Rivas J. Optical imaging spectroscopy for rapid, primary screening of SARS-CoV-2: a proof of concept. Sci Rep. 2022;12:2356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Shah S, Majmudar K, Stein A, Gupta N, Suppes S, Karamanis M, Capannari J, Sethi S, Patte C. Novel Use of Home Pulse Oximetry Monitoring in COVID-19 Patients Discharged From the Emergency Department Identifies Need for Hospitalization. Acad Emerg Med. 2020;27:681-692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 40. | McKay GN, Mohan N, Butterworth I, Bourquard A, Sánchez-Ferro Á, Castro-González C, Durr NJ. Visualization of blood cell contrast in nailfold capillaries with high-speed reverse lens mobile phone microscopy. Biomed Opt Express. 2020;11:2268-2276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Pirsalehi A, Salari S, Baghestani A, Sanadgol G, Shirini D, Baerz MM, Abdi S, Akbari ME, Bashash D. Differential alteration trend of white blood cells (WBCs) and monocytes count in severe and non-severe COVID-19 patients within a 7-day follow-up. Iran J Microbiol. 2021;13:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 42. | Leulseged TW, Hassen IS, Ayele BT, Tsegay YG, Abebe DS, Edo MG, Maru EH, Zewde WC, Naylor LK, Semane DF, Dresse MT, Tezera BB. Laboratory biomarkers of COVID-19 disease severity and outcome: Findings from a developing country. PLoS One. 2021;16:e0246087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 43. | Khaksari K, Nguyen T, Hill B, Quang T, Perreault J, Gorti V, Malpani R, Blick E, González Cano T, Shadgan B, Gandjbakhche AH. Review of the efficacy of infrared thermography for screening infectious diseases with applications to COVID-19. J Med Imaging (Bellingham). 2021;8:010901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Roblyer D. Perspective on the increasing role of optical wearables and remote patient monitoring in the COVID-19 era and beyond. J Biomed Opt. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Mishra T, Wang M, Metwally AA, Bogu GK, Brooks AW, Bahmani A, Alavi A, Celli A, Higgs E, Dagan-Rosenfeld O, Fay B, Kirkpatrick S, Kellogg R, Gibson M, Wang T, Rolnik B, Ganz AB, Li X, Snyder MP. Early Detection Of COVID-19 Using A Smartwatch. 2020 Preprint. Available from: medRxiv: 2020.07.06.20147512. [DOI] [Full Text] |
| 46. | Seshadri DR, Davies EV, Harlow ER, Hsu JJ, Knighton SC, Walker TA, Voos JE, Drummond CK. Wearable Sensors for COVID-19: A Call to Action to Harness Our Digital Infrastructure for Remote Patient Monitoring and Virtual Assessments. Front Digit Health. 2020;2:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 47. | Schuller BW, Schuller DM, Qian K, Liu J, Zheng H, Li X. COVID-19 and Computer Audition: An Overview on What Speech & Sound Analysis Could Contribute in the SARS-CoV-2 Corona Crisis. Front Digit Health. 2021;3:564906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 48. | Merchant SA, Shaikh MJS, Nadkarni P. Tuberculosis conundrum - current and future scenarios: A proposed comprehensive approach combining laboratory, imaging, and computing advances. World J Radiol. 2022;14:114-136. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 49. | Giger M. Medical imaging of COVID-19. J Med Imaging (Bellingham). 2021;8:010101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Li Y, Pei X, Guo Y. 3D CNN classification model for accurate diagnosis of coronavirus disease 2019 using computed tomography images. J Med Imaging (Bellingham). 2021;8:017502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 51. | Nadkarni P, Merchant SA. Enhancing medical-imaging artificial intelligence through holistic use of time-tested key imaging and clinical parameters: Future insights. Artif Intell Med Imaging. 2022;3:55-69. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (5)] |
| 52. | Rieke N, Hancox J, Li W, Milletarì F, Roth HR, Albarqouni S, Bakas S, Galtier MN, Landman BA, Maier-Hein K, Ourselin S, Sheller M, Summers RM, Trask A, Xu D, Baust M, Cardoso MJ. The future of digital health with federated learning. NPJ Digit Med. 2020;3:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 608] [Cited by in RCA: 682] [Article Influence: 136.4] [Reference Citation Analysis (0)] |
| 53. | McMahan B, Ramage D. Federated Learning: Collaborative Machine Learning without Centralized Training Data. Google AI Blog. 6 Apr 2017. [cited 14 November 2021]. Available from: https://starrymind.tistory.com/180. |
| 54. | Dean J, Ghemawat S. MapReduce: Simplified Data Processing on Large Clusters. Commun ACM. 2008;51:107-113. [DOI] [Full Text] |
| 55. | El Naqa I, Li H, Fuhrman J, Hu Q, Gorre N, Chen W, Giger ML. Lessons learned in transitioning to AI in the medical imaging of COVID-19. J Med Imaging (Bellingham). 2021;8:010902-010902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Manokaran J, Zabihollahy F, Hamilton-Wright A, Ukwatta E. Detection of COVID-19 from chest x-ray images using transfer learning. J Med Imaging (Bellingham). 2021;8:017503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 57. | Kusakunniran W, Karnjanapreechakorn S, Siriapisith T, Borwarnginn P, Sutassananon K, Tongdee T, Saiviroonporn P. COVID-19 detection and heatmap generation in chest x-ray images. J Med Imaging (Bellingham). 2021;8:014001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Rao K, Xie K, Hu Z, Guo X, Wen C, He J. COVID-19 detection method based on SVRNet and SVDNet in lung x-rays. J Med Imaging (Bellingham). 2021;8:017504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Chaddad A, Hassan L, Desrosiers C. Deep CNN models for predicting COVID-19 in CT and x-ray images. J Med Imaging (Bellingham). 2021;8:014502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 60. | Cho JL, Villacreses R, Nagpal P, Guo J, Pezzulo AA, Thurman AL, Hamzeh NY, Blount RJ, Fortis S, Hoffman EA, Zabner J, Comellas AP. Quantitative Chest CT Assessment of Small Airways Disease in Post-Acute SARS-CoV-2 Infection. Radiology. 2022;304:185-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 79] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 61. | Fuhrman JD, Chen J, Dong Z, Lure FYM, Luo Z, Giger ML. Cascaded deep transfer learning on thoracic CT in COVID-19 patients treated with steroids. J Med Imaging (Bellingham). 2021;8:014501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | TechCrunch BS. The future is not the Internet of Things… it is the Connected Intelligent Edge. Dec 21, 2021. [cited 22 December 2021]. Available from: https://www.nastel.com/the-future-is-not-the-internet-of-things-it-is-the-connected-intelligent-edge/. |
| 63. | Arora S. Edge Computing Vs. Cloud Computing: What are the Differences. Jun 30, 2022. [cited 8 March 2022]. Available from: https://www.simplilearn.com/edge-computing-vs-cloud-computing-article. |