Published online Aug 28, 2020. doi: 10.4329/wjr.v12.i8.172
Peer-review started: March 22, 2020
First decision: April 29, 2020
Revised: May 2, 2020
Accepted: July 1, 2020
Article in press: July 1, 2020
Published online: August 28, 2020
Processing time: 154 Days and 17.7 Hours
Prostatic artery embolization (PAE) has gained acceptance as a minimally invasive, safe and effective treatment of symptomatic benign prostatic hyperplasia. Radiologic imaging is an indispensable part of post-interventional evaluation of PAE and serves both clinical and investigational purposes. In this context, ultrasonography (US) has a central and multifaceted role. Gray-scale US is routinely utilized for measurement of significant outcome parameters (prostatic volume, intra-vesical prostatic protrusion and post-void residual volume) before and after PAE. Improvement of these parameters may become more obvious one-month post-PAE, or later. Contrast-enhanced US (CEUS) with intravenous administration of a second-generation echo-enhancer can demonstrate prostatic infarcts (as enhancement defects) immediately post-PAE and monitor their resolution over time. The volume of prostatic infarcts can also be measured and compared to prostatic volume. Prostatic infarction is a definite sign of the local efficacy of PAE and a predictor of prostate shrinkage and (at least in some patients) of clinical success. CEUS can also be performed intraoperatively in the angio-suite, for on-site evaluation of the ischemic effect; a variation of this technique, with intraarterial (instead of intravenous) administration of diluted echo enhancer, can also be applied intraoperatively, to map the embolized territory and to prevent non-target embolization. Initial experience with US-elastographic techniques (shear-wave and strain elastography) has shown that they can detect and quantify the improvement of tissue elasticity post-PAE, thus providing new insights into the therapeutic mechanisms of this treatment. With utilization of high-end equipment, experience and standardized imaging protocols, US could be the primary modality for imaging evaluation of PAE.
Core tip: Ultrasonography is a practical and affordable modality for imaging evaluation of the efficacy of prostatic artery embolization (PAE). This evaluation includes, but is not limited to, standard measurements of prostatic volume, intravesical prostatic protrusion and post-void residual volume. With contrast-enhanced ultrasound, prostatic infarcts can be easily detected shortly after PAE, and their extent appears to have clinical and prognostic value. Strain- and shear-wave elastography can depict the changes in prostatic consistency caused by PAE. A comprehensive description of all these applications is attempted in the following review.
- Citation: Moschouris H, Dimakis A, Anagnostopoulou A, Stamatiou K, Malagari K. Sonographic evaluation of prostatic artery embolization: Far beyond size measurements. World J Radiol 2020; 12(8): 172-183
- URL: https://www.wjgnet.com/1949-8470/full/v12/i8/172.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i8.172
During the last years, prostatic artery embolization (PAE) has emerged as an effective, minimally invasive endovascular treatment of symptomatic benign prostatic hyperplasia (BPH)[1-3]. Technically successful PAE entails superselective catheterization and embolization of the prostatic arteries (PAs) of at least one pelvic side with microparticles (spherical or non-spherical, with diameters of 50-500 μm)[3,4]. PAE-induced ischemia results in prostate shrinkage and in functional changes which eventually result in the relief of BPH symptoms[3,4]. Therefore, characterization of the clinical success of PAE is primarily based on symptomatic improvement. The latter is quantitatively expressed by a reduction of the International Prostate Symptom Score (IPSS)[2], with reported post-PAE mean rates of reduction of 53.9%-62.5%[1] and with an IPSS reduction of at least 25% required for characterization of the clinical success of PAE. Nevertheless, the role of imaging in assessment of the therapeutic efficacy of PAE is also essential. Radiologic imaging is expected to provide a non-invasive, reliable and reproducible evaluation of prostatic size and of the morphologic, textural and perfusional changes that occur in the prostate after PAE[5]. A straightforward demonstration of the local efficacy on the target organ is essential for a relatively new endovascular treatment like PAE. Imaging follow-up is also required to evaluate the durability of the treatment effects over time and to diagnose some of the complications of PAE. Additionally, the correlation of imaging findings with clinical, biochemical and uroflowmetric data can serve investigational purposes and can improve our understanding of the therapeutic mechanisms of PAE.
At present, magnetic resonance (MR) is considered the “gold standard” modality for imaging evaluation of PAE. By virtue of its superior soft tissue contrast resolution, MR accurately shows size and texture changes of the prostate post-PAE[5-7]; ischemic changes caused by PAE are also associated with signal alterations in unenhanced T1 and T2 sequences, although their delineation is more clear and detailed on gadolinium-enhanced T1 sequences[6,7]. However, equipment and examination costs may limit the availability and repeatability of MR and the technique (or its dedicated contrast media) may be contraindicated in some patients. Ultrasonography (US) is a more affordable, flexible and widely available modality, which has also been utilized for the study of PAE since the early applications of this treatment[8,9]. Although standard (gray-scale) US is limited to the evaluation of size and shape of the prostate before and after PAE, more advanced techniques can significantly increase the diagnostic yield of US, with an acceptable increase in the time and cost of the examination.
During the last 4 years, in our institution we have utilized US (standard and advanced techniques) as the primary modality for imaging evaluation of PAE intraoperatively, immediately post-procedure and for short- and long-term follow-up. Based on this experience and on current literature, we herein attempt to provide a comprehensive review of the technique, role, findings and limitations of US evaluation of PAE.
Gray-scale US is routinely utilized in many centers for the calculation of prostate volume (PV) before and after PAE and for evaluation of prostate shrinkage. The latter is an easily conceivable sign of the therapeutic effect of PAE. Although transrectal US (t.r.-US) is preferred by some researchers[1], transabdominal US (t.a.-US) is also acceptable. In experienced hands, PV measurements with t.a.-US correlate closely with those of t.r.-US and the former technique is more comfortable for the patient and more suitable for repeated application[10,11]. Of note, it has been demonstrated that variations in bladder volume have a negligible effect on transabdominal measurements of PV, if bladder volume is in the range of 100-400 mL[11,12]. With both approaches, the prostate is scanned in the axial, coronal and sagittal plane and PV is calculated with the ellipsoid formula (length × height × width × 0.52). A well-recognized disadvantage of this formula is its limited accuracy in larger, irregular shaped or asymmetrically enlarged prostates. Results may also depend on the experience of the operator[13]. Three-dimensional US may provide a more accurate estimate of PV[11], but, as a more complex and technically demanding method, it is not routinely utilized for follow-up post-PAE. Prostate shrinkage caused by technically successful PAE is negligible during the first days post-procedure, but becomes significant one month post-PAE and at 3, 6 and 12 mo follow-up[1,2]. Reported mean reduction rates of PV post-PAE are 31.9%, 32.1% and 37.6% at 6, 12 and 24 mo post-intervention, respectively[1]. Long-term data are limited, although there is evidence of sustained tumor shrinkage (mean PV reduction of 12.6% at 3 years post-PAE)[14].
Intra-vesical prostatic protrusion (IPP) represents the extent to which the enlarged prostate (its median lobe, lateral lobes, or both) protrudes into the urinary bladder[15]. IPP causes a ball-valve type of obstruction and disrupts the funneling shape of the bladder neck thus contributing to bladder outlet obstruction. IPP greater than 10 mm is considered significant and is associated with increased PV and decreased urinary flow rate. US (both t.a.-US and t.r.-US) is the standard modality for calculation of IPP, which is measured on the sagittal view and is defined as the greatest vertical distance from the tip of the prostate gland to the circumference of the urinary bladder[15]. PAE has proved efficient in reducing the IPP (reported reduction rates: 14.7%-25%)[16,17] and this effect has been associated with clinical improvement of treated patients.
In general, although MR is considered the gold standard for the morphologic and volumetric assessment of the prostate post-PAE, US is accepted by most researchers as a valid alternative; availability, easy application and cost-effectiveness make US particularly suitable for repeated, long-term follow-up of large PAE-patient cohorts[1,18]. Finally, gray-scale t.a.-US is the standard modality for the calculation of post-void residual volume (PVR). Reported mean rates of the reduction of PVR post-PAE are 55.6%, 67.3% and 64.7% at 6, 12 and 24 mo post-intervention, respectively[1].
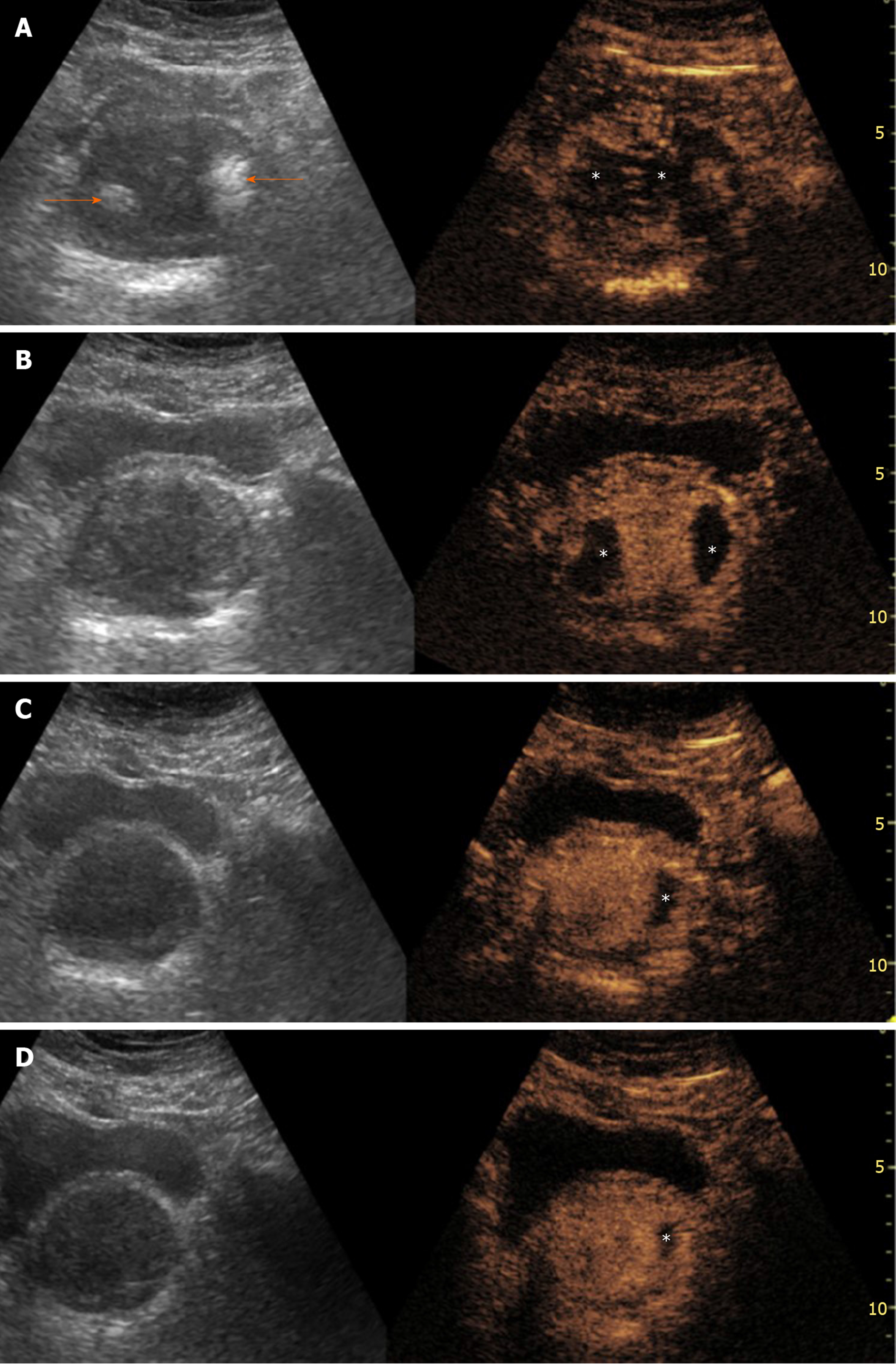
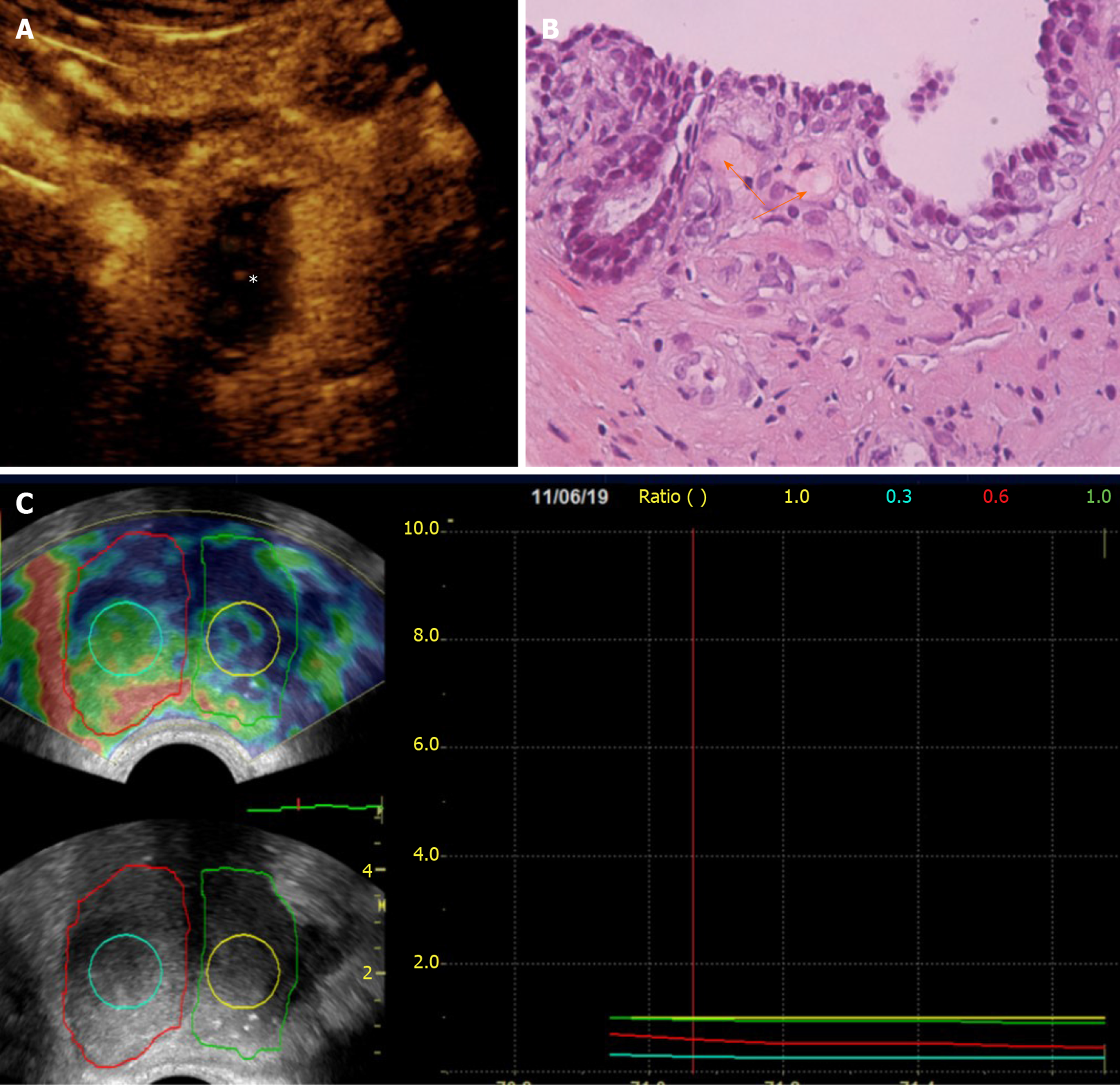
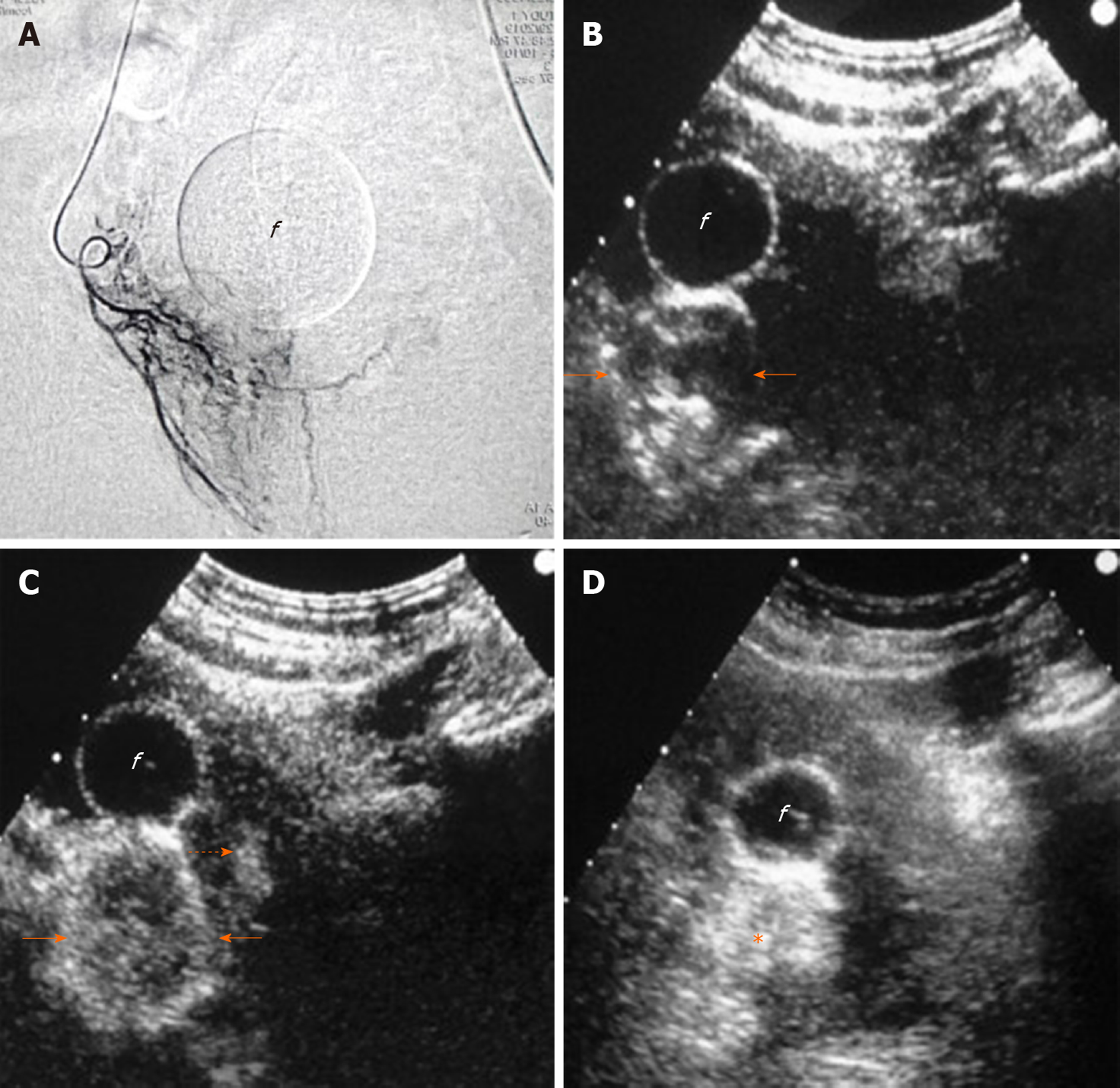
Irreversible prostatic ischemia and consequent infarction play a central role in the therapeutic action of PAE[4]. The embolized, infarcted adenomas of BPH undergo gradual shrinkage, the mass effect on the prostatic urethra is relieved and symptoms, as well as urinary flow, are improved. At present, our knowledge regarding imaging of prostatic ischemia post-PAE is derived primarily from contrast-enhanced MR (CEMR) studies[6,7,17,19]. Nevertheless, contrast-enhanced US (CEUS), as a modality of real-time and detailed imaging of micro- and macrocirculation, has also yielded promising results[8,9,20,21]. In fact, thanks to its anatomic location, relatively small size and immobility, the prostate may be an even more suitable target for CEUS study compared to the liver and other abdominal organs. Indeed, CEUS post-PAE has proved feasible, cost-effective and able to depict in detail prostatic infarcts. Similar to other abdominal applications of CEUS, a second generation ultrasound contrast agent (for example, suspension of microbubbles of sulphur hexafluoride, SonoVue, Bracco, Italy), is injected as a bolus in a forearm vein, followed by a flush of normal saline. A dedicated, contrast specific, continuous scanning, low mechanical index (MI = 0.09-0.13) technique is utilized and the prostate is scanned in three planes for 1-2 min post-injection. Both t.r.- and t.a.-CEUS can be applied, to depict prostatic infarcts, with the former approach offering a higher spatial and contrast resolution; however, comparison between these two approaches has shown insignificant differences in the measurements of prostatic infarcts post-PAE[20]. Moreover, in the clinical setting of PAE, detection of the tiniest foci of residual enhancement is not as crucial as in the oncologic, post-ablation applications of CEUS. When BPH nodules are completely infarcted, they appear as round or ovoid enhancement defects on CEUS; when incomplete infarction has occurred, the enhancement defects may be irregular in shape, or geographic[21]. Prostatic infarcts can be detected almost immediately post-PAE; however, they are more clearly delineated during the first days post-procedure. In the following weeks, prostatic infarcts become gradually smaller and the majority disappear 3-6 mo post-PAE[20,21] (Figure 1). PAE-induced prostatic infarcts have been observed in 71.4%-92.8% of patients with CEUS[20,21] and in 33%-100% with MR[7,17]. Differences in the prevalence and extent of prostatic infarcts post-PAE are explained by variations in the embolization technique: Superselective cannulation of the PAE, distal advancement of the microcatheter in the intraprostatic branches and utilization of smaller embolic agents (with diameters < 300 μm) are factors associated with more frequent and extensive infarction[4,7,22]. Prostatic infarcts usually appear only in the treated lobe of the prostate; occasionally however, bilateral infarction can occur after unilateral PAE, and this is attributed to intraprostatic anastomoses with the PA branches of the contralateral side. Of note, PAE-induced infarcts are located exclusively in the transitional zone; the peripheral zone is spared, possibly due to differences in its blood supply and microcirculation[19].
The volume of prostatic infarcts can be calculated with the ellipsoid formula (in the same manner as PV) and this is an acceptable method for regularly shaped, round or ovoid infarcts[21]. Volumetric assessment with planimetry and dedicated software result in superior accuracy, particularly in the case of multiple, irregularly shaped infarcts, but at present this technique is applied only with CEMR[17]. The volume of prostatic infarcts is divided by the PV (calculated at the same time) and the percentage of prostatic ischemia (pPI) is derived[21]. pPI correlates strongly with, and can predict subsequent prostate shrinkage (the latter being the final result of the absorption of infarcted tissue)[21]. The extent of prostatic infarction appears to be a predictor of the clinical success of PAE, at least in the subgroup of patients with BPH, urinary retention and indwelling bladder catheter (Figure 2). In a recent study with systematic CEUS evaluation post-PAE, successful removal of the catheter was achieved only in patients with pPI > 10%[21]. In a similar patient cohort, treated with PAE and studied with MR, a successful and durable bladder catheter removal was also observed only when the volume of infarcted prostatic tissue exceeded 10% of PV[7].
In brief, prostatic infarcts represent a significant imaging finding post-PAE. The early detection of prostatic infarcts with CEUS can be utilized to demonstrate the local efficacy of PAE and to predict prostate shrinkage and clinical success, which become apparent several days or weeks later.
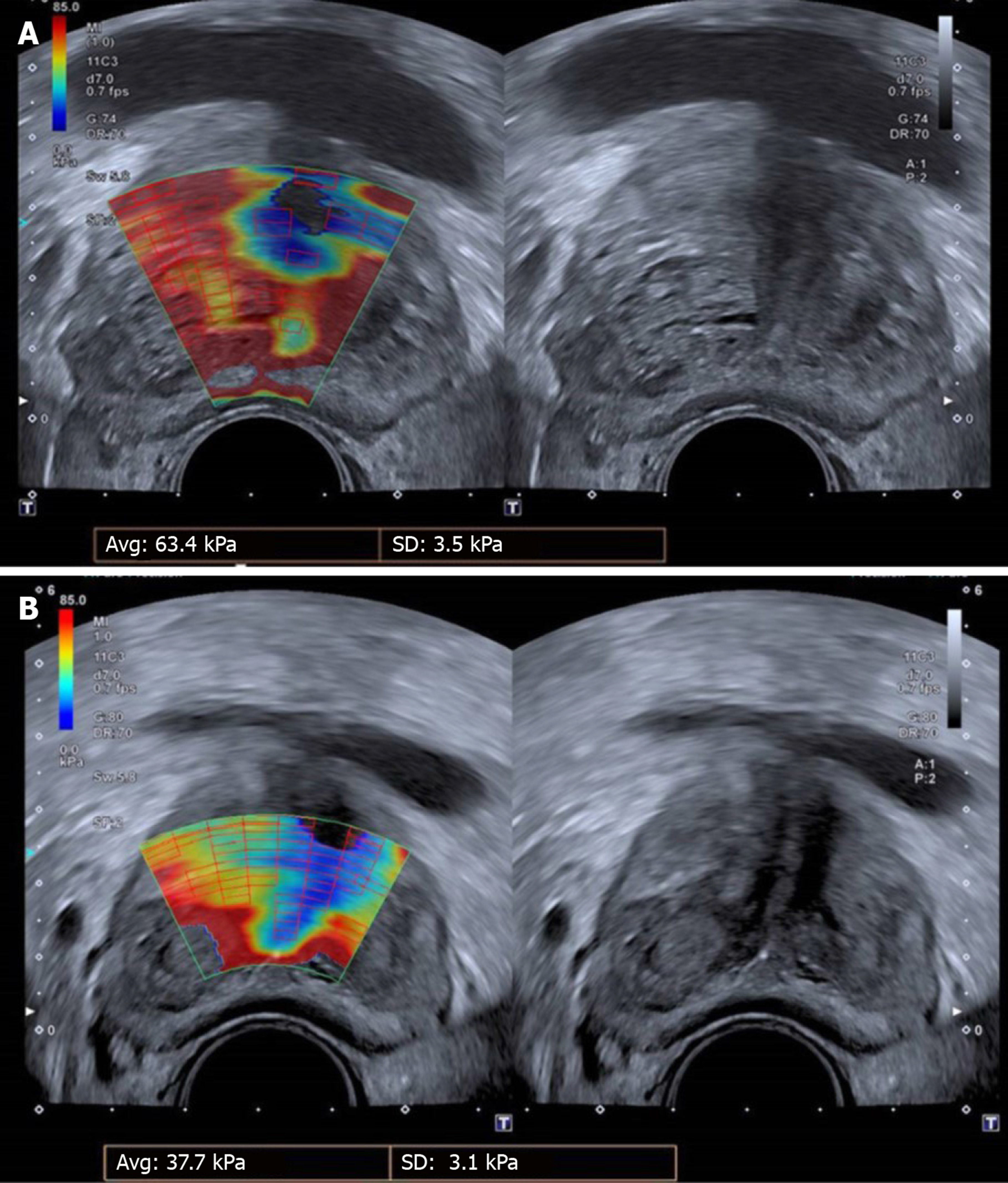
It is almost certain that infarction, followed by prostate shrinkage, is not the only therapeutic mechanism of PAE. In fact, reduction of PV and relief of the extrinsic compression to the prostatic urethra, addresses only the “static” (anatomic) component of BPH. However, there is also a “dynamic” (functional) component in the pathophysiology of BPH, related to activation of the alpha1-adrenergic receptors of the prostate and associated with reduced elasticity of the prostatic adenomatous tissue[4,23]. It is believed that PAE blocks the arrival of sympathetic mediators which are required to activate the alpha1-receptors, additionally; PAE reduces the number of the receptors themselves, thus improving the elasticity of the prostate. This potential mechanism is supported by the clinical observation of “softening” of the prostate on digital rectal examination after successful PAE. US-elastographic techniques have also been utilized to provide relevant evidence (Figures 3 and 4). Shear-wave elastography (SWE) uses a sonographic push pulse to generate a shear wave in the tissues. The velocity of the shear wave is affected by tissue stiffness (higher velocity in stiffer tissues); shear-wave velocity can be calculated and color-coded for each pixel of the respective US-image, thus creating an elastographic color map, which is overlaid on the gray-scale image[24]. In a pilot study[23], t.r.-SWE of the prostate was performed in 8 patients before and 1 month after clinically successful PAE. SWE showed a statistically significant reduction of shear-wave velocity (19.0%, P < 0.001, measured in m/s) and of the elastic modulus (29.8%, P = 0.002, measured in kPa) of the transitional prostate zone, indicating increased tissue elasticity post-PAE. Of note, no significant elastographic changes were observed in the peripheral zone. Another small group of PAE-patients were studied with t.r.-strain elastography, a semi-quantitative US-elastographic technique which measures tissue deformation caused by mechanical stimuli from the US-probe[25]. By comparing the tissue before and after compression, the degree of local tissue deformation (strain) is estimated in real time and projected on a color map. In the aforementioned work, the elasticity (E) index, was defined as the ratio of the average strain in the target area to the average strain in the entire elastogram. A statistically significant reduction of the E-index (compatible with increased tissue elasticity) was found one month post-PAE, both for the entire prostate and for the center of transitional zone. Of note, the reduction of the E-index was more marked for the transitional zone than for the entire prostate. Moreover, the distribution of elastographic changes did not correlate closely with ischemic changes (which were mapped with CEUS); this is probably a clue that ischemia and tissue stiffness reduction are two distinct therapeutic pathways of PAE. Despite these original and remarkable observations, it should be recognized that there is no standardized, widely accepted US-elastographic protocol for PAE, and there are several technical and operator-related limitations; therefore, at present, US-elastography is not a standard part of the imaging follow-up of PAE.
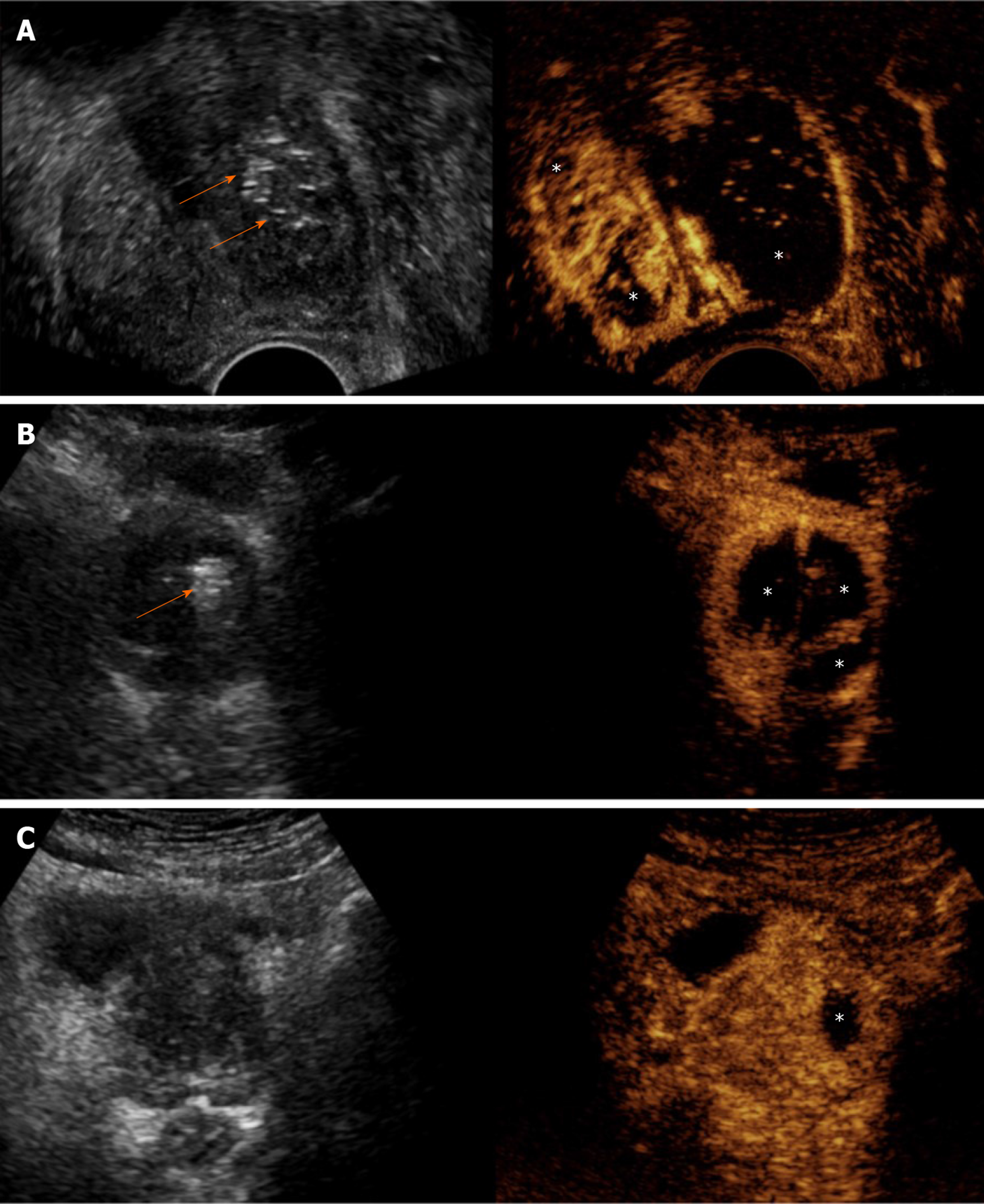
Some researchers, taking advantage of the portability and flexibility of the US-equipment, have applied US techniques in the angio-suite, where PAE is performed, for on-site evaluation of the procedure[21,26]. On gray-scale US, echogenic foci appear within the prostate immediately post-PAE and remain visible for a few days after the procedure[20,21]. This finding is probably caused by tiny bubbles of air trapped in the mixture of contrast and embolic material. These mechanisms are the same with those that cause “echogenic response” in liver tumors after transarterial (chemo) embolization[27]. The echogenic foci are more prominent and numerus in prostates with extensive infarction post-PAE[20].
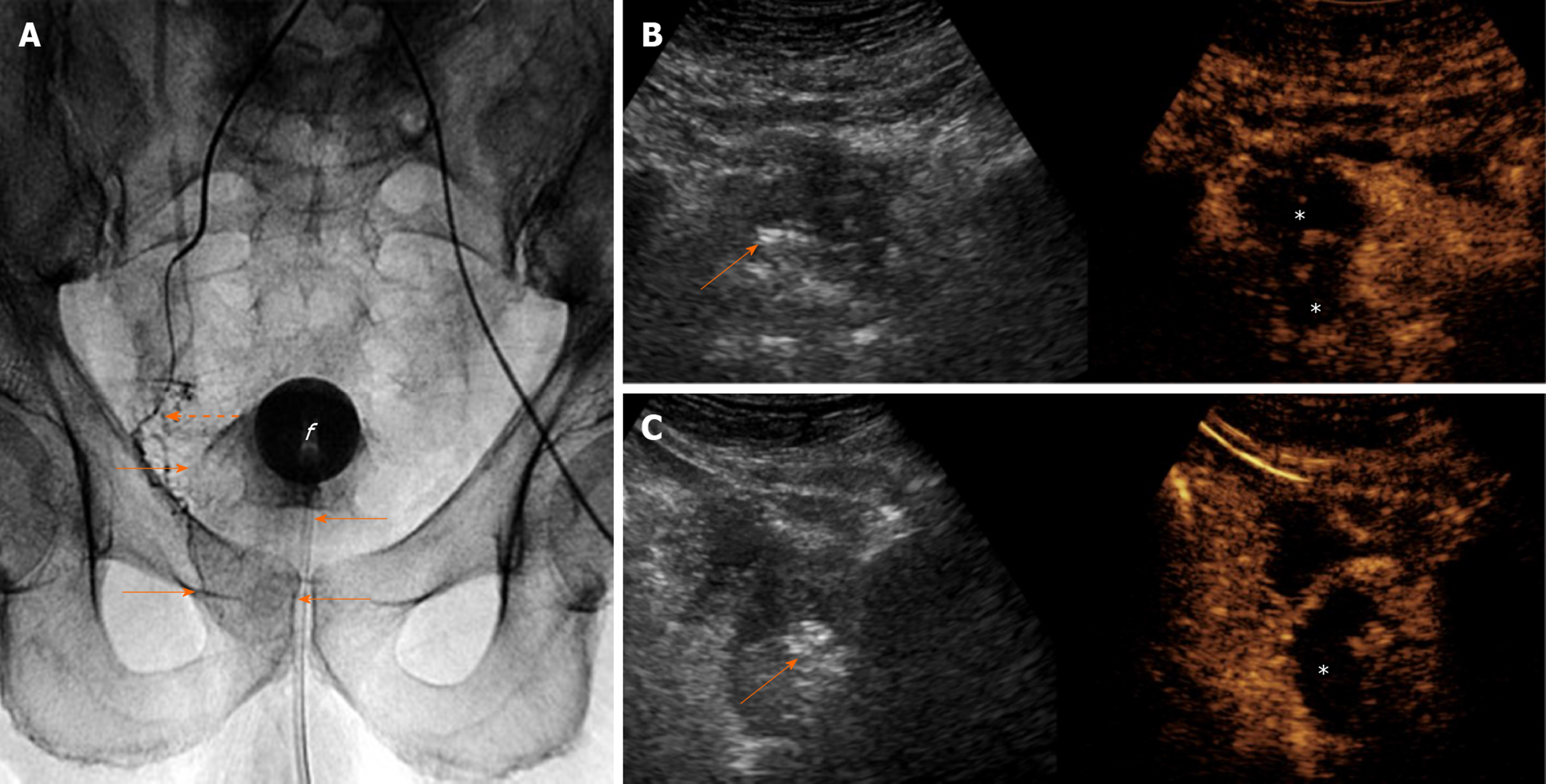
Transabdominal CEUS with the standard (intravenous) route of administration of the echo-enhancer can also be performed in the angiography suite, shortly after injection of the embolic material, to demonstrate the ischemic effect[21]. Prostatic infarcts can be detected as early as a few minutes post-embolization; however occasionally, accurate assessment of their extent at that time may be compromised by the aforementioned echogenic foci. The latter can be differentiated from the microbubbles of the echo-enhancer by comparing (on the split-screen display of the machine) the unenhanced gray-scale image with the corresponding CEUS image and by observing that the echogenic foci are stationary, while the microbubbles are constantly circulating. This immediate evaluation of the ischemic effect may guide further decisions on the procedure: If CEUS demonstrates inadequate infarction despite apparent angiographic flow stasis in the PA, a reevaluation of flow with angiography (after a waiting time of a few minutes) and a search for additional PAs should be considered. On the other hand, if CEUS demonstrates adequate devascularization of the treated lobe after standard (proximal) PAE, application of more technically demanding and time-consuming techniques of intraprostatic branch embolization (such as the PErFecTED technique) can be avoided[21] (Figure 5).
Intraarterial (i.a.) CEUS has also been utilized for intraprocedural guidance of PAE. This technique entails sonographic imaging of the prostate with CEUS algorithm, during injection of diluted echo enhancer through the microcatheter positioned in the PA. One group[26] utilized a suspension of perflutren lipid microspheres (Definity, Lantheus Medical Imaging, North Billerica, MA, United States) diluted 1:20 in saline and injected in small aliquots (0.3-0.5 mL); diluted SonoVue can also be utilized for i.a.-CEUS[28], although it should be acknowledged that this is an off-label application. Intraarterial CEUS can be performed prior to injection of the embolic, to show the part of the prostate that is fed by the selected artery (Figure 6). Enhancement of neighboring organs (for example: bladder, rectum) can also be demonstrated, if parts of these organs share the same arterial supply with the prostate. Thus, i.a.-CEUS could confirm successful selection of the PA and could prevent some cases of non-target embolization. Intraarterial CEUS could play a role in guidance of PAE, particularly when cone-beam CT equipment is not available[26]. Intraprocedural sonography may be technically challenging and acquisition of images of acceptable quality is not guaranteed: Scanning has to be performed transabdominally, under sterile drapes; the patient’s suprapubic discomfort during PAE may make compression with the transducer intolerable; the catheterized bladder is usually empty and the Foley catheter itself may be an obstacle for the ultrasound beam.
In our practice, the equipment utilized for US evaluation of PAE is located in the interventional radiology department and US studies are performed immediately after clinical evaluation of the patient. Thus, referrals of the patient to other departments are minimized and patients’ compliance with the follow-up schedule is enhanced. All US studies are performed or supervised by the interventional radiologist involved in PAE, to facilitate correlation of angiographic and technical details with US findings. Close and sincere collaboration between the interventional radiologist and urologist is one of the cornerstones of a successful PAE practice, and the presence of the referring urologist during US studies is welcome and encouraged. In this way, US/CEUS signs of the therapeutic efficacy of PAE (prostate shrinkage, prostatic infarcts) can be easily communicated to, and discussed with the clinician.
We perform an early CEUS (10-24 h post-PAE, just before the patient’s discharge) focusing on the detection of prostatic infarcts. This provides an initial impression on the efficacy of PAE. An additional CEUS study can be performed within the next few days, if the initial CEUS was compromised by echogenic artifacts. Additional follow-up with CEUS may be performed for research purposes. Such a protocol is affordable, because, with modern equipment, a diagnostic CEUS study of the prostate can be performed with only one fourth of the standard dose of the echo-enhancer (1.2 mL of the 4.8 mL content of the SonoVue vial)[21]. In the routine clinical (non-research) setting, subsequent imaging follow-up is limited to standard, unenhanced US at 1, 3, and 6 mo post-PAE and at 6-mo intervals thereafter. All studies of an individual patient are preferably performed by the same, experienced operator; this may introduce an observer bias, but reduces interobserver and experience-related variability[5]. The t.a. approach is preferred for the standard imaging schedule, as it is simpler and more comfortable than t.r. We reserve the latter for research protocols and for US-elastography.
Several shortcomings of the US techniques for PAE were reported in the previous paragraphs and they should always be taken into consideration in clinical practice. The inherent limitations of US (operator dependency, relatively small field of view, poor performance in obese patients and when gas-filled viscera are superimposed) also apply to the dedicated techniques utilized for PAE. We consider that US is not suitable for definite diagnosis of the ischemic complications of PAE. In patients with clinical suspicion of such complications, prompt evaluation with CT, MR or endoscopy is advisable.
Currently available US techniques (Table 1) enable the radiologist to perform a comprehensive evaluation of the effect of PAE. Gray-scale US is efficient for basic size measurements and is a standard part of the follow-up of PAE. With CEUS, prostatic vascularity and the ischemic effect of PAE can be readily appreciated both during and post-procedure and this information may increase the safety and efficacy of intervention and predict clinical success. It is very likely that in the near future, optimization of US-elastography and other technological advances will further enhance the role of US in the study of PAE.
| Modality | Parameter/feature evaluated | Approach | Technique/methodology | Expected finding after successful PAE |
| Gray-scale US | PV | t.a., t.r. | Measurement at 3 planes, ellipsoid formula | PV reduction |
| IPP | t.a., t.r. | Unidimensional measurement | IPP reduction | |
| PVR | t.a. | Measurement at 3 planes, ellipsoid formula | PVR reduction | |
| CEUS | Prostatic enhancement and infarction | t.a., t.r. | Bolus i.v. injection of 2nd generation US contrast agent. Measurement at 3 planes, ellipsoid formula | Prostatic infarcts preferably exceeding 10% of PV |
| US-elastography | Tissue stiffness (YM, EM, SWV) | t.r. | Shear-wave elastography | YM, EM, SWV reduction |
| Tissue stiffness Elasticity (E) index | t.r. | Strain elastography | E-index reduction | |
| Intraprocedural gray-scale US | Prostate echogenicity | t.a. | Visual assessment | Multiple strong echoes at the embolized area |
| Intraprocedural CEUS-i.a. | Distribution of contrast enhancement within and/or around prostate | t.a. | i.a. administration of diluted US contrast agent. Scanning as for CEUS post-PAE | Rapid, intense enhancement of the targeted prostatic lobe. Absence of extraprostatic enhancement |
| Intraprocedural CEUS-i.v. | As for CEUS post-PAE | t.a. | As for CEUS post-PAE | As for CEUS post-PAE |
The authors are grateful to Dr. de Assis AM, Interventional Radiologist, Interventional Radiology Department, Radiology Institute, University of Sao Paulo Medical School, for providing the SWE images and for sharing his valuable experience on US-elastography of PAE.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xia SJ S-Editor: Yan JP L-Editor: Webster JR P-Editor: Liu JH
| 1. | Kuang M, Vu A, Athreya S. A Systematic Review of Prostatic Artery Embolization in the Treatment of Symptomatic Benign Prostatic Hyperplasia. Cardiovasc Intervent Radiol. 2017;40:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clin Radiol. 2017;72:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Teichgräber U, Aschenbach R, Diamantis I, von Rundstedt FC, Grimm MO, Franiel T. Prostate Artery Embolization: Indication, Technique and Clinical Results. Rofo. 2018;190:847-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Sun F, Crisóstomo V, Báez-Díaz C, Sánchez FM. Prostatic Artery Embolization (PAE) for Symptomatic Benign Prostatic Hyperplasia (BPH): Part 2, Insights into the Technical Rationale. Cardiovasc Intervent Radiol. 2016;39:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Fernandes L, Rio Tinto H, Pereira J, Duarte M, Bilhim T, Martins Pisco J. Prostatic arterial embolization: post-procedural follow-up. Tech Vasc Interv Radiol. 2012;15:294-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Frenk NE, Baroni RH, Carnevale FC, Gonçalves OM, Antunes AA, Srougi M, Cerri GG. MRI findings after prostatic artery embolization for treatment of benign hyperplasia. AJR Am J Roentgenol. 2014;203:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Kisilevzky N, Faintuch S. MRI assessment of prostatic ischaemia: best predictor of clinical success after prostatic artery embolisation for benign prostatic hyperplasia. Clin Radiol. 2016;71:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Carnevale FC, Antunes AA, da Motta Leal Filho JM, de Oliveira Cerri LM, Baroni RH, Marcelino AS, Freire GC, Moreira AM, Srougi M, Cerri GG. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovasc Intervent Radiol. 2010;33:355-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Antunes AA, Carnevale FC, da Motta Leal Filho JM, Yoshinaga EM, Cerri LM, Baroni RH, Marcelino AS, Cerri GG, Srougi M. Clinical, laboratorial, and urodynamic findings of prostatic artery embolization for the treatment of urinary retention related to benign prostatic hyperplasia. A prospective single-center pilot study. Cardiovasc Intervent Radiol. 2013;36:978-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Ozden E, Göğüş C, Kiliç O, Yaman O, Ozdiler E. Analysis of suprapubic and transrectal measurements in assessment of prostate dimensions and volume: is transrectal ultrasonography really necessary for prostate measurements? Urol J. 2009;6:208-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Kim SH, Kim SH. Correlations between the various methods of estimating prostate volume: transabdominal, transrectal, and three-dimensional US. Korean J Radiol. 2008;9:134-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Yuen JS, Ngiap JT, Cheng CW, Foo KT. Effects of bladder volume on transabdominal ultrasound measurements of intravesical prostatic protrusion and volume. Int J Urol. 2002;9:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Choi YJ, Kim JK, Kim HJ, Cho KS. Interobserver variability of transrectal ultrasound for prostate volume measurement according to volume and observer experience. AJR Am J Roentgenol. 2009;192:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Pisco JM, Bilhim T, Pinheiro LC, Fernandes L, Pereira J, Costa NV, Duarte M, Oliveira AG. Medium- and Long-Term Outcome of Prostate Artery Embolization for Patients with Benign Prostatic Hyperplasia: Results in 630 Patients. J Vasc Interv Radiol. 2016;27:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 177] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 15. | Keqin Z, Zhishun X, Jing Z, Haixin W, Dongqing Z, Benkang S. Clinical significance of intravesical prostatic protrusion in patients with benign prostatic enlargement. Urology. 2007;70:1096-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Lin YT, Amouyal G, Thiounn N, Pellerin O, Pereira H, Del Giudice C, Déan C, Sapoval M. Intra-vesical Prostatic Protrusion (IPP) Can Be Reduced by Prostatic Artery Embolization. Cardiovasc Intervent Radiol. 2016;39:690-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Ali R, Gabr A, Mouli SK, Kallini JR, Riaz A, Mora R, Lewandowski RJ, Hohlastos E, Casalino DD, Hofer MD, Hamoui N, Miller FH, Hairston J, Salem R. MR imaging findings of the prostate gland following prostate artery embolization: results from a prospective phase 2 study. Abdom Radiol (NY). 2019;44:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Pisco JM, Rio Tinto H, Campos Pinheiro L, Bilhim T, Duarte M, Fernandes L, Pereira J, Oliveira AG. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol. 2013;23:2561-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 19. | Zhang H, Shen Y, Pan J, Wang H, Zhong Y, Wang Y, Ye H. MRI features after prostatic artery embolization for the treatment of medium- and large-volume benign hyperplasia. Radiol Med. 2018;123:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Moschouris H, Stamatiou K, Kalokairinou Motogna M, Vrakas S, Kiltenis M, Kladis-Kalentzis K, Tsavdaroglou A, Papadogeorgopoulos N, Marmaridou K, Malagari K. Early post-interventional sonographic evaluation of prostatic artery embolization. A promising role for contrast-enhanced ultrasonography (CEUS). Med Ultrason. 2018;20:134-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Moschouris H, Stamatiou K, Malagari K, Marmaridou K, Kladis-Kalentzis K, Kiltenis M, Papadogeorgopoulos N, Tsavari A, Manoloudaki K. The value of contrast-enhanced ultrasonography in detection of prostatic infarction after prostatic artery embolization for the treatment of symptomatic benign prostatic hyperplasia. Diagn Interv Radiol. 2019;25:134-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Carnevale FC, Moreira AM, Antunes AA. The "PErFecTED technique": proximal embolization first, then embolize distal for benign prostatic hyperplasia. Cardiovasc Intervent Radiol. 2014;37:1602-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | de Assis AM, Moreira AM, Carnevale FC, Marcelino ASZ, de Oliveira Cerri LM, Antunes AA, Srougi M, Cerri GG. Effects of Prostatic Artery Embolization on the Dynamic Component of Benign Prostate Hyperplasia as Assessed by Ultrasound Elastography: A Pilot Series. Cardiovasc Intervent Radiol. 2019;42:1001-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Correas JM, Tissier AM, Khairoune A, Khoury G, Eiss D, Hélénon O. Ultrasound elastography of the prostate: state of the art. Diagn Interv Imaging. 2013;94:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Moschouris H, Stamatiou K, Dimakis A, Malagari K. Re: de Assis et al. "Effects of Prostatic Artery Embolization on the Dynamic Component of Benign Prostate Hyperplasia as Assessed by Ultrasound Elastography: A Pilot Series". Cardiovasc Intervent Radiol. 2019;42:1366-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Nzekwu E, Mirakhur A, Lee A, Bakshi D. Intra-Arterial and Intravenous Contrast-Enhanced Ultrasonography in Prostate Artery Embolization: A Case Series. J Vasc Interv Radiol. 2018;29:1399-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Moschouris H, Malagari K, Kornezos I, Papadaki MG, Gkoutzios P, Matsaidonis D. Unenhanced and contrast-enhanced ultrasonography during hepatic transarterial embolization and chemoembolization with drug-eluting beads. Cardiovasc Intervent Radiol. 2010;33:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Moschouris H, Malagari K, Kalokairinou M, Stamatiou K, Marinis A, Papadaki MG. Contrast-enhanced ultrasonography with intraarterial administration of SonoVue for guidance of transarterial chemoembolization: an initial experience. Med Ultrason. 2011;13:296-301. [PubMed] |