Published online Jun 28, 2022. doi: 10.4329/wjr.v14.i6.165
Peer-review started: January 29, 2022
First decision: April 10, 2022
Revised: April 26, 2022
Accepted: June 20, 2022
Article in press: June 20, 2022
Published online: June 28, 2022
Processing time: 150 Days and 5.9 Hours
The commonly used predictors of clinically relevant postoperative pancreatic fistula (CR-POPF) following pancreaticoduodenectomy (PD) have subjective assessment components and can be used only in the postoperative setting. Also, the available objective predictors based on preoperative cross-sectional imaging were not prospectively studied.
To evaluate the accuracy of the pancreatic attenuation index (PAI) and pancreatic enhancement ratio (PER) for predicting CR-POPF following PD and its correlation with pancreatic fat fraction and fibrosis.
A prospective observational study included patients who underwent PD for benign and malignant pathology of the periampullary region or pancreatic head between February 2019 and February 2021. Patients undergoing extended or total pancreatectomy and those with severe atrophy of pancreatic tissue or extensive parenchymal calcifications in the pancreatic head and neck precluding calculation of PAI and PER were excluded from the study. Preoperatively PAI was measured in the neck of the pancreas by marking regions of interest (ROI) in the non-contrast computed tomography (CT), and PER was measured during the contrast phase of the CT abdomen. Also, the fibrosis score and fat fraction of the pancreatic neck were assessed during the histopathological examination. Demographic, clinical and preoperative radiological indices (PAI, PER) were evaluated to predict CR-POPF. Preoperative pancreatic neck CT indices were correlated with the histopathological assessment of fat fraction and fibrosis.
Of the 70 patients who underwent PD, 61 patients fulfilling the inclusion criteria were included in the analysis. The incidence of CR-POPF was 29.5% (18/61). PAI had no association with the development of CR-POPF. Of the preoperative parameters, PER (mean ± standard deviation [SD]) was significantly lower in patients developing CR-POPF (0.58 ± 0.20 vs 0.81 ± 0.44, P = 0.006). The area under the curve for the PER was 0.661 (95%CI: 0.517-0.804), which was significant (P = 0.049). PER cut-off of 0.673 predicts CR-POPF with 77.8% sensitivity and 55.8% specificity. PAI and PER had a weak negative correlation (Strength-0.26, P = 0.037). Also, PER showed a moderately positive correlation with fibrosis (Strength 0.50, P < 0.001). Patients with CR-POPF had a significantly higher incidence of the intraabdominal abscess (50% vs 2.3%, P < 0.001), delayed gastric emptying (83.3% vs 30.2, P < 0.001), and prolonged mean (± SD) postoperative hospital stay (26.8 ± 13.9 vs 9.6 ± 3.6, P = 0.001).
PER exhibited good accuracy in predicting the development of CR-POPF. PER additionally showed a good correlation with PAI and fibrosis scores and may be used as an objective preoperative surrogate for assessing pancreatic texture. However, ROI-based PAI did not show any association with CR-POPF and pancreatic fat fraction.
Core Tip: The prospective observational study evaluated the accuracy of the pancreatic computed tomography indices in predicting clinically relevant pancreatic fistula after pancreaticoduodenectomy. Though the predictive accuracy of pancreatic attenuation index (PAI) was low, pancreatic enhancement ratio (PER) exhibited good accuracy in predicting the development of clinically relevant postoperative pancreatic fistula (CR-POPF). Also, PER showed a statistically significant weak negative correlation with PAI and moderately positive correlation with fibrosis scores suggesting that PER may be an objective preoperative surrogate for assessing pancreatic texture. Preoperative quantification of PER can improve the risk stratification and management of patients at high risk of CR-POPF.
- Citation: Gnanasekaran S, Durgesh S, Gurram R, Kalayarasan R, Pottakkat B, Rajeswari M, Srinivas BH, Ramesh A, Sahoo J. Do preoperative pancreatic computed tomography attenuation index and enhancement ratio predict pancreatic fistula after pancreaticoduodenectomy? World J Radiol 2022; 14(6): 165-176
- URL: https://www.wjgnet.com/1949-8470/full/v14/i6/165.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i6.165
Pancreaticoduodenectomy (PD) has been established as the standard surgical treatment for resectable pancreatic head cancer and periampullary tumors. Advances in surgical technology and perioperative care have reduced PD-related mortality from roughly 20% to less than 5%[1]. But the morbidity following a PD continues to remain high[2]. Hence, the focus has shifted to make PD a less morbid procedure. The most feared consequence of PD is postoperative pancreatic fistula (POPF)[1,2]. POPF is frequently linked to a lengthy and challenging hospital stay that imposes a significant social and financial burden. Despite numerous novel perioperative therapies, there has been no substantial reduction in reported POPF rates[2,3].
The implications of identifying patients at risk of clinically relevant POPF (CR-POPF) are manifold. To begin, we can tailor surgical procedures to high-risk factors by making modifications that have been demonstrated to reduce the occurrence of CR-POPF. Second, high-risk patients can be closely assessed for the need for early intervention to avoid the disastrous consequences of POPF. Finally, it helps identify low-risk patients in whom the enhanced recovery after surgery (ERAS) pathway may be implemented confidently. Commonly used predictive models for POPF, such as the Fistula Risk Score (FRS), modified FRS, and Day 1 Drain Fluid Amylase estimation, can be used only in the postoperative setting[4-6]. Attenuation and enhancement patterns of pancreatic parenchyma on computed tomography (CT) were studied as preoperative predictors of CR-POPF[7-11]. While pancreatic attenuation index (PAI) can quantify pancreatic fat, pancreatic enhancement ratio (PER) has been correlated with pancreatic fibrosis. Therefore, the presence of a higher preoperative mean PER and lower PAI can be considered protective against the development of CR-POPF after PD[7-11]. However, the predictive accuracy of these indices for CR-POPF was not prospectively studied. Also, the distribution of fat and fibrosis within the pancreas varies, with pancreatic neck fat and fibrosis assuming relevance since it is the site of anastomosis, which previous studies have not addressed. Also, no previous research has prospectively correlated preoperative PAI and PER with histological pancreatic fat fraction and fibrosis, particularly in the neck. The present study aims to calculate the accuracy of the pancreatic neck PAI and PER in predicting CR-POPF and its correlation with histological pancreatic neck fat fraction and fibrosis scoring.
Patients above 18 years of age who underwent elective PD for both benign and malignant pathology involving periampullary and pancreatic head from February 2019 to February 2021 and consented to participate were assessed for inclusion in the prospective observational study. Patients undergoing extended or total pancreatectomy and those with contraindication to undergo preoperative contrast-enhanced CT (CECT) or severe atrophy of pancreatic tissue or extensive parenchymal calcifications in the pancreatic head and neck precluding calculation of PAI and PER were excluded from the study. Also, patients who died in the immediate postoperative period (< 48 h) were excluded from the analysis. The study was approved by the Institute's scientific advisory and Ethics Committee (JIP/IEC/2018/500 dated 25-01-2019).
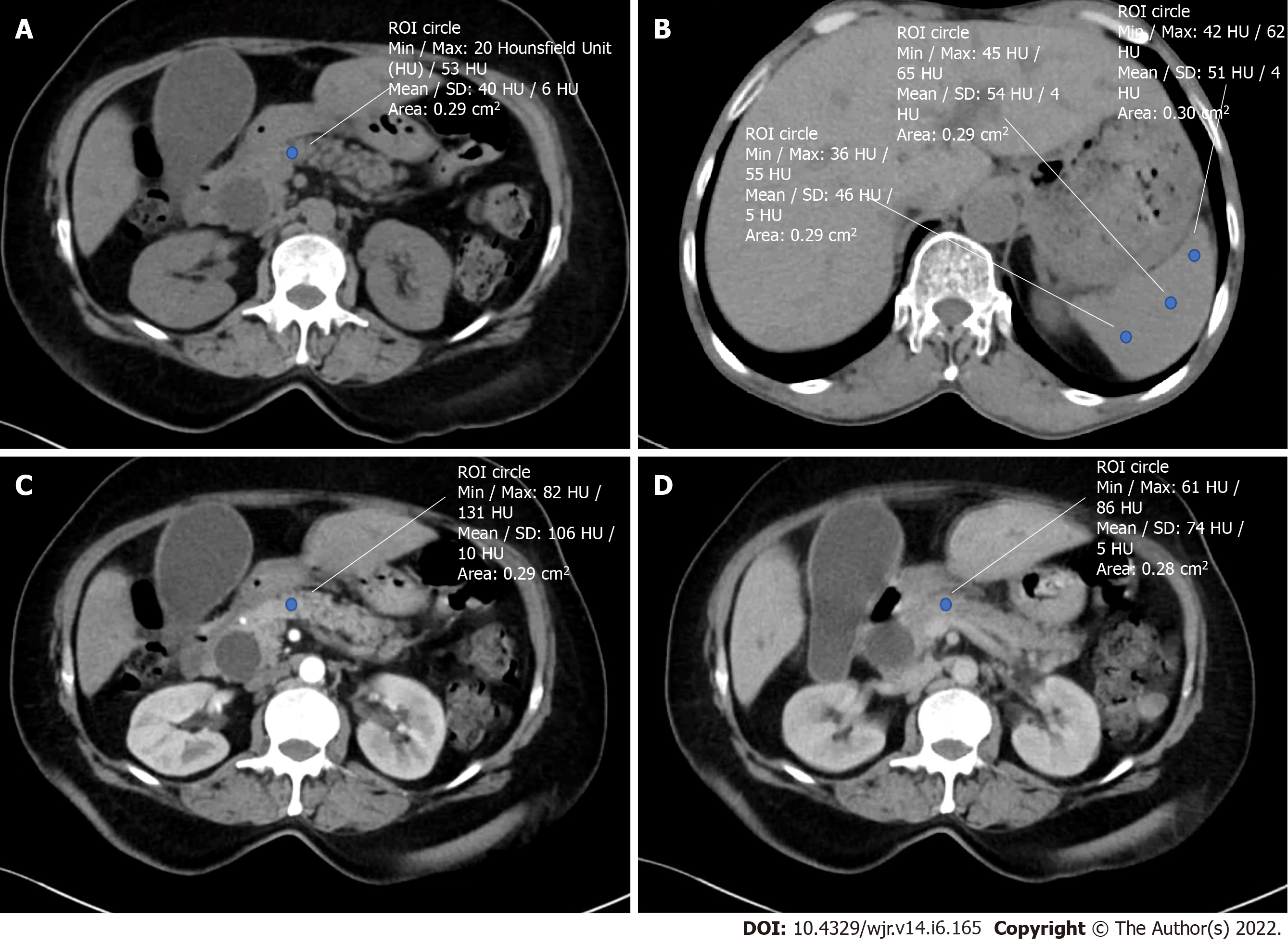
As part of the routine evaluation, all patients underwent a pancreatic protocol CECT. Non-contrast and CECT of the abdomen and pelvis were performed using a 128 slice CT scanner (SomatomTM Definition Edge, M/s Siemens, Erlangen, Germany). Intravenous iodinated contrast media Iohexol with 300mg Iodine concentration (ContrapaqueTM 300, JB chemicals and pharmaceuticals limited, India) was administered at a dose of 1.5 mL/ kg body weight at the rate of 3-4 mL/s followed by 20 mL of the saline chase at 3 mL/s. A dual head pressure injector (Medrad® Stellant D pedestal-mount with Certegra® Workstation) was used for contrast injection. Scans were triggered using the Bolus tracking technique when the threshold of 150HU was reached in the upper abdominal aorta. Contrast-enhanced scans included late arterial phase at 30-40 sec from the start of contrast injection (12-15 sec after bolus tracking), portal venous phase at 60-70 sec (25-30 secs delay after the arterial phase) and equilibrium phase at 3 min from contrast injection. The plain and contrast-enhanced images were reconstructed at 3 mm thickness and viewed in a picture archiving and communication system workstation using CentricityTM Universal Viewer Zero Footprint (GE, United States). On non-enhanced CT images, Hounsfield Units (HU) represents tissue density, while on contrast-enhanced CT images, it represents a measure of combination involving density and vascularity (18). Attenuation (HU) was measured in the neck of the pancreas and spleen, and attenuation values were calculated with regions of interest (ROI) of 0.2-0.3 cm2. The mean of 3 ROI values obtained in the neck region divided by splenic attenuation gave the PAI of the pancreatic neck (Figure 1). PER was calculated in the neck of the pancreas as (EP-Pre)/ (AP-Pre) (AP-arterial phase, pre-nonenhanced phase, EP-equilibrium phase)[11].
All patients underwent pylorus resecting PD at the surgeon's discretion using an open, laparoscopic, or robot-assisted technique. All operations were performed by three qualified surgeons with extensive experience in pancreatobiliary surgery. Pancreaticojejunostomy (PJ) was performed using modified Blumgart or a modified invagination technique depending on the size of the pancreatic duct at the surgeon's discretion. Hepaticojejunostomy (HJ) was done 15 cm distal to PJ by Blumgart Kelly technique. Antecolic Gastrojejunostomy was done about 50 cm distal to the HJ. Two abdominal drains were placed, one in the subhepatic space near HJ and the other one adjacent to the PJ. Feeding jejunostomy was done routinely for early postoperative enteral feeding.
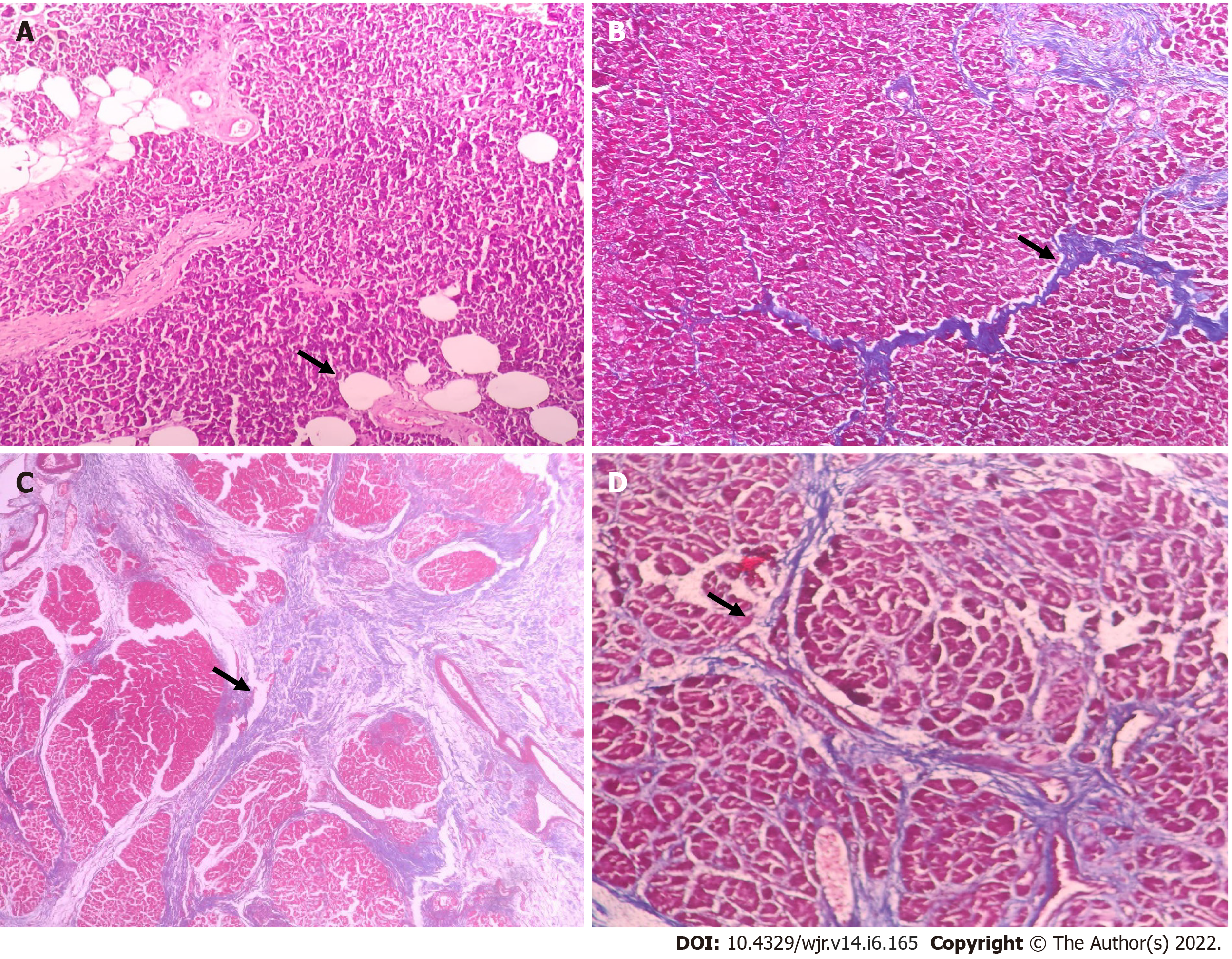
A pancreatic neck tissue specimen was sent for histopathological evaluation. The pathologist, blinded to CT data and pancreatic texture, performed histological analysis. The existence of Langerhans' islets confirmed the Pancreatic tissue. Only tissue free of inflammatory lesions and calcifications was evaluated. The histologic pancreatic fat fraction was defined as the area ratio of pancreatic intraparenchymal fat to that of the total tissue times 100% (< 5%-mildly fatty; 5-15%-moderately fatty, > 15%-heavily fatty) using hematoxylin and eosin stain[12]. The degree of fibrosis was studied using Masson's trichome stain. The extent of intralobular and interlobular fibrosis was separately measured, and the total score (0-6) was calculated (Figure 2). According to the total score, fibrosis was classified as weak (score 0-3) and heavy (score 4-6)[13].
The primary objective of this prospective observational study was to determine the predictive accuracy of PAI and PER for CR-POPF following PD. The patients' demographic and clinical data, including age, sex, body mass index, bilirubin level, preoperative biliary drainage, comorbidities (diabetes mellitus and hypertension), weight loss and radiological indices (PAI and PER) were collected to determine the preoperative factors predictive of CR-POPF. Also, the operative outcomes, including operative time, estimated blood loss, need for blood transfusion, pancreatic texture and postoperative complications, were compared between patients with and without CR-POPF. Delayed gastric emptying [DGE], Post pancreatectomy hemorrhage (PPH) and Postoperative pancreatic fistula [POPF] were graded as per the International Study Group for Pancreatic Surgery [ISGPS] definition[14-16]. To correlate preoperative CT indices (PAI and PER) with histopathological features, pancreatic neck fat fraction and fibrosis were measured in the pancreatic neck tissue specimen.
The statistical analysis was done using IBM SPSS Statistics for Windows, Version 28.0. (Armonk, NY, United States). The estimated sample size was calculated, anticipating an AUC of 0.75 for PER in predicting CR-POPF with 90% power and a 5% level of significance. The required sample size was calculated as 60. Both descriptive and inferential statistics were used to analyze the data. Baseline characteristics of the patients are presented by descriptive statistics. Categorical data (sex, clinical factors, presence or absence of DGE, CRPOPF, PPH, Intraabdominal abscess, pancreatic gland texture, pathological diagnosis) was described using percentages and frequencies and compared by using Fischer exact test or Chi-square test. The normality of continuous data was assessed by the Kolmogorov-Smirnov test. The normally distributed data were described by mean ± standard deviation (SD). Median and interquartile range was used for non-Gaussian data. Comparison of the continuous data (age, duct size, serum bilirubin) between the two groups was done by independent Student's t-test for parametric data and Mann-Whitney U-test for nonparametric data. The ability of PAI and PER to predict CR-POPF was assessed using receiver operating characteristic (ROC) analysis. A perfect test will have an AUC equaling 1. A 95% confidence interval was calculated and reported for the outcome measures. Statistical analysis was carried out at a 5% significance level, and P < 0.05 was considered statistically significant. The Pearson correlation coefficient (r) was used to examine the association of the histologic pancreatic fibrosis score and fat fraction with PAI and PER independently. A perfect positive correlation will show a value of +1, and a value of -1 indicates a perfect negative correlation.
Of the 70 patients who underwent PD during the study period, 61 patients fulfilling the inclusion criteria were included in the analysis. Five patients did not achieve the required ROI (0.2-0.3 cm2), three patients who could not undergo histopathological analysis due to insufficient or other pathological changes in the sample and one patient who died during the immediate postoperative period were excluded from the analysis.
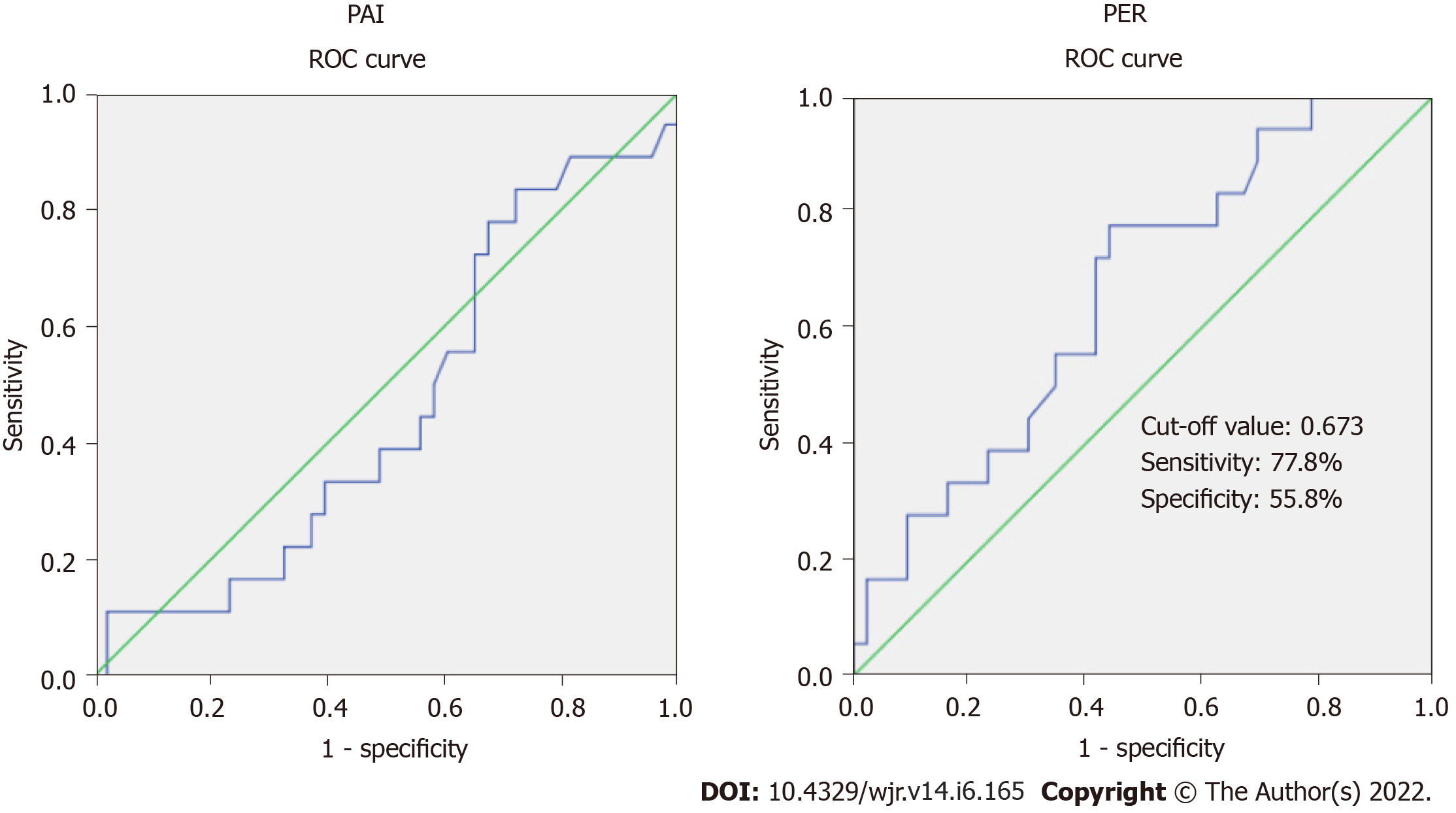
The overall incidence of CR-POPF in the study cohort was 29.5% (18/61). The demographic variables, history of weight loss, presence of comorbidities, preoperative hemoglobin, serum bilirubin and preoperative biliary drainage were comparable between groups with and without CR-POPF (Table 1). While PAI was similar between the two groups, the mean (± SD) PER was significantly lower in patients developing CR-POPF (0.58 ± 0.20 vs 0.81 ± 0.44, P = 0.006). The ROC analysis was done to determine the accuracy of PAI and PER in predicting CR-POPF (Figure 3). The area under the curve for the PAI was 0.461 (95%CI: 0.304-0.617), which was not significant (P = 0.630). At the same time, the area under the curve for the PER was 0.661 (95%CI: 0.517-0.804), which was significant (P = 0.049). We can predict whether a randomly chosen case will develop CR-POPF with a probability of 66.1%. With a cut-off of PER = 0.673, PER can predict those with CR-POPF with 77.8% sensitivity and 55.8% specificity (Figure 3).
| Parameter | CR-POPF, n = 18 | No CR-POPF, n = 43 | P value |
| Age in yr, mean ± SD | 53.7 ± 10.8 | 54.7 ± 11.5 | 0.746 |
| Sex, n (%) | |||
| Male | 10 (55.6) | 28 (65.1) | 0.567 |
| Female | 8 (44.4) | 15 (34.9) | |
| BMI in kg/m2, mean ± SD | 21.1 ± 4.4 | 20.1 ± 3.9 | 0.388 |
| Weight loss, n (%) | 15 (83.3) | 32 (74.4) | 0.525 |
| Comorbidities, n (%) | 11 (61.1) | 22 (51.2) | 0.578 |
| Hemoglobin in gm%, mean ± SD | 10.7 ± 1.4 | 10.8 ± 1.5 | 0.735 |
| Preoperative serum bilirubin (mg/dL), median (IQR) | 2 (1.8-6) | 3 (1-7) | 0.848 |
| Preoperative biliary drainage, n (%) | 10 (55.6) | 22 (51.2) | 0.786 |
| Pancreatic attenuation index, mean ± SD | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.741 |
| Pancreatic enhancement ratio, mean ± SD | 0.6 ± 0.2 | 0.8 ± 0.4 | 0.006 |
There was no significant correlation between PAI and fat fraction or fibrosis score (Table 2). Pearson correlation coefficient between PER and fibrosis score was moderately positive and statistically significant with a strength of 0.504 and a P value of < 0.001. The positive correlation between PER and fibrosis score suggests that an increase in the intraparenchymal fibrosis results in the delayed pancreatic enhancement on CT, reflected as an increased PER. The correlation coefficient between PER and PAI was low negative and statistically significant, with a strength of -0.267 and a P-value of 0.037. The negative correlation between PER and PAI signifies that as the fibrosis increases, resulting in an increased delayed pancreatic enhancement, the fat fraction within the pancreas decreases, represented by a lower PAI.
The operative time, blood loss, intraoperative blood transfusion, surgical approach and pancreatic duct size was comparable between the two groups (Table 3). The proportion of patients with soft pancreas was significantly higher in the CR-POPF group. Postoperatively patients with CR-POPF had a significantly higher incidence of delayed gastric emptying (83.3% vs 30.2%, P < 0.001) and intra-abdominal abscess (50% vs 2.3%, P < 0.001). Also, Patients with CR-POPF had a prolonged postoperative hospital stay. There was no significant difference in the pancreatic fat fraction and fibrosis score between the two groups.
| Parameter | CR-POPF, n = 18 | No CR-POPF,n = 43 | P value |
| Operative time in min, mean ± SD | 521.9 ± 123 | 463.9 ± 101.2 | 0.275 |
| Blood loss in mL, median (IQR) | 550 (350-725) | 475 (350-800) | 0.830 |
| Intraoperative blood transfusion, n (%) | 6 (33.3) | 17 (39.5) | 0.775 |
| Pancreatic texture, n (%) | |||
| Firm | 1 (5.6) | 20 (47.6) | 0.002 |
| Soft | 17 (94.4) | 22 (52.4) | |
| Pancreatic duct size in mm, mean ± SD | 2.8 ± 1.1 | 3.4 ± 1.6 | 0.169 |
| Surgical approach, n (%) | |||
| Open | 9 (50) | 24 (55.8) | |
| Laparoscopic | 6 (33.3) | 12 (27.9) | |
| Robot assisted | 3 (16.7) | 7 (16.3) | 0.927 |
| Delayed gastric emptying, n (%) | 15 (83.3) | 13 (30.2) | < 0.001 |
| Postpancreatectomy hemorrhage, n (%) | 3 (16.7) | 4 (9.3) | 0.662 |
| Intra-abdominal abscess, n (%) | 9 (50) | 1 (2.3) | < 0.001 |
| Hospital stay in d, mean ± SD | 26.8 ± 13.9 | 9.6 ±.6 | 0.001 |
| Pathology, n (%) | |||
| Malignant | 17 (94.4) | 35 (81.4) | |
| Benign | 1 (5.6) | 8 (18.6) | 0.259 |
| Fat fraction, n (%) | |||
| Absent | 6 (33.3) | 20 (46.5) | |
| Mild | 9 (50.0) | 17 (39.6) | 0.669 |
| Moderate | 3 (16.7) | 6 (13.9) | |
| Fibrosis score, n (%) | |||
| Weak | 16 (88.9) | 27 (62.8) | |
| Heavy | 2 (11.1) | 16 (37.2) | 0.063 |
The present study documents the role of preoperative CT indices, especially PER, in predicting CR-POPF. Despite improved surgical techniques and perioperative management, PD remains a morbid procedure with a 30-50% estimated morbidity rate[1,2]. POPF is the critical cause of post-PD morbidity, and pancreatic texture has been reported as an important predictive parameter for POPF[17,18]. A soft pancreatic texture has been associated with an increased risk, while a firm pancreas protects against POPF. However, intraoperative assessment of gland consistency by the surgeon's digital palpation is highly subjective[18]. In recent years, laparoscopic and robotic approaches for PD have increased globally. Assessment of pancreatic texture during minimally invasive PD, especially the robotic approach, is challenging. Hence, parameters like acinar cell density and fibrosis score on histopathological examination were evaluated as objective criteria for pancreatic texture[19]. However, these parameters are not helpful for the preoperative prediction of POPF. Preoperative CR-POPF prediction using dependable parameters can assist in implementing intraoperative and postoperative measures to reduce CR-POPF-related morbidity. Hence, attempts have been made to correlate preoperative cross-sectional imaging (CECT and MRI) with pancreatic texture[7-11,20]. Most studies evaluating PAI and PER on the CECT abdomen were retrospective, which precludes assessment and correlation of pancreatic neck fat fraction and fibrosis[7-11].
The high fat fraction in the pancreas makes the pancreas softer, which might increase the risk of POPF following PD. Liver Attenuation Index is the widely used radiological index to measure liver fat fraction[21]. Similarly, Yardimci et al[22] proposed PAI as a simple tool to assess pancreatic fat fraction based on the study of 76 patients who underwent PD. The PAI cut-off value of 0.67 was valuable for risk calculation in their research. Other studies also reported the usefulness of PAI in assessing pancreatic fat fraction[7,8]. Although PAI was proposed as a simple tool that can be quickly evaluated, the lack of adequate external validation remains the primary impediment to its widespread adoption. In the present study, PAI was not useful for predicting CR-POPF. Also, PAI did not correlate with histological pancreatic fat fraction. On the other hand, PAI correlated negatively with PER, indicating an inverse relationship between pancreatic fat content with fibrosis and pancreatic texture. According to our analysis, PAI may not accurately reflect pancreas fat fraction and softness. However, the lack of predictability and correlation may be due to the small sample size and the underpowered study.
An increase in the fibrosis of the pancreas makes the gland firmer, decreasing the incidence of POPF. It is technically straightforward to perform a pancreatoenteric anastomosis on a firmer gland. Maehira et al[9], in a retrospective analysis of 115 patients, reported that the pattern of pancreatic enhancement could be a reliable predictor for the development of CR-POPF. Also, Kang et al[11] documented that PER cut-off of 1.100 might be a valuable predictor for the risk of developing a CR-POPF following PD. In the present study, the PER cut-off value of 0.661 had a sensitivity of 78% and a specificity of 55% in predicting CR-POPF. Also, PER had a positive correlation with pancreatic fibrosis. The main drawback of using PER as a predictor for CR-POPF is that the perfusion of organs with injected contrast depends upon the patient's hemodynamic status, influencing the final indices values, unlike PAI, which is independent of contrast.
With pancreatic fibrosis known for the protection of CR-POPF and pancreatic fatty infiltration being a concern, it is prudent that radiological indices be correlated with histopathological findings to determine their predictive accuracy. While multiple studies have evaluated different CT parameters, a few have tried to link with histology. However, no previous studies have looked at both contrast and non-contrast indices and their relationship with pancreatic neck fat fraction and fibrosis. The present study results are similar to the study by Hashimoto et al[10], which reported a correlation between PER and pancreatic fibrosis. However, in contrast to the current study, bolus tracking was not used in their imaging protocol. Hence, the timing differences between the scan performance and arrival of injected contrast in the structures were not considered. Further, the iodine concentration of the contrast used could affect the magnitude of enhancement. Kang et al[11] reported that the CT enhancement ratio was a more powerful predictor of pancreatic fistula than fecal elastase-1 Levels. However, in contrast to the current study, their study was a retrospective analysis, with no reference standards of the pathological fibrosis data to correlate with the CT enhancement ratios.
Our study did not show any correlation of PAI with pancreatic fat fraction. Kim et al[12] reported a significant correlation between the PAI and histopathological fat fraction. However, the clinical parameter that was assessed was post PD glycemic control, unlike CR-POPF in our study. Though the study was able to show a positive correlation, it was a retrospective study, with a small sample size and lack of clarity on whether the histological fat fraction corresponded with the area of ROI. Hori et al[23] have recently shown that area-based assessment on unenhanced CT showed higher correlation and concordance with histopathology-based fat fraction in the pancreas than the ROI-based CT attenuation assessment. A few studies have reported the usefulness of MRI for analyzing pancreatic fat content[20]. As MRI is not widely available and routinely used for preoperative workup of patients undergoing PD, its use as a predictor tool for CR-POPF has a limited application. The different CT attenuation and enhancement values reported in the present study could be due to the calculation of CT indices precisely at the pancreatic neck. In contrast, previous studies measured randomly across the pancreas.
Our study is limited by a few factors that require attention. Firstly, the small sample size may not represent the entire patient cohort. A future study with a larger sample size is needed to determine PAI's predictability accurately. The reliable prediction of CR-POPF preoperatively is challenging in patients undergoing PD as it is a mix of a heterogeneous population of patients subjected to different heterogeneous surgical approaches. In PD, with various reconstructive options available and each Institute and each surgeon adopting a technique of their own choice, creating a standardized operative technique is nearly impossible. A homogenous population of patients and standardized uniform surgical techniques are prerequisites for any preoperative prediction models to show good predictive ability, both of which are difficult to achieve in the case of PD. The patient characteristics, the surgeon's expertise and surgical techniques are vital in deciding the risk of a patient developing CR-POPF. With all these factors coming into play, it is expected that accurate preoperative prediction of CR-POPF is not always possible. Even if some studies show a single or group of parameters as predictors for CR-POPF, external validation might not offer the same result because of the factors mentioned above.
Nevertheless, identifying potential preoperative predictors for CR-POPF is a vital step in our journey to decrease the morbidity associated with PD. Our study failed to demonstrate any association of PAI with CR-POPF and postoperative fat fraction, which may be explained apart from the small sample size to the restrictive ROI. Area-based assessment for the pancreatic fat fraction in future studies may better correlate with histopathological fat fraction.
The PER showed good accuracy in predicting the development of CR-POPF and a PER ratio of 0.673 or below increased the likelihood of CR-POPF. The positive correlation of PER with fibrosis and negative correlation with PAI suggest that PER may be an objective surrogate for assessing pancreatic texture, especially in minimally invasive surgery, where pancreatic texture assessment could be challenging. ROI-based PAI has a poor prediction for CR-POPF and does not correlate with a pancreatic fat fraction or fibrosis scores. Preoperative quantification of PER can improve the risk stratification and management of patients at high risk of CR-POPF. A multi-center trial with a larger sample size is necessary to validate PAI and PER reliably.
Postoperative pancreatic fistula is the critical cause of morbidity after pancreaticoduodenectomy. Identifying patients at risk of clinically relevant postoperative pancreatic fistula can potentially improve clinical outcomes after pancreaticoduodenectomy.
Most of the available models to predict postoperative pancreatic fistula can be used only in the postoperative setting.
To calculate the accuracy of the pancreatic neck pancreatic attenuation index (PAI) and pancreatic enhancement ratio (PER) in predicting clinically relevant postoperative pancreatic fistula and its correlation with histological pancreatic neck fat fraction and fibrosis scoring.
Patients who underwent pancreaticoduodenectomy for benign and malignant pathology of the periampullary region or pancreatic head between February 2019 and February 2021 were included in the prospective observational study. The PAI was measured in the neck of the pancreas by marking regions of interest in the preoperative non-contrast computed tomography (CT), and the PER was measured during the contrast phase of the CT abdomen. Preoperative pancreatic neck CT indices were correlated with histopathological evaluation of Fibrosis score and the fat fraction of the pancreatic neck and clinically relevant postoperative pancreatic fistula.
The PAI had no significant association with the development of clinically relevant postoperative pancreatic fistula (CR-POPF). However, PER was significantly lower in patients developing CR-POPF (0.58 ± 0.20 vs 0.81 ± 0.44, P = 0.006). Also, PER cut-off of 0.673 predicts CR-POPF with 77.8% sensitivity and 55.8% specificity. The PER showed a moderately positive correlation with fibrosis (Strength 0.50, P < 0.001).
PER showed good accuracy in predicting CR-POPF. Also, PER showed a good correlation with fibrosis scores and may be used as an objective preoperative surrogate for assessing pancreatic texture.
Quantifying PER on preoperative computed tomography can improve the risk stratification and management of patients at high risk of clinically relevant postoperative pancreatic fistula. Failure to demonstrate an association of PAI with clinically relevant postoperative pancreatic fistula and postoperative fat fraction suggests that area-based assessment for the pancreatic fat fraction may be better than the region of interest-based estimation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Indian chapter of International Hepatopancreatobiliary Association, 563; Indian Association of Surgical Gastroenterology, K-65; International Hepatopancreatobiliary association, M02056.
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Bencini L, Italy; Yang F, China A-Editor: Morozov S S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Greenblatt DY, Kelly KJ, Rajamanickam V, Wan Y, Hanson T, Rettammel R, Winslow ER, Cho CS, Weber SM. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126-2135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 2. | Tzeng CW, Katz MH, Fleming JB, Lee JE, Pisters PW, Holmes HM, Varadhachary GR, Wolff RA, Abbruzzese JL, Vauthey JN, Aloia TA. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg. 2014;18:146-55; discussion 155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Berger AC, Howard TJ, Kennedy EP, Sauter PK, Bower-Cherry M, Dutkevitch S, Hyslop T, Schmidt CM, Rosato EL, Lavu H, Nakeeb A, Pitt HA, Lillemoe KD, Yeo CJ. Does type of pancreaticojejunostomy after pancreaticoduodenectomy decrease rate of pancreatic fistula? J Am Coll Surg. 2009;208:738-47; discussion 747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 4. | Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 2013;216:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (2)] |
| 5. | Kantor O, Talamonti MS, Pitt HA, Vollmer CM, Riall TS, Hall BL, Wang CH, Baker MS. Using the NSQIP Pancreatic Demonstration Project to Derive a Modified Fistula Risk Score for Preoperative Risk Stratification in Patients Undergoing Pancreaticoduodenectomy. J Am Coll Surg. 2017;224:816-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Liu Y, Li Y, Wang L, Peng CJ. Predictive value of drain pancreatic amylase concentration for postoperative pancreatic fistula on postoperative day 1 after pancreatic resection: An updated meta-analysis. Medicine (Baltimore). 2018;97:e12487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Tranchart H, Gaujoux S, Rebours V, Vullierme MP, Dokmak S, Levy P, Couvelard A, Belghiti J, Sauvanet A. Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. 2012;256:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Lim S, Bae JH, Chun EJ, Kim H, Kim SY, Kim KM, Choi SH, Park KS, Florez JC, Jang HC. Differences in pancreatic volume, fat content, and fat density measured by multidetector-row computed tomography according to the duration of diabetes. Acta Diabetol. 2014;51:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 9. | Maehira H, Iida H, Mori H, Kitamura N, Miyake T, Shimizu T, Tani M. Computed Tomography Enhancement Pattern of the Pancreatic Parenchyma Predicts Postoperative Pancreatic Fistula After Pancreaticoduodenectomy. Pancreas. 2019;48:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Hashimoto Y, Sclabas GM, Takahashi N, Kirihara Y, Smyrk TC, Huebner M, Farnell MB. Dual-phase computed tomography for assessment of pancreatic fibrosis and anastomotic failure risk following pancreatoduodenectomy. J Gastrointest Surg. 2011;15:2193-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Kang JH, Park JS, Yu JS, Chung JJ, Kim JH, Cho ES, Yoon DS. Prediction of pancreatic fistula after pancreatoduodenectomy by preoperative dynamic CT and fecal elastase-1 Levels. PLoS One. 2017;12:e0177052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kim SY, Kim H, Cho JY, Lim S, Cha K, Lee KH, Kim YH, Kim JH, Yoon YS, Han HS, Kang HS. Quantitative assessment of pancreatic fat by using unenhanced CT: pathologic correlation and clinical implications. Radiology. 2014;271:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 146] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Klöppel G, Maillet B. Pseudocysts in chronic pancreatitis: a morphological analysis of 57 resection specimens and 9 autopsy pancreata. Pancreas. 1991;6:266-274. [PubMed] |
| 14. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2957] [Article Influence: 369.6] [Reference Citation Analysis (35)] |
| 15. | Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Traverso LW, Yeo CJ, Büchler MW. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 2327] [Article Influence: 129.3] [Reference Citation Analysis (0)] |
| 16. | Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, Neoptolemos JP, Padbury RT, Sarr MG, Yeo CJ, Büchler MW. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1411] [Cited by in RCA: 1945] [Article Influence: 108.1] [Reference Citation Analysis (0)] |
| 17. | Lin JW, Cameron JL, Yeo CJ, Riall TS, Lillemoe KD. Risk factors and outcomes in postpancreaticoduodenectomy pancreaticocutaneous fistula. J Gastrointest Surg. 2004;8:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 286] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Hu BY, Wan T, Zhang WZ, Dong JH. Risk factors for postoperative pancreatic fistula: Analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 2016;22:7797-7805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Laaninen M, Bläuer M, Vasama K, Jin H, Räty S, Sand J, Nordback I, Laukkarinen J. The risk for immediate postoperative complications after pancreaticoduodenectomy is increased by high frequency of acinar cells and decreased by prevalent fibrosis of the cut edge of pancreas. Pancreas. 2012;41:957-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Kim Z, Kim MJ, Kim JH, Jin SY, Kim YB, Seo D, Choi D, Hur KY, Kim JJ, Lee MH, Moon C. Prediction of post-operative pancreatic fistula in pancreaticoduodenectomy patients using pre-operative MRI: a pilot study. HPB (Oxford). 2009;11:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Adalı G, Bozkurt B, Ceyhan Ö, Server S, Doğusoy GB, Yüzer Y, Tokat Y. Body Mass Index and Unenhanced CT as a Predictor of Hepatic Steatosis in Potential Liver Donors. Transplant Proc. 2019;51:2373-2378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Yardimci S, Kara YB, Tuney D, Attaallah W, Ugurlu MU, Dulundu E, Yegen ŞC. A Simple Method to Evaluate Whether Pancreas Texture Can Be Used to Predict Pancreatic Fistula Risk After Pancreatoduodenectomy. J Gastrointest Surg. 2015;19:1625-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Hori M, Onaya H, Hiraoka N, Yamaji T, Kobayashi H, Takahashi M, Mutoh M, Shimada K, Nakagama H. Evaluation of the degree of pancreatic fatty infiltration by area-based assessment of CT images: comparison with histopathology-based and CT attenuation index-based assessments. Jpn J Radiol. 2016;34:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |