Published online Jul 28, 2022. doi: 10.4329/wjr.v14.i7.209
Peer-review started: March 28, 2022
First decision: May 12, 2022
Revised: June 9, 2022
Accepted: July 18, 2022
Article in press: July 18, 2022
Published online: July 28, 2022
Processing time: 120 Days and 19.6 Hours
Mucormycosis is caused by the fungi belonging to the order Mucorales and class Zygomycetes. The incidence of mucormycosis has increased with the onset of the severe acute respiratory syndrome coronavirus 2 infections leading to the coronavirus disease 2019 (COVID-19) pandemic. This rise is attributed to the use of immunosuppressive medication to treat COVID-19 infections. Authors have retrospectively collected data of our cases of mucormycosis diagnosed from April 2020 to April 2021 at our institute. A total of 20 patients with rhinocerebral mucormycosis were studied. Most of the study subjects were male patients (90%) and were of the age group 41-50 years. Most patients in the review had comorbidities (85%) with diabetes being the most common comorbidity. Para nasal sinuses were involved in all the cases. Involvement of the neck spaces was present in 60% of the cases. Involvement of the central nervous system was present in 80% of the cases. Orbital involvement was present in 90% of the cases. The authors reviewed the various imaging findings of mucormycosis on computed tomography and magnetic resonance imaging in this article.
Core Tip: Rhinocerebral mucormycosis constituted the aftermath of the coronavirus disease 2019 pandemic, leading to rapid increase in the number of cases, which were previously restricted only to few susceptible groups of patients. Rhinocerebral mucormycosis is associated with high mortality and morbidity. After clinical examination, imaging is the backbone for the diagnosis of this severe disease. Computed tomography helps in the preliminary diagnosis and helps to stage the disease. However, when orbital and intracranial extension is present, magnetic resonance imaging (MRI) is preferred because it delineates the involvement of these structures better. MRI can also delineate vascular involvement better. This article reviews the various imaging findings of mucormycosis.
- Citation: Saneesh PS, Morampudi SC, Yelamanchi R. Radiological review of rhinocerebral mucormycosis cases during the COVID-19 Pandemic: A single-center experience. World J Radiol 2022; 14(7): 209-218
- URL: https://www.wjgnet.com/1949-8470/full/v14/i7/209.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i7.209
Mucormycosis is caused by fungi belonging to the order Mucorales and class Zygomycetes[1]. Fungal spores that are present in the air constitute a source of infection. However, these fungi rarely infect healthy individuals, as normal host defense mechanisms prevent invasion by these organisms. However, when host defense mechanisms are weakened due to several factors, such as congenital disorders, acquired immunodeficiency syndrome, hematological malignancies, uncontrolled systemic illnesses, and the use of immunosuppressive medication, these organisms invade and proliferate in human tissues. The incidence of mucormycosis has increased over the past few years due to the aging population, medical comorbidities, the increase in the incidence of malignancies, and the pandemic of human immunodeficiency virus infection[2].
The situation has worsened with the onset of the severe acute respiratory syndrome coronavirus 2 infections, which has led to the coronavirus disease 2019 (COVID-19) pandemic that began in late 2019 and has continued till the present. The main pathogenesis of the complications of this viral infection is due to the excessive immunological response that leads to damage to host’s own tissues[3]. This has resulted in the use of immunosuppressive medication in the form of corticosteroids, interleukin antagonists, and various antibodies to counter the inflammatory cytokines. These drugs have proved to be efficacious in dealing with the complications and cytokine storm of COVID-19. However, they come with serious side effects of immunosuppression.
The number of cases of mucormycosis has rapidly increased in the last few months. This rise is attributed to the use of immunosuppressive medication to treat COVID-19 infections[4]. The paranasal sinuses and lungs, being the first spaces to come in contact with the fungus, are most commonly affected. Once the disease is established, it spreads to the surrounding structures, such as the orbit, brain, mediastinum, etc. The overall mortality of mucormycosis is more than 50%, and the mortality rate for disseminated disease reaches 100%[5]. The infection responds to only a few antifungals, such as amphotericin B, which are very toxic[5].
The authors of the present study have also come across many cases of mucormycosis in the last few months during the times of the COVID-19 pandemic. The following is a mini-review of the radiological findings of the rhinocerebral mucormycosis cases recorded by us in the last few months.
We have retrospectively collected data on cases of mucormycosis diagnosed from April 2020 to April 2021 at our institute, which is a tertiary care center located in the state of Kerala, India.
All adult patients above the age of 18 years who were diagnosed with mucormycosis by imaging post–COVID-19 infection and confirmed by histopathological examination were studied. Patients with an unknown medical history, absent hospital records, and unknown outcomes were excluded from the review.
Hospital databases in the radiology department of our hospital were searched with the keyword “mucormycosis,” and results were obtained. A list of cases was obtained, which was filtered to include only those cases from April 2020 to April 2021. Hospital identification numbers were then used to trace the clinical details and outcomes of the patients. The demographic details of the patients were recorded, and the COVID-19 infection history and treatment history were noted. History of various comorbidities, including malignancies, was obtained from the hospital records. The findings of the CECT scans,which were obtained using a 128-slice dual-energy CT scanner (SOMATOM Definition Flash, Siemens, Germany), were reviewed by the same radiologist to ensure uniformity in reporting. Images were acquired with 1–3 mm collimation and a pitch of up to 2:1 to allow for coverage of the area of interest in a single breath-hold.
Imaging was repeated at one-month follow-up to study the lesions.
A total of 20 patients with rhinocerebral mucormycosis were studied. Eighteen patients had isolated rhino-cerebral mucormycosis, and two patients had combined pulmonary and rhino-cerebral mucormycosis (Table 1). Most study subjects were male patients (90%). The age distribution of the subjects is as follows: 10% were between 20–30 years of age, 20% were between 31–40 years of age, 30% were between 41–50 years of age, 5% were between 51–60 years of age,20% were between 61–70 years of age, and 15% were between 71–80 years of age. Most patients in the review had comorbidities (85%): 20% had hematological malignancy, 40% had diabetes, 10% had acquired immunodeficiency syndrome, and 15% were transplant recipients on immunosuppressive medication.
| Serial No. | Age | Sex | Co-morbidities | Primary location | Cerebral involvement | Orbital involvement | Involvement of neck spaces |
| 1 | 34 | Male | Acute myeloid leukemia | Right nasal cavity, Right ethmoid, Sphenoid | ICA thrombosis, Cavernous sinus thrombosis, Leptomeningeal enhancement | No | Right pterygopalatine, Right infra temporal, Bilateral parapharangeal, Retropharyngeal space |
| 2 | 64 | Male | Renal transplant | Pan sinusitis | > Meningoencephalitis, > infiltration into left basifrontal and gangliocapsular region along olfactory fossa | Extra ocular muscles, Orbital cellulitis | No |
| 3 | 72 | Male | Diabetes | Left maxillary, Ethmoid, Frontal, Sphenoid | Ganglio-capsular infarctcavernous sinus thrombosis | Retro- orbitalfat and extra ocular muscles and orbital apex | No |
| 4 | 50 | Male | No | Right maxillary | Cavernous sinus, Subdural abscess | Orbital apex, Orbital abscess | Temporal, pterygopalatine, masticator space |
| 5 | 40 | Male | Post renal transplant | Pan sinus | Cavernous sinus thrombosis, Temporal lobe involvement | Extra conal extension, Right extra ocular muscles, Orbital floor | Infratemporal fossa, pterygopalatine |
| 6 | 45 | Male | Acquired immunodeficiency syndrome | Frontal | ICA thrombosis cavernous sinus thrombosis, Cerebral infarct epidural abscess | Left orbit, Extra ocular muscles | Left pterygopalatine |
| 7 | 67F | Female | Diabetes | Pan-sinus | Basifrontal brain parenchyma involvement, meningoencephalitis | Orbital apex, extra ocular muscles Subperiosteal abscess | Right infra temporal fossa, retropharyngeal space |
| 8 | 75 | Male | Diabetes | Pan-sinusitis | No cerebral involvement | Extraconal fat involvement | No |
| 9 | 22 | Male | ALL | Right maxillary sinus | ICA thrombosis, Cavernous sinus involvement, Cerebral infarct | Extra ocular muscle, Orbital apex, Subperiosteal abscess | Right Infratemporal fossa, Right pterygopalatine fossa |
| 10 | 50 | Male | Diabetes | Left maxillary sinus | No cerebral involvement | Left extraconal space | No |
| 11 | 50 | Male | Acquired immunodeficiency syndrome | Pan sinusitis | Cavernous sinus, Epidural abscess | Orbital Apex, Extra ocular muscles involvement, Subperiosteal abscess | Pterygopalatine fossa |
| 12 | 40 | Male | Kidney transplant | Frontal, Ethmoid, sphenoid | ICA thrombosis, Temporal lobe abscess | Orbital apex, Extra ocular muscles, Subperiosteal abscess, Cellulitis | No |
| 13 | 21 | Female | Acute lymphocytic leukemia | Right maxillary sinus and nasal cavity | ICA thrombosis, Frontal lobe abscess, Infarct | Extra ocular muscles, Intraconal fat | Pterygopalatine fossa and infratemporal fossa |
| 14 | 64 | Male | Diabetes | Pan sinusitis | Cavernous sinus, Subdural abscess | Orbital apex, Extraocular muscles, Subperiosteal abscess | No |
| 15 | 48 | Male | No | Pan sinusitis | Cavernous sinus, ICA thrombosis | Orbital apex, Extra ocular muscles, Orbital abscess | Pterygopalatine fossa, Infra temporal fossa |
| 16 | 68 | Male | Diabetes | Pan sinusitis | Not involved | Extraconal fat | Pterygopalatine fossa |
| 17 | 57 | Male | Diabetes | Pan sinusitis | Subdural abscess | Extraconal orbital fat, Extra ocular muscles, Orbital cellulitis | Infratemporal fossa |
| 18 | 71 | Male | Diabetes | Sphenoid, Ethmoid, Nasal cavity | Frontal lobe | Orbital apex, Extra ocular muscles | Para pharyngeal space, Pterygopalatine fossa |
| 19 | 48 | Male | No | Right pan sinusitis, Nasal cavity | Frontal lobe, Cavernous sinus | Extraconal fat, extra ocular muscles | No |
| 20 | 37 | Male | Lymphoma | Right sinusitis | Not involved | Extraconal orbital fat | No |
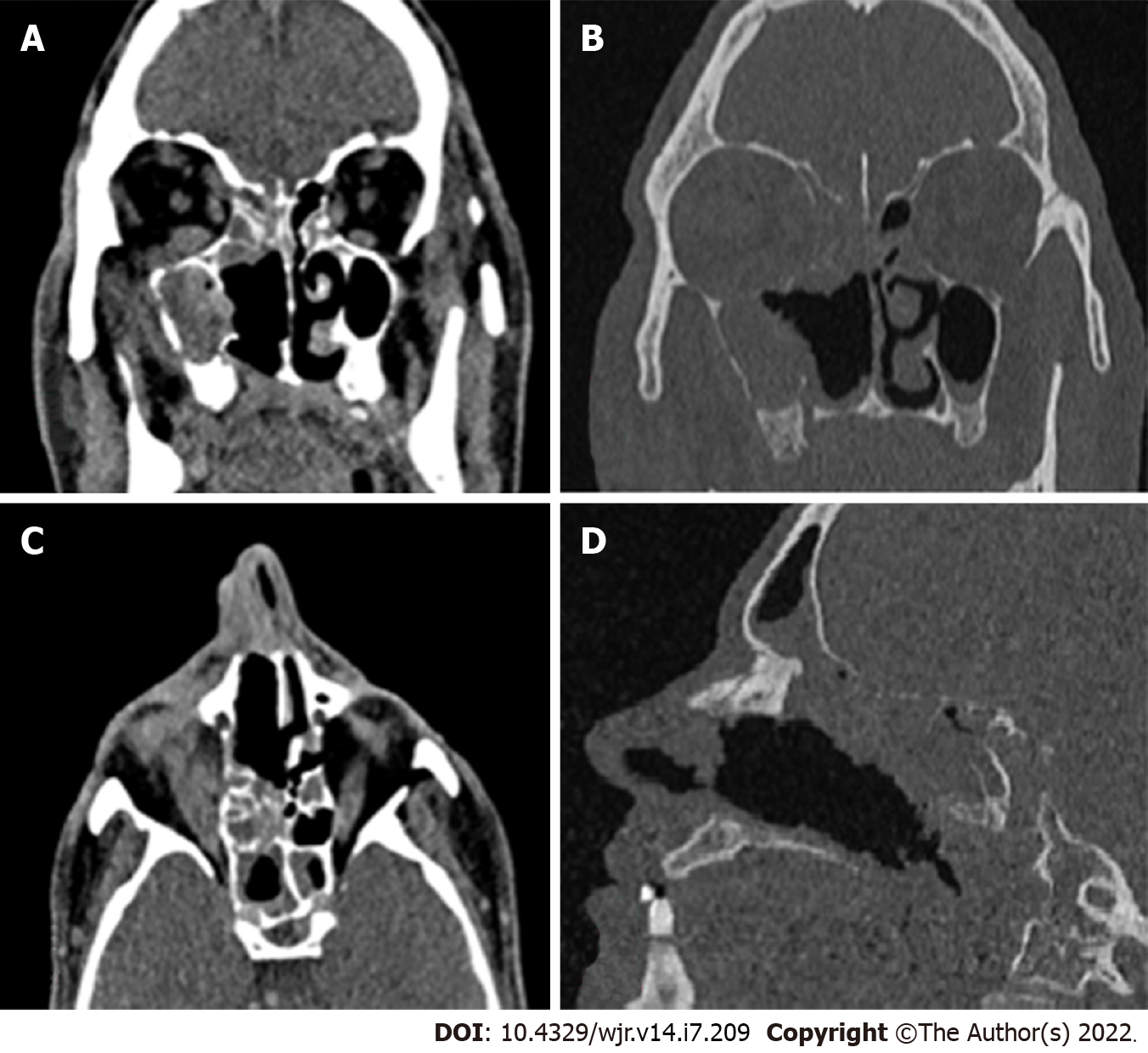
Sinuses were involved in all the cases. Unilateral involvement of the sinuses was more common than bilateral involvement. In most cases (35%), all four sinuses (maxillary, frontal, ethmoid, and sphenoid) were involved. The isolated maxillary sinus was involved in 20% of the cases. The isolated frontal sinus was involved in 5% of the cases. The ethmoid and sphenoid sinuses combined were involved in 10% of the cases. The frontal, ethmoid, and sphenoid sinuses combined were involved in 5% of the cases.
Involvement of the neck spaces was present in 60% of the cases (Table 2). The pterygopalatine space was involved in 50% of the cases. The infratemporal fossa was involved in 40% of the cases. The masticator space, retropharyngeal space, and parapharyngeal spaces were each involved in 10% of the cases.
| Complication/local invasion | N = 20 |
| Cerebral | 16/20 |
| Arterial involvement (ICA thrombosis) | 6 |
| Cavernous sinus thrombosis | 10 |
| Brain Parenchymal involvement | 11 |
| Meninges and Dural/epidural involvement | 7 |
| Orbit | 18/20 |
| Extraocular muscles | 14 |
| Orbital apex | 9 |
| Orbital fat only/orbital cellulitis/abscess | 16 |
| Neck spaces | 12/20 |
| Pterygopalatine foramen and fossa | 10 |
| Infratemporal fossa | 8 |
| Masticator space | 2 |
| Retropharyngeal space | 2 |
| Parapharyngeal space | 2 |
At one month follow-up, only 50% of the patients survived, 25% had progression of the lesions, 20% had improvement in the lesions, and 5% had static lesions.
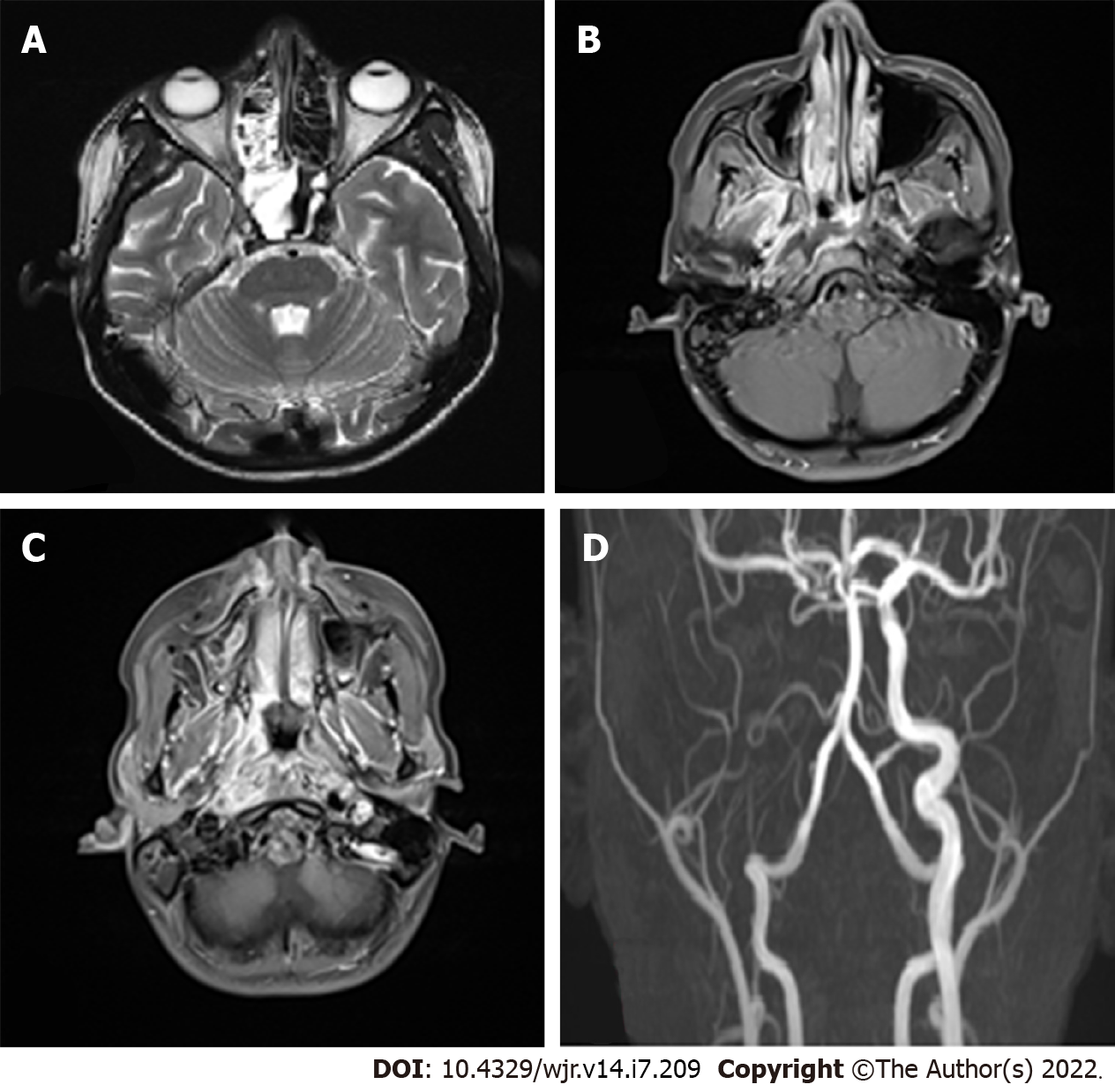
There were no cases of isolated cerebral mucormycosis. Involvement of the central nervous system (CNS) was present in 80% of the cases. The involvement of CNS included the following: leptomeningeal enhancement, meningoencephalitis, brain infarcts, brain abscesses, internal carotid artery thrombosis, cavernous sinus thrombosis, dural venous sinus thrombosis, and epidural abscesses.
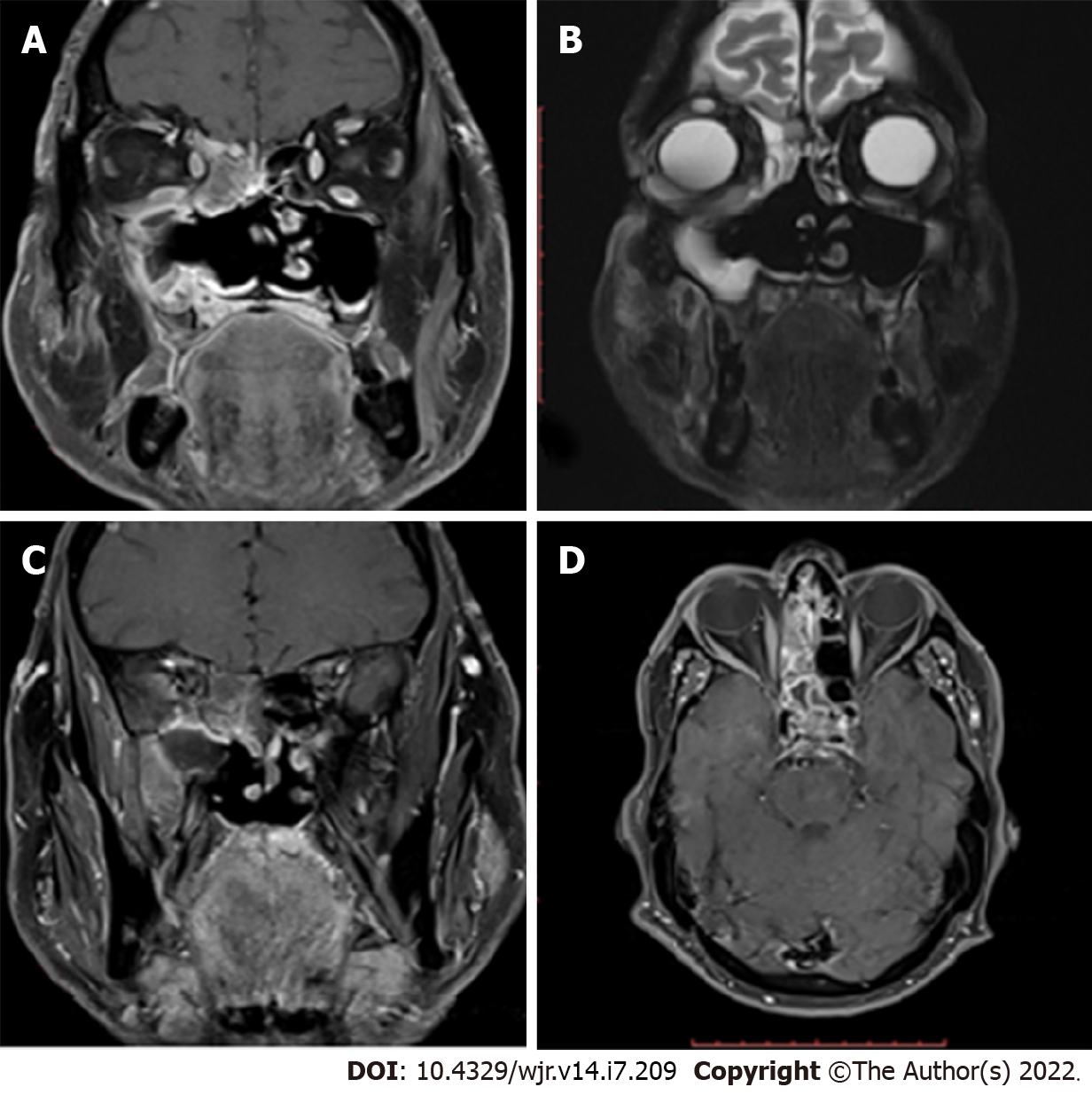
The involvement of various structures of the CNS is listed in Table 2. Vascular involvement was present in 60% of the patients in our study, with the most common lesion being cavernous sinus thrombosis. Orbital involvement, which included orbital fat involvement, extraocular muscle involvement, and orbital cellulitis, was present in 90% of the cases (Table 2).
Most cases of rhinocerebral mucormycosis occurred in males in the present review as in other previous case series and reviews[6,7]. Most patients had comorbidities, with diabetes being the predominant comorbidity as in other previous studies. In the study by Dubey et al[8], all post–COVID-19 patients diagnosed with rhinocerebral mucormycosis were diabetic. This also includes new-onset diabetes due to the usage of corticosteroids during the treatment of COVID-19, which is as high as 38.18%[8].
In the present study, similar to the study by Therakathu et al[9], unilateral involvement of the sinuses was more common. The ethmoid sinus was the most common sinus to be involved in the present study, followed by the maxillary sinus, as in the studies by Therakathu et al[9] and Patel et al[10]. In the study by Therakathu et al[9], the most common site to be involved other than the sinuses was the orbit (76%) and the face (57%), followed by the orbital apex, masticator space, pterygopalatine fossa, bone, skull base, cavernous sinus, brain, and internal carotid artery.Orbital involvement was also very common in the present study, accounting for 90% of the cases. However, in the study by Patel et al[10], orbital involvement was present in only 60% of the cases.
Infection by fungi of the order Mucorales begins in the nasal cavity mostly in the middle turbinate and starts spreading, mostly invading the sphenoethmoidal complex[11,12]. As the fungi have the ability to invade the blood vessels and the bony walls, they spread rapidly in immunocompromised hosts and those with chronic debilitating illnesses, to reach the sinus cavity[13]. The necrotic tissue formed due to vascular occlusion acts as a rich niche for the further growth of the organism. Further invasion of the orbits and brain occurs through the foramen and through the sphenopalatine and internal maxillary arteries[14].
The invasion in mucormycosis can be divided into three stages as per Rupa et al[15]:
Stage 1: The infection is localized to the nasal cavity and paranasal sinuses.
Stage 2: The infection begins to spread to the peri-sinus areas, which are completely resectable.
Stage 3: The infection spreads into the intracranial cavity and to the surrounding areas of the sinuses, which are partially resectable.
Computed tomography (CT) is usually the first investigation to be performed whenever invasive rhinocerebral mucormycosis is suspected based on clinical history and examination. The CT findings are nonspecific and include inflammatory changes in the sinuses. Early changes include mucosal thickening due to inflammation, bony erosions, and the formation of a mass lesion inside the sinuses, leading to the opacification of the sinuses. Hyperattenuation of the secretions on CT is suggestive of fungal sinusitis. The hyperdense areas seen in the sinuses are due to the presence of fungal hyphae and debris. Early changes suggestive of the spread of the infection outside the sinus include loss of normal fat density in the periantral fat (anterior, premaxillary, or retroantral) and orbit owing to edemafrom vascular congestion[16]. Superficial cellulitis is another early sign of invasion, which is not common in nonfungal sinusitis[10]. Late stages are characterized by signs suggestive of gross invasion of the structures of the orbit and the cranial cavity, which are more specific (Figure 1). Bone changes are also better visualized on CT.
The enhancement pattern of the lesions on contrast-enhanced computed tomography (CECT) varies from none to mild to heterogeneous enhancement, which was also seen in the cases in the present study[9,10]. The mild form was the most common type of enhancement observed by Therakathu et al[9]. Mucosal involvement may appear as a diffuse thickening or nodular thickening. Bone involvement was seen in the form of bone rarefaction, erosion, and permeative destruction in 40% of the cases in the study by Therakathu et al[9]. Middlebrooks et al[17]designed a CT-based model based on seven variables; this model can be used to suspect acute invasive fungal sinusitis. The variables are periantral fatinvolvement, bone dehiscence, orbital invasion, septal ulceration, pterygopalatine fossa, nasolacrimal duct, and lacrimal sac. In a study by Silverman et al[16], most cases of extra sinus invasion occurred without bony invasion, suggesting that perivascular or perineural invasion plays an important role in the spread of mucormycosis. In the same study, Silverman et al[16] noted that the presence of retroantral, facial, and orbital fat stranding was associated with a more aggressive infection.
Orbital and intracranial invasions are best seen by magnetic resonance imaging (MRI). Early changes are nonspecific. These include mucosal thickening, which appears hypointense on T1-weighted images and hyperintense on T2-weighted images. In the study by Therakathu et al[9], on a T2-weighted sequence, 37% of the lesions were isointense to mildly hypointense, 32% were heterogeneous, and 32% were hyperintense. Fungal elements are hypointense on T2-weighted images (Figure 2). The enhancement pattern is best studied on fat-suppressed post-gadolinium images and is different for different lesions. Of all the cases in the study by Therakathu et al[9], 29% showed intense homogenous enhancement, 36% showed heterogeneous enhancement, and 36% showed no enhancement.
Because of the angioinvasive nature of mucormycosis, the vessels get thrombosed. Upon injection of the contrast, the normal expected pattern of mucosal enhancement in case of inflammatory lesions may not be visible. Instead, there will be a low-signal intensity of the affected mucosa of the nasal turbinate on T2-weighted MRI images associated with an increased signal on diffusion-weighted images. This was referred to as the black turbinate sign by Safder et al[18].
Differential diagnosis on imaging for mucormycosis includes the following: Acute rhinosinusitis with complications, Wegener’s granulomatosis, and squamous cell carcinoma.
However, in the COVID-19pandemic, mucormycosis should be considered the first differential diagnosis.
Most of the imaging features correlate with the angioinvasive pattern of the mucormycosis infection. The imaging findings are nonspecific, but early diagnosis is of paramount importance because of the associated morbidity and mortality. Sen et al[19] found that cavernous sinus thrombosis and cribriform plate erosion were the commonest pathways of spread into the cranium and were present in 76% and 22% of the patients, respectively[19]. The involvement may appear as leptomeningeal inflammation, which appears as leptomeningeal enhancement and involvement of the cranial nerves with signs of meningism.This may be accompanied by cerebritis. Cerebritis appears as T2-FLAIR hyperintensity with variable enhancement and heterogeneous diffusion restriction on diffusion-weighted imaging. Invasion of the parenchyma may appear as granuloma formation or abscess formation. Fungal granulomas may show faint enhancement and surrounding edema.
In contrast to the pyogenic abscess, fungal abscesses are frequently multiple and form at the corticomedullary junction and in the basal ganglia. Isolated fungal abscesses are rare, and one should suspect intravenous drug abuse if encountered with such a situation. Fungal abscesses have crenated borders and non-enhancing, diffusion-restricting intracavitary projections[20].They are hypointense on T1 and hyperintense on T2 and show rim enhancement[20]. On diffusion-weighted imaging, Luthra et al[20] found that fungal abscesses showed restriction of diffusion in the projections and the wall, and the core of the abscess had no restriction of diffusion. In the same study, the authors found that the apparent diffusion coefficient was higher for the wall of the abscess when compared to the intracavitary projections for the fungal abscess, and it was statistically significant[20].
Vascular complications are observed in the late stages. They include both venous and arterial complications such as cavernous sinus thrombosis, arterial thrombosis, and aneurysmal dilatation (Figure 3). In the study by Mohindra et al[21], the role of MRI in the detection of vascular lesions was studied. In the study by Razek et al[22], cavernous sinus involvement in mucormycosis appears hypointense on T1 and T2 sequences with intense, inhomogeneous post-contrast enhancement. Cavernous sinus thrombosis is the most common complication in the present study, as in certain published studies.
Diabetes was one of the major predisposing factors for mucormycosis during the COVID-19 pandemic. Prakash et al[23] highlighted that rhinocerebral mucormycosis cases were predominantly present in those with uncontrolled diabetes and diabetic ketoacidosis, and few were present in immunosuppressed hosts. The findings were similar to those in other studies by Sen et al[18], John et al[24], and Hoenigl et al[25]. In the afore mentioned studies, the prevalence of diabetes was 78%-94% among patients with mucormycosis post–COVID-19 infection. In Patel et al’s study, patients with mucormycosis with poor glycemic control had a more invasive disease, which was statistically significant (P value = 0.040)[10]. The rampant use of corticosteroids and other immunomodulatory drugs to control the severity of the COVID-19 infection has further led to the increased predisposition[26]. During the peak of the pandemic, when healthcare facilities were functioning beyond their capacities, there were instances of unsupervised treatment with these immunomodulatory agents, leading to further escalation of the problem.
Rhinocerebral mucormycosis constituted the aftermath of the COVID-19 pandemic, leading to a rapid increase in the number of cases, which were previously restricted to only a few susceptible groups of patients. Rhinocerebral mucormycosis is associated with high mortality and morbidity. Hence, it should be suspected in any patient who presents symptoms of sinusitis, facial swelling, or CNS symptoms. After clinical examination, imaging is the backbone of the diagnosis of this severe disease. CT helps in the preliminary diagnosis and helps stage the disease. CT detects bony erosion better. However, when an orbital and intracranial extension is present, MRI is preferred, as it delineates the involvement of these structures better. MRI can also delineate vascular involvement better. In accessible sites, biopsy and potassium hydroxide mount help clinch the diagnosis. The treatment of rhinocerebral mucormycosis consists of debridement of the necrotic tissue along with intravenous antifungals for a prolonged duration. Control of diabetes and judicious use of corticosteroids and immunomodulatory drugs can decrease the incidence of this life-threatening disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chavan RP, India; Fan YW, China; Roushdy TM, Egypt S-Editor: Liu JH L-Editor:A P-Editor: Liu JH
| 1. | Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP. Pathogenesis of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S16-S22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 484] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 2. | Camara-Lemarroy CR, González-Moreno EI, Rodríguez-Gutiérrez R, Rendón-Ramírez EJ, Ayala-Cortés AS, Fraga-Hernández ML, García-Labastida L, Galarza-Delgado DÁ. Clinical features and outcome of mucormycosis. Interdiscip Perspect Infect Dis. 2014;2014:562610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Sweeney RM, McAuley DF. Acute respiratory distress syndrome. Lancet. 2016;388:2416-2430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 4. | Song G, Liang G, Liu W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia. 2020;185:599-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 433] [Cited by in RCA: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 5. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1969] [Article Influence: 98.5] [Reference Citation Analysis (1)] |
| 6. | Herrera DA, Dublin AB, Ormsby EL, Aminpour S, Howell LP. Imaging findings of rhinocerebral mucormycosis. Skull Base. 2009;19:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Gupta N, Dembla S. Cranial nerve involvement in mucormycosis in post-COVID patients: a case series. Egypt J Radiol Nucl Med. 2022;53:28. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Dubey S, Mukherjee D, Sarkar P, Mukhopadhyay P, Barman D, Bandopadhyay M, Pandit A, Sengupta A, Das S, Ghosh S, Adhikari S, Biswas PS, Pal P, Roy H, Patra N, Das A, Sinha P, Mondal MK, Shrivastava SR, Bhattacharya K, Mukhopadhyay M, Ahmed K, Halder TK, Saha M, Maity S, Mandal A, Chatterjee D, Saha S, Chunakar A, Saha A, Ray BK. COVID-19 associated rhino-orbital-cerebral mucormycosis: An observational study from Eastern India, with special emphasis on neurological spectrum. Diabetes Metab Syndr. 2021;15:102267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Therakathu J, Prabhu S, Irodi A, Sudhakar SV, Yadav VK, Rupa V. Imaging features of rhinocerebral mucormycosis: a study of 43 patients. Egypt J Radiol Nucl Med. 2018;49:447-452. [RCA] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 10. | Patel DD, Adke S, Badhe PV, Lamture S, Marfatia H, Mhatre P. COVID-19 associated Rhino-Orbito-Cerebral Mucormycosis: Imaging spectrum and Clinico-radiological correlation- a single Centre experience. Clin Imaging. 2022;82:172-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Gillespie MB, Huchton DM, O'Malley BW. Role of middle turbinate biopsy in the diagnosis of fulminant invasive fungal rhinosinusitis. Laryngoscope. 2000;110:1832-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Gillespie MB, O'Malley BW Jr, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg. 1998;124:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 124] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Skiada A, Pavleas I, Drogari-Apiranthitou M. Epidemiology and Diagnosis of Mucormycosis: An Update. J Fungi (Basel). 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 327] [Article Influence: 65.4] [Reference Citation Analysis (1)] |
| 14. | Bhandari J, Thada PK, Nagalli S. Rhinocerebral Mucormycosis. [Updated 2021 Nov 25]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559288/. |
| 15. | Rupa V, Maheswaran S, Ebenezer J, Mathews SS. Current therapeutic protocols for chronic granulomatous fungal sinusitis. Rhinology. 2015;53:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Silverman CS, Mancuso AA. Periantral soft-tissue infiltration and its relevance to the early detection of invasive fungal sinusitis: CT and MR findings. AJNR Am J Neuroradiol. 1998;19:321-325. [PubMed] |
| 17. | Middlebrooks EH, Frost CJ, De Jesus RO, Massini TC, Schmalfuss IM, Mancuso AA. Acute Invasive Fungal Rhinosinusitis: A Comprehensive Update of CT Findings and Design of an Effective Diagnostic Imaging Model. AJNR Am J Neuroradiol. 2015;36:1529-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 18. | Safder S, Carpenter JS, Roberts TD, Bailey N. The "Black Turbinate" sign: An early MR imaging finding of nasal mucormycosis. AJNR Am J Neuroradiol. 2010;31:771-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Sen M, Honavar SG, Bansal R, Sengupta S, Rao R, Kim U, Sharma M, Sachdev M, Grover AK, Surve A, Budharapu A, Ramadhin AK, Tripathi AK, Gupta A, Bhargava A, Sahu A, Khairnar A, Kochar A, Madhavani A, Shrivastava AK, Desai AK, Paul A, Ayyar A, Bhatnagar A, Singhal A, Nikose AS, Tenagi AL, Kamble A, Nariani A, Patel B, Kashyap B, Dhawan B, Vohra B, Mandke C, Thrishulamurthy C, Sambare C, Sarkar D, Mankad DS, Maheshwari D, Lalwani D, Kanani D, Patel D, Manjandavida FP, Godhani F, Agarwal GA, Ravulaparthi G, Shilpa GV, Deshpande G, Thakkar H, Shah H, Ojha HR, Jani H, Gontia J, Mishrikotkar JP, Likhari K, Prajapati K, Porwal K, Koka K, Dharawat KS, Ramamurthy LB, Bhattacharyya M, Saini M, Christy MC, Das M, Hada M, Panchal M, Pandharpurkar M, Ali MO, Porwal M, Gangashetappa N, Mehrotra N, Bijlani N, Gajendragadkar N, Nagarkar NM, Modi P, Rewri P, Sao P, Patil PS, Giri P, Kapadia P, Yadav P, Bhagat P, Parekh R, Dyaberi R, Chauhan RS, Kaur R, Duvesh RK, Murthy R, Dandu RV, Kathiara R, Beri R, Pandit R, Rani RH, Gupta R, Pherwani R, Sapkal R, Mehta R, Tadepalli S, Fatima S, Karmarkar S, Patil SS, Shah S, Dubey S, Gandhi S, Kanakpur S, Mohan S, Bhomaj S, Kerkar S, Jariwala S, Sahu S, Tara S, Maru SK, Jhavar S, Sharma S, Gupta S, Kumari S, Das S, Menon S, Burkule S, Nisar SP, Kaliaperumal S, Rao S, Pakrasi S, Rathod S, Biradar SG, Kumar S, Dutt S, Bansal S, Ravani SA, Lohiya S, Ali Rizvi SW, Gokhale T, Lahane TP, Vukkadala T, Grover T, Bhesaniya T, Chawla U, Singh U, Une VL, Nandedkar V, Subramaniam V, Eswaran V, Chaudhry VN, Rangarajan V, Dehane V, Sahasrabudhe VM, Sowjanya Y, Tupkary Y, Phadke Y; members of the Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC) Study Group. Epidemiology, clinical profile, management, and outcome of COVID-19-associated rhino-orbital-cerebral mucormycosis in 2826 patients in India - Collaborative OPAI-IJO Study on Mucormycosis in COVID-19 (COSMIC), Report 1. Indian J Ophthalmol. 2021;69:1670-1692. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 242] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 20. | Luthra G, Parihar A, Nath K, Jaiswal S, Prasad KN, Husain N, Husain M, Singh S, Behari S, Gupta RK. Comparative evaluation of fungal, tubercular, and pyogenic brain abscesses with conventional and diffusion MR imaging and proton MR spectroscopy. AJNR Am J Neuroradiol. 2007;28:1332-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 21. | Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. 2007;50:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Razek AA, Castillo M. Imaging lesions of the cavernous sinus. AJNR Am J Neuroradiol. 2009;30:444-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Prakash H, Chakrabarti A. Global Epidemiology of Mucormycosis. J Fungi (Basel). 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 24. | John TM, Jacob CN, Kontoyiannis DP. When Uncontrolled Diabetes Mellitus and Severe COVID-19 Converge: The Perfect Storm for Mucormycosis. J Fungi (Basel). 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 276] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 25. | Hoenigl M, Seidel D, Carvalho A, Rudramurthy SM, Arastehfar A, Gangneux JP, Nasir N, Bonifaz A, Araiza J, Klimko N, Serris A, Lagrou K, Meis JF, Cornely OA, Perfect JR, White PL, Chakrabarti A; ECMM and ISHAM collaborators. The emergence of COVID-19 associated mucormycosis: a review of cases from 18 countries. Lancet Microbe. 2022;3:e543-e552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 272] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 26. | Al-Tawfiq JA, Alhumaid S, Alshukairi AN, Temsah MH, Barry M, Al Mutair A, Rabaan AA, Al-Omari A, Tirupathi R, AlQahtani M, AlBahrani S, Dhama K. COVID-19 and mucormycosis superinfection: the perfect storm. Infection. 2021;49:833-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |