Published online Oct 28, 2021. doi: 10.4329/wjr.v13.i10.314
Peer-review started: March 28, 2021
First decision: June 3, 2021
Revised: June 12, 2021
Accepted: October 9, 2021
Article in press: October 9, 2021
Published online: October 28, 2021
Processing time: 211 Days and 22 Hours
Gastrointestinal (GI) cancers often require a multidisciplinary approach involving surgeons, endoscopists, oncologists, and interventional radiologists to diagnose and treat primitive cancers, metastases, and related complications. In this context, interventional radiology (IR) represents a useful minimally-invasive tool allowing to reach lesions that are not easily approachable with other techniques. In the last years, through the development of new devices, IR has become increasingly relevant in the context of a more comprehensive management of the oncologic patient. Arterial embolization, ablative techniques, and gene therapy represent useful and innovative IR tools in GI cancer treatment. Moreover, IR can be useful for the management of GI cancer-related complications, such as bleeding, abscesses, GI obstructions, and neurological pain. The aim of this study is to show the principal IR techniques for the diagnosis and treatment of GI cancers and related complications, as well as to describe the future perspectives of IR in this oncologic field.
Core Tip: Interventional radiology is a minimally-invasive tool for the diagnosis and treatment of different gastrointestinal cancers, representing a useful alternative to more invasive approaches such as surgery and endoscopy. Hereby, we describe the different radiological techniques for the diagnosis and treatment of gastrointestinal cancers and related complications, underlining the role of this specialty in cancer patient’s care.
- Citation: Reitano E, de'Angelis N, Bianchi G, Laera L, Spiliopoulos S, Calbi R, Memeo R, Inchingolo R. Current trends and perspectives in interventional radiology for gastrointestinal cancers. World J Radiol 2021; 13(10): 314-326
- URL: https://www.wjgnet.com/1949-8470/full/v13/i10/314.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i10.314
Gastrointestinal (GI) cancers are currently among the five most common cancers worldwide for both men and women[1]. According to the GLOBOCAN 2018, colon cancer and gastric cancer represents respectively the 3rd and 5th most common cancers[2,3]. Some GI, such as the pancreatic cancer (PC), are rarer but burdened by a high mortality rate[4]. PC represents the thirteen most common cancer and the seventh most common cause of cancer-related death[4]. The incidence of GI cancer shows significant geographical variations, with colorectal cancer incidence higher in Western Countries and North America[3,5], whereas gastric cancer incidence is higher in Asia and Africa[2]. These geographical differences are mainly linked to environmental and lifestyle factors such as nutritional habits, alcohol intake, genetics, and obesity[2,5].
Nowadays, the “gold standard” management of cancers involves a multi-specialist staff consisting of oncologists, surgeons, endoscopists, and radiologists to provide a multi-disciplinary diagnostic and treatment approach to the oncologic patient.
Interventional radiology (IR) is getting a key role in oncologic patients' cares, being an essential tool in both the initial diagnosis and the subsequent treatment, as well as in the management of the related complications[6]. IR provides adequate diagnostic samples through a minimally invasive access, which can be obtained under imagine guidance by percutaneous and needle aspiration[7]. Therapeutic applications of IR in oncology are mainly focused on local cancer treatment, including radiofrequency (RF) ablation or trans-arterial chemoembolization (TACE)[8]. Cancers complications, such as pain, bleeding, organ obstructions, or venous thrombosis can also be managed by IR, with the eventual placement of gastrostomy or jejunostomy in selected patients[9,10].
This article aims to analyse the current roles of IR in GI cancer management and provide an extensive overview of the current literature on the topic. In this article, only cancers located in the GI tract (from the esophagus to the colon) will be considered. Liver, pancreas, and biliary tract will not be taken into account, as they should require a separate discussion.
The adequate treatment of GI cancers depends on a timely definitive diagnosis and the staging of the disease[11]. Imaging techniques improved the assessment and staging of cancers, but the histological analysis represents the gold standard for the definitive diagnosis of this disease. Biopsies samples are required to assess the biomarker status of different solid GI cancers and should be performed not only for the initial diagnosis but at multiple end-points, to detect the cancer progression, predict the prognosis and guide the next-line therapy[12]. The improvement of the histological and cytological analysis, especially in the field of immunochemical examination, enables the identification of the primary tumor site and predicts the sensitivity to chemotherapeutic drugs[13].
Minimally invasive techniques have a prominent role in this contest. Endoscopy currently represents the first-level procedure for the histological diagnosis of GI cancers. However, lesions located within the submucosa or subserosa (such as lymphoma or gastrointestinal stromal tumours), may be difficult to diagnose with this approach[14]. Cancers located in the small bowel or colon could be not always reachable by the endoscope, due to their location or to stenosis of the lumen[14]. In this case, biopsies can obtain by interventional radiologists through direct visualization under image guidance of the masses, allowing the safe passage of the needle and minimising the trauma to the surrounding areas. In biopsy planning, imaging techniques help to define lesion location, accessibility, and suitability for biopsy also providing the identification of the mass to sample, in the context of multiple lesions[6]. In case of metastasis on the liver, not accessible by endoscopy, IR-biopsy can help to identify the primary tumour and define a tissue diagnosis[6].
The choice of imaging guidance modality is multifactorial and there are different options. Ultrasonography (US) is a fast and cost-effective technique, that guarantees real-time imaging, allowing the monitoring of the needle trajectory to the target lesion, without radiation exposure. US-guided percutaneous biopsy provides the diagnosis of solid abdominal organ lesions located in the spleen, pancreas, or lymph nodes, with high diagnostic accuracy and low complications and mortality rates[15]. Moreover, US is useful in guiding biopsies with intracavitary access and must be considered as a diagnostic alternative tool for the diagnosis of low rectal lesions and stromal tumours[16]. The success of US depends on different factors, such as the operator experience[16]. However, different studies suggested US superiority to computed tomography (CT)-guided biopsies, in case of lesions visible with ultrasounds[15,16]. CT-guided biopsy provides a more defined anatomical image, allowing a more precise needle localization when compared to US, showing to be particularly useful in case of pelvic or deep biopsies, which can be difficult to be performed using US. However, CT-guided biopsies have a low real-time guidance capability to track the needle and the target location, requiring intermittent sweeps of the region of interest to confirm the location of the needle during the procedure, thus increasing the biopsy time. The principal disadvantage of the procedure is clearly linked to the radiations exposure expecially for the patients, with radiation dose-related to different factors such as the total scan time, the peak tube kilovoltage (kVP), and milliamperage (mA), the part of the body that must be scanned and the size of the patient[17]. CT-fluoroscopy is an alternative method resulting from technical advantages of the common CT, which allows near real-time imaging of the needled trajectory, reducing the procedural time. Fluoroscopic images are acquired at a lower mA, reducing the radiation dose to the patient, but increasing the radiation dose to the staff, due to the proximity of the physician to the x-ray source during the procedure[18]. However, recent available fusion image guidance systems allow decreasing the radiation exposure through real-time projection during the US-guided biopsies of a needle on to pre-existing CT or magnetic resonance imaging (MRI) image, improving at the same time the accuracy of the procedure[19]. Cone-beam computed tomography (CBCT) guided biopsy, represents the last frontier in the field of IR. Although its extensive use in pleural and pulmonary masses, its virtual navigation system allowed to increase the diagnostic accuracy of the target lesion through a 3D visualization and real-time guidance of the needle trajectory[20], with initial applications also for the diagnosis of GI lesions[21].
Arterial embolization (AE) is a useful therapeutic option for hypervascular cancer treatment. Therefore, AE is widely used in liver metastasis treatment, instead of primary GI cancers[22].
Imagine-guided cancer treatment represents a minimally invasive alternative or adjunct to surgery in the management of GI tumours[23,24]. AE consists of the identification of the arterial supply of a solid tumour in CT or MRI and the devascularization of the pathological tissue through transcatheter embolization[24]. Vessels occlusion can be achieved using polyvinyl alcohol, blood clots, coils, and liquid embolic introduced into the tumour bed through fluoroscopic arterial catheterization in IR[25,26]. The interruption of the cancer supplies induced hypoxia and inhibits the tumour growth. Therefore AE can be used in conjunction with ablative treatments or as an alternative to surgery[26]. Indeed, in the case of hypervascular cancers, this technique helps to reduce operative blood loss[27]. AE has a prominent role in the treatment of hepatic metastasis, especially from colon or rectal cancer[28-30]. In this context, a modification of this technique, the TACE, allowed the infusion of a single or combination of chemotherapy agents in the hepatic pathological tissue through the selective hepatic artery embolization[31-33]. This technique reduces the systematic dose of chemotherapy agents, allowing them to reach a higher local concentration. TACE should be repeated for more sessions until the complete devascularization of the pathological tissue[32]. Finally, separate mention should be given to the radioembolization, despite its use is limited to hepatic pathological tissue. It consists of beta-radiation emitting radio-isotopes directly into the mass employing microspheres (glass or resin) resulting in selective tissue necrosis[32].
Local cancers ablation is an alternative technique for early stages or not candidate for surgical resection[34]. Tumour ablation mediated by IR allowed pathological tissue necrosis in different modalities, including RF, microwave, and cryotherapy[34]. RF ablation (RFA) is mainly applied in liver metastasis of gastric and colon cancers[35,36]. RFA consists of the administration of electrical energy to a tissue, through an electrode connected in a closed-loop circuit to a monopolar or bipolar energy source[8]. The tissue reached a temperature higher than 60 degrees Celsius with consequent thermal damage. RFA is a safe technique with a lower mortality rate (0.3%) and complication rate (2.2%)[8], with an efficacy, described also in the context of skeletal, renal, and lung metastasis with curative or palliative purpose[37-39]. Conversely to RFA, cryotherapy induces cell necrosis by applying subfreezing temperatures, using nitrogen or argon gas under high pressure[40]. The process of freezing-thawing must be repeated to obtain an effective ablation due to the mechanical stress-induced to the cell membranes[41]. CT identifies the ablated zone in real-time as a low-density area[41]. Acting by a mechanism of osmosis and necrosis, different studies suggested that the intracellular content that remains intact allows inducing an immune-specific reaction with an onco-suppressive effect outside the ablated tissue. However, these considerations are based on preclinical studies[42,43], and prospective clinical trials are needed to confirm these data. Microwave ablation is based on the application of electromagnetic energy within a range of at least 915 MHz, agitating the water molecules in target tissue and inducing cell death through coagulation necrosis[44]. Despite microwave showed equivalent or higher clinical efficacy if compared to RFA, however, RFA showed lower recurrence rates and a higher survival rate achieving extensive necrosis after few sessions, with less post-procedural pain[45,46]. In any case, the decision of which ablation methods should be used, must take into consideration several factors such as the tumour type and location (especially the proximity to vulnerable areas) and patients’ comorbidities.
Advanced in immunology and molecular oncology led to the development of gene therapy. It consists of the administration of genetic agents into a tissue in order to stimulate the immune response, reduce the oncogenic expression, modulate the angiogenesis or modify the response to chemotherapeutics[47]. The selective arterial injections of genetic agents are followed by the vessel embolization, to assure the administration of the substance directly into the mass, limiting the adverse effects and increasing the local dwell time[47]. Genetics agents are typically transferred into the cell through vector agents which allow them to cross cell membranes[48]. Vectors are usually plasmids, phospholipidic agents, or viruses like adenovirus, Epstein-Barr virus, and retroviruses (which provided a lasting genetic expression)[48]. However, clinical studies on gene therapies are very limited and, although the results look promising (especially in the treatment of liver metastases), further studies are needed to confirm the data[48,49].
IR has also a role in the minimally invasive treatments of different GI cancers complications, avoiding reoperations and allowing a speeding recovery time[50]. Therefore, IR plays a key role in the field of oncology, contributing to revolutionize the postoperative management of these patients. Indeed, IR allows management of possible complications, which would otherwise require a new surgery, in a minimally invasive way.
IR also provides a palliative treatment in advanced GI cancers stages, through diminishing pain or allowing symptoms reduction[9,51].
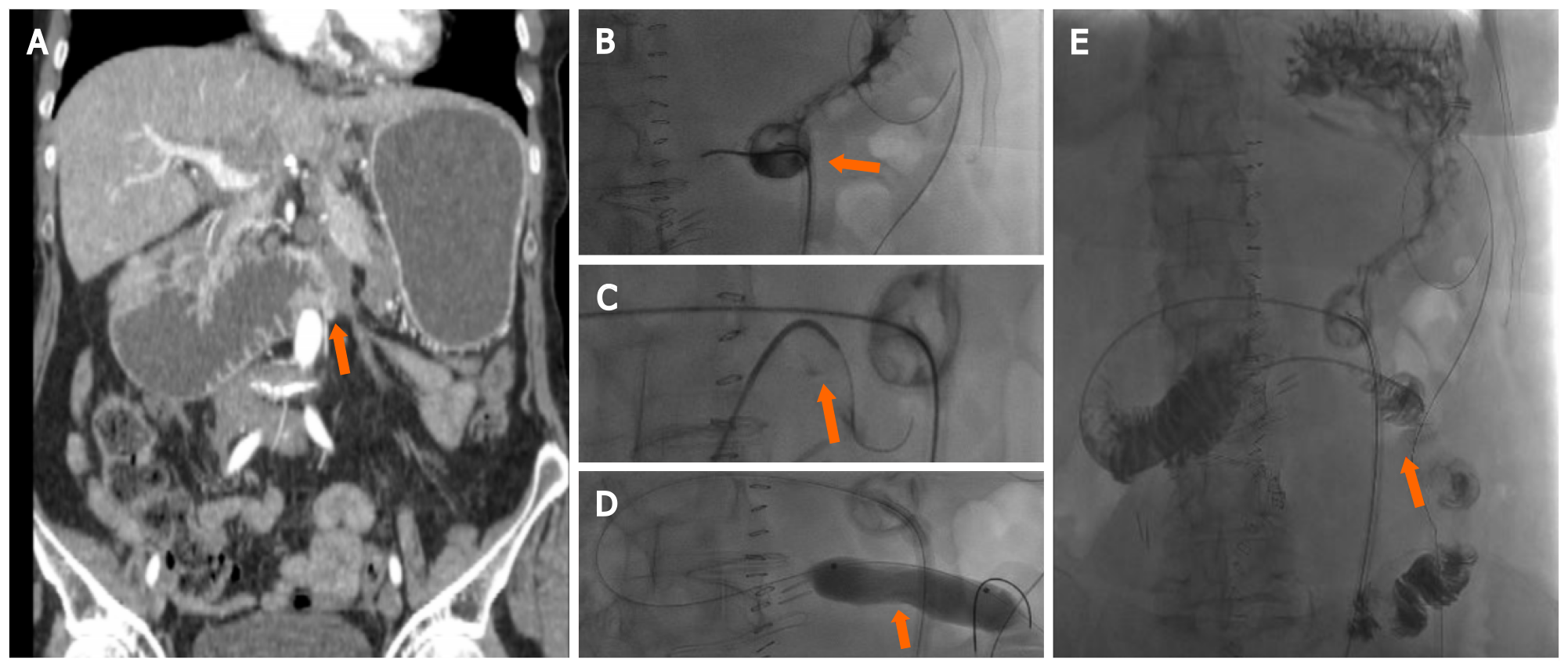
Besides the role of AE and its modification in the treatment of hepatic pathological tissues, its use in GI cancers is limited to acute bleeding treatments[23,52]. Bleeding from advanced gastric cancers accounts for 1% to 8% of the upper gastrointestinal bleedings (UGIB), causing delays in chemotherapy and increasing transfusion requirements[53,54]. Moreover, endoscopy represents the gold standard for UGIB, being able to recognize the exact source of bleeding[55]. However, in presence of profuse bleeding masking the exact source, endoscopy may fail to stop it[56,57]. Due to advances in angiography systems and haemostatic materials, IR embolization is recognized as an alternative modality in patients in whom endoscopy fails or is not indicated[58,59] IR embolization is also used in the treatment of lower gastrointestinal bleedings (LGIB), defined as bleeding originating distal to the ligament of Treitz[60]. The introduction of super-selective embolization with coaxial microcatheter systems and embolic agents (such as pledgets of absorbable gelatine sponge, polyvinyl alcohol, or other spherical particulates, micro-coils, and liquid embolic agents) represents a useful tool in LGBI[60,61]. According to the American College Guidelines[62] in the treatment of LGIB, it should be considered in high-risk patients with ongoing bleeding who do not respond adequately to the volume resuscitation and who are unlikely to tolerate bowel preparation and colonoscopy (Figure 1). Although its major complication is ischemia, it should be preferred as a first-line approached in these selected patients[63]. A new frontier for the treatment of LGIB is CBCT embolization, which allowed a fast identification of the bleeding site and simplifying the placement of the microcrater in the vessel, without requiring sequential angiography[64]. The indications and possible complications of these techniques are the same as the traditional AE, with the theoretical advantage of greater safety and efficacy due to the modern and accurate tools[64].
AE represents a useful tool also for postoperative bleeding, allowing to stop the bleeding avoiding surgical reoperation, with minimally invasive access[65]. Another possible complication of surgery is the arteriovenous or arterio-enteric fistulas, life-threatening conditions[66]. Although conventional angiography is rarely used as the first-line imaging modality for its diagnosis, angioembolization allowed minimally invasive management of the fistula and to avoid major surgery[67].
Finally, in the event of an arterial bleeding from pseudoanurysm, endovascular treatment with covered self-expanding stent-grafts placement was reported as an effective method. It is performed under local anesthesia, which avoids the need for general or locoregional anesthesia in unstable, high-risk patients[65,66].
An intrabdominal abscess could be the first cancer presentation[68] as well as a postoperative complication[50,69]. In both cases, IR is a reliable minimally invasive alternative to surgery, although the feasibility of this technique depends on the abscess location and the consistency of the contents of collections[70]. In case of deep-seated abscess or abscess located close to vulnerable structures, CT-guided percutaneous drainage is the gold standard (Figure 2). Despite the limit of a non-real-time image, it allowed the best image-depiction of the collection and the adjacent organs[7]. In the case of easily accessible abscesses, US-guided drainage must be preferred and should always be the first procedure in patients with simple abscesses[71]. US and CT can be combined with fluoroscopy to avoid guidewire kinking during the procedure and to monitor the placement of catheters[70]. The abscess can only be aspirated, or a catheter can be left in place for few days, especially when contamination or communication with the bowel or urinary tract is suspected[70]. Deep-seated abscess with interposition of organs can be drained with a surgical approach or the intervening organ can be traversed with a catheter[72]. This approach is not suitable for almost all abdominal organs, except the stomach and the liver[72,73]. Finally, transvaginal and transrectal drainage with US or CT guidance allows access to deep-seated abscesses beside the vagina or rectum, often resulting from gynecological or rectal cancers, and inaccessible with percutaneous methods[74,75]. Percutaneous abscess drain placement for abdominal and pelvic collections could be achieved also with cone-beam CT, with equivalent successful rate and radiation dose of conventional CT positioning and the advantage of reduced procedural time[76].
Oesophageal or gastric cancers determining luminal obstruction, dysphagia, or swallowing impairment, are frequently cause of intolerance of the oral intake, requiring nutritional support through a gastrostomy or gastrojejunostomy[77]. The first percutaneous radiologic gastrostomy (PRG) was performed in 1981 using fluoroscopic guidance to avoid bowel and solid organs, without the need for upper endoscopy[10].
IR showed higher technical success and safety rates, with the advantage to be performed in patients not eligible for endoscopy or surgical procedures[10]. PRG complications are similar to the percutaneous endoscopic gastrostomy (PEG), including infections (23%) and the discomfort on feeding (33%)[78,79] and less frequent complications such as haemorrhage, ileus, aspiration of feed, and tube occlusion[10].
The tube dislocation is relatively common, with the possibility of easy tube reinsertion in the same tract if this is established for more than 2 wk. Alternatively, early tube dislodgment requiring repeated gastric puncture[79]. Gastrostomy and gastrojejunostomy can be performed also in small bowel obstruction with a decompression purpose with a success rate higher than 98%[80] (Figure 3). In patients with ascites, a paracentesis must be performed to reduce the peritoneal liquid, to reduce the possibility of complications such as peritonitis or peri-catheter leakage[80,81]. Contraindications for PRG are the same as PEG, including coagulopathy as an absolute contraindication and immunosuppression as a relative one[10]. In the last years, different studies, suggested the positioning of gastroduodenal and colonic self-expanded stent under fluoroscopic-guide as a palliative treatment, in oncologic patients with no indication for surgery[82,83]. Self-expanded stent are extensively used in the palliative treatment of duodenal and rectal occlusions, as given the smallest diameter of these segments, a malignant obstruction can easily occur at these levels[82].
The positioning of the stent under fluoroscopy-guidance allowed to approach the obstruction and the safe placement of the stent, without the need of bowel preparation in case of colonic stents[82]. The use of angiographic catheters with variable head shapes and easily shapable guide-wires can facilitate passing the angulated obstruction, which is the most common cause of endoscopic failure[82,83].
Pain represents a significant source of morbidity in oncologic patients, especially in advanced stages, with an incidence ranging from 40% to 90%. According to the World Health Organization, opiates remain the first choice drugs in these patients. However, those patients with non-controlled pain or with intolerable analgesic effects could also benefit from interventional pain control techniques[84,85]. Upper abdominal visceral cancers are often poorly responsive to analgesic therapy. In these cases, nerve block or celiac ganglion neurolysis can reduce pain, especially related to pancreatic, gastric, and oesophageal cancers[86] (Figure 4). The substances most often employed in IR include local alcohol or phenol, which induce permanent nerve destruction, and triamcinolone, which reversibly blocks nocireceptors[87]. CT represents the most commonly used image-modality to guide the celiac axis block, with either an anterior or posterior approach, according to the operator experience[87]. The most frequent complications of these techniques are diarrhea (73%) and orthostatic hypotension (12%)[87].
IR showed an exponential growth in the last years and represents a useful tool in the treatment of oncologic patients. Its role in the context of GI cancers is increasingly relevant, allowing for the diagnosis and treatment of cancer and related complications, with a minimally-invasive approach. The introduction of ablation techniques and monitoring devices contributed to the effectiveness and safety of IR procedures, allowing for the treatment of lesions close to sensitive structures, often difficult to be accessed by other approaches. IR is a very useful tool also in the treatment of GI cancer complications, e.g., bleeding from the digestive tract that cannot be reached by endoscopy[56].
Given the increasing relevance of IR in GI cancers management, the inclusion of interventional radiologists in the multidisciplinary oncologic staff is considered of paramount importance. Specific training programs, also including the use of simulators, are necessary to support the IR learning curve.
IR is a medical specialty which uses minimally-invasive technique in GI cancer management. Given its prominent role, the IR specialist should always be considered as an essential player in the multidisciplinary staff responsible for the treatment of the oncologic patient.
The entire manuscript has been written and amended by a native English speaker (Martin Mariappan MD).
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kamada Y, Kanat O, Vasani J S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159:335-349.e15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1227] [Article Influence: 245.4] [Reference Citation Analysis (0)] |
| 2. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 726] [Article Influence: 103.7] [Reference Citation Analysis (1)] |
| 3. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1067] [Article Influence: 177.8] [Reference Citation Analysis (1)] |
| 4. | Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol. 2019;10:10-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 893] [Cited by in RCA: 1518] [Article Influence: 253.0] [Reference Citation Analysis (1)] |
| 5. | GBD 2017 Risk Factor Collaborators. . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923-1994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3399] [Cited by in RCA: 3033] [Article Influence: 433.3] [Reference Citation Analysis (0)] |
| 6. | Silverman SG, Khorasani R, Adams DF, Phillips MD, Sica GT, Mayer RJ. Multidisciplinary gastrointestinal cancer clinic: abdominal radiologist as active participant. Acad Radiol. 1998;5:694-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Mahnken AH, Ricke J. CT- and MR-guided interventions in radiology. Berlin: Springer Berlin Heidelberg, 2013: 11-24. |
| 8. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 932] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 9. | Campbell TC, Roenn JH. Palliative Care for Interventional Radiology: An Oncologist's Perspective. Semin Intervent Radiol. 2007;24:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Lyon SM, Pascoe DM. Percutaneous gastrostomy and gastrojejunostomy. Semin Intervent Radiol. 2004;21:181-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 11. | Abi-Jaoudeh N, Duffy AG, Greten TF, Kohn EC, Clark TW, Wood BJ. Personalized oncology in interventional radiology. J Vasc Interv Radiol. 2013;24:1083-92; quiz 1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Tam AL, Lim HJ, Wistuba II, Tamrazi A, Kuo MD, Ziv E, Wong S, Shih AJ, Webster RJ 3rd, Fischer GS, Nagrath S, Davis SE, White SB, Ahrar K. Image-Guided Biopsy in the Era of Personalized Cancer Care: Proceedings from the Society of Interventional Radiology Research Consensus Panel. J Vasc Interv Radiol. 2016;27:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Mihlon F 4th, Ray CE Jr, Messersmith W. Chemotherapy agents: a primer for the interventional radiologist. Semin Intervent Radiol. 2010;27:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | de Sio I, Funaro A, Vitale LM, Niosi M, Francica G, Federico A, Sgambato D, Loguercio C, Romano M. Ultrasound-guided percutaneous biopsy for diagnosis of gastrointestinal lesions. Dig Liver Dis. 2013;45:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Arellano RS, Maher M, Gervais DA, Hahn PF, Mueller PR. The difficult biopsy: let's make it easier. Curr Probl Diagn Radiol. 2003;32:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Sheafor DH, Paulson EK, Simmons CM, DeLong DM, Nelson RC. Abdominal percutaneous interventional procedures: comparison of CT and US guidance. Radiology. 1998;207:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Cerci JJ, Tabacchi E, Bogoni M, Delbeke D, Pereira CC, Cerci RJ, Krauzer C, Sakamoto DG, Fanti S, Vitola JV. Comparison of CT and PET/CT for biopsy guidance in oncological patients. Eur J Nucl Med Mol Imaging. 2017;44:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Paulson EK, Sheafor DH, Enterline DS, McAdams HP, Yoshizumi TT. CT fluoroscopy--guided interventional procedures: techniques and radiation dose to radiologists. Radiology. 2001;220:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 165] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 19. | Hakime A, Deschamps F, De Carvalho EG, Teriitehau C, Auperin A, De Baere T. Clinical evaluation of spatial accuracy of a fusion imaging technique combining previously acquired computed tomography and real-time ultrasound for imaging of liver metastases. Cardiovasc Intervent Radiol. 2011;34:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Lim HJ, Park CM, Yoon SH, Bae JS, Goo JM. Cone-Beam CT Virtual Navigation-Guided Percutaneous Needle Biopsy of Suspicious Pleural Metastasis: A Pilot Study. Korean J Radiol. 2018;19:872-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Choi YR, Chung JW, Kim JH, Kim HC, Jae HJ, Hur S. Cone-Beam Computed Tomography-Hepatic Arteriography as a Diagnostic Tool for Small Hypervascular Hepatocellular Carcinomas: Method and Clinical Implications. Korean J Radiol. 2020;21:306-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Takaki H, Litchman T, Covey A, Cornelis F, Maybody M, Getrajdman GI, Sofocleous CT, Brown KT, Solomon SB, Alago W, Erinjeri JP. Hepatic artery embolization for liver metastasis of gastrointestinal stromal tumor following imatinib and sunitinib therapy. J Gastrointest Cancer. 2014;45:494-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Goldstein HM, Medellin H, Ben-Menachem Y, Wallace S. Transcatheter arterial embolization in the management of bleeding in the cancer patient. Radiology. 1975;115:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Eriksson LG, Ljungdahl M, Sundbom M, Nyman R. Transcatheter arterial embolization vs surgery in the treatment of upper gastrointestinal bleeding after therapeutic endoscopy failure. J Vasc Interv Radiol. 2008;19:1413-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 25. | Amesur NB, Zajko AB, Carr BI. Chemo-embolization for unresectable hepatocellular carcinoma with different sizes of embolization particles. Dig Dis Sci. 2008;53:1400-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: A network meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0184597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 27. | Rodríguez Carvajal R, Orgaz A, Leal JI, Peinado FJ, Vicente S, Gil J, Flores A, Fontcuberta J, Buendia E, Bolufer E, Gómez A, Doblas M. Renal embolization and nephrectomy in a single surgical act in high-risk renal tumor pathology. Ann Vasc Surg. 2011;25:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Wörns MA, Galle PR. Future perspectives in hepatocellular carcinoma. Dig Liver Dis. 2010;42 Suppl 3:S302-S309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1106] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 30. | Fiorentini G, Sarti D, Nardella M, Inchingolo R, Nestola M, Rebonato A, Guadagni S. Chemoembolization Alone or Associated With Bevacizumab for Therapy of Colorectal Cancer Metastases: Preliminary Results of a Randomized Study. In Vivo. 2020;34:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Fiorentini G, Carandina R, Sarti D, Nardella M, Zoras O, Guadagni S, Inchingolo R, Nestola M, Felicioli A, Barnes Navarro D, Munoz Gomez F, Aliberti C. Polyethylene glycol microspheres loaded with irinotecan for arterially directed embolic therapy of metastatic liver cancer. World J Gastrointest Oncol. 2017;9:379-384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 33. | Pereira PL, Iezzi R, Manfredi R, Carchesio F, Bánsághi Z, Brountzos E, Spiliopoulos S, Echevarria-Uraga JJ, Gonçalves B, Inchingolo R, Nardella M, Pellerin O, Sousa M, Arnold D, de Baère T, Gomez F, Helmberger T, Maleux G, Prenen H, Sangro B, Zeka B, Kaufmann N, Taieb J. The CIREL Cohort: A Prospective Controlled Registry Studying the Real-Life Use of Irinotecan-Loaded Chemoembolisation in Colorectal Cancer Liver Metastases: Interim Analysis. Cardiovasc Intervent Radiol. 2021;44:50-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-Guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25:1691-705.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 35. | Chen J, Tang Z, Dong X, Gao S, Fang H, Wu D, Xiang D, Zhang S. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol. 2013;39:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Liu LX, Zhang WH, Jiang HC. Current treatment for liver metastases from colorectal cancer. World J Gastroenterol. 2003;9:193-200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 100] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | Rose SC, Thistlethwaite PA, Sewell PE, Vance RB. Lung cancer and radiofrequency ablation. J Vasc Interv Radiol. 2006;17:927-951; quiz 951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Thanos L, Mylona S, Galani P, Tzavoulis D, Kalioras V, Tanteles S, Pomoni M. Radiofrequency ablation of osseous metastases for the palliation of pain. Skeletal Radiol. 2008;37:189-194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Iannuccilli JD, Dupuy DE, Beland MD, Machan JT, Golijanin DJ, Mayo-Smith WW. Effectiveness and safety of computed tomography-guided radiofrequency ablation of renal cancer: a 14-year single institution experience in 203 patients. Eur Radiol. 2016;26:1656-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Beland M, Mueller PR, Gervais DA. Thermal ablation in interventional oncology. Semin Roentgenol. 2007;42:175-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Aarts BM, Klompenhouwer EG, Rice SL, Imani F, Baetens T, Bex A, Horenblas S, Kok M, Haanen JBAG, Beets-Tan RGH, Gómez FM. Cryoablation and immunotherapy: an overview of evidence on its synergy. Insights Imaging. 2019;10:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 42. | Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1145] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 43. | Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory vs suppressive immune responses. Cryobiology. 2009;58:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 44. | Hinshaw JL, Lubner MG, Ziemlewicz TJ, Lee FT Jr, Brace CL. Percutaneous tumor ablation tools: microwave, radiofrequency, or cryoablation--what should you use and why? Radiographics. 2014;34:1344-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 215] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 45. | Galandi D, Antes G. Radiofrequency thermal ablation vs other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2002;CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Ohmoto K, Yoshioka N, Tomiyama Y, Shibata N, Kawase T, Yoshida K, Kuboki M, Yamamoto S. Comparison of therapeutic effects between radiofrequency ablation and percutaneous microwave coagulation therapy for small hepatocellular carcinomas. J Gastroenterol Hepatol. 2009;24:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Biçeroğlu S, Memiş A. Gene therapy: applications in interventional radiology. Diagn Interv Radiol. 2005;11:113-118. [PubMed] |
| 48. | Yu M, Chen W, Zhang J. p53 gene therapy for pulmonary metastasis tumor from hepatocellular carcinoma. Anticancer Drugs. 2010;21:882-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Mahnken AH, Pereira PL, de Baère T. Interventional oncologic approaches to liver metastases. Radiology. 2013;266:407-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 50. | Sohn TA, Yeo CJ, Cameron JL, Geschwind JF, Mitchell SE, Venbrux AC, Lillemoe KD. Pancreaticoduodenectomy: role of interventional radiologists in managing patients and complications. J Gastrointest Surg. 2003;7:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Buss MK. The Intersection of Palliative Care and Interventional Radiology: Enhancing Understanding and Collaboration. Semin Intervent Radiol. 2017;34:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Park S, Shin JH, Gwon DI, Kim HJ, Sung KB, Yoon HK, Ko GY, Ko HK. Transcatheter Arterial Embolization for Gastrointestinal Bleeding Associated with Gastric Carcinoma: Prognostic Factors Predicting Successful Hemostasis and Survival. J Vasc Interv Radiol. 2017;28:1012-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Del Piano M, Bianco MA, Cipolletta L, Zambelli A, Chilovi F, Di Matteo G, Pagliarulo M, Ballarè M, Rotondano G; Prometeo study group of the Italian Society of Digestive Endoscopy (SIED). The "Prometeo" study: online collection of clinical data and outcome of Italian patients with acute nonvariceal upper gastrointestinal bleeding. J Clin Gastroenterol. 2013;47:e33-e37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 54. | Esrailian E, Gralnek IM. Nonvariceal upper gastrointestinal bleeding: epidemiology and diagnosis. Gastroenterol Clin North Am. 2005;34:589-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Kim YI, Choi IJ, Cho SJ, Lee JY, Kim CG, Kim MJ, Ryu KW, Kim YW, Park YI. Outcome of endoscopic therapy for cancer bleeding in patients with unresectable gastric cancer. J Gastroenterol Hepatol. 2013;28:1489-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 56. | Stanley AJ, Laine L. Management of acute upper gastrointestinal bleeding. BMJ. 2019;364:l536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 137] [Article Influence: 22.8] [Reference Citation Analysis (36)] |
| 57. | Spiliopoulos S, Inchingolo R, Lucatelli P, Iezzi R, Diamantopoulos A, Posa A, Barry B, Ricci C, Cini M, Konstantos C, Palialexis K, Reppas L, Trikola A, Nardella M, Adam A, Brountzos E. Transcatheter Arterial Embolization for Bleeding Peptic Ulcers: A Multicenter Study. Cardiovasc Intervent Radiol. 2018;41:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 58. | Lee HJ, Shin JH, Yoon HK, Ko GY, Gwon DI, Song HY, Sung KB. Transcatheter arterial embolization in gastric cancer patients with acute bleeding. Eur Radiol. 2009;19:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Inchingolo R, Nestola M, Posa A, Di Costanzo G, Nardella M. Intrastent Pseudoaneurysm following Endoscopic Biliary Stent Insertion. J Vasc Interv Radiol. 2017;28:1321-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Speir EJ, Ermentrout RM, Martin JG. Management of Acute Lower Gastrointestinal Bleeding. Tech Vasc Interv Radiol. 2017;20:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Hur S, Jae HJ, Lee M, Kim HC, Chung JW. Safety and efficacy of transcatheter arterial embolization for lower gastrointestinal bleeding: a single-center experience with 112 patients. J Vasc Interv Radiol. 2014;25:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:459-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 63. | Nykänen T, Peltola E, Kylänpää L, Udd M. Transcatheter Arterial Embolization in Lower Gastrointestinal Bleeding: Ischemia Remains a Concern Even with a Superselective Approach. J Gastrointest Surg. 2018;22:1394-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Ierardi AM, Urbano J, De Marchi G, Micieli C, Duka E, Iacobellis F, Fontana F, Carrafiello G. New advances in lower gastrointestinal bleeding management with embolotherapy. Br J Radiol. 2016;89:20150934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Chatani S, Inoue A, Ohta S, Takaki K, Sato S, Iwai T, Murakami Y, Watanabe S, Sonoda A, Nitta N, Maehira H, Tani M, Murata K. Transcatheter Arterial Embolization for Postoperative Bleeding Following Abdominal Surgery. Cardiovasc Intervent Radiol. 2018;41:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 66. | Partovi S, Trischman T, Sheth RA, Huynh TTT, Davidson JC, Prabhakar AM, Ganguli S. Imaging work-up and endovascular treatment options for aorto-enteric fistula. Cardiovasc Diagn Ther. 2018;8:S200-S207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Chiesa R, Melissano G, Marone EM, Marrocco-Trischitta MM, Kahlberg A. Aorto-oesophageal and aortobronchial fistulae following thoracic endovascular aortic repair: a national survey. Eur J Vasc Endovasc Surg. 2010;39:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 68. | Cho J, Park I, Lee D, Sung K, Baek J, Lee J. Advanced Gastric Cancer Perforation Mimicking Abdominal Wall Abscess. J Gastric Cancer. 2015;15:214-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 69. | Connell TR, Stephens DH, Carlson HC, Brown ML. Upper abdominal abscess: a continuing and deadly problem. AJR Am J Roentgenol. 1980;134:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 70. | Maher MM, Gervais DA, Kalra MK, Lucey B, Sahani DV, Arellano R, Hahn PF, Mueller PR. The inaccessible or undrainable abscess: how to drain it. Radiographics. 2004;24:717-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 71. | Men S, Akhan O, Köroğlu M. Percutaneous drainage of abdominal abcess. Eur J Radiol. 2002;43:204-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | Cronin CG, Gervais DA, Castillo CF, Mueller PR, Arellano RS. Interventional radiology in the management of abdominal collections after distal pancreatectomy: a retrospective review. AJR Am J Roentgenol. 2011;197:241-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Hawel J, McFadgen H, Stewart R, El-Ghazaly T, Alawashez A, Ellsmere J. Interventional radiology-assisted transgastric endoscopic drainage of peripancreatic fluid collections. Can J Surg. 2020;63:E254-E256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Varghese JC, O'Neill MJ, Gervais DA, Boland GW, Mueller PR. Transvaginal catheter drainage of tuboovarian abscess using the trocar method: technique and literature review. AJR Am J Roentgenol. 2001;177:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 75. | Martins BC, Marques CF, Nahas CS, Hondo FY, Pollara W, Nahas SC, Ribeiro Junior U, Cecconello I, Maluf-Filho F. A novel approach for the treatment of pelvic abscess: transrectal endoscopic drainage facilitated by transanal endoscopic microsurgery access. Surg Endosc. 2012;26:2667-2670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Partovi S, Li X, Moon E, Thompson D. Image guided percutaneous gastrostomy catheter placement: How we do it safely and efficiently. World J Gastroenterol. 2020;26:383-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (3)] |
| 77. | McKay T, Ingraham CR, Johnson GE, Kogut MJ, Vaidya S, Padia SA. Cone-Beam CT with Fluoroscopic Overlay Versus Conventional CT Guidance for Percutaneous Abdominopelvic Abscess Drain Placement. J Vasc Interv Radiol. 2016;27:52-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 78. | Gonçalves F, Mozes M, Saraiva I, Ramos C. Gastrostomies in palliative care. Support Care Cancer. 2006;14:1147-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD, Bettmann MA, Gordon SR. Percutaneous radiologic gastrostomy vs percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005;56:84-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 80. | Ryan JM, Hahn PF, Mueller PR. Performing radiologic gastrostomy or gastrojejunostomy in patients with malignant ascites. AJR Am J Roentgenol. 1998;171:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Given MF, Lyon SM, Lee MJ. The role of the interventional radiologist in enteral alimentation. Eur Radiol. 2004;14:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 82. | Katsanos K, Sabharwal T, Adam A. Stenting of the lower gastrointestinal tract: current status. Cardiovasc Intervent Radiol. 2011;34:462-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 83. | Katsanos K, Sabharwal T, Adam A. Stenting of the upper gastrointestinal tract: current status. Cardiovasc Intervent Radiol. 2010;33:690-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Caraceni A, Portenoy RK; a working group of the IASP Task Force on Cancer Pain. An international survey of cancer pain characteristics and syndromes. IASP Task Force on Cancer Pain. International Association for the Study of Pain. Pain. 1999;82:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 401] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 85. | Caraceni A, Martini C, Zecca E, Portenoy RK, Ashby MA, Hawson G, Jackson KA, Lickiss N, Muirden N, Pisasale M, Moulin D, Schulz VN, Rico Pazo MA, Serrano JA, Andersen H, Henriksen HT, Mejholm I, Sjogren P, Heiskanen T, Kalso E, Pere P, Poyhia R, Vuorinen E, Tigerstedt I, Ruismaki P, Bertolino M, Larue F, Ranchere JY, Hege-Scheuing G, Bowdler I, Helbing F, Kostner E, Radbruch L, Kastrinaki K, Shah S, Vijayaram S, Sharma KS, Devi PS, Jain PN, Ramamani PV, Beny A, Brunelli C, Maltoni M, Mercadante S, Plancarte R, Schug S, Engstrand P, Ovalle AF, Wang X, Alves MF, Abrunhosa MR, Sun WZ, Zhang L, Gazizov A, Vaisman M, Rudoy S, Gomez Sancho M, Vila P, Trelis J, Chaudakshetrin P, Koh ML, Van Dongen RT, Vielvoye-Kerkmeer A, Boswell MV, Elliott T, Hargus E, Lutz L; Working Group of an IASP Task Force on Cancer Pain. Breakthrough pain characteristics and syndromes in patients with cancer pain. An international survey. Palliat Med. 2004;18:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 222] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 86. | Midia M, Dao D. The Utility of Peripheral Nerve Blocks in Interventional Radiology. AJR Am J Roentgenol. 2016;207:718-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Akhan O, Ozmen MN, Basgun N, Akinci D, Oguz O, Koroglu M, Karcaaltincaba M. Long-term results of celiac Ganglia block: correlation of grade of tumoral invasion and pain relief. AJR Am J Roentgenol. 2004;182:891-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |