Published online Dec 28, 2021. doi: 10.4329/wjr.v13.i12.371
Peer-review started: February 10, 2021
First decision: October 17, 2021
Revised: November 25, 2021
Accepted: December 9, 2021
Article in press: December 9, 2021
Published online: December 28, 2021
Processing time: 315 Days and 10.1 Hours
Endovascular therapy is playing an increasing role in the treatment of iliofemoral venous disease. Iliac stent patency is multifactorial, and current management is based on best clinical practices, varying by institution.
To evaluate how thrombophilia influences management and outcomes of patients who undergo venous stenting for thrombotic iliac vein compression syndromes.
A retrospective observational analysis was performed on 65 patients with thrombotic iliac vein compression syndrome that underwent common iliac vein (CIV) stenting between December 2013 and December 2019 at a large academic center. Search criteria included CIV stenting and iliac vein compression. Non-thrombotic lesions and iliocaval thrombosis and/or occlusions were excluded. A total of 65 patients were selected for final analysis. Demographic information, procedural data points, and post-procedural management and outcomes were collected. Statistical analyses included Fisher's exact and Chi-square tests to compare discrete variables and the Wilcoxon rank-sum test to compare continuous variables between thrombophilia positive and negative patients.
65 patients underwent successful balloon angioplasty and CIV stenting. Of these patients, 33 (50.8%) underwent thrombophilia testing, with 16 (48.5%) testing positive. Stent patency on ultrasound did not significantly differ between thrombophilia positive and negative patients at 1 mo (92.3% vs 81.3%, P = 0.6), 6 mo (83.3% vs 80%, P > 0.9), or 12 mo (77.8% vs 76.9%, P = 0.8). Immediately after stent placement, thrombophilia patients were more likely to be placed on dual therapy (aspirin and anticoagulation) or triple therapy (aspirin, clopidogrel, and anticoagulation) (50% vs 41.2%, P > 0.9), and remain on dual therapy at 6 mo (25% vs 12.5%, P = 0.5) and 12 mo (25% vs 6.7%, P = 0.6). There was no significant difference in re-intervention rates (25% vs 35.3%, P = 0.7) or number of re-interventions (average 2.3 vs 1.3 per patient, P = 0.4) between thrombophilia positive and negative patients.
Half of patients with stented thrombotic iliac vein compression syndrome and thrombophilia testing were positive. The presence of thrombophilia did not significantly impact stent patency or re-intervention rates.
Core Tip: Endovascular therapy is playing an increasing role in the treatment of iliofemoral venous disease. Iliac stent patency is multifactorial, and current management is based on best clinical practices. Despite an underlying anatomic venous abnormality, half of our patient cohort with stented thrombotic iliac vein compression syndrome tested positive for thrombophilia. The presence of thrombophilia did not demonstrate a statistically significant difference in stent patency rates or re-intervention rates.
- Citation: Cramer P, Mensah C, DeSancho M, Malhotra A, Winokur R, Kesselman A. Prevalence of hypercoagulable states in stented thrombotic iliac vein compression syndrome with comparison of re-intervention and anticoagulation regimens. World J Radiol 2021; 13(12): 371-379
- URL: https://www.wjgnet.com/1949-8470/full/v13/i12/371.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i12.371
Iliofemoral vein thrombosis accounts for approximately 25% of all deep vein thrombosis and is associated with an increased risk of embolic and post-thrombotic complications[1]. Anticoagulation is the standard of care for the treatment of symptomatic acute deep vein thrombosis. However, despite appropriate anticoagulant therapy, the post-thrombotic syndrome (PTS) remains a frequent complication seen in 30% to 50% of patients diagnosed with iliofemoral deep vein thrombosis. The clinical manifestations of PTS include pain, swelling, heaviness, fatigue, itching, or cramping of the affected leg[1-3]. To reduce the burden of post-thrombotic symptoms, endovascular approaches with thrombolysis, thrombectomy, balloon angioplasty and stenting are being increasingly utilized in centers with expertise in these procedures[4-7]. The current C-TRACT trial is further investigating the role of endovascular intervention for chronic iliac vein obstruction. Guidelines for therapeutic anticoagulation after iliocaval stent placement remain variable by institution, however long-term anticoagulation is often recommended in patients with underlying thrombophilia[8].
Thrombophilia is an inherited or acquired condition that predisposes a person to develop a thrombotic event. Thrombophilia screening should only be done if the discovery of the thrombophilia will require extending the duration of the anticoagulation treatment. Conversely, if a thrombotic event occurred in the presence of a major transient risk factor, thrombophilia screening should not be performed. Whether or not the presence of an underlying thrombophilia increases the risk of recurrent thrombosis, particularly in-stent thrombosis in patients that have undergone venous interventional procedures, remains unknown[9]. Therefore, we sought out to identify the prevalence of thrombophilias in patients with thrombotic iliac vein compression syndrome who underwent venous stenting. We also compared if the presence of thrombophilia influenced post-procedure antithrombotic regimens, stent patency and re-intervention rates.
The institutional review board approved this study with waiver of informed consent. We performed a retrospective review of electronic medical records at a large academic medical center from December 2013 to December 2019. Search criteria included common iliac vein (CIV) stenting and iliac vein compression. Non-thrombotic lesions and iliocaval thrombosis and/or occlusions were excluded. A total of 65 patients were selected for final analysis.
Medical records were reviewed for demographic information, procedural data points, and post-procedural management and outcomes. Procedural data points included pre-intervention venous patency, stent location, stent type and diameter, and any additional endovascular procedures performed at that time. Post-procedural outcomes included subjective clinical symptom improvement, medication regimen and duration, stent patency on imaging, and re-intervention requirement. Types of antithrombotic therapy included antiplatelet, anticoagulation, single antiplatelet and anticoagulation (dual therapy), or dual antiplatelet agents and anticoagulation (triple therapy). Hematology consultations with or without thrombophilia evaluations were also reviewed. Statistical analyses included Fisher's exact and Chi-square tests to compare discrete variables and the Wilcoxon rank-sum test to compare continuous variables between thrombophilia positive and negative patients.
Baseline demographics are summarized in Table 1. Our patient population included 38 (58.5%) males and 27 (41.5%) females. Clinical symptoms included lower extremity swelling (n = 57, 87.7%), pain (n = 44, 67.7%), venous stasis ulceration (n = 7, 10.8%), varicose veins (n = 3, 4.6%), and pelvic pain (n = 2, 3.1%). Venous thromboembolism histories were reviewed for high risk features suspicious for thrombophilia; 24 (36.9%) experienced their first venous thrombosis (VTE) at a young age (less than 40 years old), 12 (18.5%) had a strong family history of thrombosis, and 16 (24.6%) were unprovoked.
| Variable | Summary (n = 65) |
| Median age in years (interquartile range) | 54 (41-63) |
| Median BMI in kg/m2 (interquartile range) | 28 (25.1-32.7) |
| Gender | |
| Male | 38 (58.5%) |
| Female | 27 (41.5%) |
| Clinical symptoms | |
| Lower extremity swelling | 57 (87.7%) |
| Lower extremity pain | 44 (67.7%) |
| Venous stasis ulceration | 7 (10.8%) |
| Varicose veins | 3 (4.6%) |
| Pelvic pain | 2 (3.1%) |
| Symptomatic side | |
| Left | 49 (75.4%) |
| Right | 14 (21.5%) |
| Bilateral | 2 (3.1%) |
| Thrombophilia risk factor | |
| Young age (< 40 yr) | 24 (35.9%) |
| Family history | 12 (18.5%) |
| Unprovoked | 16 (24.6%) |
| VTE provoking factor | |
| Prolonged immobilization | 15 (23.1%) |
| Malignancy | 13 (20.0%) |
| Recent surgery | 5 (7.7%) |
| Trauma | 5 (7.7%) |
| Pregnancy | 7 (14.6%) |
| Hormonal supplement | 4 (6.2%) |
| None | 16 (24.6%) |
A total of 33 (50.8%) underwent thrombophilia testing, with 16 (48.5%) testing positive. There were ten patients with Factor V Leiden heterozygous mutations (G1691A), four with antiphospholipid antibodies (three lupus anticoagulant, one anticardiolipin antibody), one prothrombin gene mutation G20210A, one antithrombin deficiency, and one protein S deficiency. Only one patient had two concomitant thrombophilias, comprising Factor V Leiden and lupus anticoagulant.
Procedure details are summarized in Table 2. All 65 subjects included in this study underwent venography, balloon angioplasty, and CIV stenting. The majority of interventions were left-sided (n = 50, 76.9%) with stenting extending into the external iliac vein (n = 54, 83.1%) and common femoral vein (n = 45, 69.2%).
| Variable | Summary (n = 65) |
| Pre-procedure CIV patency | |
| Stenosis | 47 (72.3%) |
| Occlusion | 16 (24.6%) |
| In-stent thrombosis | 2 (3.1%) |
| Stent location | |
| Left CIV | 50 (76.9%) |
| Right CIV | 11 (16.9%) |
| Bilateral CIV | 4 (6.2%) |
| Stent type | |
| Wallstent | 51 (78.5%) |
| Venovo | 9 (13.8%) |
| Smart | 2 (3.1%) |
| Vici | 3 (4.6%) |
| CIV stent balloon dilation diameter (mm) | |
| 12 | 1 (1.5%) |
| 14 | 14 (21.5%) |
| 16 | 28 (43.1%) |
| 18 | 19 (29.2%) |
| 20 | 3 (4.6%) |
| Additional stented segments | |
| External iliac vein | 54 (83.1%) |
| Common femoral vein | 45 (69.2%) |
| Simultaneous endovascular interventions | |
| Thrombolysis | 25 (38.5%) |
| Thrombectomy | 17 (26.2%) |
| CIV filter retrieval | 3 (4.6%) |
Technical success, defined by CIV stent placement and clearance of thrombus burden, was achieved in 65 (100%) patients. Clinical success, defined by patient reported symptom improvement, was achieved in 14 (87.5%) thrombophilia positive, 12 (70.6%) thrombophilia negative, and 21 (65.6%) untested. Median follow-up duration was 14 mo.
Antithrombotic regimens were reviewed at post-procedure day 1 (n = 65), 6 mo (n = 61), and 12 mo (n = 57). The day after stent placement, 2 (3.1%) patients were on single antiplatelet, 34 (52.3%) patients were on anticoagulation, 17 (26.2%) patients were on dual therapy, 11 (16.9%) patients were on triple therapy, and 1 (1.5%) patient was off antithrombotic medication. At 6 mo, 3 (4.9%) patients were on single antiplatelet, 34 (55.7%) patients were on anticoagulation, 17 (27.9%) patients were on dual therapy, 0 (0%) patients were on triple therapy, and 7 (11.5%) patients were off antithrombotic medication. At 12 mo, 11 (19.3%) patients were on single antiplatelet, 26 (45.6%) were on anticoagulation, 9 (15.8%) patients were on dual therapy, 0 (0%) patients were on triple therapy, and 11 (19.3%) patients were off antithrombotic medication.
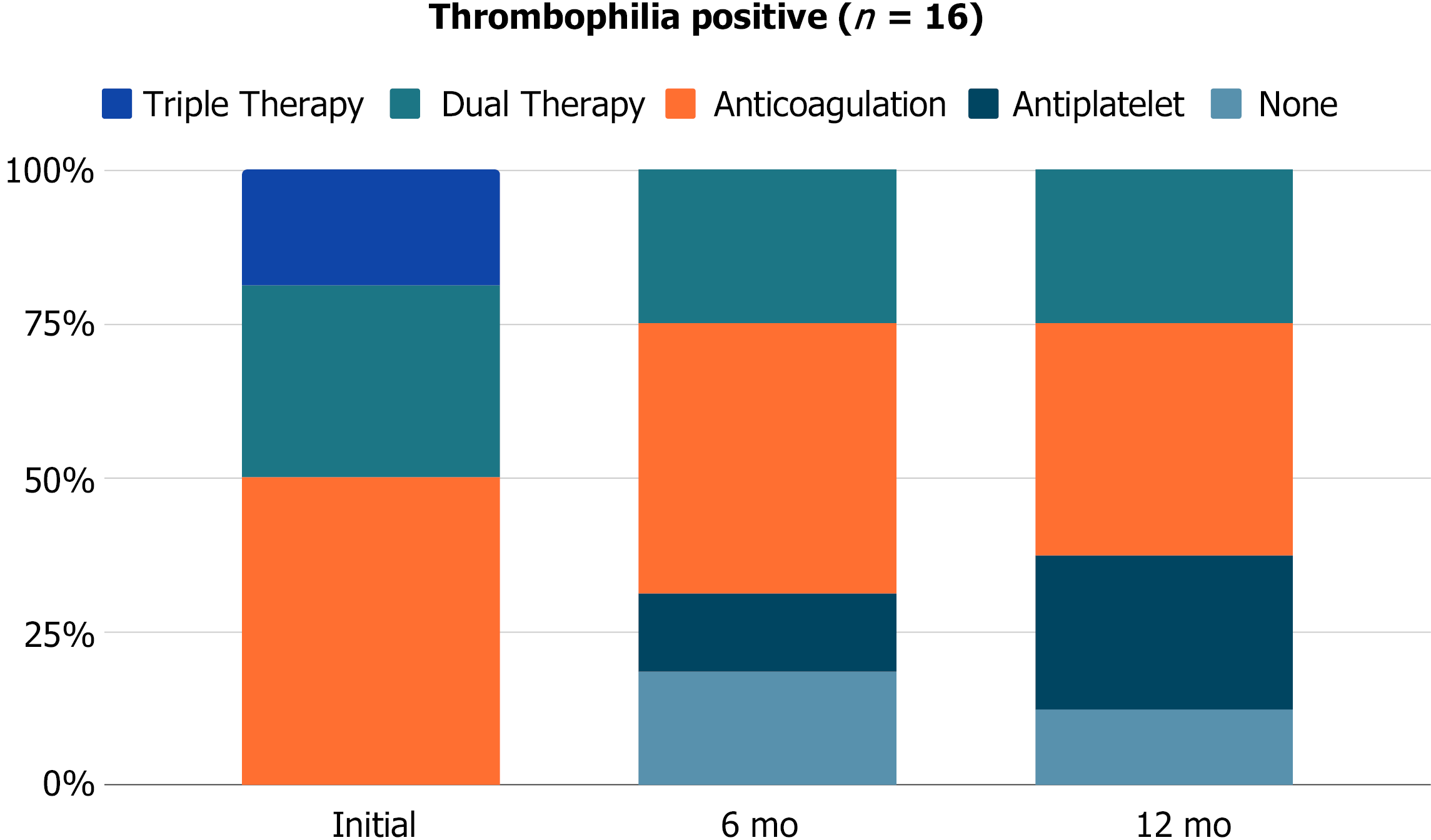
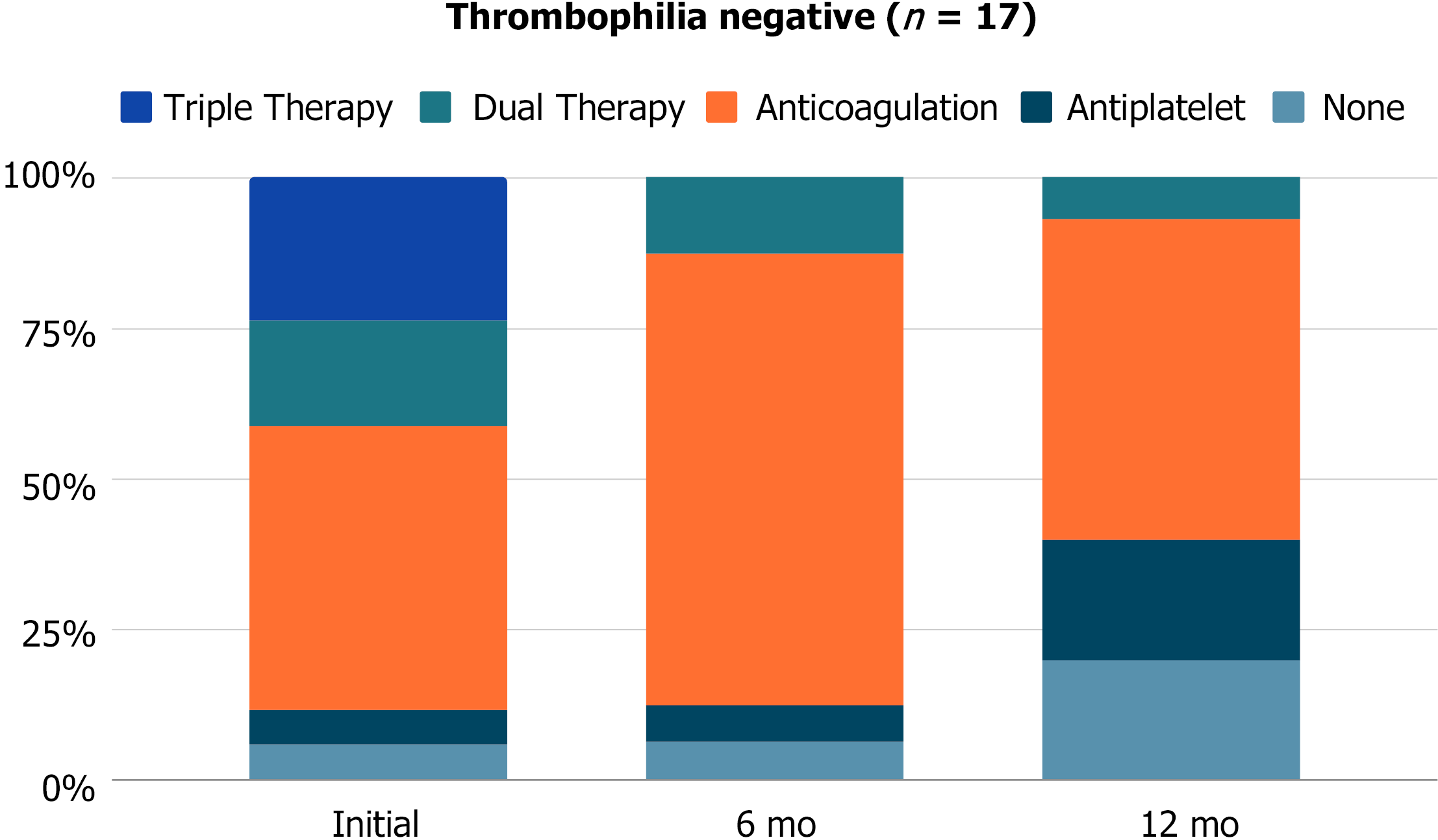
Post-stenting antithrombotic regimens are broken down by thrombophilia testing in Figures 1 and 2. Immediately after stent placement, thrombophilia patients were more likely to be placed on dual or triple therapy (50% vs 41.2%, P > 0.9) and remain on dual therapy at 6 mo (25% vs 12.5%, P = 0.5) and 12 mo (25% vs 6.7%, P = 0.6) compared to thrombophilia negative patients. Stent patency on ultrasound did not significantly differ between thrombophilia positive and negative patients at 1 mo (92.3% vs 81.3%, P = 0.6), 6 mo (83.3% vs 80%, P > 0.9), or 12 mo (77.8% vs 76.9%, P = 0.8).
Stent thrombosis occurred in 2 (12.5%) thrombophilia positive and 4 (23.5%) thrombophilia negative patients. The median time to stent thrombosis was longer in thrombophilia patients (1.1 mo vs 0.5 mo). At the time of stent thrombosis, 3 (50%) patients were on anticoagulation, 1 (16.7%) patient was on dual therapy, and 2 (33.3%) patients were off antithrombotic medication. Following thrombosis, all patients were transitioned to anticoagulation alone (n = 3) or dual therapy (n = 3). Anticoagulation therapies included full-dose direct oral anticoagulants, half-dose direct oral anticoagulants, and enoxaparin. There was no significant difference in re-intervention rates (25% vs 35.3%, P = 0.7) or number of re-interventions (average 2.3 vs 1.3 per patient, P = 0.4) between thrombophilia positive and negative patients, as seen in Table 3.
| Thrombophilia work-up (n = 33) | Positive (n = 16) | Negative (n = 17) |
| Clinical success | ||
| Stent patency | 14 (87.5%) | 12 (70.6%) |
| 1 mo | 12 of 13 (92.3%) | 13 of 16 (81.3%) |
| 6 mo | 10 of 12 (83.3%) | 12 of 15 (80%) |
| 12 mo | 7 of 9 (77.8%) | 10 of 13 (76.9%) |
| Stent thrombosis | 2 (12.5%) | 4 (23.5%) |
| Anticoagulated during stent thrombosis | 1 of 2 (50%) | 2 of 4 (50%) |
| Re-intervention rates | 4 (25%) | 6 (35.3%) |
| Number of re-interventions | ||
| 1 | 50% | 67% |
| 2 | 0% | 33% |
| 3 | 25% | 0% |
| 4 | 25% | 0% |
Bleeding complications from antithrombotic medications were seen in 14 (21.5%) patients, including ecchymoses, hematuria, rectal bleeding, epistaxis, and menorrhagia. None of these events required medication cessation or intervention. Of the patients that experienced bleeding complications, 7 (50.0%) were on anticoagulation, 4 (28.6%) were on dual therapy, and 3 (21.4%) were on triple therapy.
Endovascular therapy is playing an increasing role in the treatment of iliofemoral venous disease. Iliac stent patency is multifactorial, and current management is based on best clinical practices, varying by institution[11].
Diagnostic thrombophilia testing is recommended in patients with idiopathic or recurrent VTE, first VTE at a young age (< 40 years), VTE in the setting of a strong family history or VTE in atypical locations. There is no single laboratory test available to identify all thrombophilias and results can be affected by a variety of clinical conditions and drugs. Based on this premise, thrombophilia testing should only be performed by a coagulation specialist who knows when to do the screening, provide accurate interpretation of the results and educate the patient[9]. Our cohort demonstrated that despite having an anatomic consideration for increased thrombosis risk, 48.5% of patients who undergo venous stenting for thrombotic iliac vein compression syndrome had an underlying thrombophilia when testing was performed. This result is higher than the 32% rate of positive thrombophilia identified in 4494 patients with symptomatic VTE in the RIETE registry[12] and similar to other studies ranging from 55% to 61%[13,14]. Therefore, the decision for thrombophilia testing should be discussed by a multidisciplinary team and considered only when it will impact post-procedural medical management.
The extent of influence of inherited thrombophilia on the risk of VTE recurrence remains controversial[10]. In our cohort, stent patency and re-intervention rates were not significantly different between thrombophilia positive and negative patients. In all patients with thrombotic iliac vein compression syndrome, antithrombotic compliance and close imaging follow-up are necessary to optimize stent patency and prevent or delay re-intervention. The median time to stent thrombosis was less than one month, emphasizing the importance of the immediate post-procedural period. Immediately after stent placement, thrombophilia patients were more likely to be placed on dual or triple therapy and remain on dual therapy at 6 mo and 12 mo, although this finding was not statistically significant given the smaller sample size. Following thrombosis, all patients were transitioned to long-term anticoagulation or dual therapy, including full-dose or half-dose direct oral anticoagulants.
There is controversy around whether venous stent patency is best maintained by combined antiplatelet and anticoagulation therapy vs anticoagulation alone[15]. Antiplatelet agents did not appear to significantly increase the bleeding risk in our study, with almost half of thrombophilia patients remaining on long term antiplatelet medications and more than half on anticoagulation. The long-term management following venous stenting in thrombotic iliac vein compression syndrome is complex and patient specific. Because there was no significant difference in stent patency or re-intervention rates amongst thrombophilia positive and negative patients, the need for thrombophilia testing should be individualized and only considered when it will impact post-procedural medical management.
This study has several limitations. First it is a single center retrospective design; second, there is provider bias in choosing antithrombotic regimens based on their presumed risk of thrombosis; and third, venous stent type and extent varied, introducing confounders. Moreover, given our small sample size, our study was underpowered to obtain statistical significance for subgroups and antithrombotic regimens. Future studies focusing on anticoagulation related to venous stenting in larger cohorts would be helpful. Larger prospective randomized control trials are needed.
Despite an underlying anatomic venous abnormality, in our cohort of patients that underwent thrombophilia testing in the setting of stented common iliac thrombosis, half tested positive for thrombophilia. The presence of thrombophilia did not demonstrate a statistically significant difference in stent patency rates or re-intervention rates. The need for thrombophilia workup should be individualized and discussed by multidisciplinary teams and considered only when it will impact post-procedural medical management.
The long-term management following venous stenting in thrombotic iliac vein compression syndrome is complex and patient specific. Because there was no significant difference in stent patency or re-intervention rates amongst thrombophilia positive and negative patients, the need for thrombophilia testing should be individualized and only considered when it will impact post-procedural medical management. Future studies focusing on anticoagulation related to venous stenting in larger cohorts would be helpful.
Half of patients with stented thrombotic iliac vein compression syndrome and thrombophilia testing were positive. The presence of thrombophilia did not demonstrate a significant difference in stent patency or re-intervention rates.
65 patients underwent successful balloon angioplasty and common iliac vein (CIV) stenting. Stent patency on ultrasound did not significantly differ between thrombophilia positive and negative patients at 1 mo (92.3% vs 81.3%, P = 0.6), 6 mo (83.3% vs 80%, P > 0.9), or 12 mo (77.8% vs 76.9%, P = 0.8). Immediately after stent placement, thrombophilia patients were more likely to be placed on dual therapy (aspirin and anticoagulation) or triple therapy (aspirin, clopidogrel, and anticoagulation) (50% vs 41.2%, P > 0.9), and remain on dual therapy at 6 mo (25% vs 12.5%, P = 0.5) and 12 mo (25% vs 6.7%, P = 0.6). There was no significant difference in re-intervention rates (25% vs 35.3%, P = 0.7) or number of re-interventions (average 2.3 vs 1.3 per patient, P = 0.4) between thrombophilia positive and negative patients.
A retrospective observational analysis was performed on 65 patients with thrombotic iliac vein compression syndrome that underwent CIV stenting at a large academic center. Non-thrombotic lesions and iliocaval thrombosis and/or occlusions were excluded. Demographic information, procedural data points, and post-procedural management were compared between thrombophilia positive and negative patients.
To evaluate the prevalence and compare how thrombophilia influences management and outcomes of patients who undergo venous stenting for thrombotic iliac vein compression syndromes.
Guidelines for therapeutic anticoagulation after iliocaval stent placement remain variable by institution, however long-term anticoagulation is often recommended in patients with underlying thrombophilia. Whether or not the presence of an underlying thrombophilia increases the risk of recurrent thrombosis, particularly in-stent thrombosis in patients that have undergone venous interventional procedures, remains unknown.
Iliofemoral vein thrombosis accounts for approximately 25% of all deep vein thrombosis and is associated with an increased risk of embolic and post-thrombotic complications. Anticoagulation is the standard of care for the treatment of symptomatic acute deep vein thrombosis. However, despite appropriate anticoagulant therapy, the post-thrombotic syndrome remains a frequent complication seen in 30% to 50% of patients diagnosed with iliofemoral deep vein thrombosis. To reduce the burden of post-thrombotic symptoms, endovascular therapy is playing an increasing role in the treatment of iliofemoral venous disease.
We would like to thank Charlene Thomas for her support with our statistical analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nayak S S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh ME, Weitz JI; American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 390] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 3. | Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Duncan JR, Nieters P, Derfler MC, Filion M, Gu CS, Kee S, Schneider J, Saad N, Blinder M, Moll S, Sacks D, Lin J, Rundback J, Garcia M, Razdan R, VanderWoude E, Marques V, Kearon C; ATTRACT Trial Investigators. Pharmacomechanical Catheter-Directed Thrombolysis for Deep-Vein Thrombosis. N Engl J Med. 2017;377:2240-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 524] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 4. | Kahn SR, Julian JA, Kearon C, Gu CS, Cohen DJ, Magnuson EA, Comerota AJ, Goldhaber SZ, Jaff MR, Razavi MK, Kindzelski AL, Schneider JR, Kim P, Chaer R, Sista AK, McLafferty RB, Kaufman JA, Wible BC, Blinder M, Vedantham S; ATTRACT Trial Investigators. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 5. | Kuetting D, Luetkens J, Wolter K, Faron A, Kania A, Thomas D. Catheter-directed thrombectomy for highly symptomatic patients with iliofemoral deep venous thrombosis not responsive to conservative treatment. Cardiovasc Intervent Radiol. 2020;43:556-564. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Mabud TS, Cohn DM, Arendt VA, Jeon GS, An X, Fu J, Souffrant AD, Sailer AM, Shah R, Wang D, Sze DY, Kuo WT, Rubin DL, Hofmann LV. Lower Extremity Venous Stent Placement: A Large Retrospective Single-Center Analysis. J Vasc Interv Radiol. 2020;31:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Razavi MK, Jaff MR, Miller LE. Safety and Effectiveness of Stent Placement for Iliofemoral Venous Outflow Obstruction: Systematic Review and Meta-Analysis. Circ Cardiovasc Interv. 2015;8:e002772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 8. | Mahnken AH, Thomson K, de Haan M, O'Sullivan GJ. CIRSE standards of practice guidelines on iliocaval stenting. Cardiovasc Intervent Radiol. 2014;37:889-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Nakashima MO, Rogers HJ. Hypercoagulable states: an algorithmic approach to laboratory testing and update on monitoring of direct oral anticoagulants. Blood Res. 2014;49:85-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 10. | Rybstein MD, DeSancho MT. Hypercoagulable States and Thrombophilias: Risks Relating to Recurrent Venous Thromboembolism. Semin Intervent Radiol. 2018;35:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Attaran RR, Ozdemir D, Lin IH, Mena-Hurtado C, Lansky A. Evaluation of anticoagulant and antiplatelet therapy after iliocaval stenting: Factors associated with stent occlusion. J Vasc Surg Venous Lymphat Disord. 2019;7:527-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Roldan V, Lecumberri R, Muñoz-Torrero JF, Vicente V, Rocha E, Brenner B, Monreal M; RIETE Investigators. Thrombophilia testing in patients with venous thromboembolism. Findings from the RIETE registry. Thromb Res. 2009;124:174-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Caprini JA, Goldshteyn S, Glase CJ, Hathaway K. Thrombophilia testing in patients with venous thrombosis. Eur J Vasc Endovasc Surg. 2005;30:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Signorelli S, Fiore V, Puccia G, Mastrosimone G, Anzaldi M. Thrombophilia in patients with lower limb deep vein thrombosis (LVDT) results of a monocentric survey on 103 consecutive outpatients. Clin Appl Thromb Hemost. 2014;20:589-593. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Endo M, Jahangiri Y, Horikawa M, Kaufman JA, Schenning RC, Kolbeck KJ, Barton RE, Ohuchi Y, Liang KW, Farsad K. Antiplatelet Therapy is Associated with Stent Patency After Iliocaval Venous Stenting. Cardiovasc Intervent Radiol. 2018;41:1691-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |