Published online Jul 28, 2022. doi: 10.4329/wjr.v14.i7.229
Peer-review started: February 19, 2022
First decision: April 8, 2022
Revised: April 16, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: July 28, 2022
Processing time: 158 Days and 5 Hours
Magnetic resonance imaging (MRI) with multiparametric dynamic contrast plays a critical role in the assessment of breast lesions. Dynamic curves are a critical parameter in determining the benign or malignant nature of lesions. Dynamic curves of type 1 are known to represent benign masses, while dynamic curves of type 3 are known to identify malignant masses. Type 2 dynamic curves have a sensitivity of 42.6% and specificity of 75% for malignancy detection.
To investigate the pathological diagnosis of lesions with type 2 dynamic curves.
We evaluated breast MRI examinations performed between 2020 and 2021 retrospectively and included lesions with type 2 dynamic curves. We included 38 lesions from 33 patients. The lesions were evaluated for their pathological diagnosis and morphological characteristics.
Twenty-six lesions were malignant, while twelve were benign. The most frequently encountered benign lesion (7/12, 58.3%) was sclerosing adenosis, while the most frequently encountered malignant diagnosis was invasive ductal cancer. The presence of a type 2 dynamic curve had a sensitivity of 40.2% and specificity of 73.4% for predicting malignancy. By combining type 2 curves and morphological features, the sensitivity and specificity were increased.
The high rates of malignancy detected histopathologically among patients with type 2 dynamic curves in our study are remarkable. Type 2 dynamic curves can be detected in benign breast masses, especially in sclerosing adenosis cases. Considering morphological features can increase the diagnostic accuracy in cases with type 2 dynamic curves.
Core Tip: Dynamic contrast-enhanced magnetic resonance imaging (MRI) plays a critical role in the evaluation of breast lesions. The sensitivity and specificity of dynamic curves acquired using MRI are variable. While type 1 curves indicate more benign pathologies and type 3 curves indicate more malignant pathologies, there is a significant overlap in type 2 dynamic curves. We examined the histopathological outcomes of lesions with type 2 curves retrospectively. The histopathology results of lesions with type 2 curves were malignant at a rate of 68.4%. The presence of a type 2 dynamic curve had a sensitivity of 40.2% and specificity of 73.4% for predicting malignancy. By combining type 2 curves and morphological features, the area under the curve, sensitivity, and specificity were increased.
- Citation: Karavas E, Ece B, Aydın S. Type 2 dynamic curves: A diagnostic dilemma. World J Radiol 2022; 14(7): 229-237
- URL: https://www.wjgnet.com/1949-8470/full/v14/i7/229.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i7.229
Breast magnetic resonance imaging (MRI) is a noninvasive technique that is highly sensitive for detecting breast cancer. Breast MRI can be used in situations where mammography is insufficient, in patients with dense breast structure, for preoperative planning in breast cancer, in multifocal and multicentric cases, for detecting contralateral malignancy, for evaluating response to neoadjuvant chemotherapy, and for postoperative control[1-5]. Breast MRI provides morphological information about lesions as well as kinetic features such as perfusion and enhancement of the lesion. Additionally, breast MR imaging is less affected by dense breast tissue than other imaging modalities, allowing for a higher sensitivity in detecting lesions[6-10].
The most frequently used MRI technique for evaluating breast cancer is dynamic contrast-enhanced magnetic resonance imaging (DCE-MR). A low molecular weight contrast agent (gadolinium) is injected intravenously for DCE-MR imaging. Gadolinium uptake and washout, and thus the detection of signal changes on T1-weighted images and the differentiation of cancerous from normal breast tissue, are the foundations of DCE-MR imaging of breast cancer[11].
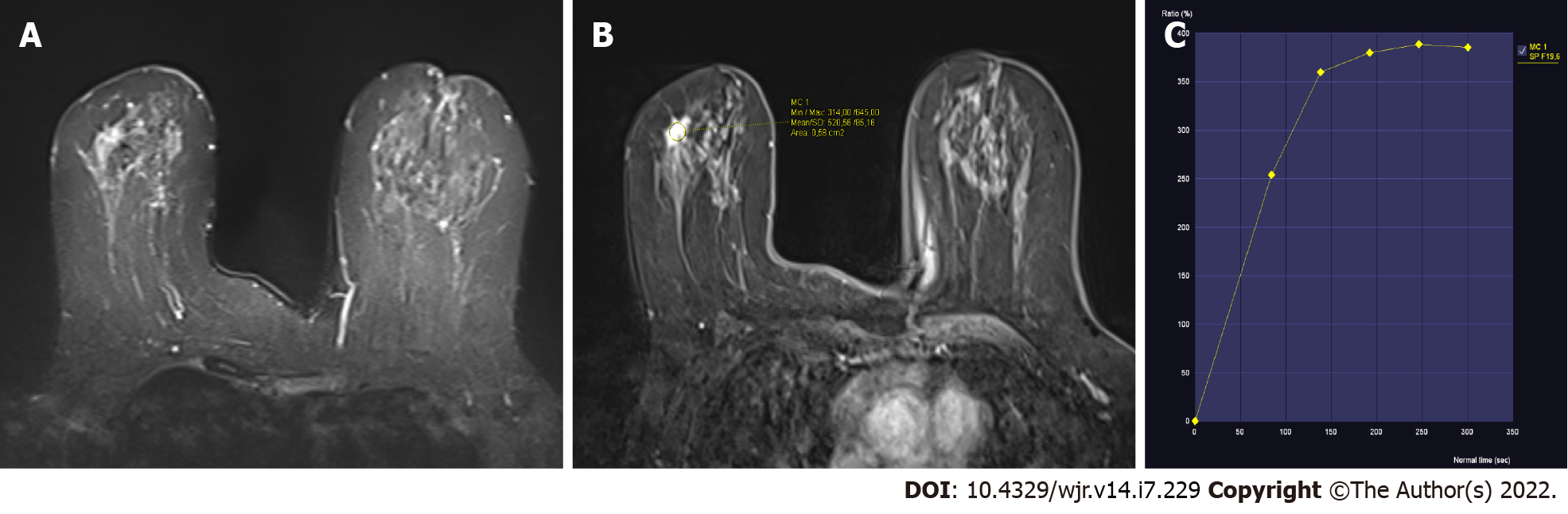
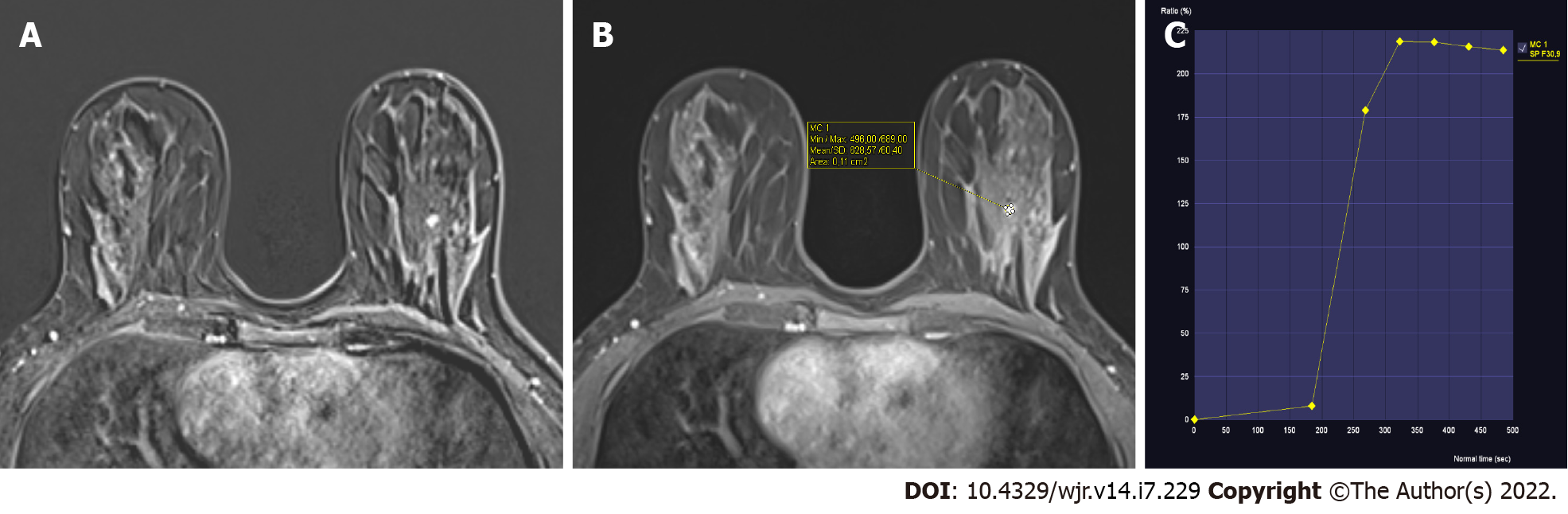
The enhancement properties are determined by examining changes in signal intensity across multiple images acquired by pre- and post-contrast repeat MRI scans. The time-signal intensity curve, also known as the kinetic curve, can be classified into three types: Type 1 (persistence), type 2 (plateau), and type 3 (washout). Dynamic curve of type 1 (persistent) exhibits a persistent increase in signal intensity following contrast agent injection. Dynamic curve of type 2 (plateau) exhibits an initial slow or rapid increase followed by a flattening. Dynamic curve of type 3 (washout) involves an initial increase and subsequent decrease in signal intensity. Between benign and malignant lesions, there is considerable overlap in dynamic curves. Various noninvasive cancers may lack washout or plateau kinetics, but various benign entities, such as fibroadenomas, fibrocystic changes, scars, sclerosing adenosis, lobular carcinoma in situ, focal fibrosis, and atypical ductal hyperplasia, may show malignant curves[9,12,13]. Therefore, dynamic curves should not be evaluated alone without considering lesion morphology.
The aim of this study was to examine the histopathological outcomes of lesions with type 2 dynamic curves, in which there is a high degree of overlap between benign and malignant entities in the kinetic analysis performed using dynamic contrast MR imaging.
Between January 2020 and January 2021, dynamic contrast enhanced breast MRI scans were evaluated retrospectively. In the research conducted from the hospital information system, there were 560 patients who underwent dynamic contrast MR examinations between January 2020 and January 2021. In the results of these patients, type 2 dynamic curve was detected in 48 lesions of 41 patients. Ten lesions in eight patients were excluded from the study due to a history of radiotherapy within the previous 6 mo, previous surgery or tru-cut biopsy, lack of histopathological results, and imaging artifacts. As a result, 38 lesions in 33 patients were included in the study.
Dynamic contrast enhanced breast MR images of the lesions included in the study were reviewed retrospectively with a consensus formed by two radiology specialists. The evaluators had more than 8 years of experience in interpreting breast MRI images. The patients' anamnesis, previous mammo
The data used for this study were collected anonymously and local ethics committee approval was obtained for this study (ethics committee number: 34336249-604.01.02-E.30236). This study adhered to the Declaration of Helsinki. Because the study was retrospective, informed consent was not obtained.
The same device and protocol were used for dynamic contrast-enhanced breast MRI examinations. One 1.5-T whole-body MRI scanner was used for breast MRI with dynamic contrast (Magnetom Avanto; Siemens Healthineers). The vendor-supplied receive-only 4-channel circularly polarized breast array coil was used. A standard protocol includes a T2-weighted rapid (fast or turbo) spin-echo (TR: 4000 ms; TE: 90 ms; ≤ 4 mm thickness) acquisition and 3D T1-weighted GRE (20/4.5; flip angle, 30°–45°; ≤ 3 mm thickness) acquisitions before and after the administration of gadolinium, with the usual dose of 0.1 mmol/kg injected as a bolus and followed by a 10–20-mL saline flush. For sagittal plane, an image matrix of 256 × 192 can be used with zero-filled interpolation to 512 × 512, a small field of view (16–18 cm), and chemical fat suppression. For bilateral axial imaging, the field of view is increased to approximately 30 cm, and high-resolution matrices (between 256 and 512) are used.
The sample size was calculated using G power analysis (alpha error: 0.05; power: 80%); the minimum number of patients was thus defined as 31. The Statistical Package for Social Sciences (SPSS) for Windows 20 software was used to analyze the data (IBM SPSS Inc., Chicago, IL, United States). The Kolmogorov-Smirnov test was used to determine whether the age data conformed to a normal distribution. Age is represented as the mean ± SD and categorical variables as number (n) and percentage values (%). To define the diagnostic efficacy of type 2 dynamic curves alone and along with morphological characteristics, receiver operating characteristic analysis was used. The chi-square test was used to compare two groups of categorical variables. Statistical significance was defined as a two-tailed value of P < 0.050.
A total of 38 lesions in 33 patients were included in the study. The mean age of the patients was 53.7 ± 10.1 years (range, 43-87 years).
The 38 lesions included in the study showed a dynamic contrast enhancement curve of type 2 (plateau) on their dynamic contrast imaging (Figures 1 and 2). The histopathological diagnoses of these lesions are shown in Table 1. As a result, 12 lesions were determined to be benign, while 26 lesions were determined to be malignant. While sclerosing adenosis was the most frequently encountered benign pathology, invasive ductal carcinoma was the most frequently encountered malignant pathology (Table 1).
| Histopathological diagnosis | n (%) | |
| Benign 12/38 (31.6%) | Sclerosing adenosis | 7 (18.4) |
| Fibroadenoma | 3 (7.9) | |
| Intraductal papilloma | 1 (2.6) | |
| Usual ductal hyperplasia | 1 (2.6) | |
| Malignant 26/38 (68.4%) | Ductal carcinoma in situ | 7 (18.4) |
| Invasive ductal carcinoma | 15 (39.5) | |
| Invasive lobular carcinoma | 4 (10.5) | |
| Total | 38 (100) |
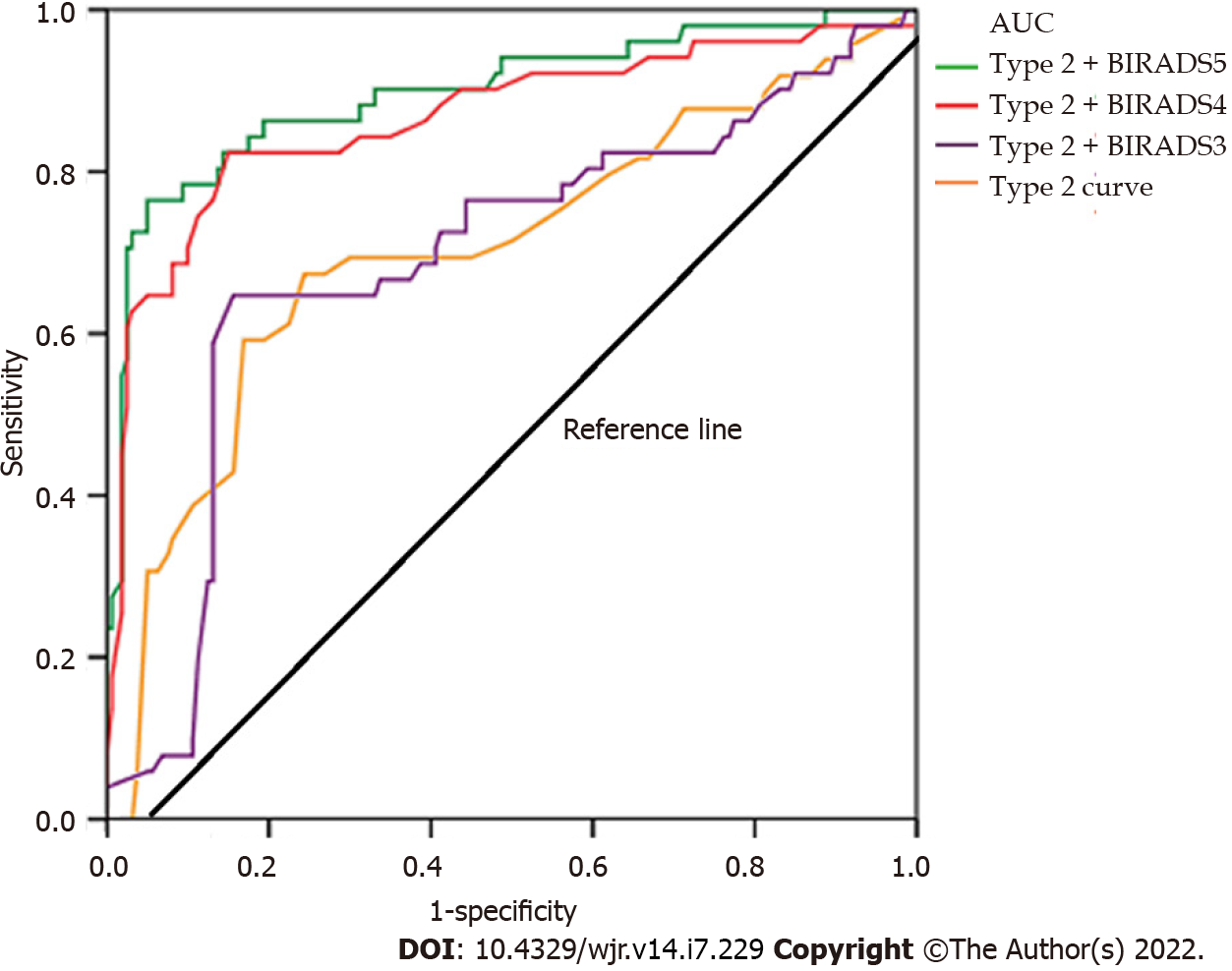
The morphological evaluation results obtained using the ACR BI-RADS classification system for the lesions are given in Table 2. Histopathological examinations of eight lesions classified as BI-RADS 3 revealed that the vast majority (5 of them) were sclerosing adenosis. One lesion in the BI-RADS 3 category was histopathologically diagnosed as invasive ductal carcinoma. The remaining two lesions were benign. Sixteen of the 18 BI-RADS 4 lesions with type 2 dynamic curve were malignant, while two were benign. All 12 lesions classified as BI-RADS 5 were found to be malignant on histopathology (Table 2).
| BI-RADS category | n (%) |
| BI-RADS-0 | 0 |
| BI-RADS-1 | 0 |
| BI-RADS-2 | 0 |
| BI-RADS-3 | 8 (21) |
| BI-RADS-4 | 18 (47.4) |
| BI-RADS-5 | 12 (31.6) |
| Total | 38 (100) |
The presence of a type 2 dynamic curve had a sensitivity of 40.2% and specificity of 73.4% for predicting malignancy. By combining type 2 curves and morphological features, the area under the curve, sensitivity, and specificity were increased (Table 3, Figure 3).
| Sensitivity | Specificity | |
| Type 2 dynamic curve | 40.2 | 73.4 |
| Type 2 dynamic curve + BI-RADS 3 category | 41.9 | 75.8 |
| Type 2 dynamic curve + BI-RADS 4 category | 95.3 | 97.7 |
| Type 2 dynamic curve + BI-RADS 5 category | 100 | 100 |
In our study, we investigated the histopathological results of type 2 dynamic curves obtained from dynamic contrast magnetic resonance imaging, which plays a critical role in the evaluation of breast lesions. We found that the type 2 dynamic curve had a sensitivity of 40.2% and specificity of 73.4% in predicting malignancy. Additionally, we found that combining type 2 dynamic curve with morphological findings increased the sensitivity and specificity.
The type 1 (persistent) dynamic curve obtained from breast MRI with dynamic contrast indicates a higher rate of benign pathologies, while the type 3 (washout) dynamic curve indicates a higher rate of malignant pathologies according to the literature[9,11]. However, it has been reported in the literature that time-signal intensity curves have a high sensitivity but relatively low specificity for breast cancer diagnosis[15-20]. It is critical to keep in mind when evaluating these kinetic images that there is considerable overlap between benign and malignant lesions[9,12,13,21]. Schnall et al[20] reported in a multicenter study of evaluating 995 breast lesions that a lesion with type 3 curve has a five times higher relative risk of cancer than a lesion with type 1 curve. According to the same study, 76% of lesions with type 3 curves were associated with malignancy. In other studies in the literature, a significant correlation was reported between malignancy and type 3 washout dynamic curve[15,16,22,23]. Durhan et al[24] in their study on young women under 40 years of age, stated that 25 of 27 malignant lesions had type 2 and type 3 dynamic curves. In contrary to the majority in the literature, Williams et al[25] found no significant difference between dynamic curves and benign and malignant lesions in their study with 41 malignant and 113 benign lesions. They stated that the reason for lack of significant difference may be due to the lower temporal resolution in their study and the different MR imaging protocols compared to other studies. Macura et al[26] stated in support of this in the literature that due to the low temporal resolution, washout may not be visible in the signal intensity-time curve because the first contrast images are acquired too late after peak formation. When these data in the literature are reviewed, it is understood that the results of dynamic curves, especially type 2 dynamic curves, may be contradictory. For this reason, we conducted a study investigating the histopathological results of type 2 curves. Our findings show that type 2 dynamic curve can be an important finding in demonstrating malignancy and supports other data in the literature in contrary to the study of Williams et al[25].
In our study, we evaluated lesions with a type 2 (plateau) dynamic curve and discovered that approximately 68% of these lesions were malignant. According to the literature, Kuhl et al[15] in their study including 101 malignant and 165 benign cases, found that the type 1 dynamic curve was observed in 83% of benign lesions and 9% of malignant lesions, type 2 dynamic curve was observed in 13% of benign lesions and 34% of malignant lesions, and type 3 dynamic curve was observed in 6% of benign lesions and 57% of malignant lesions. Bluemke et al[16] in their study including 404 malignant and 366 benign cases, found that the type 3 dynamic curve had a 20.5% sensitivity and 90.4% specificity, type 2 dynamic curve had a 42.6% sensitivity and 75% specificity, and type 1 dynamic curve had a 52.2% sensitivity and 71% specificity for detecting benignity. According to these results[15,16], our study's malignancy rate was higher than those reported in the literature, but the sensitivity and specificity rates were similar. As supported by our findings and the literature, the type 2 dynamic curve indicates an increased risk of malignant lesions. These results led us to believe that in the presence of a type 2 dynamic curve, we should exercise caution in terms of suspicion of malignancy.
Numerous studies have demonstrated that combining use of both morphological and enhancement kinetics improves the sensitivity and specificity of MRI[27-29]. Lee et al[21] stated in their study that as a reasonable strategy, the morphological features of the lesion should be evaluated before evaluating the enhancement kinetics, and in case of suspicious morphological features, further evaluation including histopathological diagnosis should be made. They also stated that if the lesion is morphologically benign or indeterminate, evaluation of the enhancement kinetics can help differentiate lesions that may require biopsy. Additionally, it should be emphasized at this point that the MR examination should be correlated with mammographic and sonographic findings to increase the accuracy of the result. According to these data, we also examined the sensitivity and specificity of type 2 dynamic curves for detecting malignancy both alone and in combination with the BI-RADS classification. Accordingly, while the sensitivity and specificity values of the type 2 dynamic curve increased significantly when combined with BI-RADS 4 and 5, no significant increase was observed when combined with BI-RADS 3. According to the ACR, radiological follow-up is recommended instead of histopathological correlation in BI-RADS 3 lesions[14]. In our study, histopathological correlation was performed on eight lesions with type 2 dynamic curves in BI-RADS 3 lesions, and one of them was found to be malignant. At this point, although there is a possibility of unnecessary histopathological correlation to BI-RADS 3 lesions, the fact that even one malignancy was detected in our results suggests that histopathological correlation may provide additional benefit in the presence of type 2 dynamic curve in BI-RADS 3 lesions. There is a need for studies examining the type 2 dynamic curve and BI-RADS 3 classification in a larger patient population.
There are some limitations to our study. The most significant limitations are the study's retrospective design and small patient population. Additionally, when the ROI method is used to create a dynamic curve, inter-rater variability may occur. In our study, two radiologists evaluated the images retrospectively with a consensus and there was no assessment of interobserver variability. Finally, the study was designed to assess only the type 2 dynamic curve in contrast-enhanced dynamic series; lesions with type 1 or type 3 dynamic curves were not included and early phase (initial) enhancement was not assessed.
In conclusion, the high rates of malignancy detected histopathologically among patients with type 2 curves in our study are remarkable. However, it is possible to detect type 2 dynamic curves in benign lesions as well. Compared to the evaluation made with only the type 2 dynamic curve, the combined evaluation with the BI-RADS categories increases the sensitivity and specificity of the type 2 dynamic curve.
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most frequently used MRI technique for evaluating breast cancer. Changes in signal intensity across multiple images acquired by pre- and post-contrast repeat MRI scans are used to determine the enhancement patterns. The time-signal intensity curve, also known as the kinetic curve, can be classified into three types: Type 1 (persistence), type 2 (plateau), and type 3 (washout). A higher rate of benign pathologies is indicated by the type 1 dynamic curve, while a higher rate of malignant pathologies is indicated by the type 3 dynamic curve. However, there is a dilemma with the type 2 curve. The aim of this study was to investigate the histopathological outcomes of lesions with type 2 dynamic curves, which have much overlap in the kinetic analysis between benign and malignant entities.
There have been several studies on type 3 and type 1 dynamic curves, but studies on type 2 dynamic curves are not sufficient. More research on type 2 dynamic curves, which have much overlap in kinetic analysis between benign and malignant entities, is needed.
The aim of this study was to examine the histopathological outcomes of lesions with type 2 dynamic curves, in which there is a high degree of overlap between benign and malignant entities in the kinetic analysis performed using dynamic contrast MRI.
Two experienced radiologists retrospectively re-evaluated lesions with type 2 dynamic curves. The included lesions were re-examined for type 2 dynamic curves by the evaluators. Additionally, the evaluators classified the lesions according to their morphological characteristics using the American College of Radiology's Breast Imaging Reporting and Data System classification. The histopathological findings of the patients were retrieved and recorded retrospectively from the hospital information system. Receiver operating characteristic analysis was done to determine the diagnostic efficacy of type 2 dynamic curves alone and in combination with morphological characteristics.
Thirty-eight lesions in 33 patients were included in the study. As a result, 12 lesions were determined to be benign, while 26 lesions were determined to be malignant. While sclerosing adenosis was the most frequently encountered benign pathology, invasive ductal carcinoma was the most frequently encountered malignant pathology. The presence of a type 2 dynamic curve had a sensitivity of 40.2% and specificity of 73.4% for predicting malignancy. By combining type 2 curves and morphological features, the area under the curve, sensitivity, and specificity were increased.
In our investigation, the significant rates of malignancy discovered histopathologically among patients with type 2 curves are remarkable. In the presence of a type 2 dynamic curve, we should exercise caution in terms of suspicion of malignancy.
Studies with larger patient populations focusing on the histopathological results of lesions with type 2 dynamic curves are needed.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chrcanovic BR, Sweden; Ma C, China S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 397] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Bedrosian I, Mick R, Orel SG, Schnall M, Reynolds C, Spitz FR, Callans LS, Buzby GP, Rosato EF, Fraker DL, Czerniecki BJ. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003;98:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999;213:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 475] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 4. | Liberman L, Morris EA, Kim CM, Kaplan JB, Abramson AF, Menell JH, Van Zee KJ, Dershaw DD. MR imaging findings in the contralateral breast of women with recently diagnosed breast cancer. AJR Am J Roentgenol. 2003;180:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Lehman CD, Gatsonis C, Kuhl CK, Hendrick RE, Pisano ED, Hanna L, Peacock S, Smazal SF, Maki DD, Julian TB, DePeri ER, Bluemke DA, Schnall MD; ACRIN Trial 6667 Investigators Group. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356:1295-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 644] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 6. | DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top Magn Reson Imaging. 2008;19:143-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Kriege M, Brekelmans CT, Boetes C, Besnard PE, Zonderland HM, Obdeijn IM, Manoliu RA, Kok T, Peterse H, Tilanus-Linthorst MM, Muller SH, Meijer S, Oosterwijk JC, Beex LV, Tollenaar RA, de Koning HJ, Rutgers EJ, Klijn JG; Magnetic Resonance Imaging Screening Study Group. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004;351:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1125] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 8. | Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220:13-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 521] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 508] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Boetes C, Veltman J. Screening women at increased risk with MRI. Cancer Imaging. 2005;5 Spec No A:S10-S15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Moon M, Cornfeld D, Weinreb J. Dynamic contrast-enhanced breast MR imaging. Magn Reson Imaging Clin N Am. 2009;17:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Rausch DR, Hendrick RE. How to optimize clinical breast MR imaging practices and techniques on Your 1.5-T system. Radiographics. 2006;26:1469-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Jansen SA, Fan X, Karczmar GS, Abe H, Schmidt RA, Newstead GM. Differentiation between benign and malignant breast lesions detected by bilateral dynamic contrast-enhanced MRI: a sensitivity and specificity study. Magn Reson Med. 2008;59:747-754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | D'Orsi C, Morris E, Mendelson E. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. American College of Radiology; 2013. Available from: https://www.scienceopen.com/document?vid=ddb508dc-e150-4b1c-aad8-1be1c031edd8. |
| 15. | Kuhl CK, Mielcareck P, Klaschik S, Leutner C, Wardelmann E, Gieseke J, Schild HH. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 934] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 16. | Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, Heywang-Köbrunner SH, Hylton N, Kuhl CK, Lehman C, Pisano ED, Causer P, Schnitt SJ, Smazal SF, Stelling CB, Weatherall PT, Schnall MD. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292:2735-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 360] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Kinkel K, Helbich TH, Esserman LJ, Barclay J, Schwerin EH, Sickles EA, Hylton NM. Dynamic high-spatial-resolution MR imaging of suspicious breast lesions: diagnostic criteria and interobserver variability. AJR Am J Roentgenol. 2000;175:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | El Khouli RH, Macura KJ, Jacobs MA, Khalil TH, Kamel IR, Dwyer A, Bluemke DA. Dynamic contrast-enhanced MRI of the breast: quantitative method for kinetic curve type assessment. AJR Am J Roentgenol. 2009;193:W295-W300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Baltzer PA, Benndorf M, Dietzel M, Gajda M, Runnebaum IB, Kaiser WA. False-positive findings at contrast-enhanced breast MRI: a BI-RADS descriptor study. AJR Am J Roentgenol. 2010;194:1658-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Schnall MD, Blume J, Bluemke DA, DeAngelis GA, DeBruhl N, Harms S, Heywang-Köbrunner SH, Hylton N, Kuhl CK, Pisano ED, Causer P, Schnitt SJ, Thickman D, Stelling CB, Weatherall PT, Lehman C, Gatsonis CA. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006;238:42-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 21. | Lee CH. Problem solving MR imaging of the breast. Radiol Clin North Am. 2004;42:919-934, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Chen W, Giger ML, Lan L, Bick U. Computerized interpretation of breast MRI: investigation of enhancement-variance dynamics. Med Phys. 2004;31:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Gilhuijs KG, Deurloo EE, Muller SH, Peterse JL, Schultze Kool LJ. Breast MR imaging in women at increased lifetime risk of breast cancer: clinical system for computerized assessment of breast lesions initial results. Radiology. 2002;225:907-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Durhan G, Azizova A, Önder Ö, Kösemehmetoğlu K, Karakaya J, Akpınar MG, Demirkazık F, Üner A. Imaging Findings and Clinicopathological Correlation of Breast Cancer in Women under 40 Years Old. Eur J Breast Health. 2019;15:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Williams TC, DeMartini WB, Partridge SC, Peacock S, Lehman CD. Breast MR imaging: computer-aided evaluation program for discriminating benign from malignant lesions. Radiology. 2007;244:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Macura KJ, Ouwerkerk R, Jacobs MA, Bluemke DA. Patterns of enhancement on breast MR images: interpretation and imaging pitfalls. Radiographics. 2006;26:1719-34; quiz 1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Liu PF, Debatin JF, Caduff RF, Kacl G, Garzoli E, Krestin GP. Improved diagnostic accuracy in dynamic contrast enhanced MRI of the breast by combined quantitative and qualitative analysis. Br J Radiol. 1998;71:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Schnall MD, Rosten S, Englander S, Orel SG, Nunes LW. A combined architectural and kinetic interpretation model for breast MR images. Acad Radiol. 2001;8:591-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Szabó BK, Aspelin P, Wiberg MK, Boné B. Dynamic MR imaging of the breast. Analysis of kinetic and morphologic diagnostic criteria. Acta Radiol. 2003;44:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |