Published online Jul 28, 2019. doi: 10.4329/wjr.v11.i7.102
Peer-review started: May 10, 2019
First decision: June 6, 2019
Revised: July 3, 2019
Accepted: July 25, 2019
Article in press: July 25, 2019
Published online: July 28, 2019
Processing time: 80 Days and 20.9 Hours
The hepatic arterial anatomy is highly variable, with the two most common variants being a replaced right hepatic artery (RHA) originating from the superior mesenteric artery (SMA) and a left hepatic artery (LHA) originating from the left gastric artery (LGA). These anatomical variants could potentially increase the risk for non-target embolization during Y90-Radioembolization due to the close proximity between hepatic and enteric vessel branches.
To evaluate the safety of Yttrium-90 radioembolization (90Y-RE) with resin microspheres in patients with a variant hepatic arterial anatomy.
In this retrospective single-center observational study, 11 patients who underwent RE with 90Y-resin microspheres via a LHA originating from the LGA, and 13 patients via a RHA originating from the SMA were included. Patient and treatment data were reviewed regarding clinical and imaging evidence of non-target embolization of 90Y-resin microspheres to the GI tract. Positioning of the tip of the microcatheter in relationship to the last hepatoenteric side branch was retrospectively analyzed using angiographic images, cone-beam CT and pre-interventional CT-angiograms.
None of the 24 patients developed clinical symptoms indicating a potential non-target embolization to the GI tract within the first month after 90Y-RE. On the postinterventional 90Y-bremsstrahlung images and/or 90Y-positron emission tomographies, no evidence of extrahepatic 90Y-activity in the GI tract was noted in any of the patients. The mean distance between the tip of the microcatheter and the last enteric side branch during delivery of the 90Y microspheres was 3.2 cm (range: 1.9-5 cm) in patients with an aberrant LHA originating from a LGA. This was substantially shorter than the mean distance of 5.2 cm (range: 2.9-7.7 cm) in patients with an aberrant right hepatic originating from the SMA.
90Y-RE via aberrant hepatic arteries appears to be safe; at least with positioning of the microcatheter tip no less than 1.9 cm distal to the last hepatoenteric side branch vessel.
Core tip: Anatomical variants of the hepatic arteries may complicate treatment with 90Y-Radioembolization (90Y-RE) due to a close proximity of hepatic and enteric vessel branches. Left hepatic arteries originating from the left gastric artery usually have a substantially shorter main stem than right hepatic arteries originating from the superior mesenteric artery. However, even a minimum distance of 1.9 cm between the tip of the microcatheter and the last hepatoenteric side branch appears to be sufficient to avoid reflux of 90Y microspheres. Therefore, 90Y-RE should be feasible and safe in most patients with aberrant hepatic arteries without a significantly increased risk for non-target embolization.
- Citation: Zimmermann M, Schulze-Hagen M, Pedersoli F, Isfort P, Heinzel A, Kuhl C, Bruners P. Y90-radioembolization via variant hepatic arteries: Is there a relevant risk for non-target embolization? World J Radiol 2019; 11(7): 102-109
- URL: https://www.wjgnet.com/1949-8470/full/v11/i7/102.htm
- DOI: https://dx.doi.org/10.4329/wjr.v11.i7.102
Radioembolization with Yttrium-90 (90Y) is a liver-directed cancer treatment which has been shown to be effective and prolong overall survival in patients with irresectable primary or metastatic liver cancer[1-6]. 90Y-Radioembolization (90Y-RE) is being increasingly used over the last couple of years, since studies have shown that it significantly prolongs time-to-progression compared to transarterial chemo-embolization in patients with hepatocellular cancer (HCC) for example, while simultaneously resulting in less toxicity[7,8]. In general, side effects after 90Y-RE are uncommon and mostly include mild post-interventional symptoms such as fatigue, abdominal pain, nausea and vomiting[9,10]. A rare, but serious complication however is non-target embolization of 90Y particles to the GI tract, which may lead to radiation- induced gastrointestinal ulceration and is thus associated with significant morbidity and mortality[11].
Non-target embolization of the GI tract during 90Y-RE may result either from hepatoenteric vessels distal to the position of the catheter tip during delivery of the 90Y microspheres, or from reflux of particles into enteric branches proximal to the location of the catheter tip. A pre-treatment mapping angiogram to assess the hepatic arterial anatomy and embolization of any hepatoenteric vessels deemed to pose a risk for non-target embolization using coils, plugs or glue is therefore routinely performed before radioembolization[12,13]. Additionally, the catheter is usually placed as distally as possible during delivery of the 90Y microspheres to minimize the risk of reflux into enteric branches.
However, patients with a variant arterial supply of the liver, such as hepatic arteries originating from the left gastric artery (LGA) or the superior mesenteric artery (SMA) for example, may have an increased risk of non-target embolization due to the close proximity between hepatic and enteric vessel branches.
Therefore, the purpose of this study is to evaluate whether 90Y-RE with resin microspheres can be safely performed via a replaced right or left hepatic artery (LHA) originating from the SMA or LGA.
This single-center retrospective study was approved by the institutional review board (IRB, internal reference no. EK 308/18).
Computed tomography (CT) angiographies and fluoroscopic angiograms of all patients that had undergone radioembolization with 90Y-resin microspheres (SIRSpheres, Sirtex Medical Ltd, Lane Cove, Australia) between 2010 and 2018 at our institution were retrospectively reviewed and screened for a variant hepatic arterial anatomy. All patients in whom a 90Y-RE was performed via a replaced right or LHA and with a minimum follow-up of one month were included in this retrospective analysis.
In general, the indication for 90Y-RE included HCC (BCLC Stage C) and liver-only or liver-dominant metastatic disease of different primary tumors (Table 1 for further details on patient characteristics). All treatment decisions were established by consensus in a multidisciplinary tumor board attended by hepatobiliary surgeons, oncologists, radiotherapists, pathologists and interventional radiologists.
| Total n = 24 | |
| Male/female | 12/12 |
| Mean age (yr) | 60 ± 10 |
| Type of tumor | |
| Hepatocellular carcinoma | 10 |
| Colorectal cancer | 4 |
| Breast cancer | 3 |
| Pancreatic cancer | 2 |
| Neuroendocrine tumor of the gastrointestinal tract | 2 |
| Endometrial carcinoma | 1 |
| Cholangiocellular carcinoma | 1 |
| Oropharyngeal cancer | 1 |
| Hepatic vascular anatomy | |
| Left hepatic artery originating from left gastric artery | 11 |
| Right hepatic artery originating from superior mesenteric artery | 13 |
| Distance between microcatheter tip and last enteric side branch (cm) | |
| Left hepatic artery originating from left gastric artery | 3.2 ± 1.0 |
| Right hepatic artery originating from superior mesenteric artery | 5.0 ± 1.7 |
| Mean administered activity (Mbq) | |
| Treatment of left hepatic lobe | 612 ± 190 |
| Treatment of right hepatic lobe | 1262 ± 540 |
Written informed consent was obtained from all patients before the procedure. All procedures were performed by interventional radiologists with at least 5 years of experience in transarterial oncologic procedures.
As part of the routine work-up before 90Y-RE, a standard mapping angiogram of the celiac axis, superior mesenteric and hepatic arterial vessels was obtained in all patients several days prior to the actual 90Y-RE to assess the hepatic vascular anatomy and identify any hepatoenteric vessels deemed at risk for non-target embolization to the GI tract. Wherever possible, these hepatoenteric vessels, e.g., a right phrenic artery arising from an aberrant left hepatic artery, were subsequently embolized using coils.
The microcatheter was then advanced as distally as possible into the respective hepatic artery to a location that was considered appropriate for subsequent delivery of the 90Y particles. At this location, an arterial phase cone beam CT (Artis Zee or ZeeGo, Siemens Healthcare, Forchheim, Germany) with undiluted contrast agent (Ultravist®-300, Bayer, Leverkusen, Germany) an injection rate of 0.8-1 mL/s with a total volume of 6.4-8 mL and an injection timing delay of 8 s was performed to screen for possible extrahepatic contrast enhancement. If no extrahepatic enhancement was seen, technetium-99m–labeled macroaggregated albumin (99mTc MAA) was injected and the patient was subsequently transferred to the Department of Nuclear Medicine for a 99mTc MAA-singe-photon emission CT/CT (99mTc-SPECT/CT) scan to determine the lung shunt fraction and to screen for the presence of extrahepatic activity.
For the eventual treatment, the tip of the microcatheter was placed at an identical position as during the 99mTc MAA-test-injection and again a cone beam CT was performed with injection of contrast material through the microcatheter to screen for possible hepatoenteric arterial communications. In 23 out of the 24 patients, 90Y-RE was performed in a lobar fashion. One patient received three segmental treatments (segments II, III and IV) via a replaced LHA at one-month intervals due to the fact that he had previously undergone right hepatic lobectomy and was therefore considered to have an increased risk of radiation-induced liver disease. Infusion of the 90Y microspheres was performed slowly, manually under intermittent fluoroscopy to ensure antegrade blood flow at all times. Complete administration of all of the calculated activity was achieved in all cases.
After completion of the procedure, each patient received post-interventional 90Y bremsstrahlung images and/or a 90Y positron emission tomography (PET) to evaluate the 90Y distribution in the liver as well as to screen for any extrahepatic activity as a result of a possible non-target embolization.
After radioembolization, all patients were routinely admitted to the nuclear medicine ward at our institution for 48 h, where they were closely monitored for any signs of acute toxicity by daily clinical examination and laboratory analysis of complete blood count, liver function tests and metabolic panel. After discharge, all patients resumed a routine schedule for follow-up with clinical examination, laboratory analysis (complete blood count, liver function tests, metabolic panel, tumor markers) and cross-sectional imaging (contrast-enhanced MRI or PET/CT) one month after treatment and then every 2-3 mo thereafter.
The primary outcome variable of this study was presence or absence of clinical or imaging evidence of non-target embolization of 90Y-microspheres to the GI tract.
Therefore, electronic medical records of all patients were reviewed for presence of nausea, vomiting, abdominal pain and fever as symptoms of potential gastrointestinal complications on days 1-3 and 4 wk after 90Y-RE. These data were graded according to the common terminology criteria for adverse events (CTCAE version 5.0); toxicities of level ≥ 3 were defined as clinically relevant. Additionally, all post-interventional 90Y bremsstrahlung images and 90Y-PETs, as well as the 99mTc MAA- SPECT/CTs and arterial cone beam CTs, were retrospectively reviewed for evidence of extra-hepatic/gastrointestinal activity or extrahepatic contrast enhancement.
Since catheter positioning is critical for target or non-target embolization, the distance between the position of the microcatheter tip during the administration of the 90Y particles and the last enteric side branch was determined using angiographic images, cone-beam CT images and pre-interventional CT angiograms (including maximum-intensity projections and curved multi-planar reconstructions whenever necessary). Continuous variables were summarized using proportions, mean and median.
Out of 158 patients who had been treated by means of 90Y-RE between 2011 and 2018 at our institution, 24 patients (12 females, 12 males, mean age of 60 ± 10 years) had been treated via an aberrant hepatic artery and were therefore included in this retrospective study. There were 11 patients with an LHA originating from the LGA and 13 patients with a right hepatic artery (RHA) originating from the SMA.
90Y-RE was successfully performed in all 24 patients. All patients were discharged as planned on the second post-interventional day and no clinically relevant toxicities (grade ≥ 3; nausea, vomiting, abdominal pain and fever) were detected during follow-up. No imaging evidence of non-target-embolization of 90Y -microspheres to the GI tract and good tumoral 90Y -uptake was noted on all of the postinterventional 90Y bremsstrahlung images and/or 90Y-PETs.
In one patient with a replaced LHA, extrahepatic activity was noted on the preliminary 99mTc MAA- SPECT/CTs along the ventral abdominal wall due to a falciforme artery arising from LHA. This falciforme artery could not be embolized due to its very small caliber, however a previous study has shown that there seems to be no absolute need for prophylactic embolization[14]. 90Y-RE was subsequently performed and resulted in non-target embolization of minor amounts of 90Y microspheres along the ventral abdominal wall; the patient remained clinically asymptomatic however.
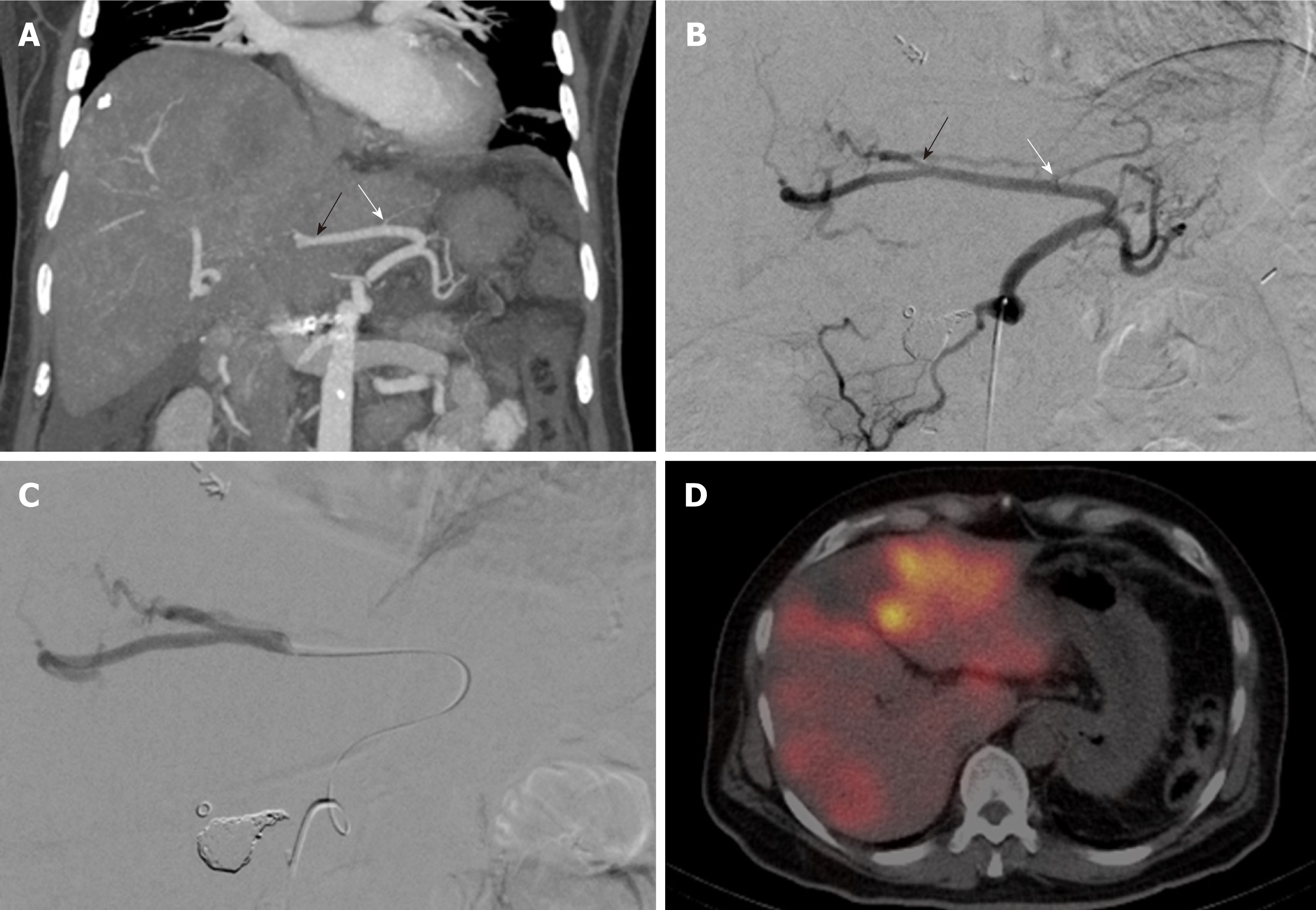
The mean distance between the tip of the microcatheter and the last enteric side branch during administration of the 90Y-microspheres was 3.2 cm (range: 1.9-5 cm) in patients with an aberrant LHA and 5.0 cm (range: 2.1-7.7 cm) in patients with an aberrant RHA (Table 1 for a summary of patient demographics and treatment characteristics). None of the arterial cone beam CTs that were performed through the microcatheter in place for treatment showed extrahepatic, gastrointestinal contrast enhancement (Figure 1A-D).
The hepatic arterial anatomy is highly variable; previous studies have shown that 39-49% of all patients have some form of variant arterial blood supply to the liver[15,16]. The two most common variants include a replaced RHA originating from the SMA with a reported prevalence of 12%-15%, and a LHA originating from the LGA with a prevalence between 4.5% and 8%[15,16]. These anatomic variants may complicate treatment by means of 90Y-RE, because of the close proximity of hepatic and enteric branches, which may increase the risk of non-target embolization of the GI tract through reflux of 90Y microspheres. This is particularly true for patients with an LHA originating from the LGA, since the distance between the origin of a replaced LHA at the LGA and the first intrahepatic side branch of the LHA is often particularly short.
In our study, the mean distance between catheter position during delivery of the 90Y particles and the last enteric side branch was shortest in patients with an LHA arising from the LGA, with the minimum distance being 1.9 cm. However, none of the patients developed clinically relevant signs and symptoms of gastrointestinal non-target embolization during follow-up and there was no evidence of 90Y activity in the GI tract on the postinterventional 90Y bremsstrahlung images or PET images in any of the patients. The results of this case series therefore suggest, that 90Y-RE with resin microspheres can be safely performed via hepatic arteries originating from either the SMA or the LGA.
Of course, the infusion rate of the 90Y particles can also significantly impact the risk of reflux and thus non-target embolization. Although administration of the 90Y particles was done manually without recording the infusion rate, we did not observe any reflux of contrast agent on the arterial cone beam CTs during the interventions. These were performed with mechanical infusion of contrast agent at a rate of 0.8-1 mL/s, and this rate could be therefore considered a safe starting point. However, hemodynamics will usually change during the procedure due to an increasing number of small vessels getting occluded by the microspheres and therefore intermittent fluoroscopy to adjust the infusion rate and verify antegrade blood flow at all times is strongly recommended. Alternatively, the use of glass microspheres (Theraspheres, BTG International, Ottawa, Ontario, Canada) instead of resin microspheres may decrease the risk for stasis and reflux of particles during treatment due to the decreased embolic load of glass microspheres compared with resin microspheres.
Several studies have explored the option of coil embolization of variant hepatic arteries before 90Y-RE as a method to redistribute and simplify the hepatic blood flow[17-19]. For example, coil embolization of an LHA arising from the LGA may be used to induce redistribution of the intrahepatic arterial blood flow to the left hepatic lobe via collaterals from the RHA and therefore facilitate whole liver treatment from a single treatment position in the RHA. While this technique can be used to avoid a potential non-target embolization to the LGA, it also eliminates the option of a selective lobar treatment in patients with a predominantly left hepatic tumor load. Additionally, due to the irreversibility of the coil embolization, it may limit future selective transarterial treatment options as well as surgical options, should the patient respond extremely well to the treatment and become a surgical candidate.
The main limitations of this study include its retrospective study design and the small patient cohort. As mentioned before, the individual hepatic arterial anatomy is highly variable and so are the number of hepatoenteric vessels and the distance between hepatic and enteric branches, which significantly impacts the risk of non-target embolization to the GI tract. Therefore, the results of this study may not be applicable to all patients and careful evaluation of the individual arterial anatomy before and during 90Y-RE is still necessary in all patients. Lastly, gastrointestinal complications after 90Y-RE are occasionally not diagnosed until several months after treatment[20]. However, this appears to be mostly attributable to misrecognition of the rather unspecific abdominal symptoms, something that appears avoidable when follow-up is performed by specialists who are familiar with these potential postinterventional complications.
In conclusion, 90Y-RE with resin microspheres via an RHA originating from the SMA and/or a LHA replaced to the LGA appears to be feasible and safe. We did not observe any evidence of non-target embolization in 24 patients with placement of the tip of the microcatheter at least 1.9 cm distal of the last enteric side branch and slow manual infusion of the 90Y-particles.
Radioembolization with Yttrium-90 (90Y) microspheres is commonly used for treatment of primary or secondary liver tumors. It is generally a well-tolerated treatment with few side effects, however non-target embolization of 90Y microspheres to the gastrointestinal tract is a severe potential complication. The risk for non-target embolization is very low in patients with a normal hepatic arterial anatomy. However, around 45% of patients have some form of variant hepatic arterial anatomy and patients with aberrant hepatic arteries might have a higher risk for reflux and non-target embolization of 90Y microspheres due to the close proximity between hepatic and enteric vessel branches.
So far, no study has specifically evaluated the safety of 90Y-Radioembolization in patients with a variant hepatic arterial anatomy. Therefore, this study aimed to evaluate whether there is an increased risk for non-target embolization during 90Y Radioembolization in this specific patient population.
To evaluate the safety of 90Y Radioembolization with resin microspheres in patients with one of the two most common hepatic arterial variants: A right hepatic artery (RHA) originating from the superior mesenteric artery (SMA) or a left hepatic artery (LHA) originating from the left gastric artery (LGA).
For this study, electronic medical records and imaging studies of 24 patients who had been treated with Radioembolization via an aberrant hepatic artery were retrospectively reviewed regarding clinical and imaging evidence of non-target embolization of 90Y-resin microspheres to the GI tract. 11 patients who underwent 90Y Radioembolization via an LHA originating from the LGA and 13 patients who underwent 90Y Radioembolization via an RHA originating from the SMA were included. Positioning of the tip of the microcatheter in relationship to the last hepatoenteric side branch was retrospectively analyzed using angiographic images, cone-beam CT and pre-interventional CT-angiograms.
None of the 24 patients developed clinical symptoms indicating a potential non-target embolization to the GI tract within the first month after 90Y-RE and there was no imaging evidence of non-target embolization on the postinterventional 90Y-bremsstrahlung images and/or 90Y-PETs in any of the patients. The distance between the tip of the microcatheter and the last enteric side branch was substantially shorter in patients with an aberrant LHA originating from a LGA (mean distance of 3.2 cm (range: 1.9-5 cm) than in those patients with an aberrant RHA originating from the SMA (mean distance of 5.2 cm (range: 2.9-7.7 cm). However even a minimum distance of 1.9 cm was sufficient to avoid reflux and non-target embolization of 90Y microspheres.
This study suggests that 90Y Radioembolization may be safely performed in patients with aberrant hepatic arteries. A minimum distance of 1.9 cm between the tip of the microcatheter and the last enteric side branch in combination with slow, manual infusion of the 90Y microspheres was sufficient to avoid reflux of microspheres and non-target embolization in this study.
Although this study provides clinical evidence that patients with aberrant hepatic arteries can generally be safely treated with 90Y Radioembolization, further studies with standardized infusion rates and catheter positions would be desirable to systematically determine exact cut-off values at which reflux and non-target embolization of 90Y microspheres occurs.
Manuscript Source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Vosmik M S-Editor: Dou Y L-Editor: A E-Editor: Ma YJ
| 1. | Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2007;69:800-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | D'Avola D, Lñarrairaegui M, Bilbao JI, Martinez-Cuesta A, Alegre F, Herrero JI, Quiroga J, Prieto J, Sangro B. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology. 2009;56:1683-1688. [PubMed] |
| 3. | Hoffmann RT, Paprottka PM, Schön A, Bamberg F, Haug A, Dürr EM, Rauch B, Trumm CT, Jakobs TF, Helmberger TK, Reiser MF, Kolligs FT. Transarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survival. Cardiovasc Intervent Radiol. 2012;35:105-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Kennedy AS, Coldwell D, Nutting C, Murthy R, Wertman DE, Loehr SP, Overton C, Meranze S, Niedzwiecki J, Sailer S. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 268] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Kennedy AS, Dezarn WA, McNeillie P, Coldwell D, Nutting C, Carter D, Murthy R, Rose S, Warner RR, Liu D, Palmedo H, Overton C, Jones B, Salem R. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol. 2008;31:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 6. | Seidensticker R, Denecke T, Kraus P, Seidensticker M, Mohnike K, Fahlke J, Kettner E, Hildebrandt B, Dudeck O, Pech M, Amthauer H, Ricke J. Matched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastases. Cardiovasc Intervent Radiol. 2012;35:1066-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Salem R, Gordon AC, Mouli S, Hickey R, Kallini J, Gabr A, Mulcahy MF, Baker T, Abecassis M, Miller FH, Yaghmai V, Sato K, Desai K, Thornburg B, Benson AB, Rademaker A, Ganger D, Kulik L, Lewandowski RJ. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;151:1155-1163.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 487] [Article Influence: 54.1] [Reference Citation Analysis (30)] |
| 8. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH, Yaghmai V, Ibrahim SM, Senthilnathan S, Baker T, Gates VL, Atassi B, Newman S, Memon K, Chen R, Vogelzang RL, Nemcek AA, Resnick SA, Chrisman HB, Carr J, Omary RA, Abecassis M, Benson AB, Mulcahy MF. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 9. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH, Sato KT, Wang E, Gupta R, Benson AB, Newman SB, Omary RA, Abecassis M, Kulik L. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 779] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 10. | Sangro B, Carpanese L, Cianni R, Golfieri R, Gasparini D, Ezziddin S, Paprottka PM, Fiore F, Van Buskirk M, Bilbao JI, Ettorre GM, Salvatori R, Giampalma E, Geatti O, Wilhelm K, Hoffmann RT, Izzo F, Iñarrairaegui M, Maini CL, Urigo C, Cappelli A, Vit A, Ahmadzadehfar H, Jakobs TF, Lastoria S; European Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY). Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology. 2011;54:868-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 505] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 11. | Yip D, Allen R, Ashton C, Jain S. Radiation-induced ulceration of the stomach secondary to hepatic embolization with radioactive yttrium microspheres in the treatment of metastatic colon cancer. J Gastroenterol Hepatol. 2004;19:347-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Lewandowski RJ, Sato KT, Atassi B, Ryu RK, Nemcek AA, Kulik L, Geschwind JF, Murthy R, Rilling W, Liu D, Bester L, Bilbao JI, Kennedy AS, Omary RA, Salem R. Radioembolization with 90Y microspheres: angiographic and technical considerations. Cardiovasc Intervent Radiol. 2007;30:571-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Paprottka PM, Jakobs TF, Reiser MF, Hoffmann RT. Practical vascular anatomy in the preparation of radioembolization. Cardiovasc Intervent Radiol. 2012;35:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Ahmadzadehfar H, Möhlenbruch M, Sabet A, Meyer C, Muckle M, Haslerud T, Wilhelm K, Schild HH, Biersack HJ, Ezziddin S. Is prophylactic embolization of the hepatic falciform artery needed before radioembolization in patients with 99mTc-MAA accumulation in the anterior abdominal wall? Eur J Nucl Med Mol Imaging. 2011;38:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Winston CB, Lee NA, Jarnagin WR, Teitcher J, DeMatteo RP, Fong Y, Blumgart LH. CT angiography for delineation of celiac and superior mesenteric artery variants in patients undergoing hepatobiliary and pancreatic surgery. AJR Am J Roentgenol. 2007;189:W13-W19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Abdelmaksoud MH, Louie JD, Kothary N, Hwang GL, Kuo WT, Hofmann LV, Hovsepian DM, Sze DY. Consolidation of hepatic arterial inflow by embolization of variant hepatic arteries in preparation for yttrium-90 radioembolization. J Vasc Interv Radiol. 2011;22:1364-1371.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Bilbao JI, Garrastachu P, Herráiz MJ, Rodríguez M, Iñarrairaegui M, Rodríguez J, Hernández C, de la Cuesta AM, Arbizu J, Sangro B. Safety and efficacy assessment of flow redistribution by occlusion of intrahepatic vessels prior to radioembolization in the treatment of liver tumors. Cardiovasc Intervent Radiol. 2010;33:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Karunanithy N, Gordon F, Hodolic M, Al-Nahhas A, Wasan HS, Habib N, Tait NP. Embolization of hepatic arterial branches to simplify hepatic blood flow before yttrium 90 radioembolization: a useful technique in the presence of challenging anatomy. Cardiovasc Intervent Radiol. 2011;34:287-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Voruganti IS, Godwin JL, Adrain A, Feller E. A Woman with Black Beads in Her Stomach: Severe Gastric Ulceration Caused by Yttrium-90 Radioembolization. Case Rep Med. 2018;2018:1413724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |