Published online Jul 28, 2022. doi: 10.4329/wjr.v14.i7.194
Peer-review started: February 22, 2022
First decision: April 28, 2022
Revised: May 17, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: July 28, 2022
Processing time: 154 Days and 16.1 Hours
Coronavirus disease 2019 (COVID-19) is caused by the novel viral pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 primarily involves the lungs. Nucleic acid testing based on reverse-transcription polymerase chain reaction of respiratory samples is the current gold standard for the diagnosis of SARS-CoV-2 infection. Imaging modalities have an established role in triaging, diagnosis, evaluation of disease severity, monitoring disease progression, extra-pulmonary involvement, and complications. As our un
Core Tip: Despite extensive global efforts, coronavirus disease 2019 (COVID-19) remains the largest public health problem of modern times. As our understanding of the disease and its manifestations improve, we must recognize and explore the potential utility of molecular imaging modalities in evaluating the long-term sequelae of COVID-19. Molecular imaging tools can be incorporated into routine clinical practice by identifying appropriate and specific indications and addressing limitations to their practical application.
- Citation: Chandekar KR, Satapathy S, Singh H, Bhattacharya A. Molecular imaging as a tool for evaluation of COVID-19 sequelae – A review of literature. World J Radiol 2022; 14(7): 194-208
- URL: https://www.wjgnet.com/1949-8470/full/v14/i7/194.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i7.194
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the novel pathogen, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1]. Having its early origins in the city of Wuhan, China, the disease was transmitted across the globe at disconcertingly rapid rates, prompting the World Health Organization (WHO) to characterize the outbreak as a pandemic in March 2020[2,3]. The resurgence of the disease in several parts of the world with identification of new mutant variants has hindered a targeted global response, owing to which, COVID-19 still remains the largest public health problem of modern times[4].
Though primarily believed to involve the lungs and the respiratory tract, the clinical spectrum of COVID-19 is diverse with potential for gastrointestinal, cardiac, renal, neurological, and hematological manifestations of varying severity[5].
SARS-CoV-2 is a single stranded RNA virus. Nucleic acid testing based on reverse-transcription polymerase chain reaction (RT-PCR) is the current gold standard for the diagnosis of SARS-CoV-2 infection. It is most commonly done with respiratory samples, such as nasopharyngeal and throat swabs[6]. Serological tests which identify antibodies to different virus proteins have also been developed. Laboratory tests, such as complete hemogram, C-reactive protein (CRP), D-dimer, prothrombin time (PT-INR), lactic dehydrogenase (LDH), ferritin, and procalcitonin, help in evaluation of disease severity and prognostication[7]. In spite of relatively low specificity and radiation exposure, imaging modalities have an established role in triaging, diagnosing, evaluating disease severity, monitoring disease progression, determining extra-pulmonary involvement, and assessing complications[8,9].
Molecular imaging modalities in current clinical practice, such as magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET), have great potential in early and sensitive disease detection, accurate delineation of disease extent, and assessing therapeutic response with the ultimate aim of personalized medicine. Novel molecular targets and tracers (metabolic agents, peptides, small molecules, receptor ligands) are being rapidly identified and developed. At present, multimodality molecular imaging is most commonly used for oncological applications. However, their role in systemic inflammatory and infectious conditions is being increasingly recognized[10,11]. The utility of molecular or functional imaging for COVID-19, in particular, has been poorly defined owing to limited availability, longer imaging times, absence of clearly defined appropriate usage criteria, lack of standardized protocols, and need for infection control. In this article, we aim to review the role of molecular imaging in evaluating the sequelae of COVID-19.
The literature search was based on three electronic databases (PubMed, Scopus, and EMBASE) using selected keywords which included, “COVID-19,” “sequelae,” “molecular imaging,” “functional imaging,” and “nuclear medicine” linked through the "AND" and "OR" Boolean operators to build specific strings for each electronic search engine. Original studies, case reports, case series, and review articles were included. No restriction was placed in terms of country or language of publication. Only full-length articles were considered. Information from websites of different professional associations and national/international organizations was searched to retrieve relevant information.
Chest radiography (CXR) and computed tomography (CT) have been extensively used for imaging evaluation of COVID-19. CXR findings in COVID-19 include consolidatory changes and bilateral ground glass and peripheral air opacities. However, these findings are non-specific and are highly dependent on duration and severity of infection at the time of acquisition[12]. The advantages of CXR over CT include its near universal availability, lower ionizing radiation exposure, ability to perform multiple repeat examinations, and portability of equipment, which reduces risk of cross-infection. CXR is limited by its lower sensitivity compared to RT-PCR and CT, particularly in early stages of the disease[13,14].
Similar to CXR, the typical findings of COVID-19 on CT are multiple ground-glass opacities (GGOs) in a posterior, subpleural, and peripheral distribution, commonly showing bilateral lung involvement. Consolidatory changes, reticular opacities, intra- and inter-lobular septal thickening, and crazy paving pattern have also been described. CT abnormalities progress rapidly after symptom onset and are reported to peak between days 6 and 13 of the illness. Late stage disease shows gradual decrease in GGOs and consolidation with appearance of signs of fibrosis[15,16]. CT findings of COVID-19 are highly non-specific and may be seen with other viral pneumonias[17,18]. A recent meta-analysis of the accuracy of diagnostic tests for COVID-19 found CT to have high sensitivity (91.9%, 95%CI: 89.8%-93.7%) and low specificity (25.1%, 95%CI: 21.0%-29.5%). Hence CT findings must be interpreted in light of clinical presentation, history of exposure, and pre-test probability[6].
At present, most consensus guidelines recommend against the routine use of CT for screening and diagnosis of COVID-19 pneumonia. However, the role of chest CT as a rapid-triage tool in resource-limited facilities (e.g., limited access to/longer processing time of RT-PCR) has also been acknowledged[19]. The use of CT has been deemed most appropriate in patients with moderate to severe respiratory symptoms or mild respiratory symptoms with risk factors for disease progression (such as presence of co-morbidities and advanced age)[20,21]. The major advantage of CT is the ability to stratify patients based on their risk for clinical decompensation and progression. To that end, different standard reporting and scoring systems have been proposed, such as COVID-19 Reporting and Data System (CO-RADS), Radiological Society of North America (RSNA) imaging classification for COVID-19, chest CT severity score (CT-SS), and total severity score (TSS)[22,23]. CT-SS is positively correlated with age, inflammatory biomarkers, clinical severity, and disease phases[24]. Lieveld et al[25] showed that the chest CT-SS was significantly positively associated with hospital and ICU admission, and in-hospital and 30 d mortality for all age groups in patients with COVID-19 and CT patterns ≥ CO-RADS 3.
MRI, owing to its lack of exposure to ionizing radiation, has been found to be useful in select patient-groups, such as pregnant women and children. Several case-reports have been published highlighting the utility of MRI in evaluation of extra-pulmonary involvement, particularly cardiac and neurological manifestations of COVID-19[26-28].
Chest ultrasonography (US) is now being advocated as a useful point-of-care (POC) imaging tool for evaluation particularly in the emergency and ICU settings. Vascular US of the limbs is useful in the diagnostic workup of patients with suspected deep vein thrombosis, a common complication of COVID-19[29,30].
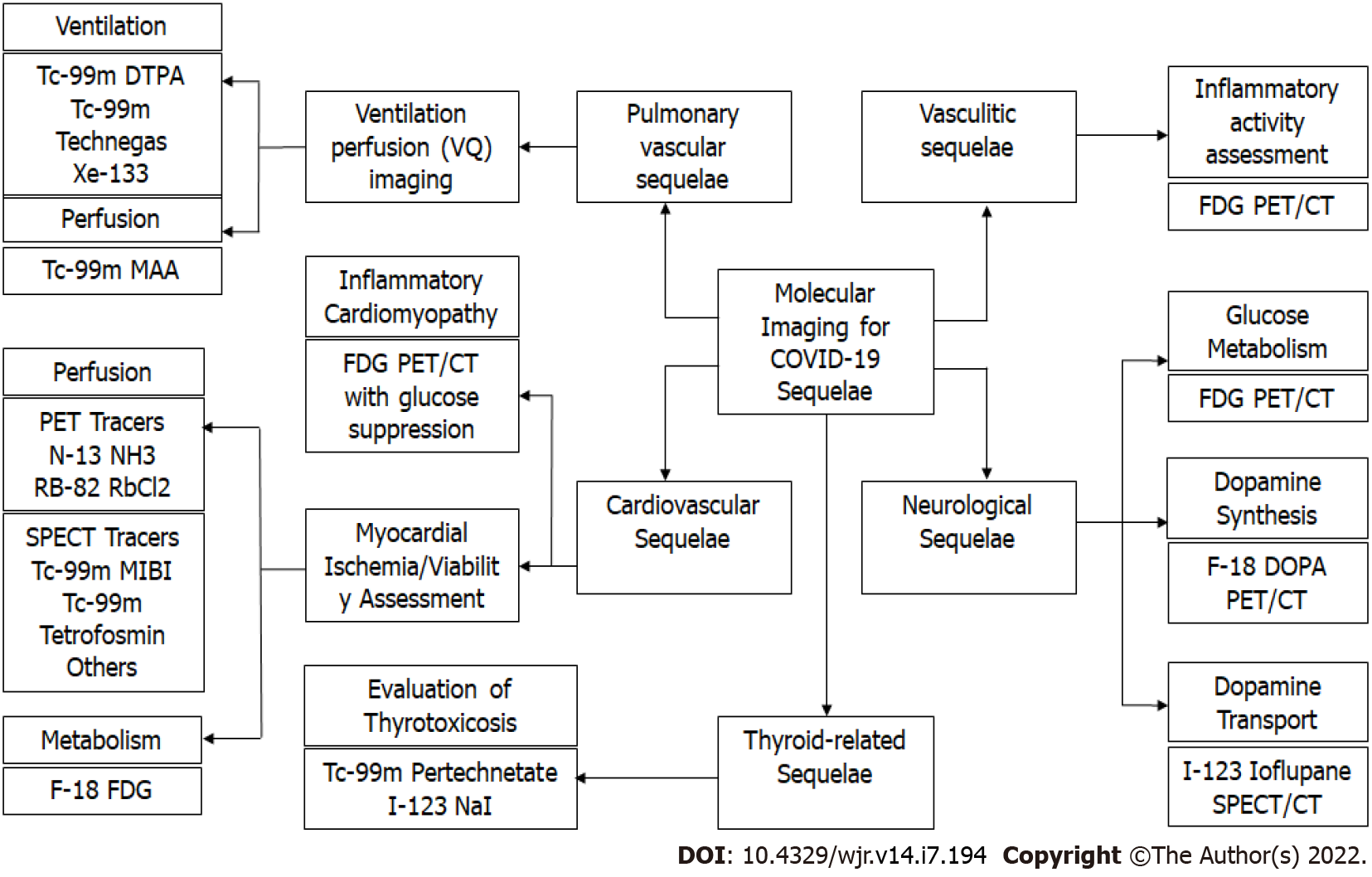
Molecular imaging is a highly sensitive modality that allows non-invasive visualization of physiological or pathological processes at the cellular or molecular level. Pathophysiological changes in affected tissues are believed to occur earlier than anatomical changes in a variety of infectious and inflammatory conditions, and hence, molecular imaging may detect these functional changes before conventional radiologic imaging modalities[31]. Different molecular imaging modalities can help in evaluating the sequelae of COVID-19 (Figure 1).
It is now well established that COVID-19 is associated with thrombotic complications, such as venous thromboembolism (VTE), myocardial infarction (MI), and ischemic stroke[32]. A recent meta-analysis found the overall prevalence of COVID-19 related VTE to be 14.7% (95%CI: 12.1%-17.6%), which was significantly higher in patients with severe systemic inflammation and respiratory failure[33-35]. However, these thromboembolic phenomena have also been documented in patients with milder forms of the disease[36].
From a histological stand-point, direct viral infection of endothelial cells with perivascular T-cell infiltration, thrombotic microangiopathy, and angiogenesis have been used to differentiate COVID-19 from other respiratory viruses[37]. Hence, both thromboembolic phenomena and in-situ thrombotic microangiopathy can be responsible for pulmonary vascular manifestations of COVID-19. Ventilation-Perfusion (VQ) imaging is the current gold-standard screening modality for evaluation of chronic thromboembolism[38]. Distal subsegmental small vessel thrombi can be missed on conventional CT pulmonary angiogram (CTPA), which is designed to visualize a luminal clot rather than assess how a clot affects lung perfusion, thereby underestimating the extent of micro-vascular injury[39]. Hence, VQ scintigraphy, a functional imaging modality which directly evaluates lung perfusion, can potentially help better identify vascular pathology and guide therapeutic decisions.
The possible patterns of perfusion defects seen in COVID-19 are closely related to their patho
Dhawan et al[42] recently proposed an algorithm to incorporate perfusion imaging instead of angiographic imaging as a first-line modality in the post-COVID recovery patients for follow-up of pulmonary vascular sequelae. It would serve as a triage tool to exclude or evaluate residual clot burden and small vessel injury.
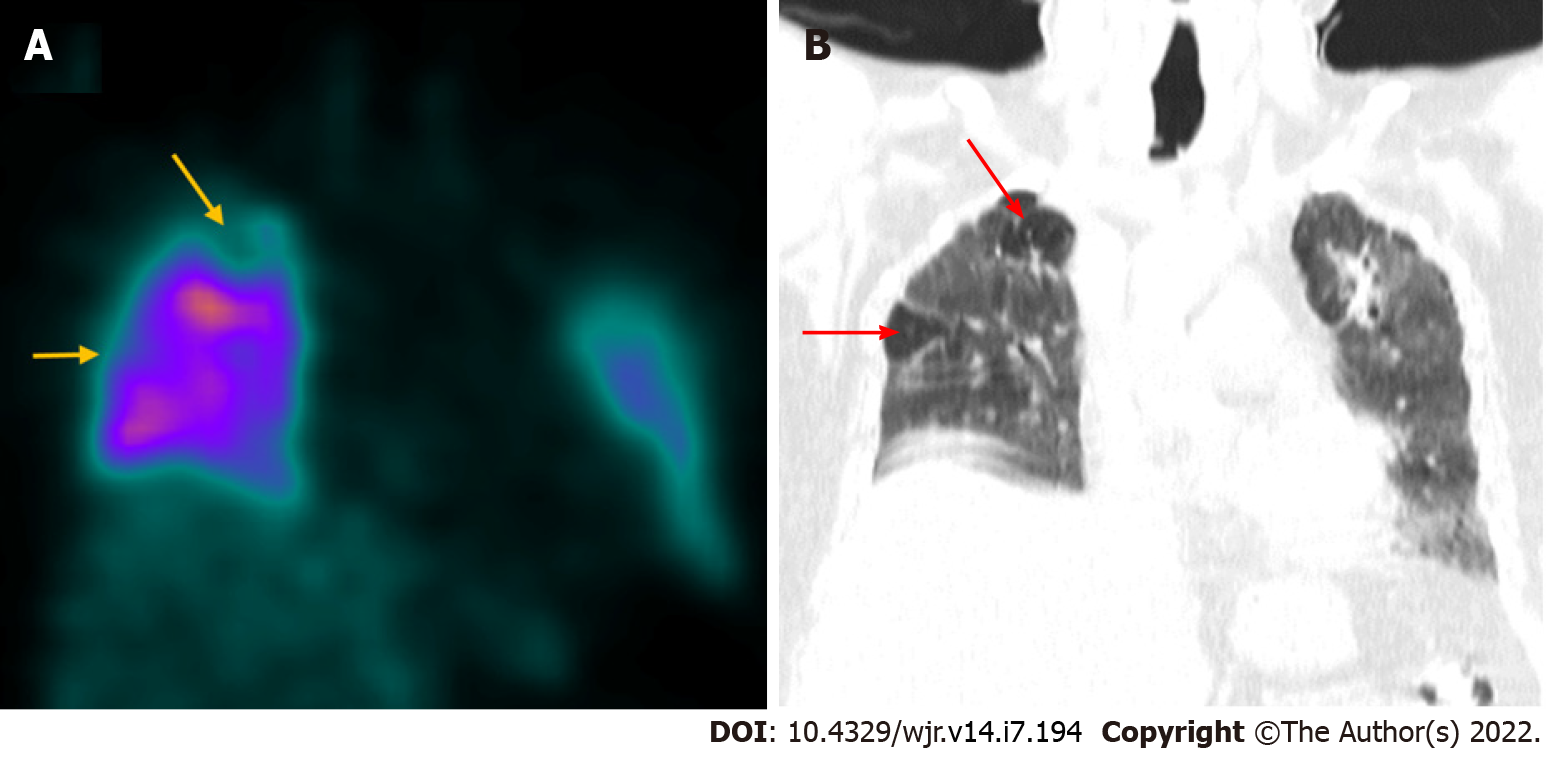
A few recent publications have discouraged the use of ventilation imaging by nuclear medicine departments to reduce the risk of cross-contamination via aerosols[43,44]. Reporting perfusion studies without concordant ventilation imaging might hinder interpretation by increasing the likelihood of false positives. However, such limitation can be significantly overcome by the use of hybrid imaging. SPECT with CT fusion (SPECT/CT) for perfusion studies can be used as a substitute for ventilation imaging by providing corroborating anatomical information about the lung parenchyma[45-47]. Perfusion-only SPECT/CT has been shown to have practical utility in the diagnosis of pulmonary embolism in COVID-19 patients with a moderate-to-high pre-test probability. A 60-year-old male underwent perfusion-only SPECT/CT (Figure 2) in our department, 2 mo following COVID-19 infection, to rule out pulmonary thromboembolism. The study revealed subsegmental mismatched defects suggestive of pulmonary thromboembolism.
The use of VQ imaging in the setting of contraindications to iodinated contrast material also makes it preferable over CTPA as a first-line imaging modality for COVID-19 related pulmonary embolism[48].
SARS-CoV-2 infection has been linked to multiple thyroid disorders, including destructive thyroiditis, autoimmune thyroid disease, central hypothyroidism and euthyroid sick syndrome[49]. The binding of the viral spike protein to the angiotensin-converting-enzyme-2 (ACE2) receptors on the surface of the thyroid follicular cells has been implicated in the etiopathogenesis of the COVID-19-related destructive thyroiditis[50]. Further, an aberrant systemic inflammatory syndrome in the wake of COVID-19 can also account for the abovementioned thyroid disorders. Thyrotoxicosis, due to destructive thyroiditis or activated/relapsed Graves’ disease, can exacerbate the cardiovascular complications and contribute to poor outcomes, especially in severe COVID-19 disease. Thyroid scintigraphy, with either 99mTc-pertechnetate or 123I-sodium iodide, can rapidly and reliably differentiate between these etiologies of thyrotoxicosis and guide the further course of treatment[49]. A 36-year-old male had complaints of painful neck swelling and fever for 1 wk with a history of COVID-19 2 mo ago. He was found to have suppressed levels (0.013 mIU/mL) of thyroid stimulating hormone (TSH). Ultrasound neck revealed a diffusely heterogeneous thyroid parenchyma with mildly increased vascularity suggestive of thyroiditis. He was referred to our department for thyroid scintigraphy to further evaluate the cause of thyrotoxicosis. Thyroid scintigraphy revealed (Figure 3) very faint heterogeneous tracer uptake in the region of the thyroid with increased background tracer activity. With the given clinical and biochemical context, scan findings were suggestive of thyroiditis, likely related to COVID-19.
18F-fluorodeoxyglucose (FDG) PET/CT is a functional imaging modality with established clinical utility in diagnosis, staging, re-staging, and therapeutic response evaluation for a variety of oncological conditions[51,52]. However, in the recent past, the role of 18F-FDG PET/CT as a hybrid imaging tool for detecting and characterizing various inflammatory disorders has also been validated. 18F-FDG PET/CT provides complimentary anatomical and functional information with the ability to non-invasively quantify inflammation[53,54]. SARS-CoV-2 viral infection results in a complex inflammatory cascade leading to release of pro-inflammatory cytokines and activation of cells such as neutrophils, monocytes, and effector T-cells. Activated inflammatory cells are highly glycolytic and hence, non-physiological FDG uptake can be reliably used as a surrogate marker for active inflammation[55].
Multiple studies have demonstrated incidental findings in otherwise asymptomatic or mildly symptomatic patients who underwent PET/CT for oncology/non-COVID related indications. Hence, nuclear medicine physicians must be aware of the radiological manifestations of COVID-19 and must nurture a high index of suspicion so that infection may be promptly identified at early stages and appropriate treatment may be initiated in such patient populations, the majority of whom may have a compromised immune status[56-60].
The currently accepted gold standard for diagnosing COVID-19 infection is RT-PCR to detect viral RNA[6]. Despite being fairly sensitive, multiple sources of false negative test results have been reported, such as insufficient viral genome, incorrect sampling technique, sampling outside the appropriate time-window for viral replication, and viral mutation. The role of 18F-FDG PET/CT has been sought to be explored at early stages when clinical symptoms are not specific and differential diagnosis is challenging[61]. An early case series by Qin et al[62] described four patients in Wuhan with strong clinical suspicion of COVID-19 who underwent 18F-FDG PET/CT during the acute phase of illness. FDG uptake was observed in regions corresponding to GGOs and/or consolidatory changes, with maximum standardized uptake (SUVmax) values ranging from 4.6 to 12.2. FDG uptake was also reported in the mediastinal and hilar lymph nodes with no obvious anatomical lymphadenopathy[62]. However, given the fact that increased FDG uptake is noted in various acute inflammatory and infectious conditions and is, hence, non-specific, 18F-FDG PET/CT is not routinely recommended for the initial evaluation of patients with known or suspicious COVID-19 infection[63].
Nevertheless, data by Qin et al[62] also raised the possibility that higher SUVmax of pulmonary lesions on 18F-FDG PET may be correlated with longer duration of healing. 18F-FDG-PET/CT could, therefore, potentially be used to monitor treatment response and predict recovery. However, these trends need to be evaluated in larger populations before meaningful conclusions can be drawn.
The cardiovascular manifestations of COVID-19 include arrhythmias, acute or fulminant myocarditis, acute coronary syndromes, and heart failure[64].
Different etiological mechanisms have been proposed to explain cardiac involvement in COVID-19 which include: (1) Direct myocardial cellular injury by the virus. The spike protein of the SARS-CoV2 virus binds to ACE2 receptors, which serves as an entry point to the cell. ACE2 is a membrane protein with documented expression on ciliated columnar respiratory epithelium, type II pneumocytes, and cardiomyocytes; (2) Severe systemic inflammatory response. High levels of proinflammatory cytokines and procoagulants result in endothelial dysfunction, microthombi formation within the coronary circulation, and increased plaque vulnerability; and (3) Mismatch of the myocardial oxygen supply and demand. Increased cardio-metabolic demand is the result of systemic inflammation and ongoing hypoxia due to severe pneumonia or acute respiratory distress syndrome[65].
A recent case report highlighted the role of multi-modality imaging for assessment of myocardial injury in COVID-19. 18F-FDG PET/CT (with 18 h prolonged fasting protocol to suppress glucose uptake) was performed in a 69-year-old woman with COVID-19 who had complaints of dyspnea and chest pain. PET showed FDG uptake in the apex, anterior wall, and septum, and 99mTc-methoxyisobutylisonitrile (MIBI) SPECT done subsequently revealed resting perfusion defects in the same segments of the left ventricular myocardium, suggestive of an acute inflammatory response to MI precipitated by COVID-19. Severe left anterior descending artery (LAD) artery disease was found on angiography performed later, confirming anterior wall MI. Segmental FDG uptake (due to inflammation) with matching perfusion defect (due to inflammatory microvascular dysfunction), though suggestive of myocarditis, can also be seen in an acute inflammatory response to MI precipitated by COVID-19, as seen in the case described above[66].
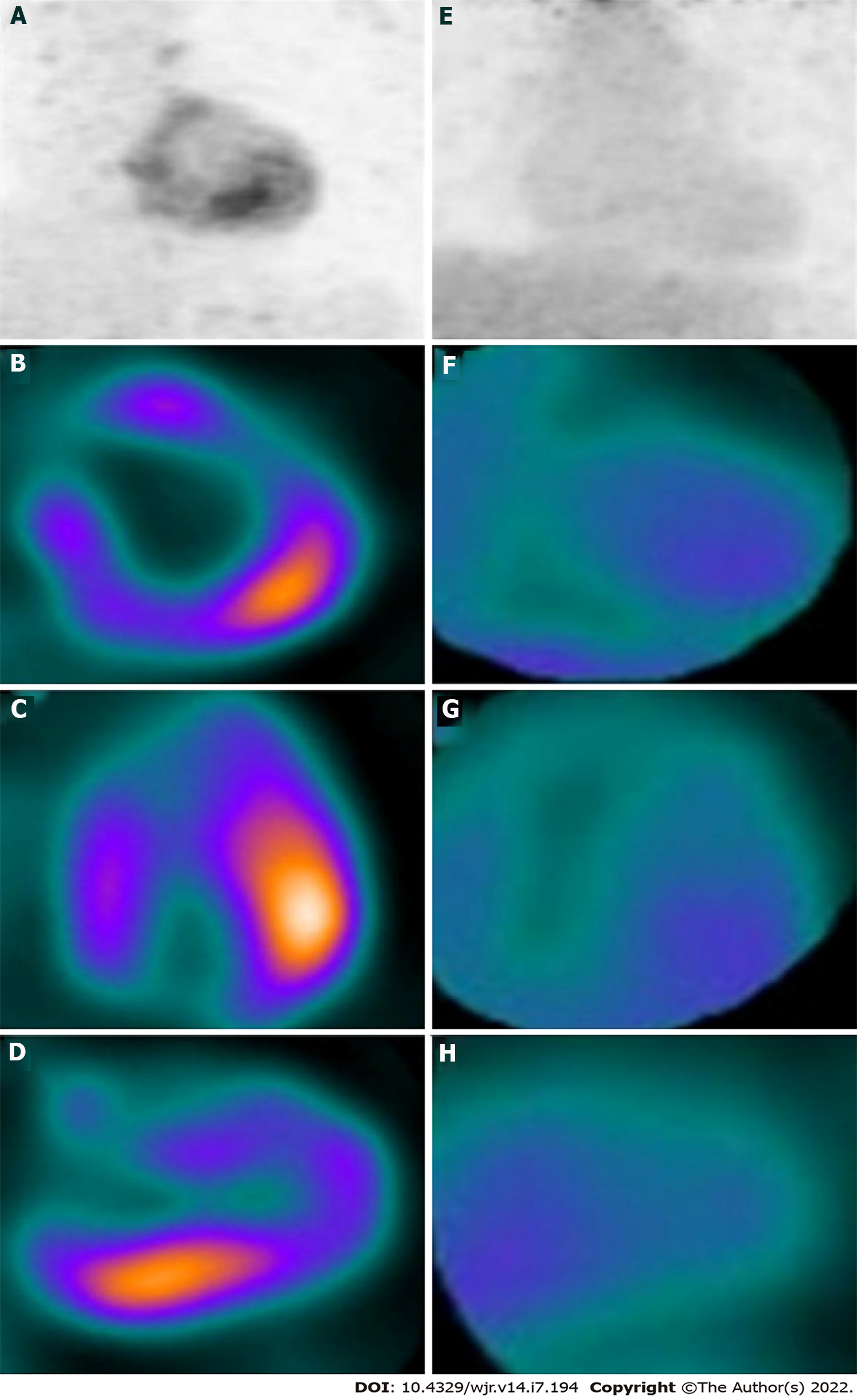
Another case report highlighted the role of 18F-FDG PET/CT in assessing myocardial inflammation in COVID-19 related multisystem inflammatory syndrome in children (MIS-C). 18F-FDG PET-CT, performed after 18 h of fasting and high-fat, low-carbohydrate diet preparation in a 14-year-old child with a clinical diagnosis of COVID-19-related MIS-C, demonstrated hypermetabolism in the inferolateral wall of the LV myocardium suggestive of active inflammation (Figure 4, attached with permission). A repeat 18F-FDG PET/CT, performed 6 wk later with the same protocol, showed resolution of hypermetabolism, consistent with clinical and biochemical improvement, thus highlighting the role of 18F-FDG PET/CT in the timely diagnosis and follow-up of this potentially life-threatening hyperinflammatory syndrome[67].
Recently there has been increasing recognition of the utility of 18F-FDG PET/CT in inflammatory disorders and, in particular, vasculitis. The European League Against Rheumatism (EULAR) recommends 18F-FDG PET/CT as an alternative imaging modality in cases of suspected large vessel vasculitis[68,69]. The advantages of 18F-FDG PET/CT are its high sensitivity and the ability to non-invasively quantify inflammatory activity. Semi-quantitative methods of grading FDG uptake have been proposed. Total vascular score (TVS) and PET vascular activity score (PETVAS), have recently been suggested as PET based global inflammatory burden parameters, with potential for differentiation of active vs non-active inflammation, predicting relapse and treatment response monitoring in large vessel vasculitis[70,71].
Sollini et al[72] recently evaluated the role of 18F-FDG PET/CT in assessing systemic inflammation in 10 patients who had recovered from COVID-19. Significantly higher target-to-blood pool ratio (a quantitative parameter of FDG uptake) was noted in COVID-19 patients, in comparison to controls in three arterial territories – thoracic aorta, right iliac artery, and femoral arteries. This study suggested that COVID-19 induces vascular inflammation, and FDG PET has a potential role for evaluation of whole body vascular inflammatory process[72].
Central nervous system (CNS) vasculitis can occur as a part of systemic vasculitides or as primary angiitis of CNS (PACNS). Viral infections, such as Varicella zoster, hepatitis C virus, West Nile virus, and human immunodeficiency virus (HIV), are known to trigger CNS vasculitis[73]. SARS-CoV-2 infection related vasculitis has also been proposed as a possible mechanism to explain COVID-19 related neurologic dysfunction and encephalopathy with clinical improvement post steroid administration[74]. MR angiography may reveal concentric vessel wall enhancement as a direct sign of regional mural inflammation[75]. However, the majority of the published literature on COVID-19 vasculitis is limited to case reports and case series. Further prospective studies are required to unequivocally establish a causal relationship between SARS-CoV-2 and vasculitis.
Neurological manifestations of COVID-19 can range from mild symptoms like headache, dizziness, and anosmia to more serious complications, such as encephalopathy, posterior reversible encephalopathy syndrome (PRES), acute demyelinating encephalomyelitis (ADEM), cerebrovascular events [including acute ischemic stroke, intracranial hemorrhage (ICH)], cortical vein and/or sinus thrombosis (CVST), and neuro-inflammatory syndromes[76].
Important neuropathological findings in COVID-19 patients include edema, gliosis with diffuse activation of microglia and astrocytes, inflammatory infiltrates, cortical and sub-cortical infarcts, hemorrhagic lesions, and arteriosclerosis. It is hypothesized that they represent a combination of direct cytopathic effects of the virus and indirect effects via the host immune-inflammatory response owing to ACE2 and heme dysregulation, along with release of pro-inflammatory cytokines. However, further studies are required to better understand the pathologic mechanisms which drive the neurological manifestations of COVID-19[77-79].
In COVID-19 patients with neurological manifestations, MRI neuroimaging may be performed to detect the underlying casual pathology, particularly if CT reveals no abnormality. The recommended basic MRI protocol includes pre- and post-contrast T1 weighted-images, T2-weighted, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted images, and hemorrhage-sensitive sequences, such as susceptibility weighted imaging (SWI)[80]. The most common neuroimaging manifestations are acute and subacute infarcts with large clot burden, ICH, microhemorrhages, asymmetrical diffuse or tumefactive T2 and FLAIR white matter hyperintensities consistent with ADEM, mesial temporal lobe, corpus callosum, and olfactory bulb involvement, and cranial nerve enhancement[81-83].
Lu et al[84] explored the role of diffusion tensor imaging (DTI) and 3D high-resolution T1 weighted sequences in assessing possible disruption of micro-structural and functional brain integrity in the recovery stages of COVID-19. They reported significantly higher bilateral gray matter volumes (GMV) in olfactory cortices, hippocampi, and insulas and changes in MRI-based measures of water diffusion in white matter of COVID-19 patients 3 mo after acute illness compared to age and sex-matched non-COVID controls, suggesting neuro-invasion potential of SARS-CoV-2[84].
High costs, long acquisition times, limited access in developing nations, and lack of specificity of nearly all reported neuroradiological findings in COVID-19 are the major limitations of MRI[85].
Delorme et al[86] reported a case series with 4 COVID-19 patients suspected to have autoimmune encephalitis. 18F-FDG PET/CT of the brain demonstrated prefrontal or orbito-frontal cortical hypometabolism and hypermetabolism in the cerebellar vermis. These findings were consistent in all 4 patients, with no specific MRI features nor significant cerebrospinal fluid (CSF) abnormalities, possibly suggesting a parainfectious cytokine storm or immune-mediated mechanism at play rather than direct neural invasion[86].
Similarly, Grimaldi et al[87] also reported diffuse cortical hypometabolism with hypermetabolism in the putamen and cerebellum in autoimmune encephalitis concomitant with SARS-CoV-2 infection. These findings, along with normal morphological data on MRI, might suggest reduced neuronal activity and functional alterations in neuro-COVID-19 patients[87]. Increased FDG uptake in the bilateral basal ganglia with T2/FLAIR hyperintensities in the bilateral hippocampi was also reported in a case of SARS-CoV-2 infection related autoimmune limbic encephalitis[88].
Further, 18F-FDG PET has also been used in the evaluation of patients with persistent functional neurological symptoms after apparent recovery from COVID-19. In a retrospective series comprising 35 such patients, 18F-FDG brain PET demonstrated hypometabolism in the bilateral rectal/orbital gyrus, including the olfactory gyrus, connected limbic/paralimbic regions, brainstem, and the bilateral cerebellar hemispheres. This metabolic picture was seen to be associated with the patients’ symptoms and could be used to reliably identify these patients from normal controls. Brain inflammation related to the neurotropism of the SARS-CoV-2 from the olfactory bulb has been suggested as a possible mechanism underlying these findings[89].
Few case reports have also highlighted the role of molecular imaging in the evaluation of parkinsonian features post COVID-19. Cohen et al[90] reported a case of parkinsonism after SARS-CoV-2 infection in a 45-year-old man. Brain CT, MRI, and EEG were normal. However, 18F-fluorodopa (18F-FDOPA) PET scan revealed decreased radiotracer uptake in both putamina (left > right) and mild decreased uptake in the left caudate nucleus. The authors reported clinical improvement of rigidity and bradykinesia after initiation of pramipexole[90].
Morassi et al[91] described consistent findings of diffuse cortical hypometabolism (with relative sparing of sensorimotor areas) associated with hypermetabolism in the brainstem, mesial temporal lobes, and basal ganglia on 18F-FDG PET/CT in two patients with COVID-19 related encephalopathy who developed a rapidly progressive form of atypical parkinsonism. 123I-ioflupane DaT-SPECT performed in one of the two patients showed asymmetrical presynaptic dopaminergic dysfunction in the bilateral putamina, more severe on the left side consistent with a parkinsonian disorder[91].
Scarlattei et al[59] have reported incidental findings of 68Ga-labelled prostate-specific membrane antigen (68Ga-PSMA) and 18F-labelled choline (18F-choline) radiotracer uptake in regions corresponding to subpleural GGOs in two patients who underwent PET/CT for evaluation of prostate cancer[59]. Understanding the exact mechanisms that lead to uptake of these radiotracers in acute infective/inflammatory pulmonary lesions offers an important research prospect.
There is ongoing research directed at identifying targets for molecular imaging of inflammation with several novel radiotracers being described in pre-clinical and early clinical studies, such as 18F-AzaFol,
Further potential targets for new radiotracers include chemokine receptor CXCR4, interleukin IL-6, fibroblast activation protein inhibitors, and inhibitors of the type 1 angiotensin-II receptor ATR1 and ACE2. A radiolabeled ACE2-receptor antagonist has already been developed for autoradiography analysis, setting in motion the potential development of a PET radiotracer[95,96]. Since SARS-CoV-2 spike proteins bind to the ACE2 receptors, novel targeted radiotracers can have potential utility in the drug development process.
Widespread utilization of molecular imaging in suspected or confirmed cases of COVID-19 is primarily limited by relatively longer imaging times (in comparison to anatomical imaging with CXR, CT, or USG) and the need for adopting infection control protocols. Further, there is a need for simultaneously ensuring optimal utilization of resources, such as finite amounts of radiotracer, which has economic ramifications. Nuclear medicine departments across the globe have to bear in mind these feasibility issues and ensure undisrupted services to patients with non-COVID-19 related indications, in particular oncological cases in which PET is mandated for crucial treatment decisions[97-99].
To summarize, the present review comprehensively highlights the tremendous potential utility of molecular imaging in evaluation of COVID-19 sequelae such as pulmonary thromboembolism, vasculitis, multi-systemic inflammation, and cardiovascular and neurological sequelae. Despite the fact that the majority of the published literature is retrospective in nature with limited sample sizes, it is clear that molecular imaging provides additional valuable information (complimentary to anatomical imaging) with semi-quantitative or quantitative parameters to define inflammatory burden and can be used to guide therapeutic strategies and assess response. The authors believe that clinical translation and appropriate utilization of molecular imaging and associated imaging biomarkers can greatly benefit both the diagnosis and management of COVID-19 sequelae.
The potential of molecular imaging and nuclear medicine as a whole, in contributing to this pandemic largely remains underutilized. Identifying appropriate indications, establishing standardized protocols, and developing structured clinical trials employing novel radiotracers will help in realizing that potential[100].
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kothan S, Thailand; Lanza G, Italy; Lukito AA, Indonesia; Purevjav E, United States S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4848] [Cited by in RCA: 4386] [Article Influence: 877.2] [Reference Citation Analysis (1)] |
| 2. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9315] [Article Influence: 1863.0] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. COVID-19 Situation Report. [cited 26 December 2021] Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10. |
| 4. | World Health Organization. COVID-19 Weekly epidemiological update- 21 December 2021. [cited 26 December 2021] Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-december-2021. |
| 5. | Macera M, De Angelis G, Sagnelli C, Coppola N; Vanvitelli Covid-Group. Clinical Presentation of COVID-19: Case Series and Review of the Literature. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Böger B, Fachi MM, Vilhena RO, Cobre AF, Tonin FS, Pontarolo R. Systematic review with meta-analysis of the accuracy of diagnostic tests for COVID-19. Am J Infect Control. 2021;49:21-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 303] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 7. | Pourbagheri-Sigaroodi A, Bashash D, Fateh F, Abolghasemi H. Laboratory findings in COVID-19 diagnosis and prognosis. Clin Chim Acta. 2020;510:475-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 8. | Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 400] [Cited by in RCA: 564] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 9. | Kooraki S, Hosseiny M, Myers L, Gholamrezanezhad A. Coronavirus (COVID-19) Outbreak: What the Department of Radiology Should Know. J Am Coll Radiol. 2020;17:447-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 280] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 10. | Pysz MA, Gambhir SS, Willmann JK. Molecular imaging: current status and emerging strategies. Clin Radiol. 2010;65:500-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 372] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 11. | Galbán CJ, Galbán S, Van Dort ME, Luker GD, Bhojani MS, Rehemtulla A, Ross BD. Applications of molecular imaging. Prog Mol Biol Transl Sci. 2010;95:237-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, Lee JCY, Chiu KW, Chung TW, Lee EYP, Wan EYF, Hung IFN, Lam TPW, Kuo MD, Ng MY. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296:E72-E78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 832] [Cited by in RCA: 826] [Article Influence: 165.2] [Reference Citation Analysis (1)] |
| 13. | Sadiq Z, Rana S, Mahfoud Z, Raoof A. Systematic review and meta-analysis of chest radiograph (CXR) findings in COVID-19. Clin Imaging. 2021;80:229-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Kim H, Hong H, Yoon SH. Diagnostic Performance of CT and Reverse Transcriptase Polymerase Chain Reaction for Coronavirus Disease 2019: A Meta-Analysis. Radiology. 2020;296:E145-E155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 15. | Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1728] [Cited by in RCA: 1596] [Article Influence: 319.2] [Reference Citation Analysis (1)] |
| 16. | Raptis CA, Hammer MM, Short RG, Shah A, Bhalla S, Bierhals AJ, Filev PD, Hope MD, Jeudy J, Kligerman SJ, Henry TS. Chest CT and Coronavirus Disease (COVID-19): A Critical Review of the Literature to Date. AJR Am J Roentgenol. 2020;215:839-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 17. | Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH. Radiographic and CT Features of Viral Pneumonia. Radiographics. 2018;38:719-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 411] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 18. | Lin L, Fu G, Chen S, Tao J, Qian A, Yang Y, Wang M. CT Manifestations of Coronavirus Disease (COVID-19) Pneumonia and Influenza Virus Pneumonia: A Comparative Study. AJR Am J Roentgenol. 2021;216:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Demirjian NL, Fields BKK, Gholamrezanezhad A. Role of Chest CT in Resource-Driven Healthcare Systems. AJR Am J Roentgenol. 2020;215:W36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Rubin GD, Ryerson CJ, Haramati LB, Sverzellati N, Kanne JP, Raoof S, Schluger NW, Volpi A, Yim JJ, Martin IBK, Anderson DJ, Kong C, Altes T, Bush A, Desai SR, Goldin J, Goo JM, Humbert M, Inoue Y, Kauczor HU, Luo F, Mazzone PJ, Prokop M, Remy-Jardin M, Richeldi L, Schaefer-Prokop CM, Tomiyama N, Wells AU, Leung AN. The Role of Chest Imaging in Patient Management During the COVID-19 Pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest. 2020;158:106-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 486] [Article Influence: 97.2] [Reference Citation Analysis (0)] |
| 21. | American College of Radiology. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection | American College of Radiology. 2020. Available from: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. |
| 22. | Prokop M, van Everdingen W, van Rees Vellinga T, Quarles van Ufford H, Stöger L, Beenen L, Geurts B, Gietema H, Krdzalic J, Schaefer-Prokop C, van Ginneken B, Brink M; COVID-19 Standardized Reporting Working Group of the Dutch Radiological Society. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology. 2020;296:E97-E104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 646] [Cited by in RCA: 589] [Article Influence: 117.8] [Reference Citation Analysis (0)] |
| 23. | Yang R, Li X, Liu H, Zhen Y, Zhang X, Xiong Q, Luo Y, Gao C, Zeng W. Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiol Cardiothorac Imaging. 2020;2:e200047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 24. | Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, Panebianco V, Andreoli C, Colaiacomo MC, Zingaropoli MA, Ciardi MR, Mastroianni CM, Pugliese F, Alessandri F, Turriziani O, Ricci P, Catalano C. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30:6808-6817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 458] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 25. | Lieveld AWE, Azijli K, Teunissen BP, van Haaften RM, Kootte RS, van den Berk IAH, van der Horst SFB, de Gans C, van de Ven PM, Nanayakkara PWB. Chest CT in COVID-19 at the ED: Validation of the COVID-19 Reporting and Data System (CO-RADS) and CT Severity Score: A Prospective, Multicenter, Observational Study. Chest. 2021;159:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 26. | Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1111] [Cited by in RCA: 1270] [Article Influence: 254.0] [Reference Citation Analysis (0)] |
| 27. | Ates OF, Taydas O, Dheir H. Thorax Magnetic Resonance Imaging Findings in Patients with Coronavirus Disease (COVID-19). Acad Radiol. 2020;27:1373-1378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Caro-Dominguez P, Shelmerdine SC, Toso S, Secinaro A, Toma P, Damasio MB, Navallas M, Riaza-Martin L, Gomez-Pastrana D, Ghadimi Mahani M, Desoky SM, Ugas Charcape CF, Almanza-Aranda J, Ucar ME, Lovrenski J, Gorkem SB, Alexopoulou E, Ciet P, van Schuppen J, Ducou le Pointe H, Goo HW, Kellenberger CJ, Raissaki M, Owens CM, Hirsch FW, van Rijn RR; Collaborators of the European Society of Paediatric Radiology Cardiothoracic Task Force. Thoracic imaging of coronavirus disease 2019 (COVID-19) in children: a series of 91 cases. Pediatr Radiol. 2020;50:1354-1368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 29. | Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46:849-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 509] [Article Influence: 101.8] [Reference Citation Analysis (1)] |
| 30. | Amatya Y, Rupp J, Russell FM, Saunders J, Bales B, House DR. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med. 2018;11:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 31. | James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 754] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 32. | Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1298] [Cited by in RCA: 1535] [Article Influence: 307.0] [Reference Citation Analysis (0)] |
| 33. | Tan BK, Mainbourg S, Friggeri A, Bertoletti L, Douplat M, Dargaud Y, Grange C, Lobbes H, Provencher S, Lega JC. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax. 2021;76:970-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 34. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3488] [Cited by in RCA: 3407] [Article Influence: 681.4] [Reference Citation Analysis (0)] |
| 35. | Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Höchter D, Keppler O, Teupser D, Zwißler B, von Bergwelt-Baildon M, Kääb S, Massberg S, Pekayvaz K, Stark K. Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy. Circulation. 2020;142:1176-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 294] [Cited by in RCA: 415] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 36. | Overstad S, Tjonnfjord E, Garabet L, Fronas S, Bergan J, Aballi S, Ghanima W. Venous thromboembolism and coronavirus disease 2019 in an ambulatory care setting - A report of 4 cases. Thromb Res. 2020;194:116-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1748] [Article Influence: 349.6] [Reference Citation Analysis (0)] |
| 38. | Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 39. | Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA, Pleasance S, Le Gal G. Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies. J Thromb Haemost. 2010;8:1716-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, Huisman MV, Klok FA. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: A different phenotype of thrombotic disease? Thromb Res. 2020;193:86-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 41. | Cavagna E, Muratore F, Ferrari F. Pulmonary Thromboembolism in COVID-19: Venous Thromboembolism or Arterial Thrombosis? Radiol Cardiothorac Imaging. 2020;2:e200289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 42. | Dhawan RT, Gopalan D, Howard L, Vicente A, Park M, Manalan K, Wallner I, Marsden P, Dave S, Branley H, Russell G, Dharmarajah N, Kon OM. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med. 2021;9:107-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 43. | Zuckier LS, Moadel RM, Haramati LB, Freeman LM. Diagnostic Evaluation of Pulmonary Embolism During the COVID-19 Pandemic. J Nucl Med. 2020;61:630-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 44. | McFarland GA, Johnson SG. Nuclear Medicine Clinical Practice in the United States During the COVID-19 Era and Beyond. J Nucl Med Technol. 2020;48:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Narechania S, Renapurkar R, Heresi GA. Mimickers of chronic thromboembolic pulmonary hypertension on imaging tests: a review. Pulm Circ. 2020;10:2045894019882620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Gutte H, Mortensen J, Jensen CV, Johnbeck CB, von der Recke P, Petersen CL, Kjaergaard J, Kristoffersen US, Kjaer A. Detection of pulmonary embolism with combined ventilation-perfusion SPECT and low-dose CT: head-to-head comparison with multidetector CT angiography. J Nucl Med. 2009;50:1987-1992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Bhatia KD, Ambati C, Dhaliwal R, Paschkewitz R, Hsu E, Ho B, Young A, Emmett L. SPECT-CT/VQ versus CTPA for diagnosing pulmonary embolus and other lung pathology: Pre-existing lung disease should not be a contraindication. J Med Imaging Radiat Oncol. 2016;60:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Das JP, Yeh R, Schöder H. Clinical utility of perfusion (Q)-single-photon emission computed tomography (SPECT)/CT for diagnosing pulmonary embolus (PE) in COVID-19 patients with a moderate to high pre-test probability of PE. Eur J Nucl Med Mol Imaging. 2021;48:794-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Giovanella L, Ruggeri RM, Petranović Ovčariček P, Campenni A, Treglia G, Deandreis D. SARS-CoV-2-related thyroid disorders: a synopsis for nuclear medicine thyroidologists. Eur J Nucl Med Mol Imaging. 2021;48:1719-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, Villani L, Magri F, Latrofa F, Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest. 2021;44:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 51. | Basu S, Alavi A. Unparalleled contribution of 18F-FDG PET to medicine over 3 decades. J Nucl Med. 2008;49:17N-21N, 37N. [PubMed] |
| 52. | Hess S, Blomberg BA, Zhu HJ, Høilund-Carlsen PF, Alavi A. The pivotal role of FDG-PET/CT in modern medicine. Acad Radiol. 2014;21:232-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Basu S, Zhuang H, Torigian DA, Rosenbaum J, Chen W, Alavi A. Functional imaging of inflammatory diseases using nuclear medicine techniques. Semin Nucl Med. 2009;39:124-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 54. | Katal S, Amini H, Gholamrezanezhad A. PET in the diagnostic management of infectious/inflammatory pulmonary pathologies: a revisit in the era of COVID-19. Nucl Med Commun. 2021;42:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Kung BT, Seraj SM, Zadeh MZ, Rojulpote C, Kothekar E, Ayubcha C, Ng KS, Ng KK, Au-Yong TK, Werner TJ, Zhuang H, Hunt SJ, Hess S, Alavi A. An update on the role of 18F-FDG-PET/CT in major infectious and inflammatory diseases. Am J Nucl Med Mol Imaging. 2019;9:255-273. [PubMed] |
| 56. | Albano D, Bertagna F, Bertoli M, Bosio G, Lucchini S, Motta F, Panarotto MB, Peli A, Camoni L, Bengel FM, Giubbini R. Incidental Findings Suggestive of COVID-19 in Asymptomatic Patients Undergoing Nuclear Medicine Procedures in a High-Prevalence Region. J Nucl Med. 2020;61:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 57. | Amini H, Divband G, Montahaei Z, Dehghani T, Kaviani H, Adinehpour Z, Akbarian Aghdam R, Rezaee A, Vali R. A case of COVID-19 lung infection first detected by [18F]FDG PET-CT. Eur J Nucl Med Mol Imaging. 2020;47:1771-1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Setti L, Kirienko M, Dalto SC, Bonacina M, Bombardieri E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020;47:1649-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 59. | Scarlattei M, Baldari G, Silva M, Bola S, Sammartano A, Migliari S, Graziani T, Cidda C, Sverzellati N, Ruffini L. Unknown SARS-CoV-2 pneumonia detected by PET/CT in patients with cancer. Tumori. 2020;106:325-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 60. | Mattoli MV, Taralli S, Pennese E, D'Angelo C, Angrilli F, Villano C. Atypical Presentation of COVID-19 Incidentally Detected at 18F-FDG PET/CT in an Asymptomatic Oncological Patient. Clin Nucl Med. 2020;45:e383-e385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 61. | Tang YW, Schmitz JE, Persing DH, Stratton CW. Laboratory Diagnosis of COVID-19: Current Issues and Challenges. J Clin Microbiol. 2020;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 721] [Cited by in RCA: 803] [Article Influence: 160.6] [Reference Citation Analysis (0)] |
| 62. | Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 63. | Treglia G. The role of 18F-FDG PET for COVID-19 infection: myth versus reality. Clin Transl Imaging. 2020;8:125-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart. 2020;106:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 65. | Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 653] [Cited by in RCA: 886] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 66. | Yousefi-Koma A, Naghashzadeh F, Figtree GA, Patel S, Karimi Galougahi K. Multi-modality imaging of inflammation and ischemia for assessment of myocardial injury in Covid-19. J Nucl Cardiol. 2021;28:3100-3103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 67. | Satapathy S, Kumar R, Kavanal AJ, Krishnaraju VS, Ramachandran A, Deo P, Dhir V, Mittal BR. COVID-19 related multisystem inflammatory syndrome in children (MIS-C): Role of 18F-FDG PET/CT to assess myocardial involvement. J Nucl Cardiol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Slart RHJA; Writing group; Reviewer group; Members of EANM Cardiovascular; Members of EANM Infection & Inflammation; Members of Committees, SNMMI Cardiovascular; Members of Council, PET Interest Group; Members of ASNC; EANM Committee Coordinator. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur J Nucl Med Mol Imaging. 2018;45:1250-1269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 339] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 69. | Dejaco C, Ramiro S, Duftner C, Besson FL, Bley TA, Blockmans D, Brouwer E, Cimmino MA, Clark E, Dasgupta B, Diamantopoulos AP, Direskeneli H, Iagnocco A, Klink T, Neill L, Ponte C, Salvarani C, Slart RHJA, Whitlock M, Schmidt WA. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis. 2018;77:636-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 637] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 70. | Kang F, Han Q, Zhou X, Zheng Z, Wang S, Ma W, Zhang K, Quan Z, Yang W, Wang J, Zhu P. Performance of the PET vascular activity score (PETVAS) for qualitative and quantitative assessment of inflammatory activity in Takayasu's arteritis patients. Eur J Nucl Med Mol Imaging. 2020;47:3107-3117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Blockmans D, de Ceuninck L, Vanderschueren S, Knockaert D, Mortelmans L, Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 449] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 72. | Sollini M, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, Gelardi F, Chiti A. Vasculitis changes in COVID-19 survivors with persistent symptoms: an [18F]FDG-PET/CT study. Eur J Nucl Med Mol Imaging. 2021;48:1460-1466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 73. | Mansueto G, Lanza G, Fisicaro F, Alaouieh D, Hong E, Girolami S, Montella M, Feola A, Di Napoli M. Central and Peripheral Nervous System Complications of Vasculitis Syndromes From Pathology to Bedside: Part 1-Central Nervous System. Curr Neurol Neurosci Rep. 2022;22:47-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 74. | Vaschetto R, Cena T, Sainaghi PP, Meneghetti G, Bazzano S, Vecchio D, Pirisi M, Brustia D, Barini M, Cammarota G, Castello L, Della Corte F. Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci. 2020;79:71-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 75. | Lersy F, Anheim M, Willaume T, Chammas A, Brisset JC, Cotton F, Kremer S. Cerebral vasculitis of medium-sized vessels as a possible mechanism of brain damage in COVID-19 patients. J Neuroradiol. 2021;48:141-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 76. | Whittaker A, Anson M, Harky A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol Scand. 2020;142:14-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 77. | Fisicaro F, Di Napoli M, Liberto A, Fanella M, Di Stasio F, Pennisi M, Bella R, Lanza G, Mansueto G. Neurological Sequelae in Patients with COVID-19: A Histopathological Perspective. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 78. | Pajo AT, Espiritu AI, Apor ADAO, Jamora RDG. Neuropathologic findings of patients with COVID-19: a systematic review. Neurol Sci. 2021;42:1255-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 79. | Cosentino G, Todisco M, Hota N, Della Porta G, Morbini P, Tassorelli C, Pisani A. Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: A critical systematic review. Eur J Neurol. 2021;28:3856-3865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 80. | Kremer S, Gerevini S, Ramos A, Lersy F, Yousry T, Vernooij MW, Anzalone N, Jäger HR. Neuroimaging in patients with COVID-19: a neuroradiology expert group consensus. Eur Radiol. 2022;32:3716-3725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Kremer S, Lersy F, de Sèze J, Ferré JC, Maamar A, Carsin-Nicol B, Collange O, Bonneville F, Adam G, Martin-Blondel G, Rafiq M, Geeraerts T, Delamarre L, Grand S, Krainik A, Caillard S, Constans JM, Metanbou S, Heintz A, Helms J, Schenck M, Lefèbvre N, Boutet C, Fabre X, Forestier G, de Beaurepaire I, Bornet G, Lacalm A, Oesterlé H, Bolognini F, Messié J, Hmeydia G, Benzakoun J, Oppenheim C, Bapst B, Megdiche I, Henry Feugeas MC, Khalil A, Gaudemer A, Jager L, Nesser P, Talla Mba Y, Hemmert C, Feuerstein P, Sebag N, Carré S, Alleg M, Lecocq C, Schmitt E, Anxionnat R, Zhu F, Comby PO, Ricolfi F, Thouant P, Desal H, Boulouis G, Berge J, Kazémi A, Pyatigorskaya N, Lecler A, Saleme S, Edjlali-Goujon M, Kerleroux B, Zorn PE, Matthieu M, Baloglu S, Ardellier FD, Willaume T, Brisset JC, Boulay C, Mutschler V, Hansmann Y, Mertes PM, Schneider F, Fafi-Kremer S, Ohana M, Meziani F, David JS, Meyer N, Anheim M, Cotton F. Brain MRI Findings in Severe COVID-19: A Retrospective Observational Study. Radiology. 2020;297:E242-E251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 306] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 82. | Gulko E, Oleksk ML, Gomes W, Ali S, Mehta H, Overby P, Al-Mufti F, Rozenshtein A. MRI Brain Findings in 126 Patients with COVID-19: Initial Observations from a Descriptive Literature Review. AJNR Am J Neuroradiol. 2020;41:2199-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 83. | Moonis G, Filippi CG, Kirsch CFE, Mohan S, Stein EG, Hirsch JA, Mahajan A. The Spectrum of Neuroimaging Findings on CT and MRI in Adults With COVID-19. AJR Am J Roentgenol. 2021;217:959-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, Jia T, Zhao Y, Wang D, Xiao A, Yin B. Cerebral Micro-Structural Changes in COVID-19 Patients - An MRI-based 3-month Follow-up Study. EClinicalMedicine. 2020;25:100484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 418] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 85. | Sklinda K, Dorobek M, Wasilewski PG, Dreżewski K, Dȩbicka M, Walecki J, Mruk B. Radiological Manifestation of Neurological Complications in the Course of SARS-CoV-2 Infection. Front Neurol. 2021;12:711026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 86. | Delorme C, Paccoud O, Kas A, Hesters A, Bombois S, Shambrook P, Boullet A, Doukhi D, Le Guennec L, Godefroy N, Maatoug R, Fossati P, Millet B, Navarro V, Bruneteau G, Demeret S, Pourcher V; CoCo-Neurosciences study group and COVID SMIT PSL study group. COVID-19-related encephalopathy: a case series with brain FDG-positron-emission tomography/computed tomography findings. Eur J Neurol. 2020;27:2651-2657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 87. | Grimaldi S, Lagarde S, Harlé JR, Boucraut J, Guedj E. Autoimmune Encephalitis Concomitant with SARS-CoV-2 Infection: Insight from 18F-FDG PET Imaging and Neuronal Autoantibodies. J Nucl Med. 2020;61:1726-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 88. | Pizzanelli C, Milano C, Canovetti S, Tagliaferri E, Turco F, Verdenelli S, Nesti L, Franchi M, Bonanni E, Menichetti F, Volterrani D, Cosottini M, Siciliano G. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: Case-report and review of the literature. Brain Behav Immun Health. 2021;12:100210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 89. | Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, Million M, Raoult D, Cammilleri S, Eldin C. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48:2823-2833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 296] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 90. | Cohen ME, Eichel R, Steiner-Birmanns B, Janah A, Ioshpa M, Bar-Shalom R, Paul JJ, Gaber H, Skrahina V, Bornstein NM, Yahalom G. A case of probable Parkinson's disease after SARS-CoV-2 infection. Lancet Neurol. 2020;19:804-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 158] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 91. | Morassi M, Palmerini F, Nici S, Magni E, Savelli G, Guerra UP, Chieregato M, Morbelli S, Vogrig A. SARS-CoV-2-related encephalitis with prominent parkinsonism: clinical and FDG-PET correlates in two patients. J Neurol. 2021;268:3980-3987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 92. | Müller C, Schibli R, Maurer B. Can Nuclear Imaging of Activated Macrophages with Folic Acid-Based Radiotracers Serve as a Prognostic Means to Identify COVID-19 Patients at Risk? Pharmaceuticals (Basel). 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Iking J, Staniszewska M, Kessler L, Klose JM, Lückerath K, Fendler WP, Herrmann K, Rischpler C. Imaging Inflammation with Positron Emission Tomography. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 94. | Fan Z, Calsolaro V, Atkinson RA, Femminella GD, Waldman A, Buckley C, Trigg W, Brooks DJ, Hinz R, Edison P. Flutriciclamide (18F-GE180) PET: First-in-Human PET Study of Novel Third-Generation In Vivo Marker of Human Translocator Protein. J Nucl Med. 2016;57:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 95. | Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9:558-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 262] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 96. | Linares A, Couling LE, Carrera EJ, Speth RC. Receptor Autoradiography Protocol for the Localized Visualization of Angiotensin II Receptors. J Vis Exp. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Czernin J, Fanti S, Meyer PT, Allen-Auerbach M, Hacker M, Sathekge M, Hicks R, Scott AM, Hatazawa J, Yun M, Schöder H, Bartenstein P, Herrmann K. Nuclear Medicine Operations in the Times of COVID-19: Strategies, Precautions, and Experiences. J Nucl Med. 2020;61:626-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 98. | Azam SA, Myers L, Fields BKK, Demirjian NL, Patel D, Roberge E, Gholamrezanezhad A, Reddy S. Coronavirus disease 2019 (COVID-19) pandemic: Review of guidelines for resuming non-urgent imaging and procedures in radiology during Phase II. Clin Imaging. 2020;67:30-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Nakajima K, Kato H, Yamashiro T, Izumi T, Takeuchi I, Nakajima H, Utsunomiya D. COVID-19 pneumonia: infection control protocol inside computed tomography suites. Jpn J Radiol. 2020;38:391-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 100. | Juengling FD, Maldonado A, Wuest F, Schindler TH. The Role of Nuclear Medicine for COVID-19: Time to Act Now. J Nucl Med. 2020;61:781-782. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |