Published online May 28, 2020. doi: 10.4329/wjr.v12.i5.48
Peer-review started: February 26, 2020
First decision: April 22, 2020
Revised: May 5, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: May 28, 2020
Processing time: 91 Days and 9.6 Hours
Vascular depression (VD) as defined by magnetic resonance imaging (MRI) has been proposed as a unique subtype of late-life depression. The VD hypothesis posits that cerebrovascular disease, as characterized by the presence of MRI-defined white matter hyperintensities, contributes to and increases the risk for depression in older adults. VD is also accompanied by cognitive impairment and poor antidepressant treatment response. The VD diagnosis relies on MRI findings and yet this clinical entity is largely unfamiliar to neuroradiologists and is rarely, if ever, discussed in radiology journals. The primary purpose of this review is to introduce the MRI-defined VD construct to the neuroradiology community. Case reports are highlighted in order to illustrate the profile of VD in terms of radiological, clinical, and neuropsychological findings. A secondary purpose is to elucidate and elaborate on the measurement of cerebrovascular disease through visual rating scales and semi- and fully-automated volumetric methods. These methods are crucial for determining whether lesion burden or lesion severity is the dominant pathological contributor to VD. Additionally, these rating methods have implications for the growing field of computer assisted diagnosis. Since VD has been found to have a profile that is distinct from other types of late-life depression, neuroradiologists, in conjunction with psychiatrists and psychologists, should consider VD in diagnosis and treatment planning.
Core tip: This manuscript provides an overview of the vascular depression construct, discusses the methods used to measure cerebrovascular disease on magnetic resonance imaging in older adults, and presents the profile of vascular depression in terms of radiological, neuropsychological, and clinical findings. The goal of this paper is to inform the neuroradiology community of the vascular depression diagnosis and to instill the importance of considering this diagnosis when evaluating magnetic resonance imaging scans of older adults.
- Citation: Rushia SN, Shehab AAS, Motter JN, Egglefield DA, Schiff S, Sneed JR, Garcon E. Vascular depression for radiology: A review of the construct, methodology, and diagnosis. World J Radiol 2020; 12(5): 48-67
- URL: https://www.wjgnet.com/1949-8470/full/v12/i5/48.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i5.48
Vascular depression (VD) is a unique subtype of late-life depression (LLD)[1-4]. The VD construct consists of a group of patients with late-onset depression (LOD), structural brain changes characterized by higher rates of hyperintensities on T2-weighted brain magnetic resonance imaging (MRI)[3,5,6], and greater neuropsychological impairment as compared to early-onset depression (EOD)[6-8]. Greater severity of MRI hype-rintensities are associated with poor response to antidepressant treatment[3] and a more chronic course of depression[9]. Therefore, the theory of VD suggests that LOD is a product of damage to corticostriatal circuits by cerebrovascular disease, which leads to executive dysfunction and poor antidepressant treatment response[1,2,4,5,8].
The VD subtype proposes that cerebrovascular disease is related to geriatric depressive syndromes, such that vascular risk factors lead to altered regional brain functioning, which leads to depression. However, there may be additional biological or genetic factors that are also determinants of VD. Indeed, there may be a strong genetic influence in the development of white matter hyperintensities (WMHs) in the elderly, and heritability of WMHs is estimated to be between 55% and 80%[10-13]. Furthermore, there is an association between vascular risk factor burden and new onset of depression in the elderly population[14]. LLD and cardiovascular disease share etiopathogenetic risk factors, such as high homocysteine levels and inflammation, which are also implicated in cognitive impairment[15]. A meta-analysis found a significant association between vascular risk and LLD, with diabetes, heart disease, and stroke identified as strong individual correlates of LLD[16], and a recent study identified midlife cerebrovascular burden as a significant predictor of LLD, even after controlling for a history of depressive symptoms[17]. A review of potential mechanisms underlying the VD construct proposed three hypotheses by which LLD is mediated: White matter disease leading to disrupted connections between brain areas associated with depression and cognition, inflammatory and immune processes leading to neurodegeneration, and reduced cerebral blood flow[18]. However, the etiology of white matter hyperintensities in older depressed adults remains unclear. Depressed elders may have poorer blood flow to prefrontal tissue as cerebrovascular disease progresses[8,19,20]. Additionally, the hippocampus is vulnerable to aging[21,22], and elders also experience reduced metabolism in limbic regions[21].
There is a consistent relationship between depression, vascular disease, and cerebrovascular risk factors[23-26]. Indeed, cerebrovascular disease is commonly seen in MRI scans of depressed elders[5,27-29]. In addition, considerable research has shown that ischemic cerebrovascular disease processes contribute to excessive deep WMHs (DWMHs)[19,30,31]. Thomas et al[18] found that all DWMHs in an elderly group with depression were ischemic, while less than a third of DWMHs in an elderly group with no depression were ischemic. However, Thomas et al[18] suggested that the high frequency of ischemic lesions in depressed patients is not due to vascular disease, but instead due to a change in DWMHs from nonischemic to ischemic once a certain threshold for ischemic damage is surpassed. The mechanisms underlying the ischemic disease connected to DWMHs are nonspecific; potential pathways include poor endothelial functioning and increased atherosclerosis[32,33]. Age-related changes in cerebral autoregulation and regulation of blood pressure may also lead to WMHs[34,35]. These findings further promote the idea that subtle microvascular changes may be one reason why some people develop their first episode of depression later in life.
White matter disease is thought to damage white matter tracts and play a major role in the etiology of LOD by affecting the processes involved in emotional regulation. A meta-analysis of 30 studies found that individuals with LOD were over four times more likely to have significant white matter changes and greater lesion severity than those with EOD[36]. These findings have also been replicated in a more recent meta-analysis[37]. Vascular changes, particularly subcortical white and gray matter lesions, are associated with more severe depressive symptoms. Specifically, in elderly patients with late-onset as opposed to early-onset depressive disorders, deep white matter and basal ganglia hyperintensities are more prevalent[5,38-41] and are associated with more severe symptoms and poorer prognosis[3,5,42-44]. The cortico-striato-pallido-thalamo-cortical pathways have been implicated as areas particularly affected by vascular disease in LLD[45]. Additionally, Bella et al[46] suggest that patients exhibiting a VD profile have disruptions in the frontal subcortical circuits connecting the dorsolateral prefrontal cortex and dorsal section of the head of the caudate nucleus.
Vascular risk factors contribute not only to the pathogenesis of depression but also complicate its treatment. Several studies have suggested that older adults with greater WMH volume show a poorer response to antidepressants[3,30,47-49]. In clinical samples, progression of white matter lesions has occurred alongside a lack of response to antidepressants and recurrence of depression. Patients with LLD with lacunar lesions, as opposed to diffuse white matter lesions, have shown a better response to antidepressant treatment, supporting the view that VD associated with white matter lesions is treatment-resistant[46]. It is hypothesized that the pathology caused by the disruption of prefrontal systems related to white matter lesions is a central mechanism to why these patients do not respond to the typical antidepressant treatment[1]. However, some studies have failed to find a connection between WMH severity and treatment outcomes[28,50,51].
Along with poorer response rates to antidepressants, white matter hyperintensity progression has been shown to predict poorer outcomes[52], higher rates of relapse[27,42,53], and difficulty achieving remission[42]. Indeed, in a study of severely depressed psychiatric inpatients, those who responded to treatment showed significantly less white matter lesion burden[3]. The presence of subcortical gray matter lesions predicted poor outcome up to 64 months following antidepressant monotherapy[53]. Steffens et al[30] found that lesions in the basal ganglia and in the cerebral cortex were associated with the persistence of depressive symptoms, while subcortical white matter lesions were associated with worsening of depressive symptoms over time. Additionally, patients with VD are more likely to suffer from earlier relapse, have a more chronic pattern of depression, and have progressive cognitive decline[3].
Since current pharmacological treatments for depression have been primarily ineffective in VD patients, there is growing interest in controlling lesion progression. If white matter lesions can predict poor antidepressant response, targeting the cerebrovascular processes themselves might be more effective. Most studies of vascular depression are correlative and cannot determine causation. It is unknown if depression puts one at greater risk for developing white matter lesions, if the presence of microvascular ischemia makes one more susceptible to depression, or if both are the result of a common pathological pathway. Another possibility is that untreated depression itself quickens lesion progression and results in additional vascular disease, such as vascular dementia. In a retrospective cohort study of patients in primary care spanning 13 years, Köhler et al[54] found that new onset of depression was associated with twice the likelihood of developing dementia, especially in those with incident vascular disease or whose depression was preceded by stroke or hypertension. Since the current standard psychopharmacologic therapy is ineffective and there are few placebo-controlled trials examining the effect of hyperintensities on antidepressant treatment response, there is a clear need for more careful clinical assessment and follow-up of those with lesion burden.
There is an established connection between vascular disease and the development of cognitive impairment (vascular dementia), which co-occurs with MDD at a rate of approximately 20%[55]. Midlife vascular risk factors are also associated with elevated amyloid deposition in late-life[56]. Furthermore, new onset of depression in adults with mild cognitive impairment was found to be associated with deep subcortical WMH severity[57]. Puglisi and colleagues[58] found that VD patients exhibit hemodynamic dysfunction, which may contribute to the development of cognitive impairment. Pennisi and colleagues[59] found differences in intracortical facilitation between patients with VD and patients with vascular cognitive impairment at baseline but not at a two-year follow-up. While the vascular cognitive impairment group did not cognitively decline over the two years, the VD group did show evidence of cognitive decline. Thus, the researchers proposed that the hyperfacilitation observed in the vascular cognitive impairment group may have contributed to the preservation of cognitive functioning as compared to the VD group. They concluded that the risk of dementia in VD may be due to lack of compensatory functional cortical changes or subcortical vascular lesions[59]. Thus, vascular depression might fall on a continuum that would include vascular disease, vascular depression itself, and vascular dementia. Indeed, case studies have reported progression from vascular depression to vascular dementia[60,61].
Vascular depression has been associated with cognitive impairment, most notably in executive functioning and psychomotor speed. Patients with MRI-defined VD were shown to have significantly more psychomotor retardation and significantly worse performance on the Stroop Color-Word Interference Test[62,63] and the Initiation/Perseveration subtest of the Mattis Dementia Rating Scale[63] than non-VD patients. Poor performance on the interference component of the Stroop Color-Word Interference Test exemplifies that response inhibition is low for these patients. Since the inception of the concept of vascular depression, Krishnan and colleagues have redefined their idea into Subcortical Ischemic Depression (SID)[64] and Alexopoulos and colleagues reconceptualized their model into the Depression-Executive Dysfunction Syndrome (DED)[65]. Both of these subtypes reflect different aspects of VD, and shed light on the neuropsychological deficits that arise when VD is defined by MRI or clinical presentation, respectively. Potter et al[66] found that patients with MRI-defined SID had an overall worse neuropsychological profile than depressed older adults without SID. Specifically, SID patients performed worse on measures of working memory and nonverbal memory, after controlling for age, cardiovascular risk, and depression symptoms[66]. Alexopoulos et al[67] found that patients with DED had reduced fluency and visual naming, and psychomotor retardation. Interestingly, both SID and DED definitions predict functional disability at similar rates[68].
Similar patterns of cognitive impairment have been found for constructs that share symptomology with VD. Depressed elderly patients with white matter hyperintensities demonstrate deficits in processing speed and executive functioning[7]. White matter abnormalities in depressed geriatric patients have also been associated with poor response inhibition as measured by the Stroop Color-Word Interference Test[69]. Geriatric depressed antidepressant non-responders show deficits in verbal learning[70], psychomotor speed[70,71], and initiation and perseveration[71]. Impairment in initiation and perseveration has also been associated with relapse and recurrence of LLD[72]. Additionally, a meta-analysis of patients with LLD found that the executive functioning domains of planning and organization were associated with poor antidepressant treatment response[73]. Vascular burden in adults with LLD was predictive of poor processing speed functioning after controlling for age, education, and depressive severity[74].
Patients with VD also display characteristics nonspecific to neuropsychological assessment. Older adults with VD have a lower rate of family history of mood disorders than older adults with depression and no vascular disease[2,63]. Patients with vascular dysfunction and depression have demonstrated higher levels of aggressive and auto-aggressive tendencies and increased alexithymia[75]. Additionally, VD patients have been shown to have more disability with activities of daily living than depressed geriatric patients without vascular disease[68,76]. Other features of VD include apathy, lack of insight, and difficulties at work[46]. Older adult women with high cerebrovascular burden and probable depression have been shown to be at a higher risk for later frailty[77] and subsequent shortened lifespan[78].
Furthermore, VD patients exhibit distinct physiological profiles. This includes specific patterns of motor cortex excitability[79,80], higher concentrations of inflammatory markers[81], and higher levels of plasma homocysteine[82,83]. Research into the etiopathology of VD indicates that a genetic variation at aquaporin 4 (AQP4) locus may play a role[84]. Sleep status between VD, non-vascular depression, and normal subjects has also been shown to differ, such that VD patients had higher rates of sleep-related breathing disorders, daytime sleepiness, and disorders of 24-hour sleep structure[85].
In clinical practice, radiologists are often tasked to evaluate the brain MRI of patients with dementia for structural damages. Although the MRI findings alone are non-specific, there are certain patterns of cerebral atrophy that may lead to additional investigations (e.g., PET, fMRI) in addition to the clinical and biological presentations for a definitive diagnosis. We believe that a similar approach can be done in vascular depression if a methodologic assessment of the cerebral white matter signal intensity on FLAIR is performed by the advised radiologists at the request of their referral practitioners.
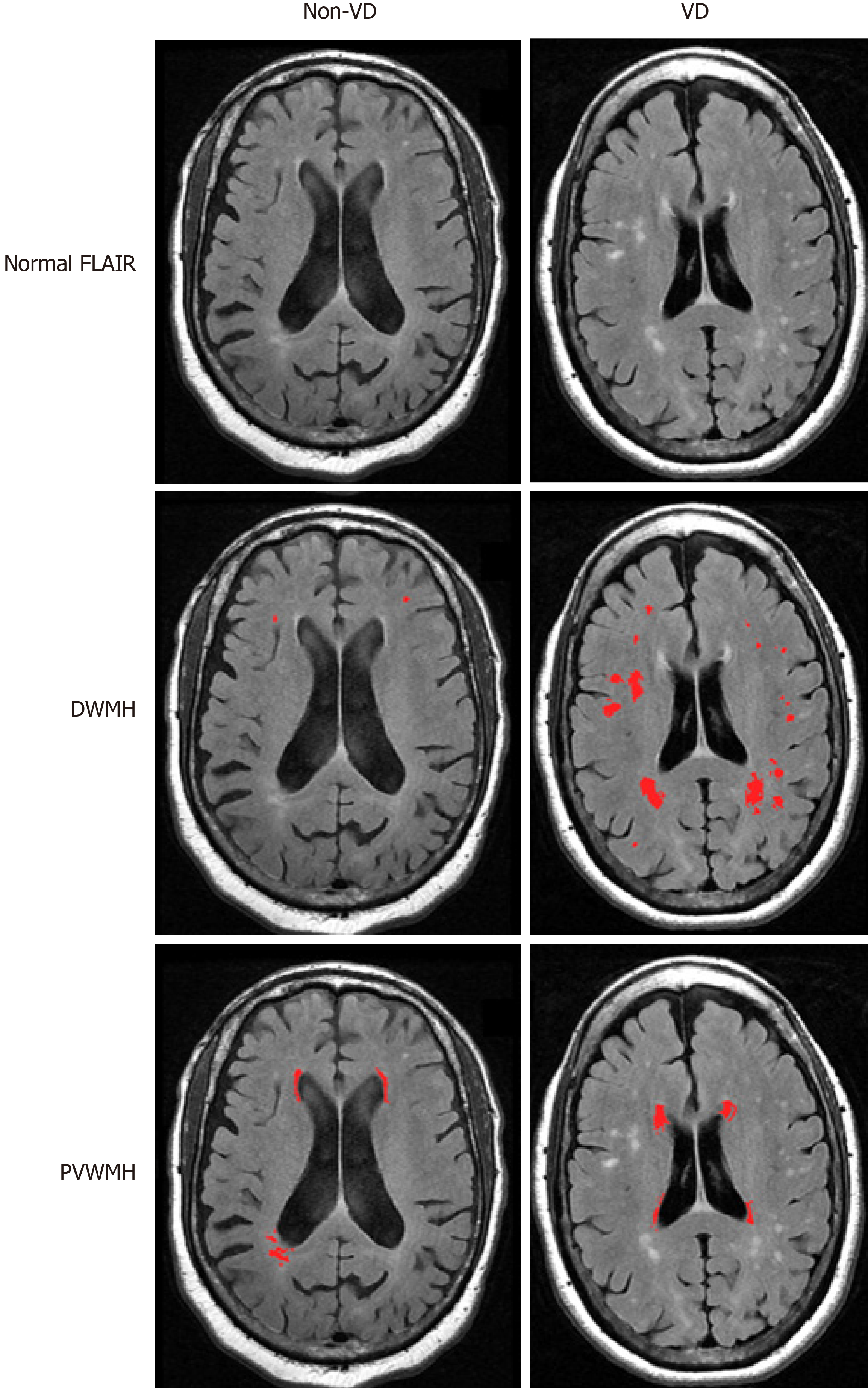
The quantification and localization of white matter hyperintensities is critical for research into the risk factors, etiology, and pathogenesis of VD. Thus, the validity of MRI-defined VD as a distinct diagnostic entity depends on the methodology that is used to characterize lesions. WMHs are typically analyzed using either semi-quantitative ratings or quantitative volumetric analysis. Past research has primarily focused on WMH visual ratings or volume measurement without distinguishing among anatomically distinct WMHs, while recent studies have increasingly used semi-automated or fully automated methods to calculate WMH volume and classify WMHs into localized categories such as periventricular white matter hyperintensities (PVWMHs) and DWMHs (Figure 1). MRI hyperintensities most often have been scored for severity using visual rating scales including the Fazekas-modified Coffey rating scale[39,86], the Scheltens rating scale[87], the Boyko Pathology Rating Scale[88], and the Virchow-Robin spaces[89,90].
A fundamental issue in standardizing MRI-defined VD is how lesions in these patients are defined. For example, subcortical ischemic disease can involve white or deep gray matter, but VD research focuses primarily on white matter lesions. Hyperintensities can be divided according to location: Periventricular on the periphery of the lateral ventricles, deep white matter, or subcortical gray matter. It has been shown that deep white matter hyperintensities, though having a uniform appearance on conventional imaging, are histologically heterogeneous[86,91]. WMHs may be considered periventricular if they are located within a certain distance from the lateral ventricles or remain contiguous as they spread away from the ventricles. Clear criteria for hyperintensities need to be operationalized so that research is conducted consistently.
The Fazekas-modified Coffey rating scale is commonly used in research evaluating VD[5,51,64,92]. This scale measures hyperintensities in three locations on T2-weighted images: Deep white matter, subcortical gray matter, and periventricular spaces. Deep white matter hyperintensities in the frontal, parietal, temporal, or occipital lobes are scored as 0 (absent), 1 (punctate foci), 2 (beginning confluence of foci), and 3 (large confluent areas). Subcortical gray matter hyperintensities are abnormalities in the caudate nucleus, putamen, globus pallidus, thalamus, and internal capsule, and are scored as 0 (absent), 1 (punctate), 2 (multipunctate), and 3 (diffuse). Periventricular hyperintensities are abnormalities immediately adjacent to the lateral ventricles and are scored as 0 (absent), 1 (caps), 2 (smooth halo), and 3 (irregular and extending into the deep white matter). However, the Fazekas scale does not allow for detailed volumetric analysis of white matter hyperintensities.
The Scheltens rating scale[87] is based on the size and number of lesions in four separate regions: Periventricular white matter, deep white matter, basal ganglia, and infratentorial area. The periventricular white matter of the frontal caps and occipital caps and bands have ratings of 0 (none), 1 (smooth halo, > 1-5 mm), and 2 (large confluent lesions, 5-10 mm). The same rating system is used for the remaining three areas: The deep white matter of the frontal, parietal, occipital, and temporal lobes; the basal ganglia including the caudate nucleus, putamen, globus pallidus, thalamus, and internal/external capsule; and the infratentorial area consisting of the cerebellum, mesencephalon, pons, and medulla. The rating system ranges from 0 to 6 with the following descriptors: 0 (none), 1 (≤ 4 mm and n ≤ 5), 2 (≤ 4 mm and n > 5), 3 (4-10 mm and n ≤ 5), 4 (4-10 mm and n > 5), 5 (≥ 10 mm and n ≥ 1), and 6 (confluent). However, this scale may underestimate the significance of punctate lesions since a score of 2 allows for infinite smaller lesions, whereas one large isolated lesion would be rated as a 5. Scheltens rating scales have a greater range than the Fazekas scale, yet Van Straaten et al[93] suggest that the Fazekas scale is most appropriate for classifying different WMH groups.
Visual rating scales of WMHs are widely used because they are relatively easy to conduct and there are several scales available with demonstrated reliability and validity. In addition, visual rating scales allow expert raters to utilize clinical judgment to evaluate the specific lesion presentations and patterns. Van Straaten et al[93] confirmed correlations among WMH volume and the three most commonly used visual rating scales, but found greater variability and less correlation among scores in patients who had greater WMH burden.
Despite the advantages, visual scales are not well suited for measuring changes in lesions, and cannot quantify lesion volume. Visual scales can be time consuming, making them a poor choice for large datasets, and rely on clinical judgment, which can introduce issues concerning interrater reliability and validity. In addition, visual scales often do not include size and uniform location, making scores from different rating scales incomparable. Although visual scales allow differentiation between periventricular, deep white, and subcortical gray hyperintensity ratings, such classifications are inherently broad and imprecise. For example, WMHs adjacent to the left anterior horn of the lateral ventricle are combined with WMHs located in the right posterior horn of the lateral ventricle in the periventricular classification. Lesions in such disparate regions may have vastly different influences on cognition.
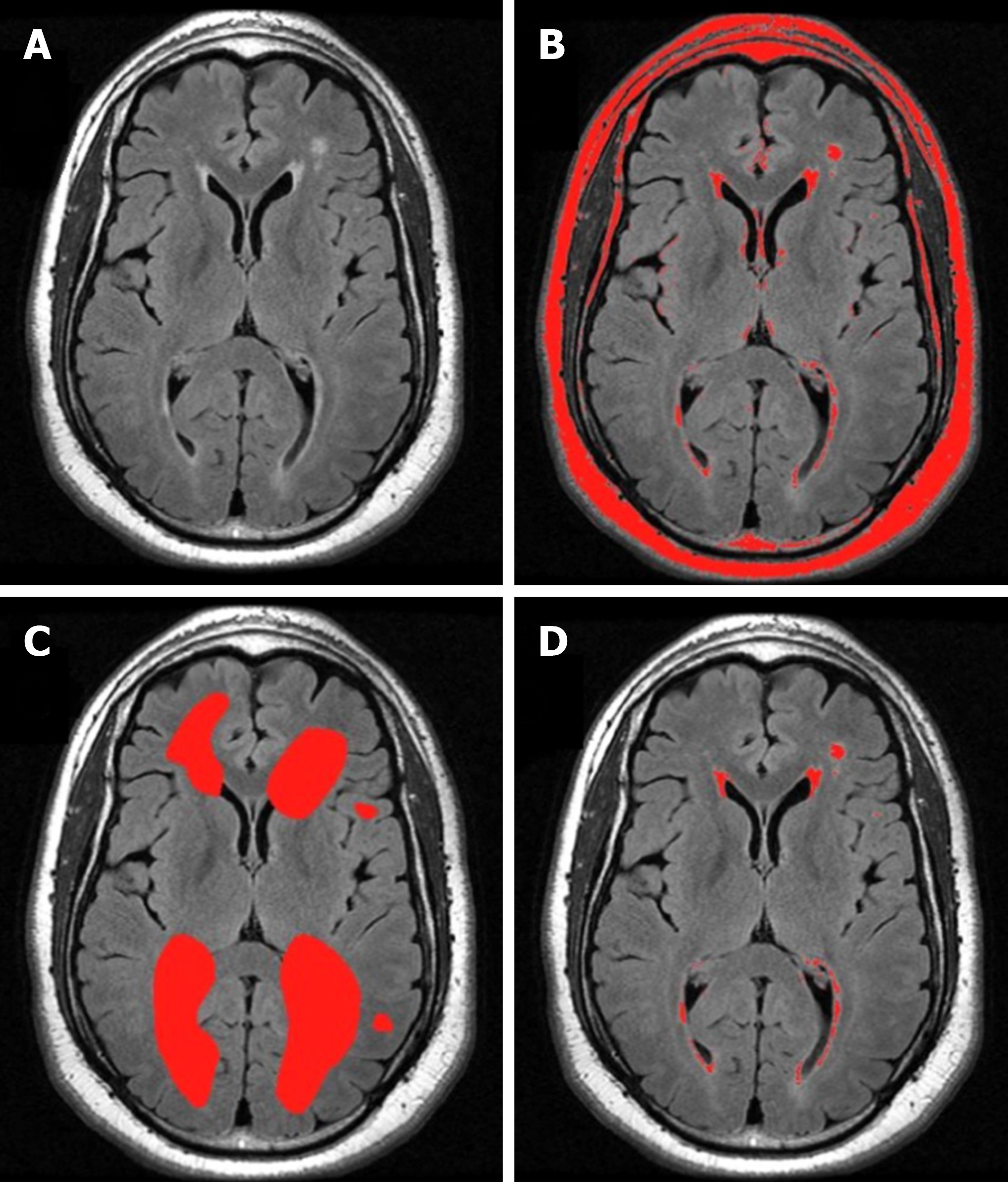
Recent imaging techniques have been developed to enable the quantitative volumetric analysis of signal hyperintensities that use semi-automated methods and provide more information on location and size, as well as continuous data[94-98]. One method uses the software MRIcro[99] to create a region of interest map by semiautomatic intensity thresholding[100] (Figure 2). Separate region of interest (ROI) maps for different hyperintensity categories (deep white, periventricular, subcortical gray) are created by gross manual outlining of hyperintensities. The overlap of the intensity ROI and each hyperintensity category ROI is then used to compute the volume and location of the final lesion map. A principle advantage of semi-automated tracing is the ability to exclude areas that are typically hyperintense (e.g., pyramidal tracts and hippocampus) from total volume estimates. However, it is more time consuming than visual rating scales. For these reasons, it may be advantageously applied for medium, but not large datasets, where a fully-automated procedure may be more appropriate.
Fully-automated segmentation techniques[101-106] eliminate the interrater reliability problem because methods of lesion quantification are standardized and require little user intervention. Additional advantages include the production of quantitative data, the ability to analyze large data sets quickly, and the ability to recognize patterns. Quantitative ratings also allow for more precise measurement, and thus greater sensitivity to smaller lesions and different criteria for classification of white matter lesions. Since fully-automated segmentation techniques take place within MRI analysis software, the finalized lesion maps are amenable to realignment with standardized anatomical atlases. This facilitates ROI analyses that can be more spatially specific than analyses performed on qualitatively scored data. With these advantages, automated methods are potentially superior to visual rating scales for quantifying signal hyperintensities on MRI. Indeed, visual ratings may be less sensitive with the potential for ceiling effects and low discrimination of absolute lesion volumes[93]. Disadvantages of automated methods include requiring computational sophistication, the correct software, and a level of user intervention and troubleshooting.
Fully-automated segmentation approaches also need to be evaluated for validity concerns. Validity may be in question because of the potential for interference effects by radiological artifacts. These artifacts may interfere with the segmentation process or with the steps needed to perform regional analyses. For example, fully-automated approaches rely on the brightness of adjacent pixels to identify lesion boundaries. This could lead to a problem in distinguishing pathological lesions from normal anatomical areas that are typically hyperintense (e.g., pyramidal tracts and hippocampus). Additionally, motion artifacts may invalidate fully automated analyses on scans that could otherwise be assessed manually on a qualitative rating scale. Therefore, the validity of MRI-defined VD relies on the reliability and validity of the rating scales and techniques used, which currently can still be inconsistent among research studies.
More recently, diffusion tensor imaging (DTI) has been used to examine the structural integrity of white matter areas relevant to LLD. In fact, it has been suggested that DTI may be more sensitive in identifying white matter damage than T2-weighted MRI[107]. Fractional anisotropy has shown that white matter changes occur with aging, cognitive dysfunction, expressions of psychopathology[21], and LLD[108]. Lower fractional anisotropy in the anterior thalamic radiation and superior longitudinal fasciculus, both projecting to the frontal lobe, has been associated with LOD[109], while lower fractional anisotropy in cortico-striato-limbic areas has been found in older adults who remained depressed after an antidepressant trial[21]. DTI has revealed microstructural changes in the anterior cingulate cortex, superior and middle frontal gyrus, and right parahippocampal gyrus in association with LLD[110,111]. Functional MRI (fMRI) has also been used to examine the relationship between white matter hyperintensities and LOD. Indeed, white matter lesions have been associated with altered functional connectivity in the brain in LLD[112-114]. This research suggests that the use of DTI and fMRI techniques may be beneficial in examining vascular depression. Other newer imaging techniques such as MR perfusion have not yet been utilized in this population.
Vascular depression is often viewed solely from a geriatric psychiatry perspective. Typically, a clinical diagnosis is made after careful evaluations that include full physical and neuropsychological examinations, despite the possibility that a specific depression symptom profile might not constitute a clinical marker for VD[115]. Vascular depression depends on the assessment of brain lesions and thus is ultimately a neuroradiological diagnosis.
A number of researchers have proposed diagnostic criteria to define the VD subtype[1,2,64,65,67], but these have not always been consistent. Without consistent criteria, it is difficult to interpret conflicting findings[116], and since we do not know to what degree the possibly different patient groups overlap, there is no way to evaluate discrepant findings. To provide more clarity on this issue, Sneed et al[92] evaluated patients with LLD and found that the vascular group was most accurately identified by DWMHs. Those in the vascular group were defined by a high probability of having MRI hyperintensities (DWM and periventricular), executive dysfunction, and late age-of-onset. These findings provide empirical evidence that VD is a unique subtype of LLD and the first empirically-based diagnostic criteria for VD[92].
WMH burden can be presented as volume, or as a proportion of the total white matter or intracranial volume, depending on the focus of the study. However, it remains unclear whether quantity or severity better accounts for predictive ability. Taylor et al[27] found that the overall percent increase in white matter hyperintensity volume had greater predictive significance of depressive symptoms and poor outcome than baseline volume. Others have reported similar results wherein greater changes in white matter presentation show greater symptoms and a more chronic course of illness[9,117,118]. This suggests that total lesion volume at any particular time point is not nearly as important as the course of progression, and thus perhaps it may be more important to look at the specific areas in which the lesions are occurring. Quantity of lesions versus a certain pattern of lesions may also be related to clinical expression. Van Straaten et al[93] quantified individual lesions to determine WMH severity, but the number of lesions was not related to depressive symptoms, suggesting that perhaps size of lesions is more relevant.
The significance of defined WMH locations has not been agreed upon, as it is unclear whether severity of overall lesion burden or lesion location is the dominant pathological contributor to vascular depression. Differences in WMH load between those with LOD and those with EOD have not consistently been found[119], suggesting that location of lesions, rather than quantity, might be more important in the differential diagnosis. Simpson et al[42] found that hyperintensities were predictive of depressive symptomology when looking at specific areas such as the frontal lobes, basal ganglia, and pons, as opposed to total volume. On the other hand, a three-year follow-up study of a community cohort concluded that WMH severity was a strong predictor of depression risk, with half of MDD elderly meeting criteria for VD[120]. Similarly, in a 12-wk antidepressant nonrandomized controlled trial, WMH severity was found to be associated with depression severity as well as vascular risk factors[121]. Hickie et al[3] reported no correlations between specific lesion locations and outcome, but rather that the burden of total white matter hyperintensity volume led to a poorer outcome in depressed patients. In addition, a two-year longitudinal study found that for every 1% increase in WMH volume, there was a 7% increased risk of poor prognosis[27].
The most consistently reported abnormality described in LLD is the increased number and/or severity of white matter hyperintensities, which mainly occur in subcortical gray and frontal white matter areas[21,122,123]. It is thought that these changes represent vascular pathology in clinically relevant areas, though the debate about lesion severity and location remains. Rabins et al[114] showed a particular dominance of lesions in the frontal lobes and basal ganglia. The locations of these lesions fit with functional imaging changes suggesting frontal and caudate abnormalities in depression, and further support the role of frontostriatal dysfunction in the vascular depression hypothesis. Many studies have focused on the significance of the frontal lobe in the physiopathology of LOD[67,76,124-126]. MacFall et al[127] further localized significant WMHs in depressed elders to the medial orbital prefrontal cortex. Other studies found that hyperintensities in the basal ganglia, particularly the putamen, predicted depressive symptoms and poorer treatment outcome[48,124]. Lesions in the basal ganglia and subcortical gray matter were also shown to be predictive of failure to respond to treatment[128]. Alexopoulos et al[129] localized WMHs to the frontal white matter lateral to the anterior cingulate cortex (ACC), which predicted lower remission rates after antidepressant treatment. More recently, increased low-theta activity in the subgenual ACC was found to be a possible predictor of antidepressant treatment response to repetitive transcranial magnetic stimulation in patients with treatment-resistant VD[130].
Vascular depression may affect different groups of older adults differently, and is likely overrepresented among African American older adults. In a sample of older adult depressed patients, 61% of African Americans and 10% of Caucasians were classified as having VD[63]. Additional evidence arises from research that shows that the rates of cardiovascular disease risk factors are significantly higher in African Americans compared to Whites. For instance, the rate of hypertension is significantly higher in African Americans (60%) than Whites (38%)[131,132]. African Americans are also more likely to have diabetes[133,134] and significant health concerns for obesity[135]. Indeed, cardiovascular disease is the leading cause of death in African Americans[136]. People with diabetes, hypertension, or individuals that smoke are 2 to 4 times more likely to develop stroke than those without diabetes or hypertension, or nonsmokers[133,137]. Additionally, stroke and stroke-related mortality rates are higher in African Americans compared to Whites across the lifespan[136,138]. Stroke increases the risk for dementia, and in particular, vascular dementia[139]. Indeed, the rate of vascular dementia is higher in African Americans relative to Whites[140]. All of these cardiovascular risk factors combine to suggest that African Americans are at high risk for vascular depression.
Neuroradiologists examining older adult scans do not typically consider vascular depression as part of the differential diagnosis. Mild and moderate white matter lesions are common in healthy elderly people and have unclear significance, but severe changes are not part of normal aging. White matter changes on MRI independently are of little clinical value to predict depressive symptoms. However, when viewed as a part of an entire clinical presentation, they raise suspicion for further depressive symptoms. For example, the rate of MRI hyperintensities has been found to be higher in populations with LLD compared to age-matched controls, while DWMHs are specifically prevalent in those with LOD[141]. Thus, in practice it would be of clinical utility to take account of the presence of white matter changes on MRI when attempting to predict future depressive symptoms. This may influence decisions regarding the frequency of clinical monitoring and the need for prophylactic antidepressants or other treatments.
Neuropsychological assessments can also play a role in the diagnosis of VD. Impairments on tests that measure executive functioning, such as the Trail Making Test[142] and the Stroop Color-Word Interference Test (Stroop Test)[143], have been observed in individuals with diagnoses of VD. Trail A of the Trail Making Test assesses visual scanning, attention, and processing speed. Trail B of the Trail Making Test requires both sequencing and set shifting, and is used to assess divided attention and cognitive flexibility[144]. Patients with lesions in the dorsolateral frontal areas have shown impairment as compared to control participants on the Trail Making Test[145], implicating executive dysfunction corresponding with the disruption of frontal areas. The Stroop Test is used to measure selective attention and response inhibition[144]. Poor performance on the Stroop Test, especially on the interference condition, has been linked to frontal lobe dysfunction[144]. However, it is possible that the Stroop Test activates a more distributed network of brain areas, including the middle frontal gyrus, parietal lobe regions, and temporal lobe regions[146]. More distinguished evidence has been found specifically for anterior cingulate cortex activation during the interference condition[146,147].
The typical use of neuroimaging in psychiatry is to exclude structural lesions or other neurological pathology as causes for clinical presentation; neuroimaging has not yet played a major role in psychiatric diagnoses. Disagreement regarding which hyperintense signals should be viewed as pathological and how they should be graded may contribute to inconsistent diagnoses in this area. However, the evidence pointing to specific neuroimaging correlates of VD imply that a neuroradiological diagnosis or recommendation for a diagnosis can be made. Despite the possibility that radiologists might not have access to a patient’s depressive symptomatology, neuropsychological performance, or history of illness, they can contribute to the diagnosis through their understanding of the nature of lesions in addition to the VD presentation. We offer some recommendations based on radiologists’ comments on MRI scans that, associated with other data, can be labeled as possible VD symptomatology. For example, a radiologist might report “mild diffuse periventricular white matter T2/FLAIR hyperintensity with scattered hyperintense flair signal in the deep and subcortical white matter,” for a scan with a rating of 3 for DWMHs on the Fazekas scale, associated with a score of 24 on the Beck Depression Inventory, Second Edition (BDI-II) and 23 on the Hamilton Depression Rating Scale (HAM-D), and evidence of poor neuropsychological performance. In this case, it is possible that the diagnosis is VD. Table 1 provides additional examples of such scenarios that are described below.
| ID | Neurorad-iologist comments | Fazekas DWMH rating | Fazekas PV-WMH rating | Depression scores | NP tests | Ethnicity | Age | Other Features |
| 1 | Multifocal white matter T2/FLAIR hyperintense lesions, the largest of which are located in the left corona radiata and left centrum semiovale, some of which have T1 hyperintensity, most consistent with a condition of microvascular ischemic disease. | 2 | 3 | HAM-D = 17; BDI-II = 12 | Trail A = 45” (9%ile); Trail B = 240” (< 1%ile); Stroop = 0.67 (6%ile) | African American | 56 | CIRS-G = 8; MMSE = 29 |
| 2 | Moderate periventricular and deep white matter foci of hyperintense FLAIR signal, more confluent in the right greater than left frontal lobe, most likely due to microvascular ischemia in this age group, accounting for concomitant cerebral/cerebellar atrophy and ventricular dilatation. | 3 | 3 | HAM-D = 22; BDI-II = 22 | Trail A = 50” (63%ile); Trail B = 146” (53%ile); Stroop = 0.52 (19%ile) | Caucasian | 81 | CIRS-G = 5; MMSE = 29 |
| 3 | Scattered deep and subcortical punctate foci of hyperintense FLAIR signal in the bilateral frontal, parietal and temporal lobes. Nonspecific patterns likely to represent sequela of migraine headaches, Lyme infection, vasculitis or microvascular ischemia. | 2 | 3 | HAM-D = 20; BDI-II = 14 | Trail A = 68” (16%ile); Trail B = 148” (34%ile); Stroop = 0.91 (1%ile) | African American | 77 | CIRS-G = 6; MMSE = 29 |
| 4 | Diffuse periventricular and deep white matter foci of hyperintense FLAIR signal in the bilateral frontal parietal and temporal lobes, some of which presenting an ovoid shape perpendicular to the long axis of the lateral ventricle. Primary consideration is multiple sclerosis in the appropriate clinical setting. Other possibilities include microvascular ischemia, vasculitis or prior infection. | 3 | 3 | HAM-D = 25; BDI-II = 21 | Trail A = 92” (< 1%ile); Trail B = 286” (< 1%ile); Stroop = 0.44 (32%ile) | African American | 65 | CIRS-G = 6; MMSE = 29 |
| 5 | Mild periventricular and pontine white matter hyperintense FLAIR signal; additional punctate foci of hyperintense FLAIR in the deep and subcortical frontal-parietal white matter. Nonspecific likely due to microvascular ischemia, migraine headaches, or vasculitis. | 2 | 2 | HAM-D = 41; BDI-II = 43 | Trail A = 30” (55%ile); Trail B = 120” (< 1%ile); Stroop = 0.53 (18%ile) | African American | 53 | CIRS-G = 5; MMSE = 28 |
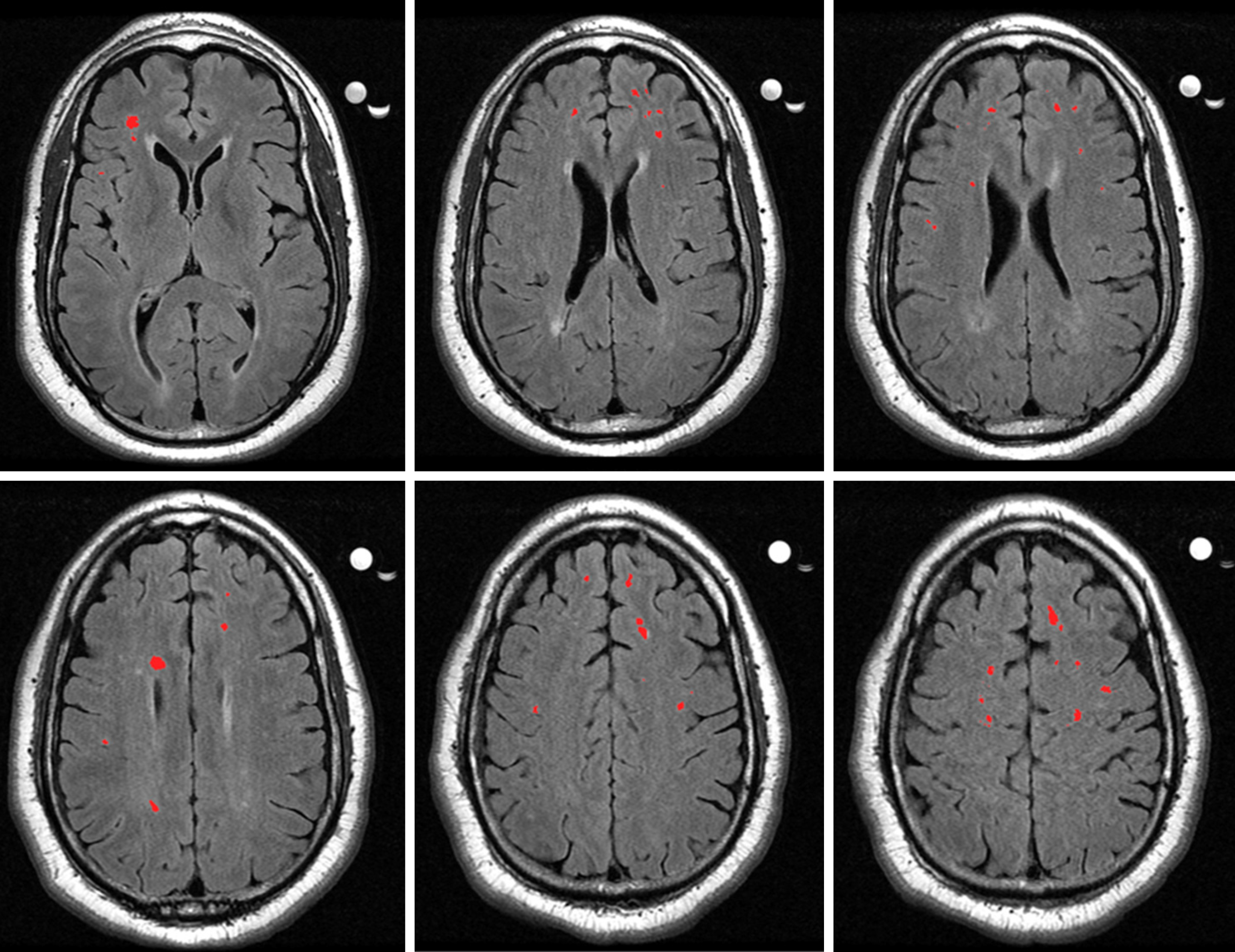
Figure 3 shows brain scans from a patient who was diagnosed with VD. Patient 1 is a 56-year-old African American female with a HAM-D score of 17 (moderate depression) and a BDI-II score of 12 (minimal depression). She had a Cumulative Illness Rating Scale – Geriatrics (CIRS-G) score of 8. The CIRS-G assesses the severity of disorders in a geriatric population and consists of 14 medical problems, with each problem rated from 0 being no problem to 4 being extremely severe, for a total score range of 0 to 56[148]. She scored a 29 out of 30 on the Mini Mental Status Exam (MMSE), indicating intact mental status[149]. Her performance on a task of processing speed was in the low average range, while performance on a task of cognitive flexibility was in the impaired range[150]. Her performance on the Stroop Test, as compared to the sample mean from the VD research study she was involved in[63], was in the borderline range, indicating poorer response inhibition than the sample. Using the Fazekas scale, the MRI scan was rated a DWMH score of 2 and a PVWMH score of 3. The radiologist’s comment for this patient is as follows: “Multifocal white matter T2/FLAIR hyperintense lesions, the largest of which are located in the left corona radiata and left centrum semiovale, some of which have T1 hyperintensity, most consistent with a condition of microvascular ischemic disease.”
Patient 2 is an 81-year-old Caucasian male with a HAM-D score of 22 (severe depression) and a BDI-II score of 22 (moderate depression). He had a CIRS-G rating of 5 and intact mental status (MMSE = 29). His performance on tasks of processing speed and cognitive flexibility was in the average range[150], and his performance on a task of response inhibition was in the low average range[63]. Using the Fazekas scale, the MRI scan was rated a DWMH score of 3 and a PVWMH score of 3. The radiologist’s comment for this patient is as follows: “Moderate periventricular and deep white matter foci of hyperintense FLAIR signal, more confluent in the right greater than left frontal lobe, most likely due to microvascular ischemia in this age group, accounting for concomitant cerebral/cerebellar atrophy and ventricular dilatation.”
Patient 3 is a 77-year-old African American female with a HAM-D score of 20 (severe depression) and a BDI-II score of 14 (mild depression). She had a CIRS-G rating of 6 and intact mental status (MMSE = 29). Performance on a task of processing speed was in the low average range, while performance on a task of cognitive flexibility was in the average range[150]. Performance on a task of response inhibition was impaired[63]. The MRI scan was rated a Fazekas DWMH rating of 2 and a PVWMH rating of 3, and the radiologist commented “Scattered deep and subcortical punctate foci of hyperintense FLAIR signal in the bilateral frontal, parietal and temporal lobes. Nonspecific patterns likely to represent sequela of migraine headaches, Lyme infection, vasculitis or microvascular ischemia.”
Patient 4 is a 65-year-old African American male with a HAM-D score of 25 (very severe depression) and a BDI-II score of 21 (moderate depression). He had a score of 6 on the CIRS-G and intact mental status (MMSE = 29). On tasks of processing speed and cognitive flexibility, performance was in the impaired range[150], while on a task of response inhibition, performance was in the average range[63]. Fazekas ratings of 3 were given for both DWMH and PVWMH. The radiologist commented “Diffuse periventricular and deep white matter foci of hyperintense FLAIR signal in the bilateral frontal parietal and temporal lobes, some of which presenting an ovoid shape perpendicular to the long axis of the lateral ventricle. Primary consideration is multiple sclerosis in the appropriate clinical setting. Other possibilities include microvascular ischemia, vasculitis or prior infection.”
Patient 5 is a 53-year-old African American female with a HAM-D score of 41 (very severe depression) and a BDI-II score of 43 (severe depression). She had a score of 5 on the CIRS-G and intact mental status (MMSE = 28). Performance on a task of processing speed was in the average range while performance on a task of cognitive flexibility was in the impaired range[150]. Performance on a task of response inhibition was in the low average range[63]. The MRI scan was rated a Fazekas DWMH rating of 2 and PVWMH rating of 2. The radiologist noted “Mild periventricular and pontine white matter hyperintense FLAIR signal; additional punctate foci of hyperintense FLAIR in the deep and subcortical frontal-parietal white matter. Nonspecific likely due to microvascular ischemia, migraine headaches, or vasculitis.”
All of the above cases represent patients who should be considered for a diagnosis of VD. All patients had elevated depression indices and neuropsychological test performance often showed evidence of impairment in areas of executive function. All patients also had intact mental states and relatively low ratings of general illness severity. Combined with Fazekas ratings of 2 and 3, the presentations indicate VD as a possible diagnosis. As such, treatments should be selected with this diagnosis in mind and vascular depression should be included in probable differential diagnoses.
In addition to the case series, Table 2 depicts primary and secondary features of VD for radiologists to have a reference guide for creating a database of potential VD cases. Importantly, radiologists will need access to information about patients beyond neuroanatomical scans in order to form such a database. Such information as described in Table 2 should be provided in referral questions and records from psychiatrists and psychologists.
| Primary features |
| Neuroimaging findings consistent with cerebrovascular disease including MRI findings of white matter hyperintensities |
| Presence of cerebrovascular risk factors |
| Cognitive impairment, particularly in areas of processing speed and/or executive functioning |
| Secondary features |
| Age > 50 |
| Poor antidepressant treatment response |
| Psychomotor retardation |
| Marked disability in activities of daily living |
| Lower rate of family history of mood disorders |
As the vascular depression hypothesis becomes more refined, the need to identify and treat this population intensifies. In fact, Gonzalez et al[151] estimated that approximately 2.64 million American adults aged 50 years or older meet criteria for VD. Furthermore, Gonzalez et al[151] found that in adults with lifetime major depression, approximately 22% of participants met criteria for the VD subtype. Combined with the increasing aging population, this high incidence rate depicts the importance of accurate identification of these patients.
White matter hyperintensities have been shown to have clinical importance in VD, as they predict a poor response to treatment and increased relapse rate, but their cause remains unclear. Interventions to slow lesion progression may ultimately be able to improve depression outcomes. With accurate identification early on in this disorder, treatment options other than antidepressants can be evaluated, since antidepressants are often not effective in VD patients. Alternative treatment options should be considered, such as those that target frontostriatal and planning/ organization networks[73]. Psychosocial treatments such as Problem Solving Therapy have been effective in helping depressed older adults with executive dysfunction[152]. Cognitive remediation strategies that target these areas may also prove to be effective. For example, in a preliminary study, Morimoto et al[153] instituted a frontostriatal-targeted computerized cognitive remediation program with depressed older adult antidepressant non-responders and found that depression scores were significantly lower after four weeks for those with more executive dysfunction.
As imaging techniques become increasingly sophisticated, WMHs will be able to be measured with greater accuracy than ever before. However, it is unlikely that this will replace semi-quantitative scoring systems in the foreseeable future. For research purposes, it would be beneficial for there to be a standardized way of scoring VD brains to improve replicability.
The importance of white matter hyperintensities in VD also indicates an increased need for the assistance of neuroradiologists in identifying these potential patients. Not only is it crucial for neuroradiologists to consider this diagnosis when evaluating MRI scans of older depressed adults, but it is important to increase the involvement of psychiatrists and psychologists in imaging decisions. The access to neuroimaging needs to be improved such that mental health providers are able to request neuroimaging. Additionally, it would be helpful for mental health providers to inform neuroradiologists of each patient’s symptomatology, so that neuroradiologists have background information that may influence possible diagnoses when evaluating imaging. As imaging techniques become more advanced, it becomes more likely that imaging will be brought into the scope of psychiatry and psychology, especially with depressive disorders. The use of imaging within psychiatry and psychology has the power to influence the management of symptoms in a critical way. Psychiatrists, psychologists, and neuroradiologists can and should work together, especially when interpreting subtle white matter changes on MRI. Both lesion severity and location should be considered important pieces of information, until such time when one is delineated as being more influential than the other for treatment or disease progression purposes. In the assessment of an older adult patient who has depression, VD should be included as a rule-out for psychiatrists, psychologists, and neuroradiologists. With clinical, neuropsychological, and radiological indicators that separate vascular depression from other depressive disorders, it is critical that patients be evaluated for this disorder.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Loeffler-Stastka H S-Editor: Gong ZM L-Editor: A E-Editor: Liu MY
| 1. | Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. 'Vascular depression' hypothesis. Arch Gen Psychiatry. 1997;54:915-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1206] [Cited by in RCA: 1149] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 2. | Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 199] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B. Subcortical hyperintensities on magnetic resonance imaging: clinical correlates and prognostic significance in patients with severe depression. Biol Psychiatry. 1995;37:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 274] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Krishnan KR, McDonald WM. Arteriosclerotic depression. Med Hypotheses. 1995;44:111-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 506] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 6. | Salloway S, Malloy P, Kohn R, Gillard E, Duffy J, Rogg J, Tung G, Richardson E, Thomas C, Westlake R. MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology. 1996;46:1567-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 7. | Lesser IM, Boone KB, Mehringer CM, Wohl MA, Miller BL, Berman NG. Cognition and white matter hyperintensities in older depressed patients. Am J Psychiatry. 1996;153:1280-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 176] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Silbersweig D, Charlson M. Clinically defined vascular depression. Am J Psychiatry. 1997;154:562-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 337] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999;7:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Turner ST, Jack CR, Fornage M, Mosley TH, Boerwinkle E, de Andrade M. Heritability of leukoaraiosis in hypertensive sibships. Hypertension. 2004;43:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Atwood LD, Wolf PA, Heard-Costa NL, Massaro JM, Beiser A, D'Agostino RB, DeCarli C. Genetic variation in white matter hyperintensity volume in the Framingham Study. Stroke. 2004;35:1609-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 184] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Carmelli D, Reed T, DeCarli C. A bivariate genetic analysis of cerebral white matter hyperintensities and cognitive performance in elderly male twins. Neurobiol Aging. 2002;23:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Assareh A, Mather KA, Schofield PR, Kwok JB, Sachdev PS. The genetics of white matter lesions. CNS Neurosci Ther. 2011;17:525-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Adams S, Conner S, Himali JJ, Beiser A, Vasan RS, Seshadri S, Pase MP. Vascular risk factor burden and new-onset depression in the community. Prev Med. 2018;111:348-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Santos M, Kövari E, Hof PR, Gold G, Bouras C, Giannakopoulos P. The impact of vascular burden on late-life depression. Brain Res Rev. 2009;62:19-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Valkanova V, Ebmeier KP. Vascular risk factors and depression in later life: a systematic review and meta-analysis. Biol Psychiatry. 2013;73:406-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Scott R, Paulson D. Cerebrovascular burden and depressive symptomatology interrelate over 18 years: support for the vascular depression hypothesis. Int J Geriatr Psychiatry. 2018;33:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 604] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 19. | Thomas AJ, O'Brien JT, Davis S, Ballard C, Barber R, Kalaria RN, Perry RH. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 273] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 20. | Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 870] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 21. | Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S, Lim KO, Hoptman MJ. Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry. 2008;165:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 22. | Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biol Psychiatry. 2000;48:791-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 302] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 23. | Bos MJ, Lindén T, Koudstaal PJ, Hofman A, Skoog I, Breteler MM, Tiemeier H. Depressive symptoms and risk of stroke: the Rotterdam Study. J Neurol Neurosurg Psychiatry. 2008;79:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Larson SL, Owens PL, Ford D, Eaton W. Depressive disorder, dysthymia, and risk of stroke: thirteen-year follow-up from the Baltimore epidemiologic catchment area study. Stroke. 2001;32:1979-1983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Sanders ML, Lyness JM, Eberly S, King DA, Caine ED. Cerebrovascular risk factors, executive dysfunction, and depression in older primary care patients. Am J Geriatr Psychiatry. 2006;14:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Simons LA, McCallum J, Friedlander Y, Simons J. Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke. 1998;29:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, Krishnan KR. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Salloway S, Boyle PA, Correia S, Malloy PF, Cahn-Weiner DA, Schneider L, Krishnan KR, Nakra R. The relationship of MRI subcortical hyperintensities to treatment response in a trial of sertraline in geriatric depressed outpatients. Am J Geriatr Psychiatry. 2002;10:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Salloway S, Correia S, Boyle P, Malloy P, Schneider L, Lavretsky H, Sackheim H, Roose S, Krishnan KR. MRI subcortical hyperintensities in old and very old depressed outpatients: the important role of age in late-life depression. J Neurol Sci. 2002;203-204:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Steffens DC, Krishnan KR, Crump C, Burke GL. Cerebrovascular disease and evolution of depressive symptoms in the cardiovascular health study. Stroke. 2002;33:1636-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Thomas AJ, Perry R, Kalaria RN, Oakley A, McMeekin W, O'Brien JT. Neuropathological evidence for ischemia in the white matter of the dorsolateral prefrontal cortex in late-life depression. Int J Geriatr Psychiatry. 2003;18:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 32. | Thomas AJ, Ferrier IN, Kalaria RN, Woodward SA, Ballard C, Oakley A, Perry RH, O'Brien JT. Elevation in late-life depression of intercellular adhesion molecule-1 expression in the dorsolateral prefrontal cortex. Am J Psychiatry. 2000;157:1682-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Tiemeier H, van Dijck W, Hofman A, Witteman JC, Stijnen T, Breteler MM. Relationship between atherosclerosis and late-life depression: the Rotterdam Study. Arch Gen Psychiatry. 2004;61:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Paranthaman R, Greenstein AS, Burns AS, Cruickshank JK, Heagerty AM, Jackson A, Malik RA, Scott ML, Baldwin RC. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 335] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 37. | Salo KI, Scharfen J, Wilden ID, Schubotz RI, Holling H. Confining the Concept of Vascular Depression to Late-Onset Depression: A Meta-Analysis of MRI-Defined Hyperintensity Burden in Major Depressive Disorder and Bipolar Disorder. Front Psychol. 2019;10:1241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Coffey CE, Figiel GS, Djang WT, Cress M, Saunders WB, Weiner RD. Leukoencephalopathy in elderly depressed patients referred for ECT. Biol Psychiatry. 1988;24:143-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 125] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Coffey CE, Figiel GS, Djang WT, Saunders WB, Weiner RD. White matter hyperintensity on magnetic resonance imaging: clinical and neuroanatomic correlates in the depressed elderly. J Neuropsychiatry Clin Neurosci. 1989;1:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 121] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 40. | Coffey CE, Figiel GS, Djang WT, Weiner RD. Subcortical hyperintensity on magnetic resonance imaging: a comparison of normal and depressed elderly subjects. Am J Psychiatry. 1990;147:187-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 227] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Figiel GS, Krishnan KR, Doraiswamy PM, Rao VP, Nemeroff CB, Boyko OB. Subcortical hyperintensities on brain magnetic resonance imaging: a comparison between late age onset and early onset elderly depressed subjects. Neurobiol Aging. 1991;12:245-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 123] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Simpson SW, Jackson A, Baldwin RC, Burns A. 1997 IPA/Bayer Research Awards in Psychogeriatrics. Subcortical hyperintensities in late-life depression: acute response to treatment and neuropsychological impairment. Int Psychogeriatr. 1997;9:257-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 72] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | O'Brien J, Ames D, Chiu E, Schweitzer I, Desmond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. BMJ. 1998;317:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Baldwin R, Jeffries S, Jackson A, Sutcliffe C, Thacker N, Scott M, Burns A. Treatment response in late-onset depression: relationship to neuropsychological, neuroradiological and vascular risk factors. Psychol Med. 2004;34:125-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 45. | Kalayam B, Alexopoulos GS, Kindermann S, Kakuma T, Brown GG, Young RC. P300 latency in geriatric depression. Am J Psychiatry. 1998;155:425-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Bella R, Pennisi G, Cantone M, Palermo F, Pennisi M, Lanza G, Zappia M, Paolucci S. Clinical presentation and outcome of geriatric depression in subcortical ischemic vascular disease. Gerontology. 2010;56:298-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression--a longitudinal evaluation. Biol Psychiatry. 1997;42:367-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 140] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Simpson S, Baldwin RC, Jackson A, Burns AS. Is subcortical disease associated with a poor response to antidepressants? Neurological, neuropsychological and neuroradiological findings in late-life depression. Psychol Med. 1998;28:1015-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Gunning-Dixon FM, Walton M, Cheng J, Acuna J, Klimstra S, Zimmerman ME, Brickman AM, Hoptman MJ, Young RC, Alexopoulos GS. MRI signal hyperintensities and treatment remission of geriatric depression. J Affect Disord. 2010;126:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 50. | Janssen J, Hulshoff Pol HE, Schnack HG, Kok RM, Lampe IK, de Leeuw FE, Kahn RS, Heeren TJ. Cerebral volume measurements and subcortical white matter lesions and short-term treatment response in late life depression. Int J Geriatr Psychiatry. 2007;22:468-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 51. | Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007;15:553-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Yanai I, Fujikawa T, Horiguchi J, Yamawaki S, Touhouda Y. The 3-year course and outcome of patients with major depression and silent cerebral infarction. J Affect Disord. 1998;47:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Steffens DC, Pieper CF, Bosworth HB, MacFall JR, Provenzale JM, Payne ME, Carroll BJ, George LK, Krishnan KR. Biological and social predictors of long-term geriatric depression outcome. Int Psychogeriatr. 2005;17:41-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Köhler S, Buntinx F, Palmer K, van den Akker M. Depression, vascular factors, and risk of dementia in primary care: a retrospective cohort study. J Am Geriatr Soc. 2015;63:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 55. | Newman SC. The prevalence of depression in Alzheimer's disease and vascular dementia in a population sample. J Affect Disord. 1999;52:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 56. | Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH. Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA. 2017;317:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 477] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 57. | Kim S, Woo SY, Kang HS, Lim SW, Choi SH, Myung W, Jeong JH, Lee Y, Hong CH, Kim JH, Na H, Carroll BJ, Kim DK. Factors related to prevalence, persistence, and incidence of depressive symptoms in mild cognitive impairment: vascular depression construct. Int J Geriatr Psychiatry. 2016;31:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Puglisi V, Bramanti A, Lanza G, Cantone M, Vinciguerra L, Pennisi M, Bonanno L, Pennisi G, Bella R. Impaired Cerebral Haemodynamics in Vascular Depression: Insights From Transcranial Doppler Ultrasonography. Front Psychiatry. 2018;9:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 59. | Pennisi M, Lanza G, Cantone M, Ricceri R, Spampinato C, Pennisi G, Di Lazzaro V, Bella R. Correlation between Motor Cortex Excitability Changes and Cognitive Impairment in Vascular Depression: Pathophysiological Insights from a Longitudinal TMS Study. Neural Plast. 2016;2016:8154969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Steffens DC, Taylor WD, Krishnan KR. Progression of subcortical ischemic disease from vascular depression to vascular dementia. Am J Psychiatry. 2003;160:1751-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 61. | Loganathan S, Phutane VH, Prakash O, Varghese M. Progression of vascular depression to possible vascular dementia. J Neuropsychiatry Clin Neurosci. 2010;22:451-t.e34-451.e35. [PubMed] [DOI] [Full Text] |
| 62. | Pimontel MA, Reinlieb ME, Johnert LC, Garcon E, Sneed JR, Roose SP. The external validity of MRI-defined vascular depression. Int J Geriatr Psychiatry. 2013;28:1189-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Reinlieb ME, Persaud A, Singh D, Garcon E, Rutherford BR, Pelton GH, Devanand DP, Roose SP, Sneed JR. Vascular depression: overrepresented among African Americans? Int J Geriatr Psychiatry. 2014;29:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Krishnan KR, Taylor WD, McQuoid DR, MacFall JR, Payne ME, Provenzale JM, Steffens DC. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;55:390-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 65. | Alexopoulos GS. "The depression-executive dysfunction syndrome of late life": a specific target for D3 agonists? Am J Geriatr Psychiatry. 2001;9:22-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Potter GG, McQuoid DR, Steffens DC, Welsh-Bohmer KA, Krishnan KR. Neuropsychological correlates of magnetic resonance imaging-defined subcortical ischemic depression. Int J Geriatr Psychiatry. 2009;24:219-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 67. | Alexopoulos GS, Kiosses DN, Klimstra S, Kalayam B, Bruce ML. Clinical presentation of the "depression-executive dysfunction syndrome" of late life. Am J Geriatr Psychiatry. 2002;10:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 68. | Chang KJ, Hong CH, Kim SH, Lee KS, Roh HW, Kang DR, Choi SH, Kim SY, Na DL, Seo SW, Kim DK, Lee Y, Chung YK, Lim KY, Noh JS, Son SJ. MRI-defined versus clinically-defined vascular depression; comparison of prediction of functional disability in the elderly. Arch Gerontol Geriatr. 2016;66:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 69. | Murphy CF, Gunning-Dixon FM, Hoptman MJ, Lim KO, Ardekani B, Shields JK, Hrabe J, Kanellopoulos D, Shanmugham BR, Alexopoulos GS. White-matter integrity predicts stroop performance in patients with geriatric depression. Biol Psychiatry. 2007;61:1007-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 70. | Culang ME, Sneed JR, Keilp JG, Rutherford BR, Pelton GH, Devanand DP, Roose SP. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatry. 2009;17:881-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56:713-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 177] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 72. | Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, Sirey JA, Hull J. Executive dysfunction and long-term outcomes of geriatric depression. Arch Gen Psychiatry. 2000;57:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 288] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 73. | Pimontel MA, Rindskopf D, Rutherford BR, Brown PJ, Roose SP, Sneed JR. A Meta-Analysis of Executive Dysfunction and Antidepressant Treatment Response in Late-Life Depression. Am J Geriatr Psychiatry. 2016;24:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 74. | Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 289] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 75. | Turk BR, Gschwandtner ME, Mauerhofer M, Löffler-Stastka H. Can we clinically recognize a vascular depression? The role of personality in an expanded threshold model. Medicine (Baltimore). 2015;94:e743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Licht-Strunk E, Bremmer MA, van Marwijk HW, Deeg DJ, Hoogendijk WJ, de Haan M, van Tilburg W, Beekman AT. Depression in older persons with versus without vascular disease in the open population: similar depressive symptom patterns, more disability. J Affect Disord. 2004;83:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 77. | Paulson D, Lichtenberg PA. Vascular depression: an early warning sign of frailty. Aging Ment Health. 2013;17:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Paulson D, Lichtenberg PA. Vascular depression and frailty: a compound threat to longevity among older-old women. Aging Ment Health. 2013;17:901-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Concerto C, Lanza G, Cantone M, Pennisi M, Giordano D, Spampinato C, Ricceri R, Pennisi G, Aguglia E, Bella R. Different patterns of cortical excitability in major depression and vascular depression: a transcranial magnetic stimulation study. BMC Psychiatry. 2013;13:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Bella R, Ferri R, Cantone M, Pennisi M, Lanza G, Malaguarnera G, Spampinato C, Giordano D, Raggi A, Pennisi G. Motor cortex excitability in vascular depression. Int J Psychophysiol. 2011;82:248-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 81. | Jeon SW, Kim YK. The role of neuroinflammation and neurovascular dysfunction in major depressive disorder. J Inflamm Res. 2018;11:179-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 82. | Bell IR, Edman JS, Selhub J, Morrow FD, Marby DW, Kayne HL, Cole JO. Plasma homocysteine in vascular disease and in nonvascular dementia of depressed elderly people. Acta Psychiatr Scand. 1992;86:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 83. | Nilsson K, Gustafson L, Hultberg B. Elevated plasma homocysteine level in vascular dementia reflects the vascular disease process. Dement Geriatr Cogn Dis Extra. 2013;3:16-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Westermair AL, Munz M, Schaich A, Nitsche S, Willenborg B, Muñoz Venegas LM, Willenborg C, Schunkert H, Schweiger U, Erdmann J. Association of Genetic Variation at AQP4 Locus with Vascular Depression. Biomolecules. 2018;8:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Chen RH, Liu N, Zhou YC, Xiao YC. Investigation and analysis of sleep status in patients with vascular depression. Int J Gerontol. 2018;12:299-302. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 86. | Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1032] [Cited by in RCA: 1142] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 87. | Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, Steinling M, Valk J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 630] [Cited by in RCA: 703] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 88. | Boyko OB, Alston SR, Fuller GN, Hulette CM, Johnson GA, Burger PC. Utility of postmortem magnetic resonance imaging in clinical neuropathology. Arch Pathol Lab Med. 1994;118:219-225. [PubMed] |
| 89. | Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512-1520. [PubMed] |
| 90. | Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. J Neurol. 2008;255:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 91. | Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am J Roentgenol. 1988;151:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 92. | Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008;64:491-497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | van Straaten EC, Fazekas F, Rostrup E, Scheltens P, Schmidt R, Pantoni L, Inzitari D, Waldemar G, Erkinjuntti T, Mäntylä R, Wahlund LO, Barkhof F; LADIS Group. Impact of white matter hyperintensities scoring method on correlations with clinical data: the LADIS study. Stroke. 2006;37:836-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 94. | Firbank MJ, Lloyd AJ, Ferrier N, O'Brien JT. A volumetric study of MRI signal hyperintensities in late-life depression. Am J Geriatr Psychiatry. 2004;12:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Gurol ME, Irizarry MC, Smith EE, Raju S, Diaz-Arrastia R, Bottiglieri T, Rosand J, Growdon JH, Greenberg SM. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 272] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 96. | MacFall JR, Taylor WD, Rex DE, Pieper S, Payne ME, McQuoid DR, Steffens DC, Kikinis R, Toga AW, Krishnan KR. Lobar distribution of lesion volumes in late-life depression: the Biomedical Informatics Research Network (BIRN). Neuropsychopharmacology. 2006;31:1500-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 97. | Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, Wilkins CH, Snyder AZ, Couture L, Schechtman K, McKinstry RC. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 98. | Wu M, Rosano C, Butters M, Whyte E, Nable M, Crooks R, Meltzer CC, Reynolds CF, Aizenstein HJ. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res. 2006;148:133-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 99. | Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1813] [Cited by in RCA: 2030] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 100. | Sneed JR, Culang-Reinlieb ME, Brickman AM, Gunning-Dixon FM, Johnert L, Garcon E, Roose SP. MRI signal hyperintensities and failure to remit following antidepressant treatment. J Affect Disord. 2011;135:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Anbeek P, Vincken KL, van Osch MJ, Bisschops RH, van der Grond J. Probabilistic segmentation of white matter lesions in MR imaging. Neuroimage. 2004;21:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 204] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 102. | Maillard P, Delcroix N, Crivello F, Dufouil C, Gicquel S, Joliot M, Tzourio-Mazoyer N, Alpérovitch A, Tzourio C, Mazoyer B. An automated procedure for the assessment of white matter hyperintensities by multispectral (T1, T2, PD) MRI and an evaluation of its between-centre reproducibility based on two large community databases. Neuroradiology. 2008;50:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Brickman AM, Sneed JR, Provenzano FA, Garcon E, Johnert L, Muraskin J, Yeung LK, Zimmerman ME, Roose SP. Quantitative approaches for assessment of white matter hyperintensities in elderly populations. Psychiatry Res. 2011;193:101-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 104. | Payne ME, Fetzer DL, MacFall JR, Provenzale JM, Byrum CE, Krishnan KR. Development of a semi-automated method for quantification of MRI gray and white matter lesions in geriatric subjects. Psychiatry Res. 2002;115:63-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Ramirez J, Gibson E, Quddus A, Lobaugh NJ, Feinstein A, Levine B, Scott CJ, Levy-Cooperman N, Gao FQ, Black SE. Lesion Explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 106. | Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59:3774-3783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 902] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 107. | Lamar M, Charlton RA, Morris RG, Markus HS. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18:634-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 108. | Tadayonnejad R, Yang S, Kumar A, Ajilore O. Multimodal brain connectivity analysis in unmedicated late-life depression. PLoS One. 2014;9:e96033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, Mäurer M, Bradley KM, Mackay CE, Ebmeier KP. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69:680-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 110. | Taylor WD, MacFall JR, Payne ME, McQuoid DR, Provenzale JM, Steffens DC, Krishnan KR. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am J Psychiatry. 2004;161:1293-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Yang Q, Huang X, Hong N, Yu X. White matter microstructural abnormalities in late-life depression. Int Psychogeriatr. 2007;19:757-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 112. | Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. 2011;194:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 113. | Andreescu C, Tudorascu DL, Butters MA, Tamburo E, Patel M, Price J, Karp JF, Reynolds CF, Aizenstein H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. 2013;214:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 114. | Rabins PV, Pearlson GD, Aylward E, Kumar AJ, Dowell K. Cortical magnetic resonance imaging changes in elderly inpatients with major depression. Am J Psychiatry. 1991;148:617-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 148] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Naarding P, Veereschild M, Bremmer M, Deeg D, Beekman AT. The symptom profile of vascular depression. Int J Geriatr Psychiatry. 2009;24:965-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 116. | Sneed JR, Roose SP, Sackeim HA. Vascular depression: A distinct diagnostic subtype? Biol Psychiatry. 2006;60:1295-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 117. | Lesser IM, Hill-Gutierrez E, Miller BL, Boone KB. Late-onset depression with white matter lesions. Psychosomatics. 1993;34:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Nebes RD, Reynolds CF, Boada F, Meltzer CC, Fukui MB, Saxton J, Halligan EM, DeKosky ST. Longitudinal increase in the volume of white matter hyperintensities in late-onset depression. Int J Geriatr Psychiatry. 2002;17:526-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Paranthaman R, Burns AS, Cruickshank JK, Jackson A, Scott ML, Baldwin RC. Age at onset and vascular pathology in late-life depression. Am J Geriatr Psychiatry. 2012;20:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Park JH, Lee SB, Lee JJ, Yoon JC, Han JW, Kim TH, Jeong HG, Newhouse PA, Taylor WD, Kim JH, Woo JI, Kim KW. Epidemiology of MRI-defined vascular depression: A longitudinal, community-based study in Korean elders. J Affect Disord. 2015;180:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 121. | Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67:277-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 122. | Taylor WD, MacFall JR, Payne ME, McQuoid DR, Steffens DC, Provenzale JM, Krishnan RR. Greater MRI lesion volumes in elderly depressed subjects than in control subjects. Psychiatry Res. 2005;139:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 123. | O’Brien J, Barber B. Neuroimaging in dementia and depression. Adv Psychiatr Treat. 2000;6:109-119. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 124. | Greenwald BS, Kramer-Ginsberg E, Krishnan KR, Ashtari M, Auerbach C, Patel M. Neuroanatomic localization of magnetic resonance imaging signal hyperintensities in geriatric depression. Stroke. 1998;29:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 125. | Kumar A, Thomas A, Lavretsky H, Yue K, Huda A, Curran J, Venkatraman T, Estanol L, Mintz J, Mega M, Toga A. Frontal white matter biochemical abnormalities in late-life major depression detected with proton magnetic resonance spectroscopy. Am J Psychiatry. 2002;159:630-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 126. | Navarro V, Gastó C, Lomeña F, Mateos JJ, Marcos T, Portella MJ. Normalization of frontal cerebral perfusion in remitted elderly major depression: a 12-month follow-up SPECT study. Neuroimage. 2002;16:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 127. | MacFall JR, Payne ME, Provenzale JE, Krishnan KR. Medial orbital frontal lesions in late-onset depression. Biol Psychiatry. 2001;49:803-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 128. | Patankar TF, Baldwin R, Mitra D, Jeffries S, Sutcliffe C, Burns A, Jackson A. Virchow-Robin space dilatation may predict resistance to antidepressant monotherapy in elderly patients with depression. J Affect Disord. 2007;97:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159:1929-1932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 214] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 130. | Narushima K, McCormick LM, Yamada T, Thatcher RW, Robinson RG. Subgenual cingulate theta activity predicts treatment response of repetitive transcranial magnetic stimulation in participants with vascular depression. J Neuropsychiatry Clin Neurosci. 2010;22:75-84. [PubMed] [DOI] [Full Text] |
| 131. | Kramer H, Han C, Post W, Goff D, Diez-Roux A, Cooper R, Jinagouda S, Shea S. Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens. 2004;17:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 242] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 132. | Geronimus AT, Bound J, Keene D, Hicken M. Black-white differences in age trajectories of hypertension prevalence among adult women and men, 1999-2002. Ethn Dis. 2007;17:40-48. [PubMed] |
| 133. | Centers for Disease Control and Prevention. National diabetes statistics report 2017. [Accessed 10 January 2020]. Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. |
| 134. | Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 364] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 135. | Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2990] [Cited by in RCA: 2637] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 136. | Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, Johnston KC, Johnston SC, Khalessi AA, Kidwell CS, Meschia JF, Ovbiagele B, Yavagal DR; American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015;46:3020-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1579] [Article Influence: 157.9] [Reference Citation Analysis (0)] |
| 137. | Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 208] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 138. | Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R, Broderick J. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 139. | Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke. 2002;33:2254-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 196] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 140. | Kuller LH, Lopez OL, Jagust WJ, Becker JT, DeKosky ST, Lyketsos C, Kawas C, Breitner JC, Fitzpatrick A, Dulberg C. Determinants of vascular dementia in the Cardiovascular Health Cognition Study. Neurology. 2005;64:1548-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 141. | Sneed JR, Culang-Reinlieb ME. The vascular depression hypothesis: an update. Am J Geriatr Psychiatry. 2011;19:99-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 142. | Reitan RM, Wolfson D. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. 2nd ed. Tucson (AZ): Neuropsychology Press, 1993. |
| 143. | MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 144. | Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological assessment. 5th ed. New York (NY): Oxford University Press, 2012. |
| 145. | Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: a study in focal lesion patients. Psychol Assess. 2001;13:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 146. | Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1166] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 147. | Ravnkilde B, Videbech P, Rosenberg R, Gjedde A, Gade A. Putative tests of frontal lobe function: a PET-study of brain activation during Stroop's Test and verbal fluency. J Clin Exp Neuropsychol. 2002;24:534-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Miller MD, Towers A. A manual of guidelines for scoring the Cumulative Illness Rating Scale for Geriatrics (CIRS-G). Pittsburgh (PA): University of Pittsburgh, 1991. |
| 149. | Iverson GL. Interpretation of Mini-Mental State Examination scores in community-dwelling elderly and geriatric neuropsychiatry patients. Int J Geriatr Psychiatry. 1998;13:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 150. | Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1807] [Cited by in RCA: 2167] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 151. | González HM, Tarraf W, Whitfield K, Gallo JJ. Vascular depression prevalence and epidemiology in the United States. J Psychiatr Res. 2012;46:456-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Areán PA, Raue P, Mackin RS, Kanellopoulos D, McCulloch C, Alexopoulos GS. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction. Am J Psychiatry. 2010;167:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 153. | Morimoto SS, Gunning FM, Wexler BE, Hu W, Ilieva I, Liu J, Nitis J, Alexopoulos GS. Executive Dysfunction Predicts Treatment Response to Neuroplasticity-Based Computerized Cognitive Remediation (nCCR-GD) in Elderly Patients with Major Depression. Am J Geriatr Psychiatry. 2016;24:816-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |