Published online Oct 28, 2020. doi: 10.4329/wjr.v12.i10.213
Peer-review started: July 5, 2020
First decision: September 17, 2020
Revised: September 29, 2020
Accepted: October 12, 2020
Article in press: October 12, 2020
Published online: October 28, 2020
Processing time: 115 Days and 10.9 Hours
The importance of fluoroscopy as an imaging modality has been minimized relative to other cross-sectional modalities, including high-resolution computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound. Fluoroscopy examinations have decreased in clinical practice due to reduced appreciation of its usefulness, insufficient training of residents, fewer staff with adequate expertise, and poor reimbursements relative to other modalities. We revisit and build upon the prior literature and history of this decreased utilization. We then seek to prove continued value, through categorized examples and within multiple subspecialties, wherein fluoroscopy plays an integral part toward clinical diagnoses as well as optimizing patient outcomes. This is particularly true for motility and esophageal disorders, where structure and function with real-time evaluation is essential. We additionally show several post-operative cases where the synergy of fluoroscopy with CT and endoscopy is apparent. The fluoroscopic radiologist also has the unique ability to vary patient positioning, as opposed to traditional CT or MRI, where orthogonal views are employed without positional or temporal changes. We turn attention to the modern era, with synergistic and novel cases demonstrating that fluoroscopy remains instrumental toward achieving a diagnosis alongside other modalities. Our cases stress the need to maintain expertise in fluoroscopy skill, and underline its continued importance in residency training programs. We conclude that fluoroscopy is a relatively inexpensive modality that is often under-appreciated in diagnostic radiology. We suggest that competency in fluoroscopy is crucial for future generations of radiologists to both work with their peers, as well as to aid clinicians in the optimal treatment of patients.
Core Tip: Although the use of fluoroscopy has diminished with the advent of high-resolution computed tomography (CT), magnetic resonance imaging (MRI) and ultrasound, we show how fluoroscopy is essential in the diagnostic algorithm and should remain an integral part of residency training. We demonstrate in this review that fluoroscopy remains an essential modality, and build upon and reference prior concerns that outline the challenges facing fluoroscopy. We address such concerns with an optimistic outlook and case examples. We not only highlight the routine uses of fluoroscopy, but more significantly state that fluoroscopy now works synergistically with CT, MR, and other cross-sectional modalities, even in the modern age.
- Citation: Shalom NE, Gong GX, Auster M. Fluoroscopy: An essential diagnostic modality in the age of high-resolution cross-sectional imaging. World J Radiol 2020; 12(10): 213-230
- URL: https://www.wjgnet.com/1949-8470/full/v12/i10/213.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i10.213
Fluoroscopy remains a valuable modality, even in the age of high-resolution cross-sectional imaging modalities such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound. It is a unique and essential tool within diagnostic radiology, and must remain an integral part of practice and residency training.
We review the past literature alongside the nature of fluoroscopy itself, beginning with the routine use of fluoroscopy as an initial diagnostic modality, underlining its utility even in the age of alternative imaging techniques. We then build upon concern expressed in the past literature regarding the apparent decline of fluoroscopy. We then present several cases in which fluoroscopy acts as an adjunct to other cross-sectional modalities, and facilitates the determination of a correct diagnosis. Finally, we show more novel examples of pathologies discovered using fluoroscopy, without which a relevant finding may have been entirely undiagnosed. Our goal is to review and reaffirm the essential role of fluoroscopy in all such categories, while reviewing the state of the modality in current radiology practice.
As described in a 2009 review by Levine et al[1], the volume of fluoroscopic examinations (particularly barium studies) has been declining for decades. This trend has been partially attributed to the increasing availability of advanced cross-sectional imaging and interventional modalities such as CT, MRI, and endoscopy. Specifically, concern was raised for the future of barium fluoroscopy in that review, with two possible end scenarios presented: The first was the eventual obsolescence of the gastrointestinal (GI) fluoroscopic examination; the second scenario was the possible stabilization and/or increase in the utilization of fluoroscopy as a cost-effective diagnostic examination[1], which relates to the objectives in this review.
There are several explanations for the apparent decline of fluoroscopy. The first is a plethora of other imaging options: modalities such as CT, MRI, and ultrasound are now used in tandem with manometry, endoscopy, and even direct clinical intervention (such as with empiric medication) particularly for GI complaints like epigastric pain or dyspepsia. Abdominal CT, focused ultrasound, and MRI enterography may now visualize pathologies previously seen with a fluoroscopic small bowel series. Even the realm of medical screening now utilizes more advanced cross-sectional imaging, with CT colonography a prime example (although public awareness of this procedure still remains under discussion, and is still considered “low” at near 1% utilization)[2].
It is not only the availability of additional modalities that poses a challenge to fluoroscopy. Prior reviews demonstrate that economic factors1 play a large part, as fluoroscopic examinations bring lower reimbursements than CT or MRI[3], particularly as based on the relative value unit (RVU) system[4]. Additionally, many emerging professionals may deem fluoroscopy as a “lower tech” form of imaging, as compared to MRI or CT. Thus, it has become increasingly difficult to find younger radiologists interested in performing these examinations. Additionally, radiology programs have not sufficiently emphasized the importance of fluoroscopic procedures. Finally, fluoroscopy is an operator-dependent modality, and unlike others (such as CT and MRI) where a technologist is primarily responsible for image acquisition, it is often the radiologists themselves that must both perform and interpret a fluoroscopic examination.
In the 2009 review, the above was noted to have negative repercussions on radiology residency training in the “art” of fluoroscopy. The number of faculty and fellows assigned to GI fluoroscopy is often lower than that of body CT, ultrasound, or MRI. Residency training may be limited to several weeks in the first year, and then afterwards only during the pediatric and night call resident rotations. As noted, insufficient training often results in the “the blind leading the blind” among senior to junior residents, which subsequently compounds errors. This is particularly a challenge when many fluoroscopic examinations are performed by trainees. In a retrospective analysis of 17966 examinations over 5.5 years, trainees had significantly more radiation exposure than that of faculty[5]. The investigators also felt that better awareness and understanding of that discrepancy may aid training programs in developing benchmarks, protocols, and focused teaching in the safe use of fluoroscopy for patients and operators.
By its very nature, fluoroscopy remains a hands-on experience, requiring direct training in the fluoroscopy suite. Faculty with working knowledge of the “applicable medical physics, patient positioning, and techniques for optimal visualization of abnormalities on single and double contrast examinations”[6] are an essential aspect of a well-rounded curriculum. Patient safety requires at least a working knowledge of fluoroscopic technique. Leaders and faculty within departments must believe in the intrinsic value of the modality, while learners must remain enthusiastic, with a curriculum that is clear and thorough.
In this review, we discuss basic fluoroscopy and present select cases of the modality working in tandem with other modalities. Our goal is not to preserve the modality simply to “keep it alive”. We must also recognize its unique benefits in radiology practice even today.
First, and most significantly, a benefit of fluoroscopy is its ability to obtain a real-time evaluation with true temporal resolution, including the ability to reposition a patient during an exam, which is often not available on traditional CT or MRI. Second, fluoroscopy exposes patients to potentially lower doses of ionizing radiation than CT, with the average dose approximately 10-50 mGy/min for normal fluoroscopy with a typical fluoroscopy suite setup[7] and with total exam times often under 1 minute. This is compared to the approximately 12 mSv effective dose for a sample CT abdomen/pelvis, and up to 24 mSv for a multiphase CT examination of the same region[8]. Third, fluoroscopy provides the ability for focused and functional examination of a particular region of interest. Lastly, contrast administration with agents including barium and non-ionic compounds can be evaluated in real time.
On the technical side, novel techniques have also been proposed to facilitate assessment on sequential studies and for different RIS/PACS systems that will reduce ambiguities and misinterpretations, such as real-time labeling of sequential fluoroscopic swallowing studies[9].
For the purposes of this review, we have categorized select fluoroscopic cases to include: (1) Those cases in which fluoroscopy is performed routinely per protocol, as an initial or mainstay modality (such as in the workup of esophageal abnormalities, post-operative gastrointestinal examinations, or in the pediatric setting where CT is less preferred due to radiation dose); (2) Those cases in which fluoroscopy provides synergistic value to other modalities such as CT in achieving diagnoses, therefore showing its enduring utility; and (3) Several illustrative cases in which a novel diagnosis is made with fluoroscopy, underlining its unique properties.
Routine use of fluoroscopy (i.e., as an initial workup or preferred diagnostic modality) is used to show both structural and functional anatomy. This is the solid basis for the use of fluoroscopy in the clinical setting, and it accentuates the utility of fluoroscopy remaining part of any radiology practice and residency curriculum.
The full range of fluoroscopic pathologies and radiologic findings are well described elsewhere. Levine et al[10] for example, have previously shown the multitude of structural and morphologic abnormalities delineated on routine esophageal fluoroscopic examinations. While the entirety of such cases is beyond the scope of a review, “routine” fluoroscopy in radiology departments performed today is as relevant now as it was for Levine and colleagues in 2009.
Fluoroscopy, besides being an adjunct to other higher-cost modalities, is by itself an excellent diagnostic tool appropriate for the elucidation of both benign and malignant esophageal pathology, as well as for the pre- and post-operative evaluation of patients. It is useful for functional as well as structural esophageal evaluation, with the esophagram and upper GI series (UGI) remaining both noninvasive and relatively inexpensive. Exams may be performed with or without the assistance of a trained speech pathologist, and in that setting, may also be used to elucidate a variety of abnormalities[11]. In addition, fluoroscopic evaluation may show morphologic abnormities in place of endoscopy[12], or perhaps demonstrate pathology such as gastroesophageal reflux in the place of pH monitoring[13]. We show the utility of routine fluoroscopy as an initial-choice modality for esophageal disorders. Particularly in the on-call setting, familiarity with the spectrum of esophageal disorders seen fluoroscopically should be required for both residents and radiologists in current practice.
Achalasia: Primary achalasia occurs idiopathically, and secondary achalasia is caused by external factors, including malignancy. It is defined as an incomplete relaxion of the lower esophageal sphincter (LES) with absent/decreased esophageal peristalsis. Primary achalasia, in particular, is seen on UGI examination by a characteristically dilated esophagus with a tapered, beak-like narrowing at the distal aspect (Figure 1).
Esophageal pseudodiverticulosis: Esophageal intramural pseudodiverticulosis is defined by small outpouchings within the esophageal wall, with many causative factors to include chronic esophagitis, gastroesophageal reflux, strictures, and malignancy (Figure 2).
Malignancy and stricture: Fluoroscopy may be utilized to visualize an esophageal malignancy, as well as to follow up a stricture in the setting of treatment. The esophagram is a useful tool for detecting stricture[14], as well as to differentiate between a begin and malignant stricture[15]. Figure 3 show a case of a 64-year-old male with squamous cell carcinoma of the esophagus and long-segment stenosis, subsequently treated with stent and chemotherapy, and ultimately developing a more prominent stricture, easily visible on serial fluoroscopic examinations.
The utility of real-time evaluation—a non-propulsive esophagus with incomplete passage of ingested material: A nonpropulsive esophagus is presented in Figure 4, demonstrating the value of real-time and functional evaluation. Incomplete passage of a barium tablet is seen, with only a narrow channel later visualized at the LES after oral contrast. These findings were found to be compatible with secondary achalasia, with the patient undergoing a successful peroral endoscopic myotomy (POEM) procedure. CT was not at all necessary for this diagnosis, and would have been of limited value toward the establishment of decreased peristalsis as compared to a functional examination.
Fluoroscopy is routinely used as an initial modality in the post-operative setting. This may be performed to evaluate for complications, including post-operative leak (Figure 5), fistula formation, and/or those sequalae which may present in the less acute setting, such as patients who are status post fundoplication[16]. The UGI examination is commonly used to this end. While CT may show findings such as extraluminal gas as a sign of perforation, fluoroscopic studies are more ideal for demonstrating the site and location of leaks[17] and of possible recurrence. Other conditions, such as gastro-gastric fistula formation, can indeed be seen in the post-operative setting, although they are discussed in a later aspect of this review for case-related reasons.
While it has been shown by Xu et al[18] that computed tomography may be superior to fluoroscopy in specific post-operative cases, fluoroscopy remains essential in an initial evaluation, often prior to CT.
In that regard, the actual “hospital course” of a radiologic workup must also be considered in the post-procedural setting. Immediate evaluation and/or stat requests from the emergency department to confirm gastric tube placement (including the gastrostomy and gastrojejunostomy subtypes) in both the pediatric and adult populations are essential fluoroscopic examinations[19]. While it is well known that alternate radiographic images may be utilized for gastric tube placement, fluoroscopy remains ideal (1) in order to evaluate real-time findings (including those that are incidental); and (2) to better evaluate for anatomic localization as well as possible and highly concerning gastric outlet obstruction (GOO) which may occur in the setting of G-tube placement[20].
As these studies may be requested from both the ED and pediatric/GI services, residents (particularly those on call) and practitioners must have training. This includes protocolling for appropriateness, as well as actual performance and interpretation.
Also known as a videofluoroscopic swallowing study (VFSS), the modified barium swallow (MBS) is often considered the procedure of choice for swallowing evaluations and evaluation of associated pathology, primarily because it permits the functional visualization of different consistencies of contrast bolus flow in relation to structural movement throughout the upper aerodigestive tract and in real-time[21]. Additionally, sequential VFSS exams are used to evaluate a patient’s progress if there is a known finding. The use of different per-oral (PO) consistencies, combined with the real-time ability to evaluate for aspiration and muscular dysfunction using fluoroscopy, is intrinsic and therefore unique to this modality (Figure 6).
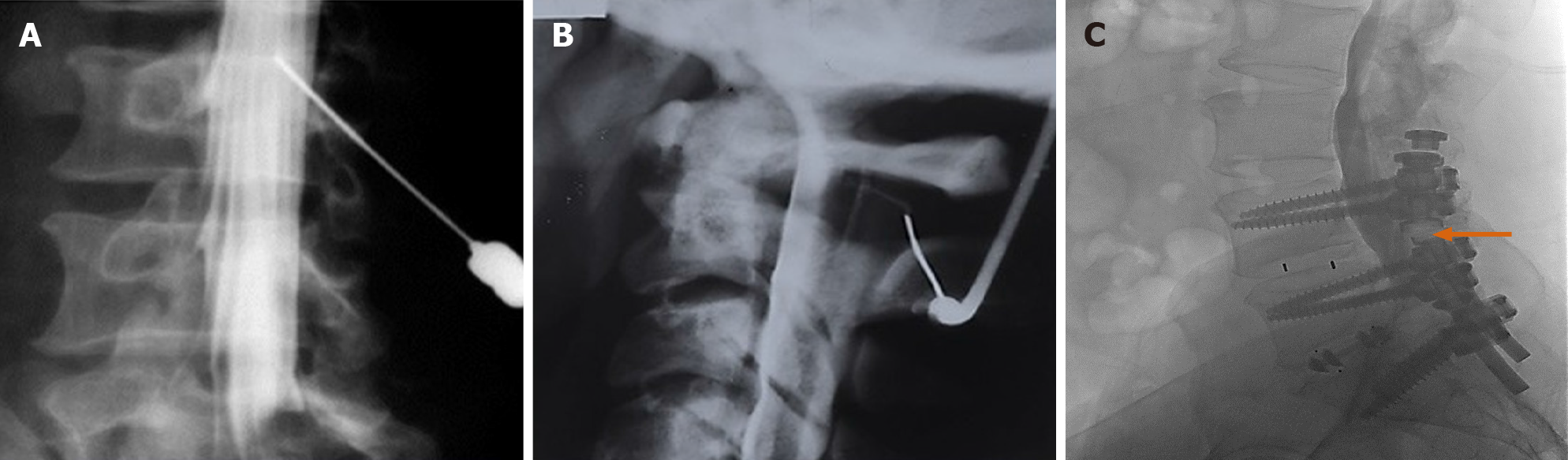
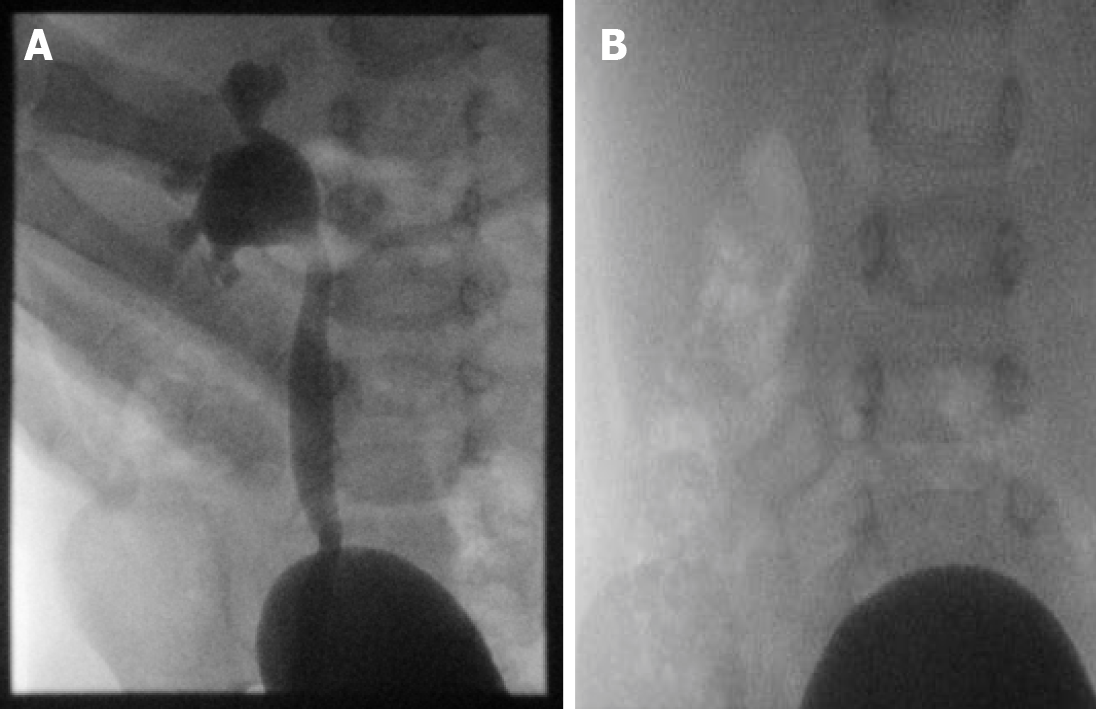
Outside of the gastrointestinal tract, fluoroscopy is a first step in CT myelography and remains an invaluable tool in the evaluation of cerebrospinal fluid (CSF) leaks or nerve root avulsions, even in the age of MRI[22]. Real-time fluoroscopy is used to guide lumbar or cervical needle placement for the subsequent introduction of myelographic contrast into the intra-thecal space, and to monitor that same contrast administration in real-time (Figure 7). Additionally, because surgical hardware can cause significant artifact on MRI, myelography is still the gold standard for evaluation of the post-operative spine after hardware fusion. As training programs increasingly employ residents on neurologic lumbar puncture rotations, this remains an essential skill.
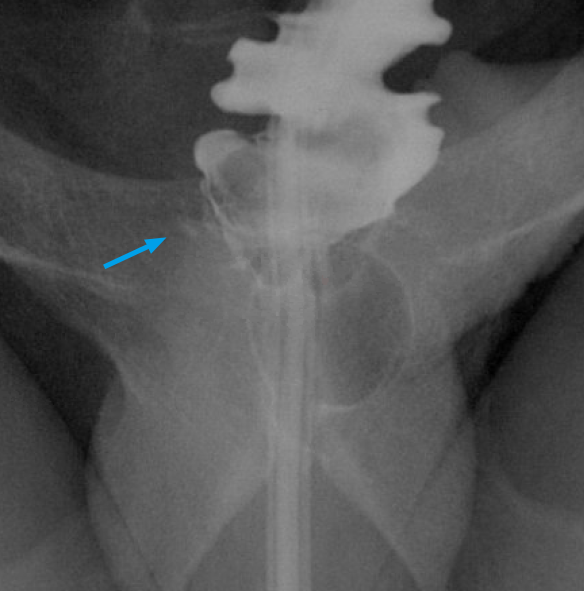
Hysterosalpingography (HSG) remains an important fluoroscopic procedure in the investigation of infertility, especially tubal patency (Figure 8). Essure® placements (note: No longer on the market) and their subsequent complications have been appropriately evaluated with fluoroscopy. It has been shown that HSG demonstrates the morphology of the uterine cavity and the patency of the fallopian tubes, with a variety of abnormalities visible that would not be so easily seen on other modalities[23]. HSG is considered to have a high sensitivity and specificity for the evaluation of tubal patency, shown in a prospective series to 92.1% and 85.7%, respectively[24].
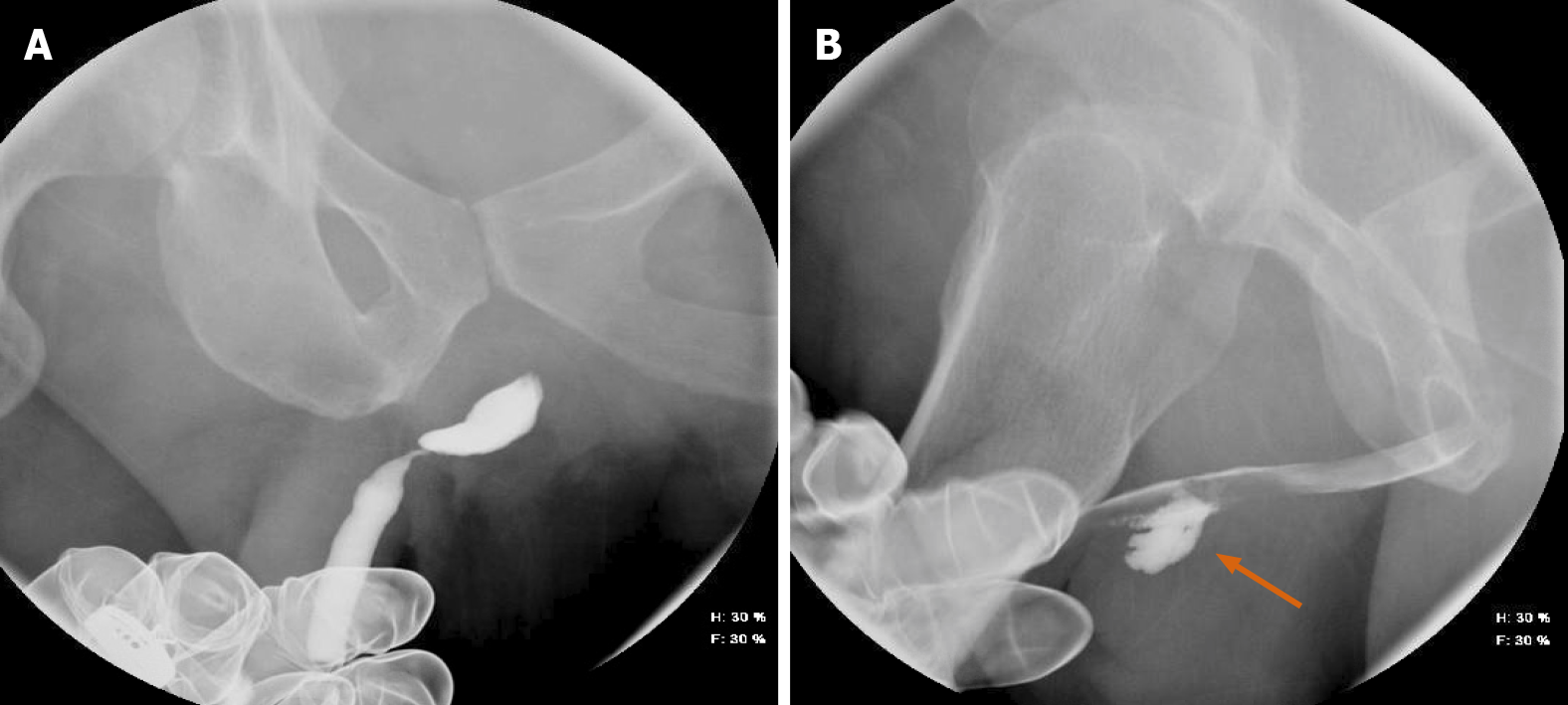
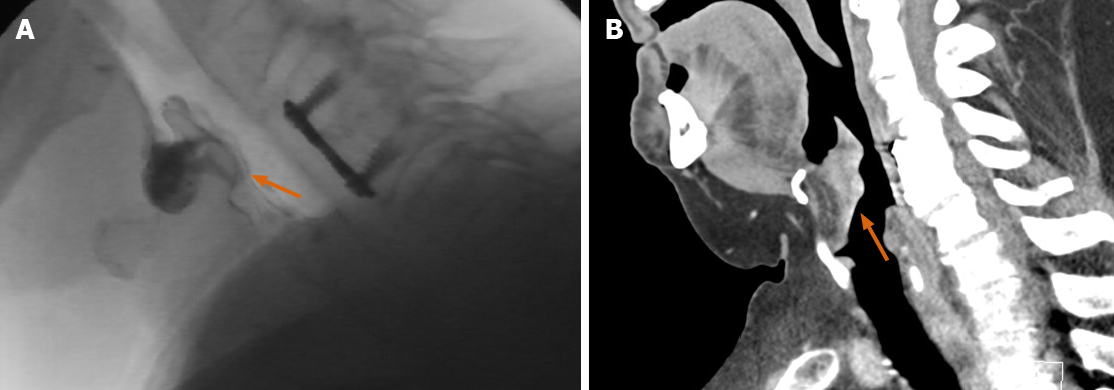
Retrograde urethrocystoscopy is invaluable for evaluating structural abnormalities such as urethral strictures, as well as post-operative complications such as leaks (Figure 9).
Dose amounts are of strong consideration in the pediatric settings, and CT is therefore not a preferred modality. In addition to a range of UGI examinations (for the evaluation of esophageal reflux, tracheoesophageal fistula, intestinal malrotation, and other disorders), pediatric examinations may include fluoroscopic small bowel series, diagnostic and/or therapeutic barium enemas (for example, for the evaluation of Hirschsprung’s Disease), voiding cystourethrograms, and more “interventional” therapeutic applications, such as the acute reduction of an ileocolic intussusception or enteric tube placement. Residents and attending staff should be competent in performing these procedures. Several cases are demonstrated:
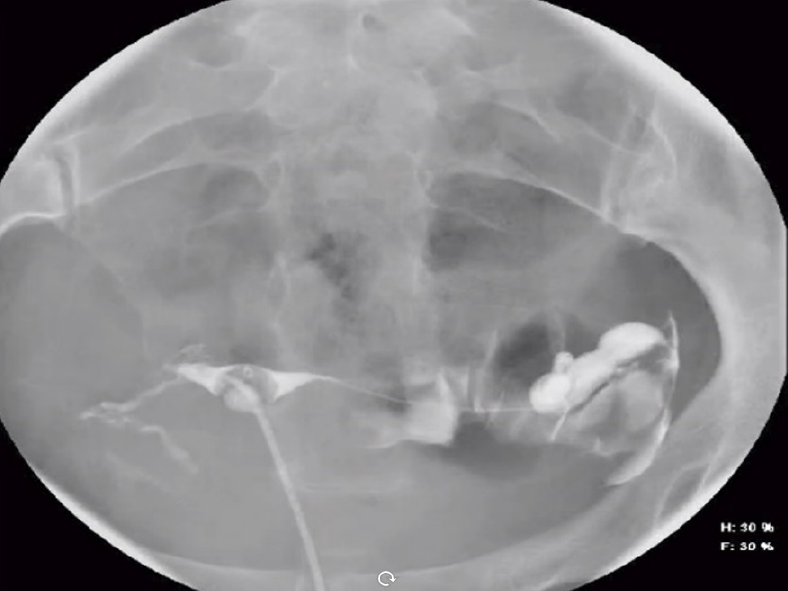
Voiding cystourethrogram: The fluoroscopic voiding cystourethrogram (VCUG) is often used in combination with ultrasound in the evaluation of reflux, persistent urinary tract infection (UTI), duplication of the collecting systems, and abnormal bladder morphology. Functional evaluation is key, and while it has been shown that non-fluoroscopic imaging modalities in the evaluation of vesicoureteral reflux (such as the Radionuclide Cystogram, or RNC) may also be useful from a functional perspective, it is also accepted that for the initial evaluation of anatomy, and particularly the grading of reflux, fluoroscopic VCUG is preferred, (although ultrasound may be gaining increased usage)[25]. Given the prevalence of reflux in children, especially among those children with concurrent UTI[26], the utility of fluoroscopy in this setting cannot be overemphasized (Figure 10).
Tracheoesophageal fistula: Tracheoesophageal fistula (TEF) represents one of the most common congenital anomalies seen in major pediatric surgical centers[27]. Specifically, we present a case of a 10-mo-old female showing a fistulous tract between the proximal esophagus and proximal trachea, located above the carina. Upon fluoroscopic evaluation, contrast was seen to follow the tract, and then to strikingly outline both the proximal trachea as well as the right and left mainstem bronchi. Findings were compatible with an H-type fistula. Strong note is made that repositioning with real-time fluoroscopy in both lateral and supine positions assisted in this diagnosis. (Figure 11).
Intestinal malrotation: Intestinal malrotation is a congenital anomaly resulting in the incomplete rotation of the fetal intestine around the axis of the superior mesenteric artery. Symptoms usually present in early childhood, and diagnosis is often made in the pediatric radiology setting. Fluoroscopic upper gastrointestinal series is the most commonly used investigative modality for the diagnosis of malrotation and midgut volvulus, with proper fluoroscopic technique (such as positioning) essential to maximize diagnostic accuracy[28]. Common fluoroscopic findings include (1) the duodenojejunal (DJ) junction failing to cross the midline of the left-sided vertebral pedicle, (2) the DJ junction lying inferior to the duodenal bulb, and (3) segments D2 and D3 of the duodenum not seen to lie posteriorly in the expected retroperitoneal position. We present as case of 4-wk-old male, status post gastroschisis repair, and unable to feed. UGI series was performed, and easily identified the classic features of malrotation of the small bowel, with the duodenal structures appearing to the right of the midline. Follow up examination showed similar findings 3 d later (Figure 12).
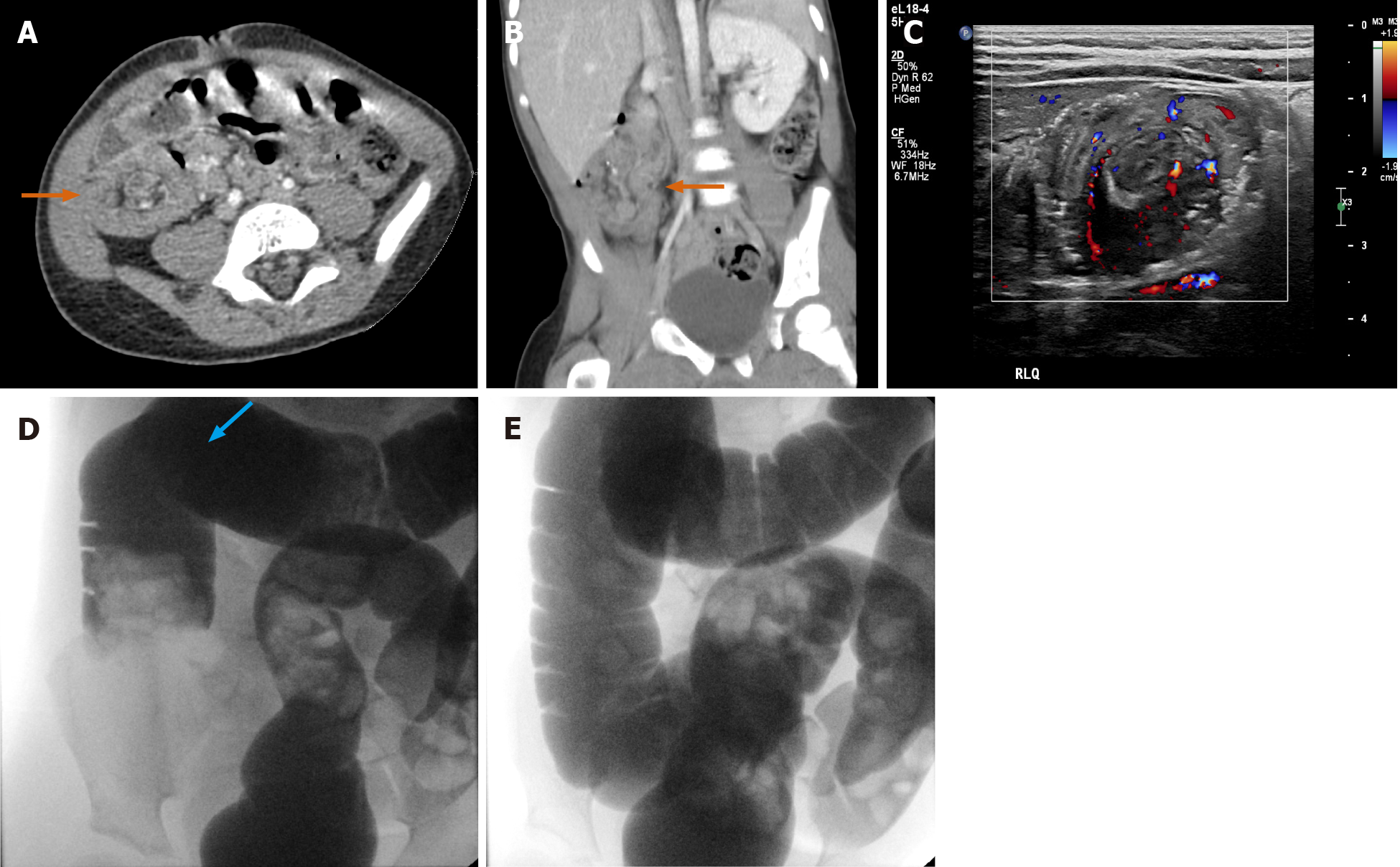
Intussusception Reduction: With intussusception, the real-time nature of fluoroscopy, as well as the skill of the operator, is of paramount importance. Intussusception is a common pediatric emergency and fluoroscopy-guided (often hydrostatic) reduction is an equally common nonoperative management strategy for treatment[29]. Multiple modalities are often used toward diagnosis and treatment, requiring the multimodal skill of sometimes a single operator (such as a resident on night call) to understand findings across CT and/or ultrasound, and ultimately fluoroscopy: including the images showing successful intervention and reduction.
We present a night call case of a 2-year-old male with ileocolic intussusception, first seen on outside CT, then confirmed on in-house ultrasound, and finally reduced (overnight) using fluoroscopically guided intervention. (Figure 13).
Such cases strongly reflect the importance of fluoroscopy in residency training. Here, the overnight resident was involved across all modalities, and without the proper fluoroscopy training, would have been of limited value in the case of a classic and hazardous pediatric emergency.
The cases of basic fluoroscopy discussed above, shown across several anatomic systems, illustrates that the modality still has strong basic utility in the current age, and confirms that ongoing and concrete instruction in the protocolling, performance, and interpretation of routine procedures must be considered both by practitioners and by radiology training programs.
While the previous categorization applied to the more basic or routine uses of fluoroscopy in the age of CT, MRI, and ultrasound, we will now build upon the concerns of prior reviews and focus now on how fluoroscopy is used synergistically with these increasingly utilized modalities. The future of fluoroscopy depends on such synergistic use, and we underline the importance of the practitioner’s understanding that fluoroscopy may be called upon to further elucidate findings in these settings.
Certain cases, such as gastro-gastric fistula (and the case of caustic ingestion, discussed later), may also be seen as an initial fluoroscopic workup. They are discussed here as they reflect a more pertinent case-specific relationship with ancillary modalities.
Multimodal cases include those in which initial (often cross-sectional) imaging was either insufficient or unsuccessful in establishing a diagnosis. In many of these cases, the utility of fluoroscopy, primarily with the unique ability to analyze in real-time as well as reposition a patient during an exam is illustrated.
Initial CT evaluation from the ED may be limited. This may be related to an undesired initial patient position or incorrect initial CT protocol. Failure of contrast bolus timing may also be a culprit during emergencies. In these cases, repeat CT may not be possible (due to dosage issues, allergies, etc.), and often a real-time component (where such would assist toward the diagnosis) is also not performed. In such cases, a causative diagnosis for a patient may not be initially established, and further evaluation with fluoroscopy is required. It is also important to note that oral (PO) contrast is not routinely used on many initial CT protocols, especially in the ED[30]. The reasons are varied and beyond the scope of this discussion; however, in cases where diagnosis is in doubt (particularly in GI cases), PO contrast for further evaluation will be needed in some form. The practitioner’s choices will therefore include repeat CT with PO contrast, or perhaps real-time fluoroscopy of the area in question.
We demonstrate, through several cases, the utility of fluoroscopy as an adjunct toward achieving a diagnosis not initially made on preliminary cross-sectional imaging, and build upon previous reviews expressing concern in this regard.
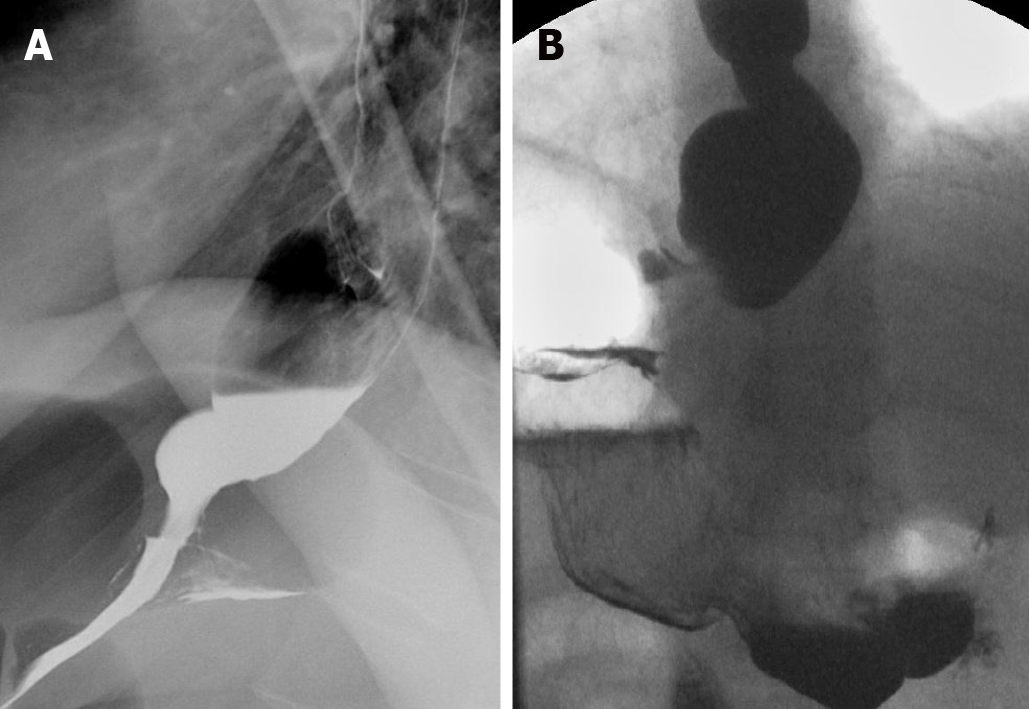
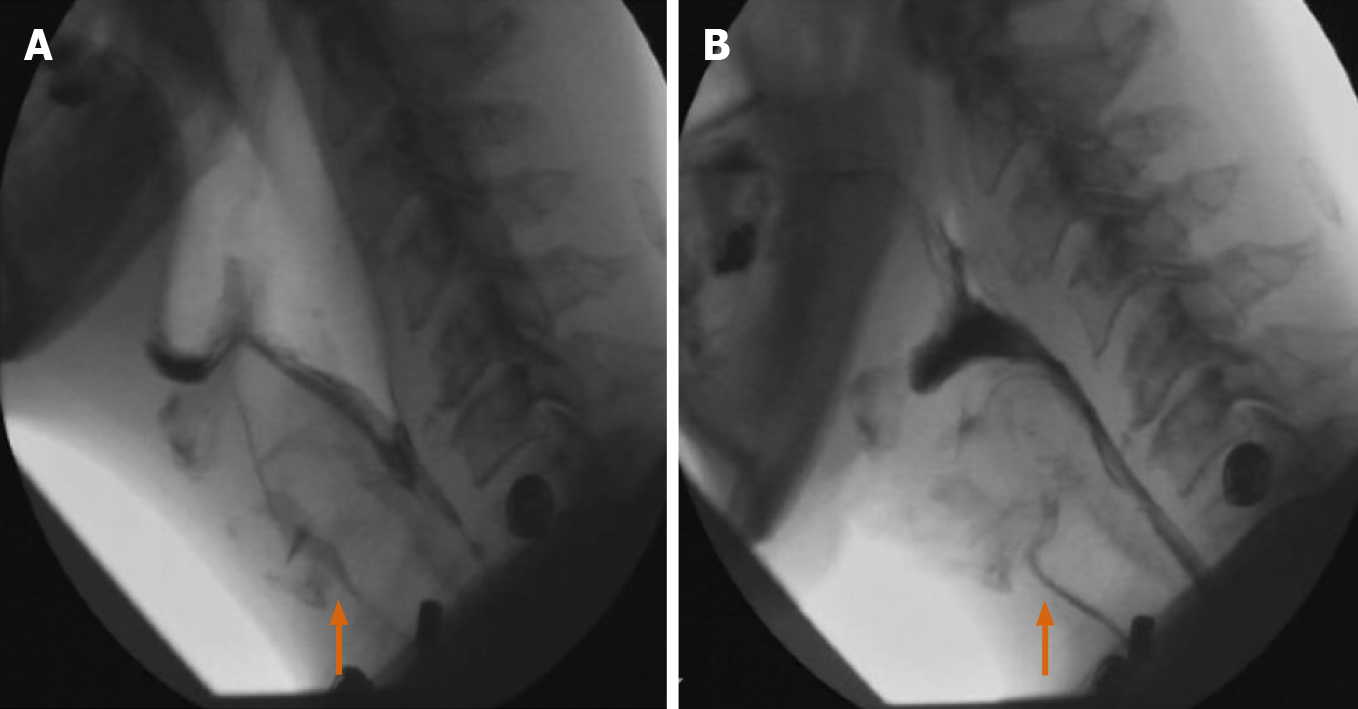
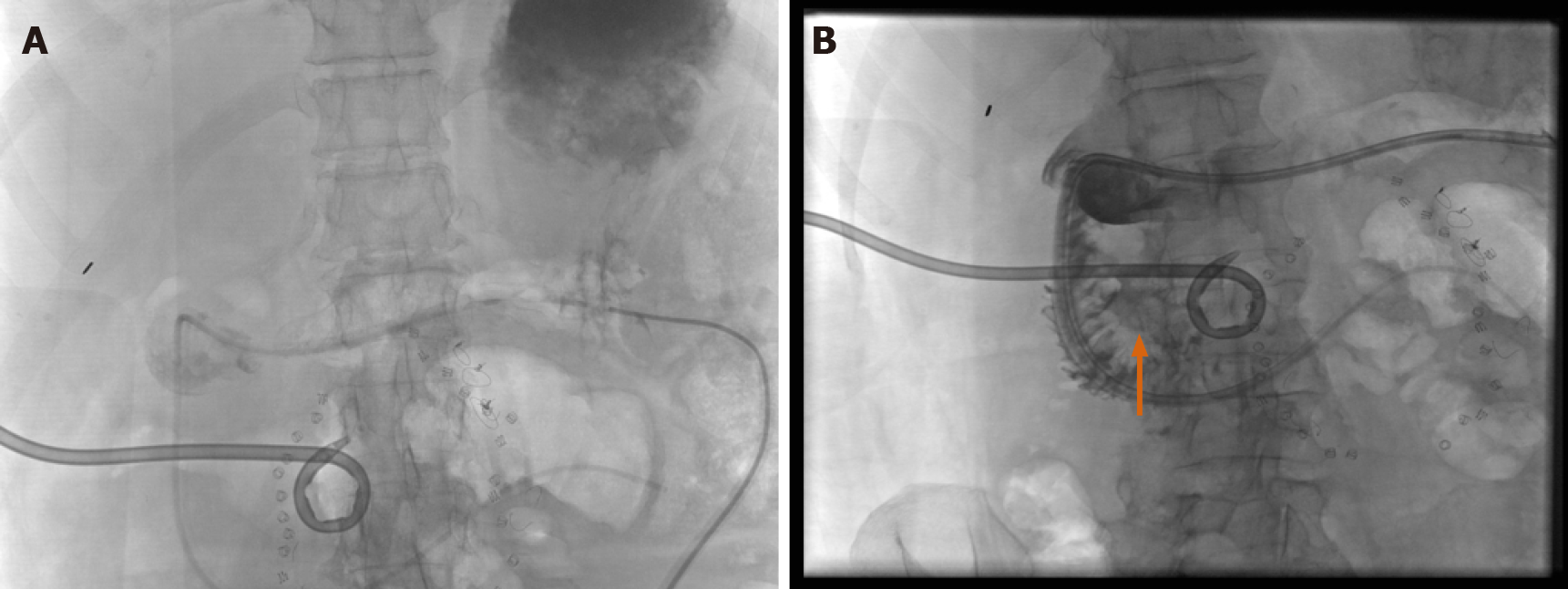
The supine position is most commonly utilized in CT. However, real-time repositioning of the patient under fluoroscopy is commonly used to see otherwise unnoticed abnormalities. The ability to reposition a patient to additional positions (such as lateral, etc.) is illustrated through a sample case (Figure 14A and B) showing a fluoroscopically discovered fistulous communication with anterior abscess in the duodenal sweep, notably not initially seen on CT.
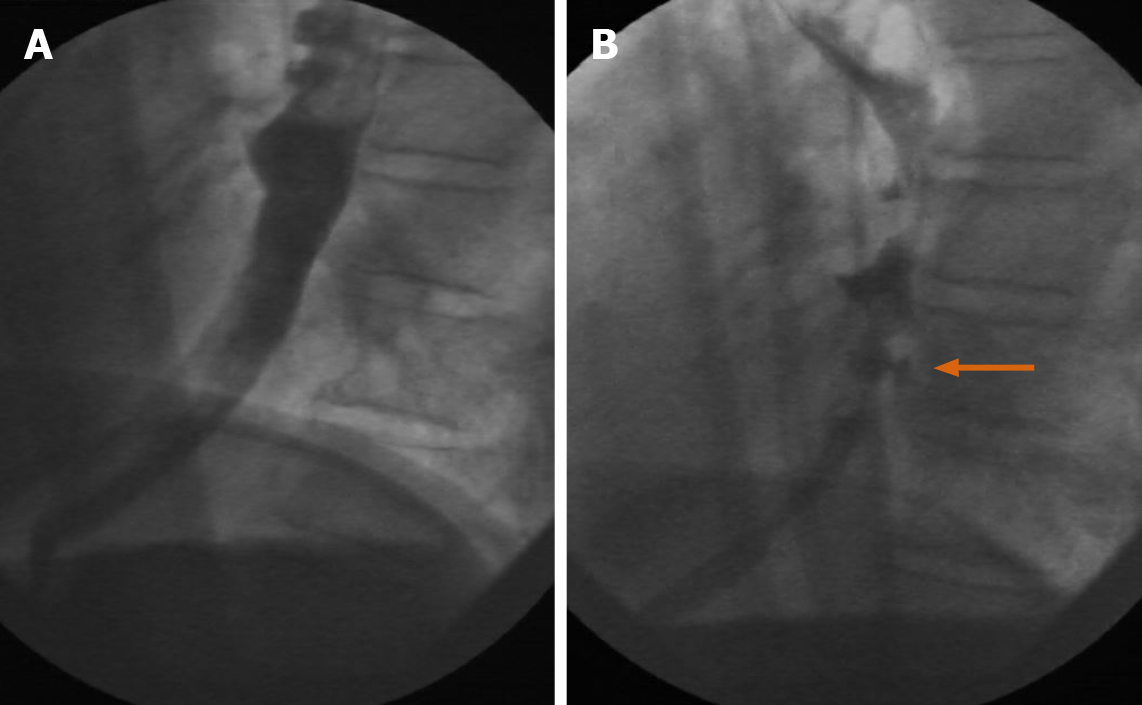
Gastrointestinal leak is a known complication after both gastric bypass (GB) and sleeve gastrectomy (SG). The reported incidence, in a large published case series of open and laparoscopic cases, is between 0.1% and 8.3% after GB and 0% and 7% after SG[31]. Specifically, enteric leakage was shown to remain as a significant risk after Roux-en-Y gastric bypass, with a retrospective analysis showing that 5.25% of patients developed a leak out of a total of 400, and with differing treatment approaches[32]. CT and fluoroscopy often work in tandem toward the evaluation of such leaks. In those cases where initial CT was performed and read as negative, fluoroscopy remains invaluable toward a quick and focused method to elucidate the diagnosis. Figure 15 therefore demonstrate a case of a 34-year-old female patient status post gastric sleeve procedure, complicated by a leak and converted to a Roux-en-Y bypass. Ongoing leak was seen following this procedure, with later attempt at an open revision of the gastrojejunostomy. Initial CT with IV contrast was read as negative. However, follow-up fluoroscopy with steep oblique and delay imaging elucidated contrast passing into an aerated structure seen just below the diaphragm, confirming a contained leak.
Gastro-gastric fistula occurs in up to 6% of Roux-en-Y gastric bypass procedures, with multiple theories for their formation. They may result as a technical complication from the incomplete division of the stomach during the creation of the pouch, or perhaps occur after a staple-line failure, then developing a leak with an abscess, which then drains into the distal stomach forming the fistula[33].
In cases of gastro-gastric fistula, even CT with PO contrast may not make a definitive diagnosis, due to the specific bolus timing and repositioning requirement. For CT, it has been shown that when performed properly, the relative attenuation ratio of oral contrast in the excluded stomach vs the gastric pouch on imaging may be a reliable tool in differentiating GG fistula from oral contrast reflux. After taking relative attenuation ratios into account in the excluded stomach and gastric pouch, radiologists' final conclusions were seen to achieve higher sensitivity (58.3%) and specificity (100%)[34]. However, PO contrast is sometimes not utilized in the initial workup, and also must be performed with correct bolus timing. If either is deficient, alternative modalities such as fluoroscopy serve as excellent and often quick ancillary examinations to cross-sectional imaging.
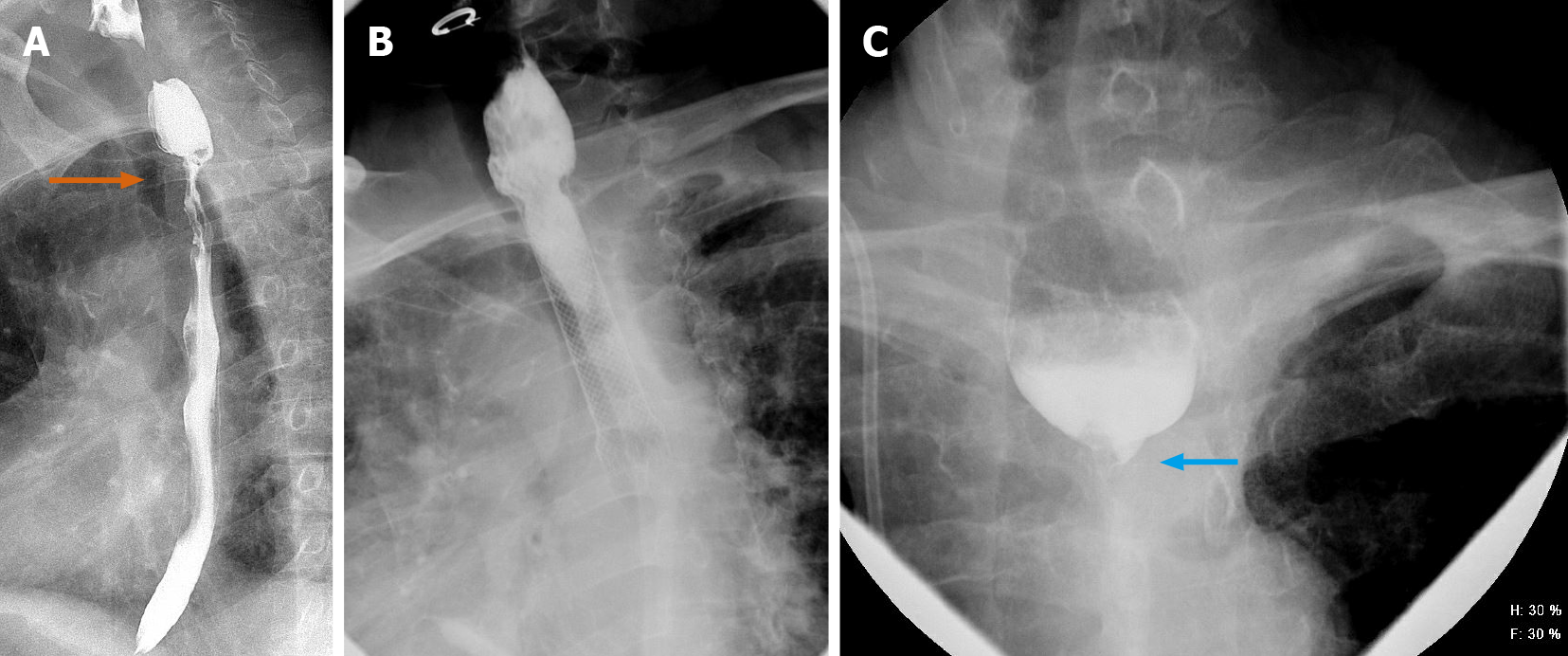
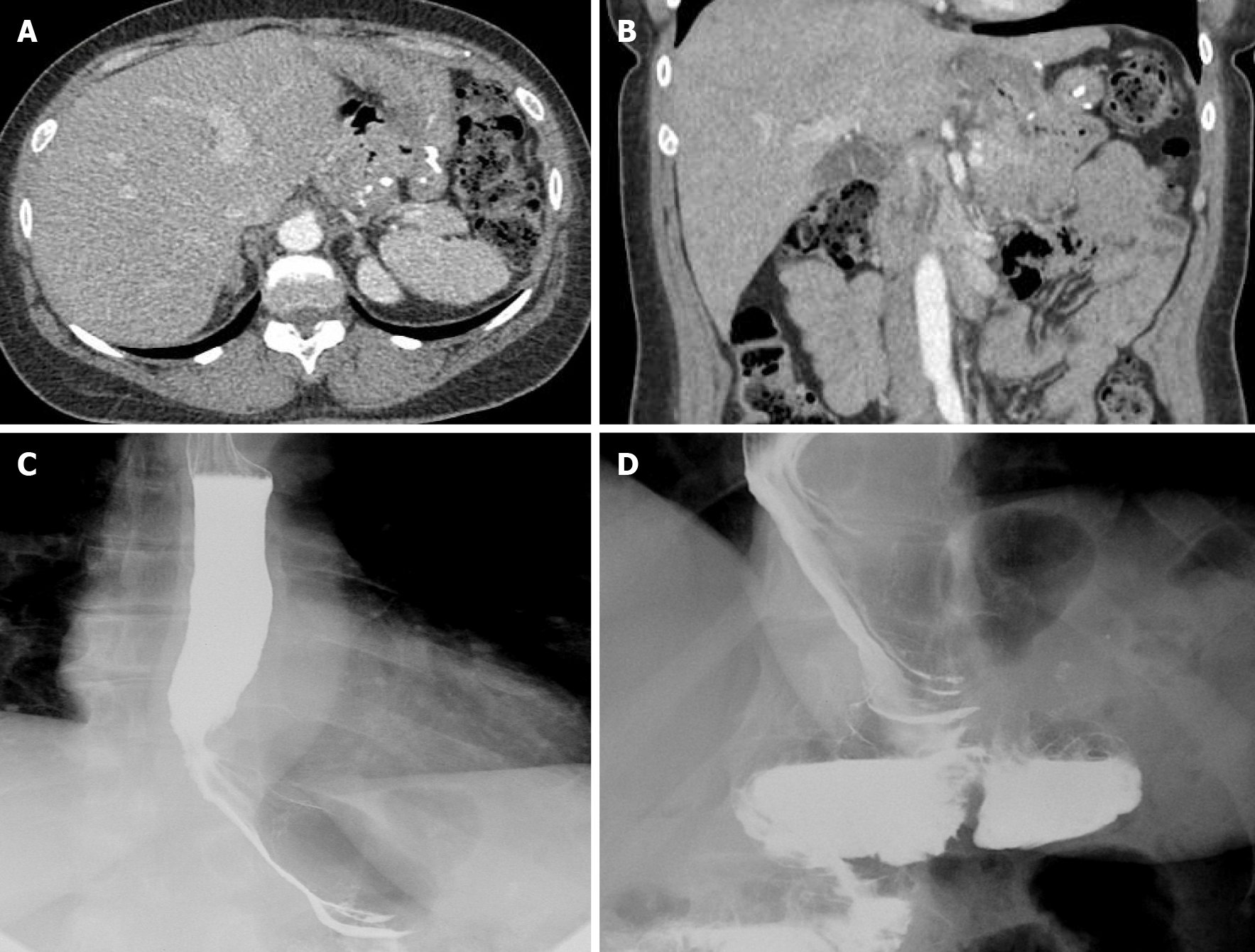
We present a case of a 50-year-old female presenting with upper abdominal pain, with history of a 5 cm × 5 cm perigastric collection, status post previous gastric sleeve bypass surgery with conversion to Roux-en-Y bypass. Initial CT of the abdomen and pelvis with intravenous contrast was read as negative for complications (Figure 16A and B). Subsequent fluoroscopy was then performed, with right anterior oblique (RAO) views demonstrating a contained walled off area of extravasation from the normal path of contrast (Figure 16C and D). Follow-up upper endoscopy was obtained, which confirmed evidence of gastro-gastric fistula. Pathology in this case was also examined, demonstrating gastric mucosa and confirming the diagnosis.
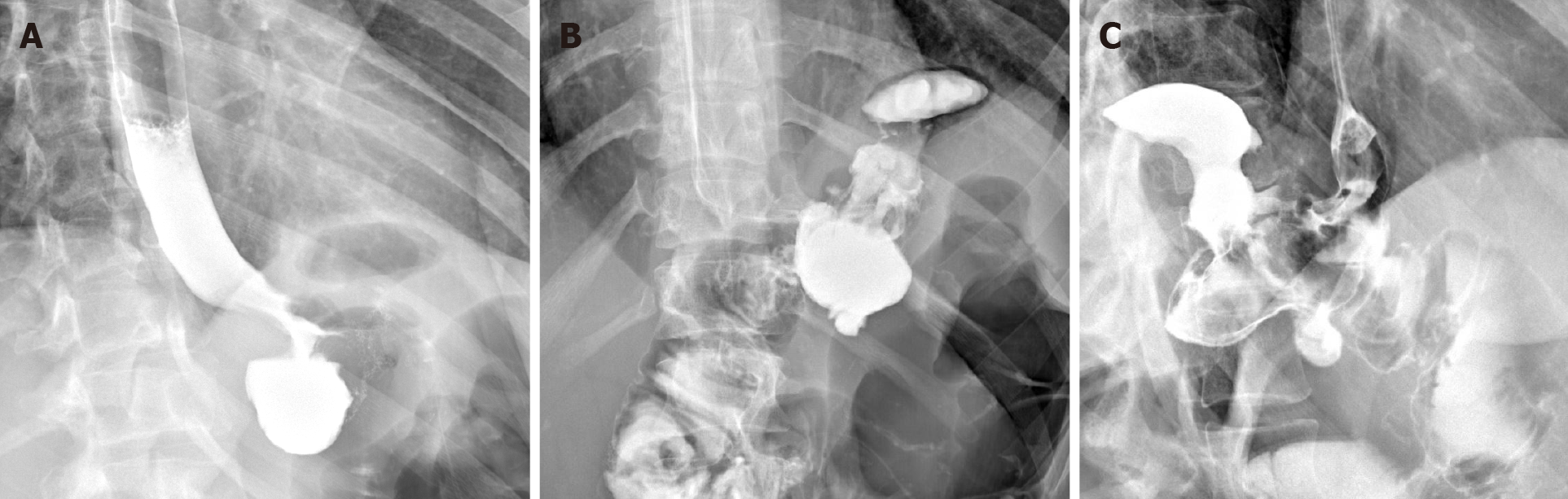
Corrosive agents cause severe damage to the gastrointestinal tract, with significant morbidity and mortality in these patients. Upper GI endoscopy remains the test of choice for assessing severity in the acute phase. However, modalities such as CT and fluoroscopy commonly demonstrate distinctive radiologic patterns. Esophageal strictures may be seen, with either short-segment or long-segment luminal narrowing and stenosis. This is presented in detailed pictorial reviews such as by Kamat et al[35] While these findings may be elucidated on an initial workup using fluoroscopy alone, we present here a case where fluoroscopy was both essential and ancillary to CT.
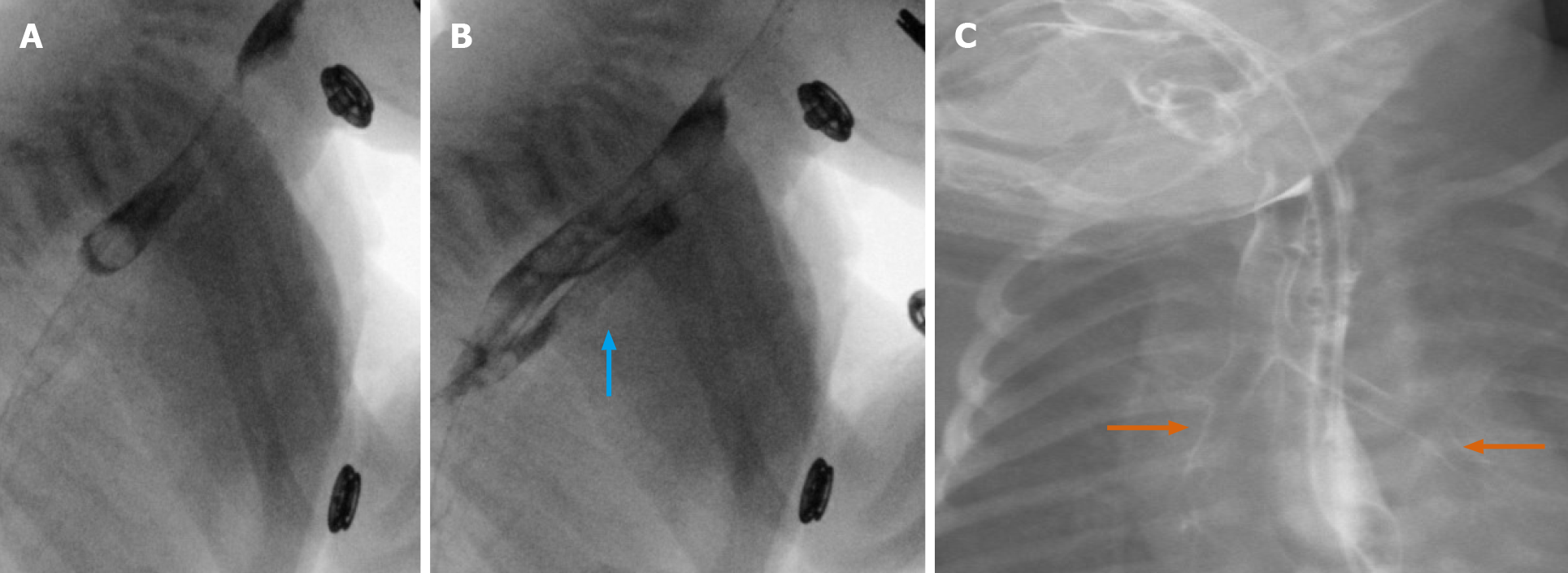
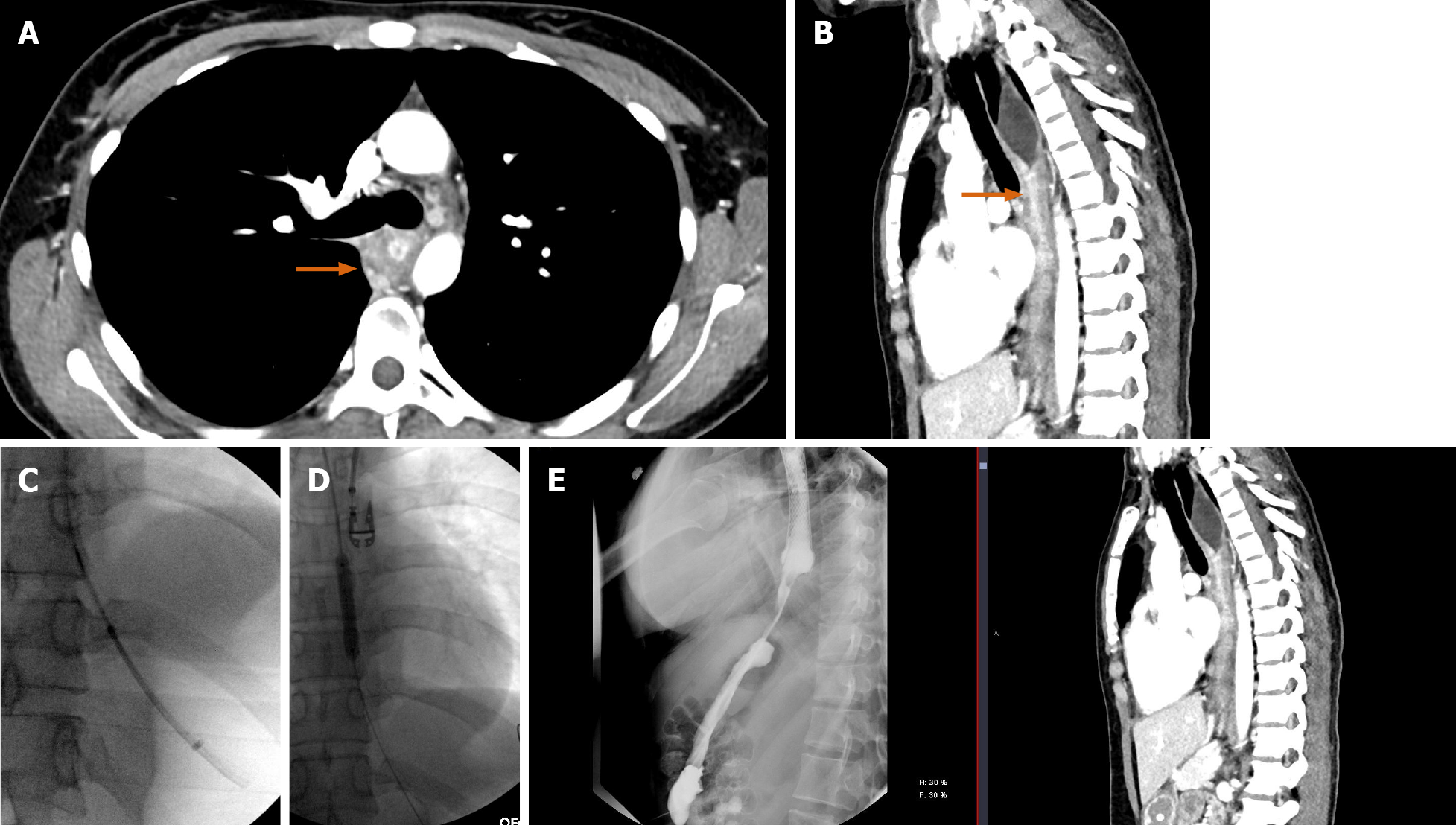
We present a 26-year-old male, with history of caustic ingestion. Initial CT demonstrated long segment luminal stenosis of the esophagus (Figure 17A and B). Upper endoscopy was performed, and was then aborted due to a non-traversable stricture. Next, upper endoscopy was re-attempted, this time with fluoroscopic guidance, and was successful for stent placement (Figure 17C and D). Ultimately, a final UGI fluoroscopic examination showed successful contrast passage through the now stented esophagus (with direct comparison to initial CT shown in the sagittal plane, (Figure 17E). This case powerfully shows the synergistic utilization of CT, upper endoscopy, and fluoroscopy to achieve a successful outcome.
While these following cases are not “routine” in the sense of an initial workup, perhaps the most novel utilization of fluoroscopy is toward the “de novo” diagnosis of pathology. While relatively rare, we include two such cases to emphasize that such evaluations rely heavily on the functional-imaging and temporal aspects of fluoroscopy, and how these aspects can highlight incidental findings beyond the basic indication of an exam.
We have already discussed the versatility of fluoroscopy in “functional” evaluation. There arise occasions, however, where more non-routine diagnoses can be established, sometimes requiring multiple fluoroscopic evaluations, medication administration and collaboration with clinicians.
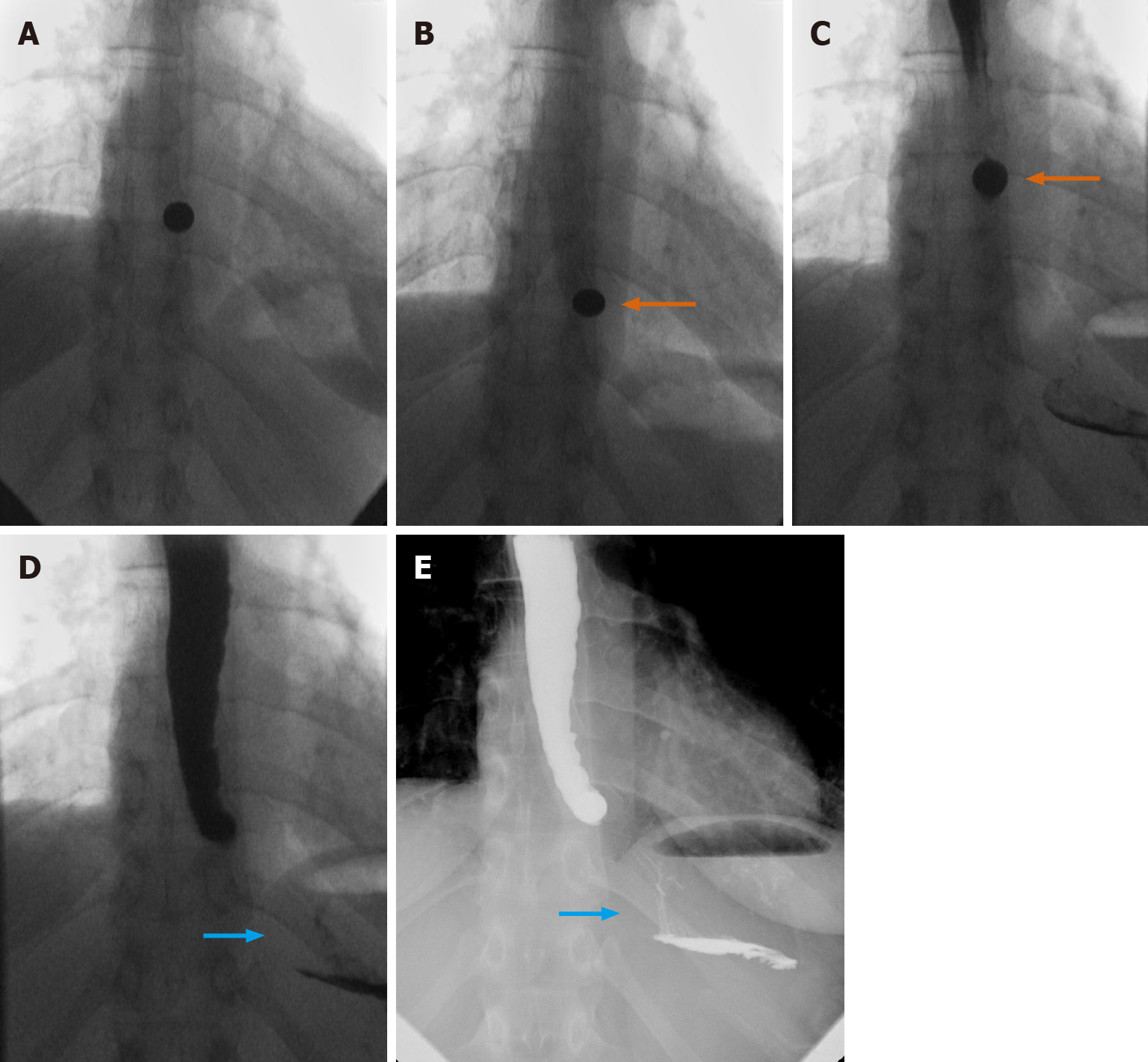
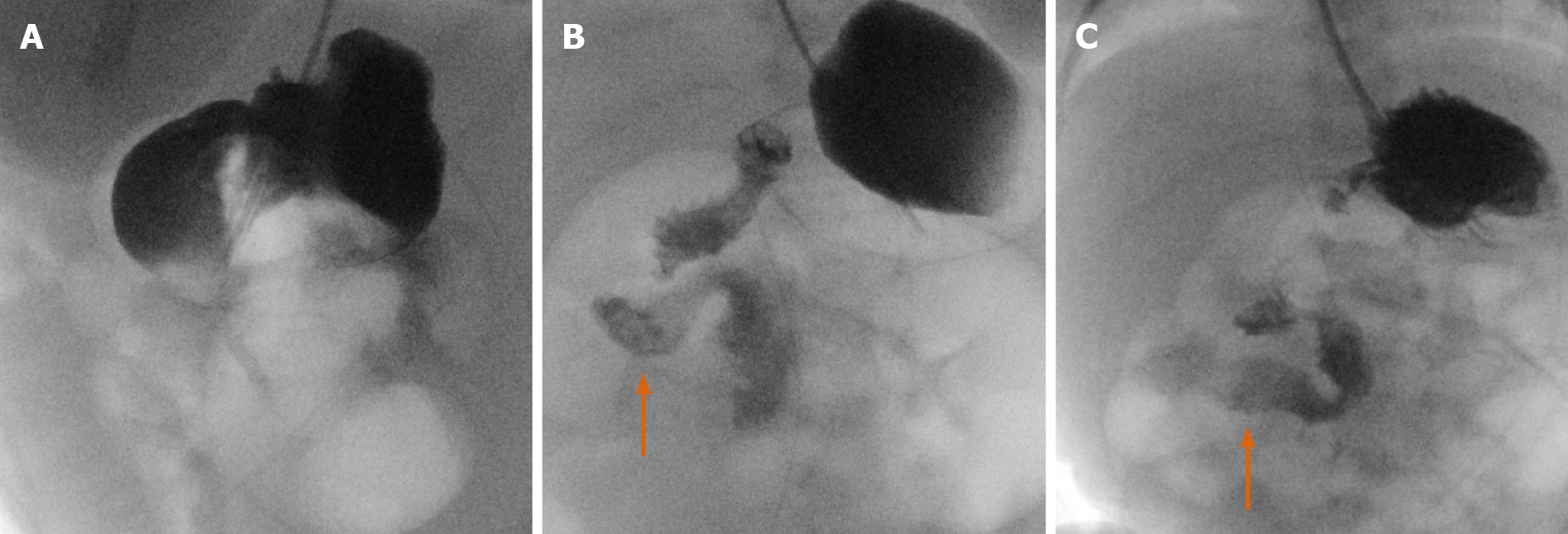
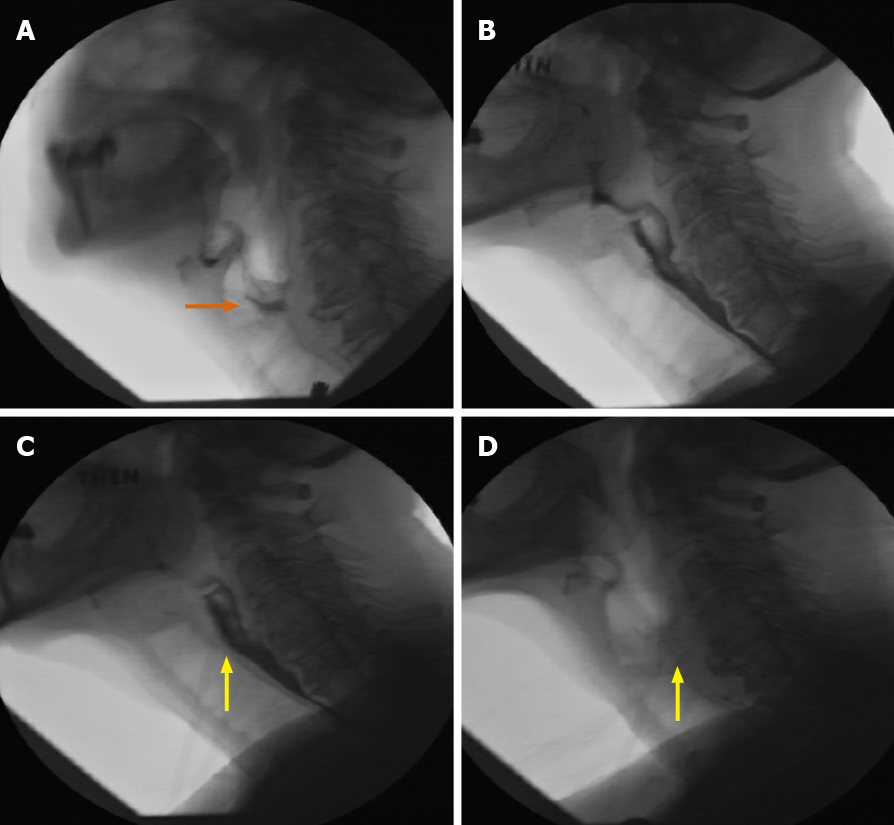
The neurological setting provides a unique example for the novel use of fluoroscopy. We present a case of a 69-year-old female, initially admitted for chronic obstructive pulmonary disease (COPD) exacerbation, with additional concern regarding dysarthria, dysphasia, lip/tongue numbness, and fatigability. Initial MBS was performed with multiple consistencies, with the ultimate findings of laryngeal penetration to the level of vocal cords and a disorganized swallow pattern (Figure 18A).
Neurology consultation was obtained, and two subsequent fluoroscopic evaluations were performed. A repeat MBS demonstrated mildly better results with thin liquid showing faint flash penetration (Figure 18B).
Finally, the use of neostigmine prior to repeat MBS was proposed and utilized to great effect, with the final swallow demonstrating even more improvement, with better organization in sequence and with no significant aspiration or penetration at multiple consistencies (Figure 18C and D). The combination of serial fluoroscopic findings under different circumstances, including neostigmine administration, was considered by neurology as highly supportive of the diagnosis of myasthenia gravis in this patient.
We present a case of MBS performed for a 64-year-old male, with history of dysphagia for 1 wk for solids and pill medications. Initial indication was for evaluation of possible aspiration. While thin liquids showed penetration and aspiration, on review, incidental note was made of a prominent soft tissue bulge at the inferior aspect of the epiglottis (Figure 19A).
Follow-up CT of the neck showed an enlarged, irregular, and enhancing epiglottis concerning for primary mucosal malignancy (Figure 19B).
Pathologic sample of the laryngeal surface of the epiglottis showed fragments of squamous cell carcinoma with basaloid features and necrosis, with additional samples from the trachea, base of tongue, and lymph nodes showing focal and metastatic squamous cell carcinoma.
This case highlights that discovered pathologies may expand beyond the initial findings and indications for exam (in this case, indication as penetration and aspiration).
The above categorical and historical discussion describes the utility of fluoroscopy across several settings, and also describes the many benefits inherent to the modality. However, no modality is without limitation. Limitations to fluoroscopy exist on both the patient and operator sides. Patient compliance with positioning and ability to take PO contrast remain an issue, particularly in intensive care unit (ICU) patients or those requiring ancillary staff, such as respiratory therapy, to monitor and remain present during the examination. Fluoroscopy also relies on significant operator skill (including the radiologist and technologist), particularly in terms of correct positioning and optimal image acquisition. Finally, radiation dose parameters and time of exam must remain within safety limits. As shown in a 2015 analysis by Wambani et al[36], the majority of the fluoroscopic examinations in their selected cohort were performed with both longer fluoroscopy time and with patient dose values with mean values above the international diagnostic reference levels. This again shows that proper training for the responsible personnel is essential, and emphasizes that correct fluoroscopy technique be an integral part of teaching for radiology residency programs.
We demonstrate in this review that fluoroscopy remains an essential modality, even in the age of high-resolution CT, MRI, and ultrasound. We build upon prior concerns that outline the challenges facing fluoroscopy, and have emphasized the need to highlight these procedures in our training programs. We agree with the 2009 findings of Levine et al[1] that radiology departments must hone the skill of their residents, and recruit/retain radiologists with strong interest and expertise in fluoroscopic procedures.
In addition, we not only highlight the routine uses of fluoroscopy, but show that fluoroscopy works synergistically with CT, MRI, and other cross-sectional modalities in the age of high-resolution diagnostic algorithms.
Fluoroscopy will continue to be an essential modality for diagnostic radiology and clinical medicine, alongside those of high-resolution CT, MRI, and ultrasound. It must also be recognized as an important therapeutic modality, such as in the treatment of intussusceptions and small bowel obstructions.
While the future of advanced modalities does not appear all in doubt, with continued improvements both technologically and in terms of sub-specialization, the future of fluoroscopy remains tenuous.
The challenges facing fluoroscopy are: (1) Its poor reimbursements, relative to other radiology examinations; (2) Increased physician time required to perform examinations, compared to other diagnostic procedures that are performed at a workstation either on-site or miles (possibly continents) away from the patient; and (3) The relative dearth of mentors in training programs able to share the skills necessary to perform examinations and teach the nuances of fluoroscopy to trainees.
The future will likely see more physician supervision of Physician Assistants and Radiology Assistants who will perform more protocolized fluoroscopy exams. Alternatively, interventional radiology (IR) may take the lead, given the procedure-based orientation of IR. This could be discussed on a local level, depending on individual training programs.
Residency programs have evolved over the years, alongside a significant increase in scientific knowledge as well as an increase in the capabilities of new and improved modalities. Further sub-specialization and advanced fellowship training is now common. We suggest that fluoroscopy be maintained as an integral part of general radiology residency programs, and even suggest the possibility of an advanced fellowship for dedicated training.
The authors thank Hittman JM (University of Maryland Medical Center, Department of Pathology) for providing both editorial assistance as well as pathologic consultation for this review.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chow J, Tang G S-Editor: Gao CC L-Editor: A P-Editor: Wu YXJ
| 1. | Levine MS, Rubesin SE, Laufer I. Barium studies in modern radiology: do they have a role? Radiology. 2009;250:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Narayan AK, Lopez DB, Kambadakone AR, Gervais DA. Nationwide, Longitudinal Trends in CT Colonography Utilization: Cross-Sectional Survey Results From the 2010 and 2015 National Health Interview Survey. J Am Coll Radiol. 2019;16:1052-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | American College of Radiology. Medicare Physician Fee Schedule. [Cited 2020 May 24]. Available from: https://www.acr.org/Advocacy-and-Economics/Radiology-Economics/Medicare-Medicaid/MPFS. |
| 4. | Relative value units (RVUs). Natl Health Policy Forum. [Published 2015 January 12]. Available from: https://www.nhpf.org/Library/the-basics/Basics_RVUs_01-12-15.pdf. |
| 5. | DeSimone AK, Post A, Duszak R Jr, Duong PT. Radiology Trainee vs Faculty Radiologist Fluoroscopy Time for Imaging-Guided Procedures: A Retrospective Study of 17,966 Reports Over a 5.5-Year Period. Curr Probl Diagn Radiol. 2018;47:233-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Levine MS, Rubesin SE, Herlinger H, Laufer I. Double-contrast upper gastrointestinal examination: technique and interpretation. Radiology. 1988;168:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Mahesh M. Minimizing Risks From Fluoroscopy [Slide Presentation]. 2019. Available from: https://Lms14.learnshare.com/. |
| 8. | Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de González A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1844] [Cited by in RCA: 1712] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 9. |
Hung RK. Real-Time Radiographic Identification of Contrast Consistency in Modified Barium Swallow Studies: An Alternative Technique.
|
| 10. | Levine MS, Rubesin SE. History and Evolution of the Barium Swallow for Evaluation of the Pharynx and Esophagus. Dysphagia. 2017;32:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Levine MS, Rubesin SE, Laufer I. Barium esophagography: a study for all seasons. Clin Gastroenterol Hepatol. 2008;6:11-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | DiPalma JA, Prechter GC, Brady CE 3rd. X-ray-negative dysphagia: is endoscopy necessary? J Clin Gastroenterol. 1984;6:409-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Pan JJ, Levine MS, Redfern RO, Rubesin SE, Laufer I, Katzka DA. Gastroesophageal reflux: comparison of barium studies with 24-h pH monitoring. Eur J Radiol. 2003;47:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Ott DJ, Gelfand DW, Lane TG, Wu WC. Radiologic detection and spectrum of appearances of peptic esophageal strictures. J Clin Gastroenterol. 1982;4:11-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Gupta S, Levine MS, Rubesin SE, Katzka DA, Laufer I. Usefulness of barium studies for differentiating benign and malignant strictures of the esophagus. AJR Am J Roentgenol. 2003;180:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Carbo AI, Kim RH, Gates T, D'Agostino HR. Imaging findings of successful and failed fundoplication. Radiographics. 2014;34:1873-1884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Javors BR, Wolf EL. Radiology of the postoperative GI tract. New York, NY: Springer, Verlag, 2002. |
| 18. | Xu T, Rosculet N, Steele K, Auster M. Comparison of upper gastrointestinal fluoroscopy vs computed tomography for evaluation of post-operative leak in a bariatric surgery patient. BJR Case Rep. 2017;3:20160076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Soscia J, Friedman JN. A guide to the management of common gastrostomy and gastrojejunostomy tube problems. Paediatr Child Health. 2011;16:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Acord M, Pollock A. Gastric Outlet Obstruction From a Button-Type Percutaneous Gastrostomy Tube. Pediatr Emerg Care. 2017;33:522-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Martin-Harris B, Jones B. The videofluorographic swallowing study. Phys Med Rehabil Clin N Am. 2008;19:769-785, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Ozdoba C, Gralla J, Rieke A, Binggeli R, Schroth G. Myelography in the Age of MRI: Why We Do It, and How We Do It. Radiol Res Pract. 2011;2011:329017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Chalazonitis A, Tzovara I, Laspas F, Porfyridis P, Ptohis N, Tsimitselis G. Hysterosalpingography: technique and applications. Curr Probl Diagn Radiol. 2009;38:199-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Foroozanfard F, Sadat Z. Diagnostic value of hysterosalpingography and laparoscopy for tubal patency in infertile women. Nurs Midwifery Stud. 2013;2:188-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Darge K. Diagnosis of vesicoureteral reflux with ultrasonography. Pediatr Nephrol. 2002;17:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Chand DH, Rhoades T, Poe SA, Kraus S, Strife CF. Incidence and severity of vesicoureteral reflux in children related to age, gender, race and diagnosis. J Urol. 2003;170:1548-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. |
| 28. | Kumbhar SS, Qi J. Fluoroscopic Diagnosis of Malrotation: Technique, Challenges, and Trouble Shooting. Curr Probl Diagn Radiol. 2020;49:476-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Esposito F, Ambrosio C, De Fronzo S, Panico MR, D'Aprano M, Giugliano AM, Noviello D, Oresta P. Fluoroscopy-guided hydrostatic reduction of intussusception in infancy: role of pharmacological premedication. Radiol Med. 2015;120:549-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Basile J, Kenny JF, Khodorkovsky B, Youssef E, Ardolic B, Chacko J, Hahn B. Effects of eliminating routine use of oral contrast for computed tomography of the abdomen and pelvis: A pilot study. Clin Imaging. 2018;49:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Kim J, Azagury D, Eisenberg D, DeMaria E, Campos GM; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS position statement on prevention, detection, and treatment of gastrointestinal leak after gastric bypass and sleeve gastrectomy, including the roles of imaging, surgical exploration, and nonoperative management. Surg Obes Relat Dis. 2015;11:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 32. | Marshall JS, Srivastava A, Gupta SK, Rossi TR, DeBord JR. Roux-en-Y gastric bypass leak complications. Arch Surg. 2003;138:520-523; discussion 523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 33. | Filho AJ, Kondo W, Nassif LS, Garcia MJ, Tirapelle Rde A, Dotti CM. Gastrogastric fistula: a possible complication of Roux-en-Y gastric bypass. JSLS. 2006;10:326-331. [PubMed] |
| 34. | Gao G, Nezami N, Mathur M, Balcacer P, Israel G, Spektor M. Diagnosis of gastrogastric fistula on computed tomography: a quantitative approach. Abdom Radiol (NY). 2018;43:1329-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Kamat R, Gupta P, Reddy YR, Kochhar S, Nagi B, Kochhar R. Corrosive injuries of the upper gastrointestinal tract: A pictorial review of the imaging features. Indian J Radiol Imaging. 2019;29:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Wambani JS, Korir GK, Tries MA, Korir IK, Sakwa JM. Patient radiation exposure during general fluoroscopy examinations. J Appl Clin Med Phys. 2014;15:4555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |