Published online Nov 28, 2020. doi: 10.4329/wjr.v12.i11.261
Peer-review started: August 31, 2020
First decision: September 15, 2020
Revised: September 30, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 28, 2020
Processing time: 90 Days and 16.5 Hours
The integrated clinical, laboratory and ultrasound approach is essential for the diagnosis, evaluation and monitoring of the patient's therapy in coronavirus disease 2019 pneumonia. The ideal imaging approach in this context is not yet well defined. Chest X-ray is characterized by low sensitivity in identifying earlier lung changes. The "bedside" pulmonary ultrasound has an undeniable series of advantages in the patient at high infectious risk and can provide incremental data in the respiratory intensive care for the serial control of the individual patient as well as for the home delivery of the stabilized subjects. Pulmonary computed tomography shows high sensitivity but should not be routinely performed in all patients, because in the first 48 h it can be absolutely negative and in the late phase the imaging findings may not change the therapeutic approach. Echocardiography should be limited to patients with hemodynamic instability to assess ventricular function and pulmonary pressures.
Core Tip: Coronavirus disease 2019 (COVID-19) pneumonia needs an integrated clinical, laboratory and ultrasound approach. The "bedside" pulmonary ultrasound has many advantages in the patient at high infectious risk and can provide incremental data in the respiratory intensive care. Chest X-ray is characterized by low sensitivity in identifying earlier lung changes. Pulmonary computed tomography shows high sensitivity but should not be routinely performed in all patients, because in the first 48 h it can be absolutely negative. We discuss the role of imaging approach in the evaluation of patients with COVID-19 pneumonia.
- Citation: D'Andrea A, Radmilovic J, Carbone A, Forni A, Tagliamonte E, Riegler L, Liccardo B, Crescibene F, Sirignano C, Esposito G, Bossone E. Multimodality imaging in COVID-19 patients: A key role from diagnosis to prognosis. World J Radiol 2020; 12(11): 261-271
- URL: https://www.wjgnet.com/1949-8470/full/v12/i11/261.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i11.261
On December 31, 2019, the World Health Organization was alerted by the Public Health Commission of the Province of Hubei, China regarding some cases of severe pneumonia, of unknown etiology, characterized by symptoms such as fever, malaise, dry cough, dyspnea and respiratory failure, which occurred in the urban area of Wuhan[1]. A new coronavirus, severe acute respiratory syndrome coronavirus 2, was identified as responsible for the lung infection and is now called coronavirus disease 2019 (COVID-19). Since then, as we well know, there has been an exponential growth of contagions, which initially involved neighboring countries, such as Japan and South Korea, and then affected extra-continental countries, with the first documented cases in Europe, United States and Africa between January and February 2020. On January 30, 2020, the World Health Organization declared the epidemic a global emergency. The epidemic exploded in Italy on February 21, 2020, while the pandemic was declared on March 10, 2020. Currently, the main sources of infection are people themselves infected with severe acute respiratory syndrome coronavirus 2. Even asymptomatic carriers can become a source of diffusion. Transmission takes place by aerosol via “droplets”, but even if direct and indirect transmissions are less important, they must be taken into account. An early diagnosis of the virus carriers is one of the key points to contain the spread, the morbidity and the mortality of the pandemic. The definitive diagnosis takes place through specific tests, while the exact role of imaging in the care pathway of the patient with suspected or confirmed COVID infection is still to be defined.
The subject of this review is to underline precisely the role of imaging in the evaluation of these patients.
According to current epidemiological investigations, the incubation lasts from 1 to 14 d, usually from 3 to 7 d. The clinical manifestations are very heterogeneous, and the main ones are fever, dry cough and asthenia[2-4]. In a minority of patients, nasal obstruction, rhinorrhea, pharyngodynia, myalgia and diarrhea are present. Common neurological symptoms include anosmia and dysgeusia and mental confusion in marked hypoxemia. Dyspnea and/or hypoxemia occur in patients with a severe form of the disease often 1 wk after the onset of the disease, while rapid progression to acute respiratory distress syndrome (ARDS), septic shock, metabolic acidosis, coagulation deficiency and finally multiple organ failure are possible in critically/very serious patients. Some children and infants may instead present an atypical clinical profile, which consists of gastrointestinal symptoms, such as vomiting, diarrhea, drowsiness or hypersomnia and tachypnea.
Of interest, as underlined by numerous position papers of national and international scientific societies, coronavirus infection can have implications in the cardiological field for the following reasons: Patients with COVID-19 infection can develop cardiac complications, although not very frequent; cardiovascular diseases continue to be predominant in the general population and the same patients who present with an acute coronary syndrome can at the same time be infected or carriers of COVID-19[5-7]. Reverse transcription-polymerase chain reaction and/or next generation sequencing can detect the presence of viral nucleic acids in oro-pharyngeal swabs, sputum and other samples from the lower respiratory tract, blood and stool. The samples of choice for greater accuracy of the test are those from the lower respiratory tract (sputum or bronchoaveolar lavage). Once the sample is collected, it should be analyzed as soon as possible. It represents the gold standard for diagnosis; but, being burdened by numerous false negatives in the initial stages of the disease, it must be repeated in suspicious cases at 72 h to confirm the absence of disease[7].
In a context of non-specific symptoms and extremely variable clinical pictures, such as severity and progression, the contribution of integrated imaging can be crucial not only for the diagnosis of pneumonia but also for therapy monitoring and obtaining prognostic information.
Currently, the optimal imaging strategy is still uncertain. The discriminating parameter to hospitalize or send home a patient with suspected COVID-19 is the presence of respiratory failure, linked to the pulmonary infection that may be in progress (respiratory failure, even subclinical, given the progressively rapid worsening of the pathology). The functional data can be easily obtained by performing a blood gas analysis (a simple, rapid procedure that does not require patient access to hospital routes with the risk of further infections) or by a 6-min walking test, while the anatomical/morphological data- are linked to the execution of imaging tests (procedures that require the patient's access within the hospital and exposure to the virus of other personnel and other environments) (Table 1).
| Lung ultrasound | Chest X-ray | Chest CT, HCRT | |
| Lung thickening | Nd | Lung thickening | |
| Confluent B lines | Aspect of white cottony lung infiltrate | Images of lung infiltrates | |
| Small peripheral consolidations | Increase in hyperechoic areas | Subpleural consolidations | |
| Translobar and non-translobar consolidations | Large translobar confluent and white thickening | Translobar consolidations | |
| Multilobar distributionFront and posterior multiple sector scans (if possible) | Cotton aspect of multilobar and bilateral interstitial pathology | Anomalies with distribution on more than two lobes and bilateral | |
| Rare pleural effusion | Rare pleural effusion | Rare pleural effusion | |
| Clinical aspect of the patient and three techniques compared | |||
| Early stage | Focal B lines | It may be negative or with few interstitial lobular changes | Few multilobar ground glass areas |
| Mild infection phase | Focal B lines with pleural thickening | Multilobar interstitial alterations with cottony aspect | Confluent opacities with ground glass areas |
| Severe infection stage | Parenchymal consolidations (hepatization of the parenchyma) | Increase in interstitial consolidations with multilobar thickening phenomena | Interstitial alveolar syndrome consolidations air bronchogram |
Interstitial pneumonia is the main clinical manifestation of COVID-19. Pulmonary interstice is defined as a particular entity located between the alveolus and capillaries and is investigated mainly with radiological techniques[7]. Interstitial pneumonia is characterized by the presence of edema and inflammatory cell infiltrate in the interstitial spaces (alveolar walls), more than in the alveolar cavities. It is only in the more advanced stages of the disease do they begin to fill the alveolar spaces, first in part [“ground glass” (GG)] and then total (consolidation). COVID-19 interstitial pneumonia does not have a specific manifestation, and we cannot define a patient as positive based only on the imaging picture. Pneumonia is in fact composed of a series of characteristics comparable with many other pneumonia or interstitial manifestations[8].
Standard chest radiographic examination (X-ray): Standard chest radiographic examination (X-ray) is characterized by low sensitivity in identifying the earliest lung changes of COVID-19, which is characterized by GG opacities. Therefore, it is not the most suitable radiological examination, except for the initial diagnosis of exclusion of other bacterial alveolar pneumonia. However, it is necessary to consider that, in many of the community-acquired lung infections, the changes become manifest on chest X-ray within a time interval usually 12 h from the beginning of the symptomatology; and, therefore, the examination can be negative if done too early.
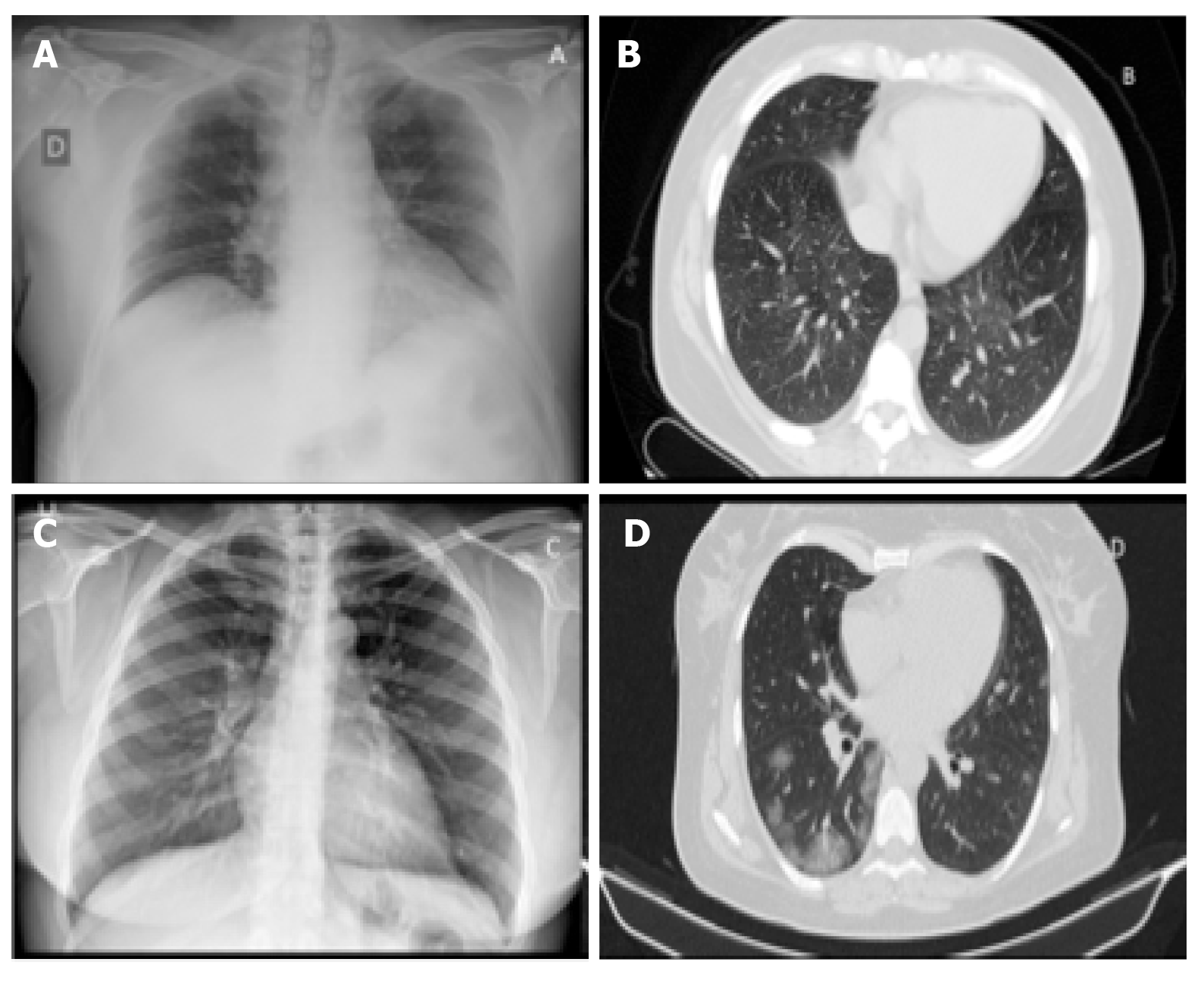
In the more advanced stages of the infection, the chest X-ray examination shows bilateral multifocal alveolar opacities, which tend to confluence up to the complete opacification of the lung, with possible small associated pleural effusion[8] (Figures 1 and 2).
Chest computed tomography: In the early stages of the morbid process, chest computed tomography (CT) and particularly high resolution CT (HRCT) have a high diagnostic sensitivity. COVID-19 pneumonia, however, shows a varied and non-specific HRCT patterns, similar to other lung infections, such as influenza A (H1N1), cytomegalovirus, other coronavirus (severe acute respiratory syndrome, Middle East respiratory syndrome), streptococcus and pneumonia from atypical germs (chlamydia, mycoplasma).
HRCT is performed with a high resolution method (gold standard of interstitial disease), which allows to have a slice thickness between 0.625 mm and 1.25 mm, capable of showing the conditions of the interstitium or the hexagon formed by the secondary lobule.
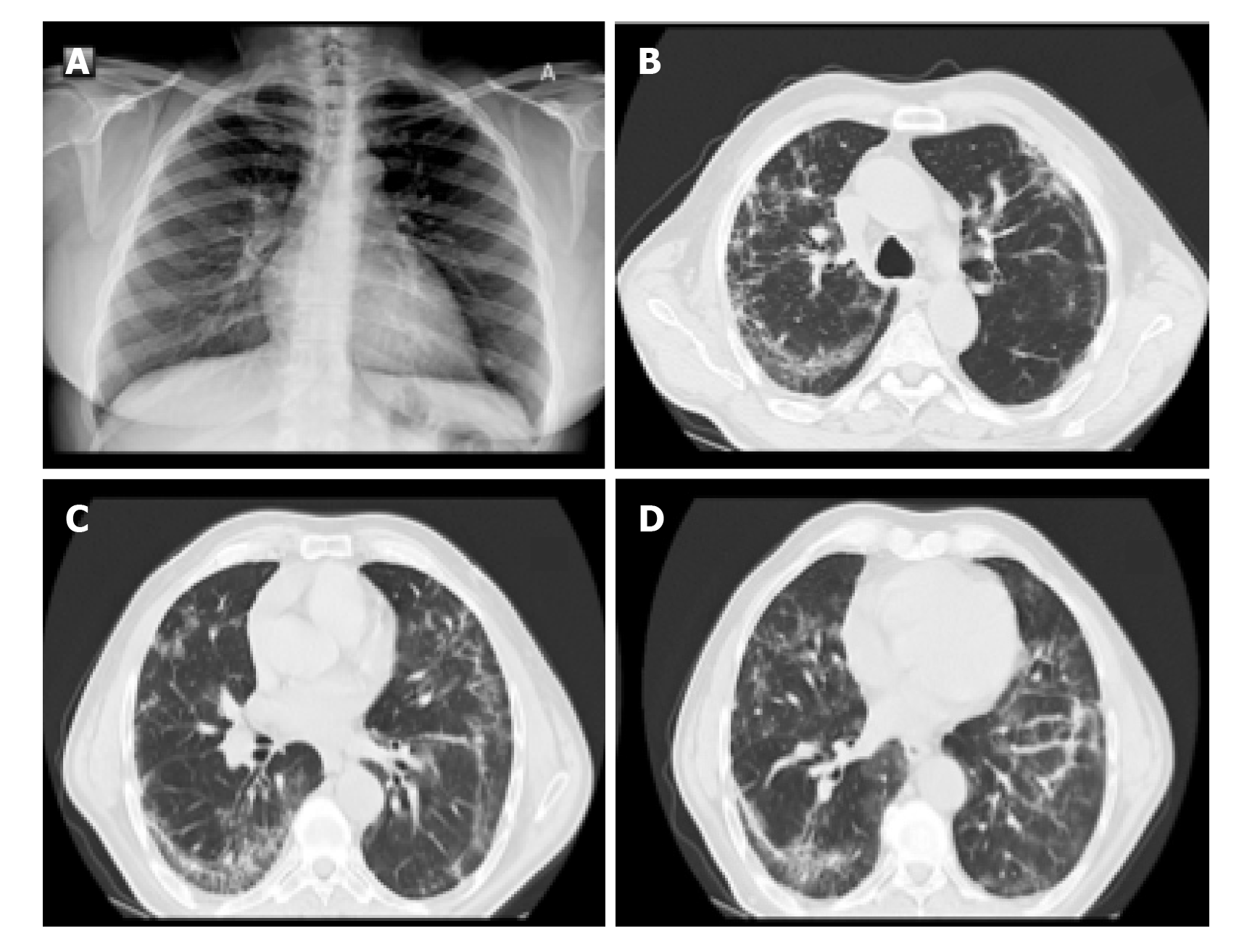
The most common findings in HRCT were the multifocal bilateral GG areas, associated with consolidation areas with patchy distribution, mainly peripheral/subpleural, and with greater involvement of the posterior regions and lower lobes[9-13]. As recently documented in a joint document of various scientific societies (Italian Society for Radiological Medicine, Italian Society for Ultrasound in Medicine and Biology and FISM)[14,15], the "pure" focal or multifocal GG pattern and the "crazy paving" pattern were also observed in COVID-19 pneumonia, characterized by the presence of GG areas superimposed on smooth thickening of the interlobular and intralobular interstitium. Less commonly described are the presence of exclusive consolidations, the "reversed halo sign" (focal area of GG delimited by a more or less complete peripheral ring of consolidation) and cavitations, calcifications, lymphadenopathies and pleural effusion.
In addition to the early stages of diagnosis, HRCT is useful in assessing the course and severity of the disease; and, therefore, in guiding the patient's clinical management. While in the initial stages there are bilateral peripheral GG opacities, in more advanced phases of the disease, the segments involved increase in number, so that the GG extends and involves an increasing percentage of the parenchyma, passing from peripheral to more and more centralized. Furthermore, the areas previously GG increase in density, up to the final parenchymal consolidation. The evolution is characteristic of this specific pathology, since coronavirus is able to determine a primary viral pneumonia. In fact, while the other viral pneumonias consolidate when a bacterial infection overlaps, in COVID-19 infection, the same pathogen involves the most peripheral branches of the bronchial tree and so determines a parenchymal consolidation without bacterial superinfection (primary viral pneumonia)[12-15].
The progression of the disease correlates with the increase in the number, size and density of the GG areas in HRCT examinations, with the appearance of widespread and bilateral parenchymal consolidations with air bronchogram in the context. CT, therefore, does not have a defined role in the initial phase, and perhaps not even in the final stages of the morbid process, but rather in the intermediate phase of the patient's diagnostic path to understand how necessary it is to be aggressive, fast and therapeutically impacting. Even the American College of Radiology states that CT should not be used for screening or as a first radiological line but only in hospitalized, symptomatic patients or in subjects with specific clinical indications[8] (Figures 1-3).
Hence, CT shows an incremental power only when it can have an effective impact on the management and on the background therapy. For example, if we want to evaluate if and how much the pulmonary thickenings have increased to decide eventually to anticipate intubation, then CT is appropriate. If, on the other hand, we simply want to acknowledge that the patient is getting worse, but the therapy is already maximized, it does not make sense to perform the CT, as it is important to avoid unnecessary infectious risks in transporting the patient to the radiology department.
Bedside pulmonary echography in a clinical setting such as COVID pneumonia has an undeniable set of benefits, such as improved ease of disinfection, less contact area with patients and the ability to perform the exam without moving the patient on a stretcher, thus reducing the risk of spreading the virus while respecting the appropriate protection measures[16,17]. The inability to maintain a minimum doctor-patient safety distance makes it one of the tests that puts health professionals at risk.
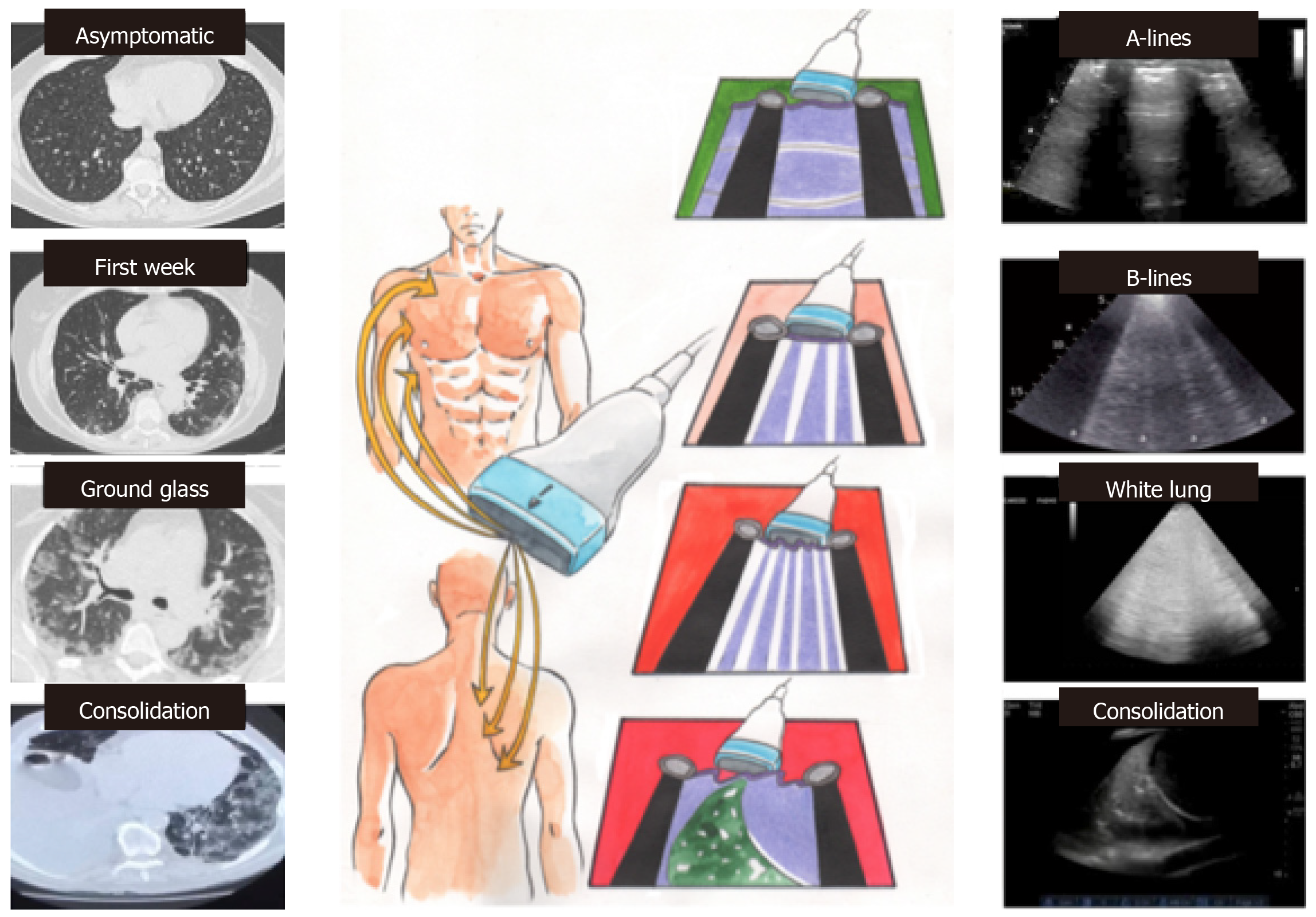
To increase significantly the sensitivity of the lung ultrasound, a thorough examination is necessary, adopting an antero-posterior window and trying to visualize the lung as much as possible in a 12-segment approach (each lung is divided in three areas by the anterior and posterior axillary lines: Front, lateral and posterior). The high frequency linear probe may be preferable to obtain high resolution images of the pleural line (the first horizontal line encountered in the scan, 1-2 mm thick depending on the probe used) to distinguish between a regular and normal sliding pleural line (“sliding”) with respect to a thickened, irregular or interrupted pleural line or with no sliding (as occurs in pneumothorax).
The key aspects to distinguish in lung scan are: (1) A lines: Normal reverberation artifacts of the ultrasound beam on the pleura/pulmonary air interface; hyperechoic (white) lines horizontal and parallel to each other and to the pleural line, typical of the healthy lung; (2) B lines (“comet tail”): Pathological artifacts typical of the interstitial disease (from pulmonary oedema to interstitial pneumonia); vertical hyperechoic lines perpendicular to the pleural line, which provide from the pleural line itself (not from the skin), direct themselves in depth and mask the A lines, preventing their visualization. The more these lines are numerous, close and coalescent, the worse is the involvement of the interstitium; (3) Pulmonary consolidation: When the degree of pulmonary aeration is reduced and the air in the alveolar spaces is replaced by exudate/inflammatory cells, the ultrasound beam is able to penetrate the parenchyma showing a “consolidation” with an ecostructural pattern similar to that of the liver (hepatization of the lung); and (4) Pleural effusion: Pulmonary ultrasound is certainly the gold standard in the diagnosis of pleural effusion. In this condition, the investigation has a very high sensitivity and specificity and allows to evaluate correctly the volume of the effusion and the echogenicity (transudate vs corpuscular exudate).
Pulmonary ultrasound results in COVID pneumonia seem to correlate very well with the results of chest CT. With the increasing severity of the disease, the following evolution has been observed: (1) Presymptomatic phase: Few areas of hyperdensity with GG at CT, mainly in the lower and posterior fields, related to the echographic B lines alternating with areas with normal A lines (“patchy”); reduced mobility of the diaphragms from the early days; (2) First week of symptoms: bilateral GG opacities more confluent on CT, related to the coalescing B lines that form “patches of white” (“waterfall sign”), and wrinkled pleural line; (3) Second week: Small peripheral subpleural bilateral consolidation on CT and echography, with evolution in “dry lung” and dense and fixed B lines with respect to pleural sliding; and (4) In the most severe form, the volume of the consolidated lung progressively increases, with the absence of color at the color-doppler of the consolidations, and the possible evolution towards ARDS (Figure 3). Within the consolidated lung there are hyperechoic branched consolidations (those that are aerial bronchograms on CT)[17-20].
Other interesting features are: Peripheral pulmonary anomalies can cause interruption and thickening of the pleural line; areas of normal lung (with an A line pattern) can be observed at the beginning of the disease or during recovery; small pleural effusions may be observed, while larger pleural effusions are uncommon.
The chest echography is highly indicated for the daily evaluation of the lung picture and the monitoring of therapy. In a prognostic sense we can distinguish two fundamental frameworks: Pattern 1: Beginning interstitial disease with B lines spread also to the anterior fields, usually in the “positive end-expiratory pressure (PEEP) responders”; Pattern 2: Ventilated front areas (A lines), thickened posterior areas (“white lung”), responsive to the pronation.
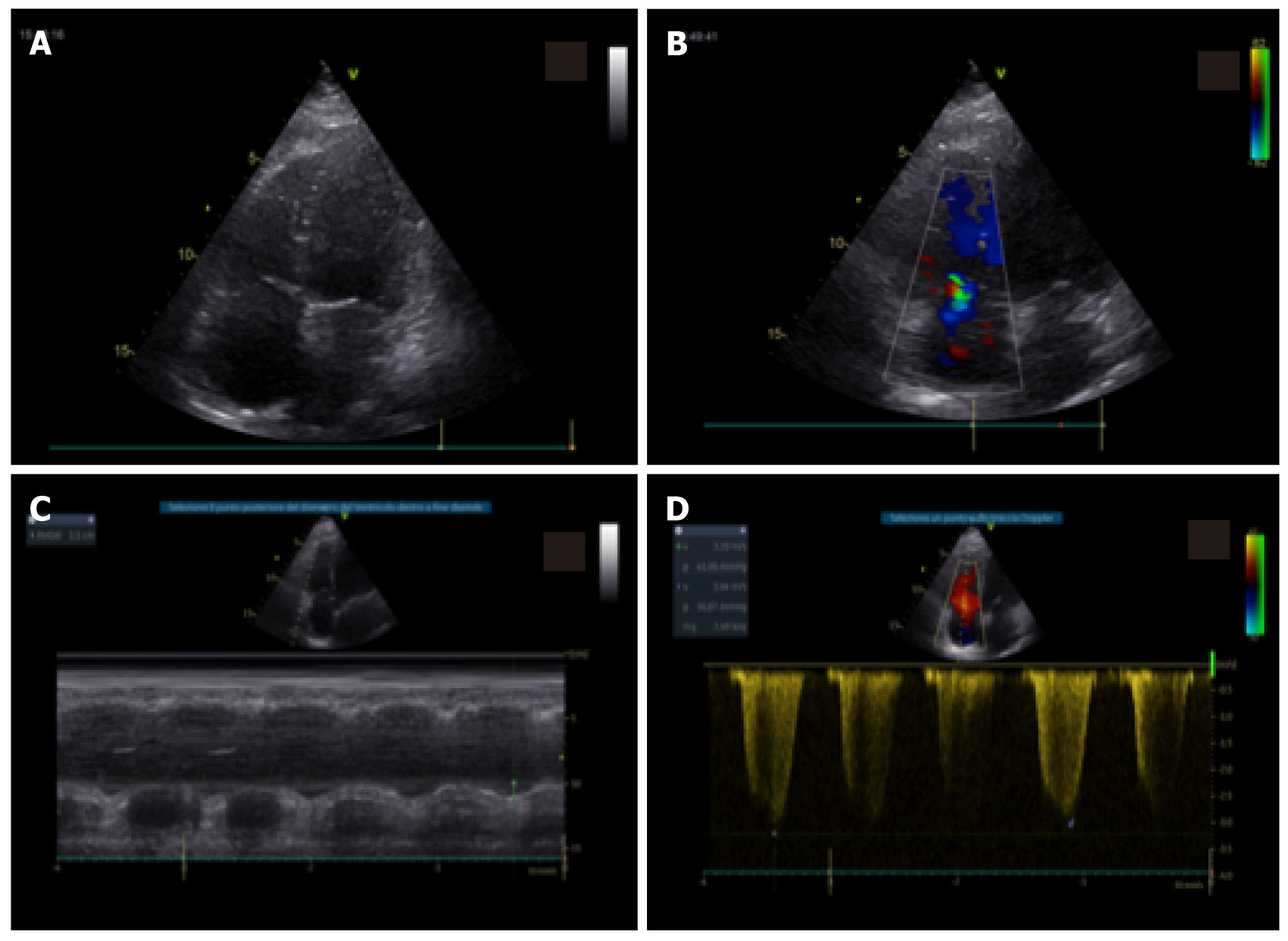
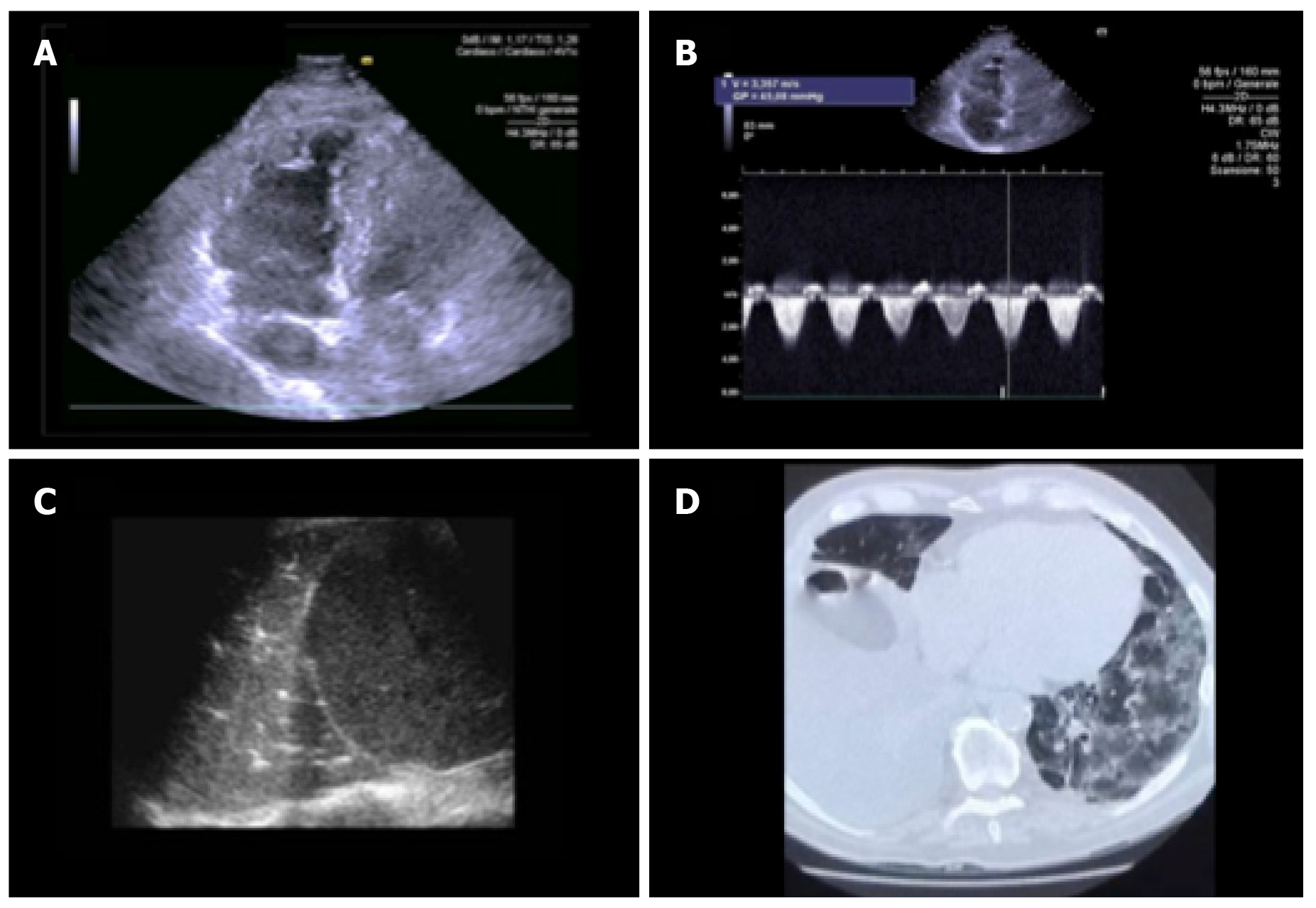
The echocardiogram in this clinical context can be useful in selected cases in detecting regional or global anomalies of the contractility of the left ventricle and in documenting the possible acute overload of the right ventricle, in case of ARDS, especially in the presence of hemodynamic instability. It should be noted that acute myocardial damage (significant increase in troponin or ischemic electrocardiograph abnormalities) was found to be, in two recent analyses with a total of more than 600 patients with established COVID infection, significantly associated with the fatal outcome, while the prognosis of patients with underlying chronic heart disease but without acute myocardial damage was relatively favorable[21]. Inflammation can be a potential determinant mechanism for myocardial injury, and an aggressive therapeutic approach can be considered for patients at high risk of myocardial injury.
The National Society for Hospital Cardiologists like other societies, such as the Italian Society of Cardiology and Italian Society of Cardiovascular Imaging, given the importance of the health phenomenon, has recently disclosed documents aimed at highlighting the importance of a correct procedure and execution of cardiology consultancy and echocardiography, especially in pandemic COVID-19 pneumonia[17]. All cardiological scientific societies believe it is important to ensure the execution of urgent and non-deferrable exams in the patient with hemodynamic instability, maintaining the possibility for the cardiologist to be able to refuse the execution of tests not considered appropriate in his own judgement and favoring the different and possible forms of remote electrocardiograph and imaging consultations, thus limiting access to infected areas.
Even the American Society of Echocardiography recently underlined how it is necessary in COVID-19 infections to select the indications for the echocardiogram, limiting it to urgent patients, to be carried out with personal protective equipment (PPE) and the necessary hygiene rules[22]. In particular, a single echocardiograph should be identified, preferably portable and of reduced dimensions to facilitate sanitation, when possible using probe covers, and the examination should be carried out by a single operator, of sufficient experience (not in training), with a scan that is a good compromise between adequacy and speed of execution (limited ultrasound sections; bimodal responses with high clinical impact; image registration and subsequent “off line” review). Particular attention is then placed on the execution of a possible transesophageal echocardiogram, the procedure itself “generating aerosol” and therefore putting those at greater infectious risk, to be limited in non-urgent indications and to be carried out with complete PPE (e.g., mask at least N95 according to American classification or FFP2 according to the European classification). The need to ensure the training of health personnel on the correct methodologies for wearing and above all removing PPE is also underlined (Figures 4 and 5).
At present, there are no echocardiographic studies conducted specifically in COVID-19 patients. However, the role of the echocardiogram in monitoring biventricular function and septic shock is well known. In the past 10 years, most studies on acute myocardial dysfunction during sepsis have been conducted in patients admitted to intensive care and subjected to mechanical ventilation, using transesophageal echocardiography[23,24]. From these studies it emerged that about 30%-40% of septic patients develop reduction of the ejection fraction of the left ventricle (LV), impaired LV diastolic function and possible impairment of the function of the right sections, which is currently less characterized but present above all during ARDS[25,26]. Another aspect that should be underlined is the definite hemodynamic effect of ventilation on ventricular performance[24]. Positive end-expiratory pressure, increasing lung volumes, can cause an increase in pulmonary vascular resistance (PVR) due to the compression of alveolar and extra-alveolar capillaries, with consequent increase in afterload and right ventricle (RV) volume, shift of the interventricular septum to the left and consequent reduction of compliance and filling of LV. However, since the increase in PVR is countered by the elimination of hypoxia-induced vasoconstriction, the net increase in PVR becomes relevant only for high PEEP values. The increase in intrathoracic pressure also determinates an increase in the resistance of the intrathoracic veins, a mechanism that reduces the gradient and venous return for RV, especially in conditions of true hypovolemia (anemia, dehydration, etc. in a healthy heart) or relative hypovolemia (severe RV dysfunction). This phenomenon, however, is counterbalanced by the increase in abdominal pressure due to the lowering of the diaphragm with the effect of “squeezing” on the liver and the spleen. Therefore, in clinical practice, the potential negative hemodynamic effects of PEEP (hypotension secondary to reduced preload and increased RV afterload) are mainly confined to patients highly dependent on preload and can be partially balanced by carrying out a preventive volemic filling[27].
The sensitivity of chest X-ray in reported COVID-19 cases was 59%, compared to 86% for lung CT, especially in detecting subtle opacities. The real sensitivity of pulmonary ultrasound in this context is not clearly defined. It will depend on several factors (in particular the severity of the disease, the presence of obesity or high acoustic impedance of the chest, the completeness of the scan and the experience of the operator). A complete lung ultrasound examination should exhibit an intermediate sensitivity between CT scan and chest radiography. There is no established data yet, but it is reasonable to extrapolate the experiences in other types of pneumonia. Specificity is certainly low. An irregular B-line or consolidation pattern can be observed in any pneumonia or interstitial lung disease. Therefore, a clinical correlation is absolutely necessary (e.g., evaluation of previous chest imaging studies to evaluate if chronic abnormalities are present). Also, it should be note that hospitalized supine patients may have B-lines and consolidation in a posterior and inferior distribution due to atelectasis. Hence, pulmonary echography can have the greatest sensitivity and specificity among outpatient or first aid patients (Table 1).
However, the serial control of the individual patient could be very useful in the context of respiratory intensive care. The evolution of a focal “white lung” in a “dry lung” with widespread sub-pleural consolidations is an indication of an unfavorable evolution of the pathology, with the consequent need for more aggressive treatment with oral-tracheal intubation rather than non-invasive ventilation. Pulmonary echography also allows checking for the presence of pneumothorax (absence of pleural sliding), new thickenings from bacterial over-infection and pleural effusion, all obviously without having to move the patient.
The integrated clinical, laboratory, radiological and ultrasound approach to the patient’s bed is fundamental in the diagnosis, monitoring and evaluation of the therapy of the patient with severe acute pneumonia due to COVID-19. Thoracic echography is a very important and well developed diagnostic path, especially in Italy, which allows to avoid unnecessary CT scans and, at the same time, highlight and monitor parenchymal damage without moving the patient. CT should not be performed routinely for all patients, because in the first 48 h it can be absolutely negative, and in the late phase the imaging finding may not change the therapeutic approach.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: MD LV S-Editor: Zhang L L-Editor: Filipodia P-Editor: Wang LYT
| 1. | Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med. 2020;382:1199-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11224] [Cited by in RCA: 9319] [Article Influence: 1863.8] [Reference Citation Analysis (0)] |
| 2. | Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8473] [Cited by in RCA: 7602] [Article Influence: 1520.4] [Reference Citation Analysis (0)] |
| 3. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19202] [Cited by in RCA: 18878] [Article Influence: 3775.6] [Reference Citation Analysis (7)] |
| 4. | Wu JT, Leung K, Leung GM. Nowcasting and forecasting the potential domestic and international spread of the 2019-nCoV outbreak originating in Wuhan, China: a modelling study. Lancet. 2020;395:689-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3012] [Cited by in RCA: 2504] [Article Influence: 500.8] [Reference Citation Analysis (0)] |
| 5. | Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wölfel R, Hoelscher M. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med. 2020;382:970-971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2799] [Cited by in RCA: 2491] [Article Influence: 498.2] [Reference Citation Analysis (0)] |
| 6. | Bonow RO, Fonarow GC, O'Gara PT, Yancy CW. Association of Coronavirus Disease 2019 (COVID-19) With Myocardial Injury and Mortality. JAMA Cardiol. 2020;5:751-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 396] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 7. | Xiong TY, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41:1798-1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 493] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 8. | Herold CJ, Sailer JG. Community-acquired and nosocomial pneumonia. Eur Radiol. 2004;14 Suppl 3:E2-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 23] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Albarello F, Pianura E, Di Stefano F, Cristofaro M, Petrone A, Marchioni L, Palazzolo C, Schininà V, Nicastri E, Petrosillo N, Campioni P, Eskild P, Zumla A, Ippolito G; COVID 19 INMI Study Group. 2019-novel Coronavirus severe adult respiratory distress syndrome in two cases in Italy: An uncommon radiological presentation. Int J Infect Dis. 2020;93:192-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 10. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1761] [Article Influence: 352.2] [Reference Citation Analysis (0)] |
| 11. | Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV). Radiology. 2020;295:202-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1827] [Cited by in RCA: 1696] [Article Influence: 339.2] [Reference Citation Analysis (0)] |
| 12. | Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, Ling Y, Jiang Y, Shi Y. Emerging 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 903] [Cited by in RCA: 784] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 13. | Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306-3309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 632] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 14. | Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1728] [Cited by in RCA: 1597] [Article Influence: 319.4] [Reference Citation Analysis (1)] |
| 15. | Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296:E15-E25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1106] [Cited by in RCA: 952] [Article Influence: 190.4] [Reference Citation Analysis (1)] |
| 16. | Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46:849-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 509] [Article Influence: 101.8] [Reference Citation Analysis (1)] |
| 17. | Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, Perlini S, Torri E, Mariani A, Mossolani EE, Tursi F, Mento F, Demi L. Is There a Role for Lung Ultrasound During the COVID-19 Pandemic? J Ultrasound Med. 2020;39:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 18. | Vetrugno L, Bove T, Orso D, Barbariol F, Bassi F, Boero E, Ferrari G, Kong R. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 19. | Orso D, Federici N, Copetti R, Vetrugno L, Bove T. Infodemic and the spread of fake news in the COVID-19-era. Eur J Emerg Med. 2020;27:327-328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 20. | Guarracino F, Vetrugno L, Forfori F, Corradi F, Orso D, Bertini P, Ortalda A, Federici N, Copetti R, Bove T. Lung, Heart, Vascular, and Diaphragm Ultrasound Examination of COVID-19 Patients: A Comprehensive Approach. J Cardiothorac Vasc Anesth. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 21. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2516] [Cited by in RCA: 2842] [Article Influence: 568.4] [Reference Citation Analysis (0)] |
| 22. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3009] [Article Influence: 601.8] [Reference Citation Analysis (1)] |
| 23. | Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Féger F, Rouby JJ. Acute left ventricular dilatation and shock-induced myocardial dysfunction. Crit Care Med. 2009;37:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 24. | Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36:1701-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 384] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 25. | Furian T, Aguiar C, Prado K, Ribeiro RV, Becker L, Martinelli N, Clausell N, Rohde LE, Biolo A. Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality. J Crit Care 2012; 27: 319.e9-319. 15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Parker MM, McCarthy KE, Ognibene FP, Parrillo JE. Right ventricular dysfunction and dilatation, similar to left ventricular changes, characterize the cardiac depression of septic shock in humans. Chest. 1990;97:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 227] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | D'Andrea A, Martone F, Liccardo B, Mazza M, Annunziata A, Di Palma E, Conte M, Sirignano C, D'Alto M, Esposito N, Fiorentino G, Russo MG, Bossone E, Calabrò R. Acute and Chronic Effects of Noninvasive Ventilation on Left and Right Myocardial Function in Patients with Obstructive Sleep Apnea Syndrome: A Speckle Tracking Echocardiographic Study. Echocardiography. 2016;33:1144-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |