Published online Nov 28, 2019. doi: 10.4329/wjr.v11.i11.134
Peer-review started: June 29, 2019
First decision: August 2, 2019
Revised: August 26, 2019
Accepted: September 25, 2019
Article in press: September 25, 2019
Published online: November 28, 2019
Processing time: 148 Days and 17 Hours
Diffusion-weighted imaging (DWI) has become a useful tool in the detection, characterization, and evaluation of response to treatment of many cancers, including malignant liver lesions. DWI offers higher image contrast between lesions and normal liver tissue than other sequences. DWI images acquired at two or more b-values can be used to derive an apparent diffusion coefficient (ADC). DWI in the body has several technical challenges. This include ghosting artifacts, mis-registration and susceptibility artifacts. New DWI sequences have been developed to overcome some of these challenges. Our goal is to evaluate 3 new DWI sequences for liver imaging.
To qualitatively and quantitatively compare 3 DWI sequences for liver imaging: free-breathing (FB), simultaneous multislice (SMS), and prospective acquisition correction (PACE).
Magnetic resonance imaging (MRI) was performed in 20 patients in this prospective study. The MR study included 3 separate DWI sequences: FB-DWI, SMS-DWI, and PACE-DWI. The image quality, mean ADC, standard deviations (SD) of ADC, and ADC histogram were compared. Wilcoxon signed-rank tests were used to compare qualitative image quality. A linear mixed model was used to compare the mean ADC and the SDs of the ADC values. All tests were 2-sided and P values of < 0.05 were considered statistically significant.
There were 56 lesions (50 malignant) evaluated in this study. The mean qualitative image quality score of PACE-DWI was 4.48. This was significantly better than that of SMS-DWI (4.22) and FB-DWI (3.15) (P < 0.05). Quantitatively, the mean ADC values from the 3 different sequences did not significantly differ for each liver lesion. FB-DWI had a markedly higher variation in the SD of the ADC values than did SMS-DWI and PACE-DWI. We found statistically significant differences in the SDs of the ADC values for FB-DWI vs PACE-DWI (P < 0.0001) and for FB-DWI vs SMS-DWI (P = 0.03). The SD of the ADC values was not statistically significant for PACE-DWI and SMS-DWI (P = 0.18). The quality of the PACE-DWI ADC histograms were considered better than the SMS-DWI and FB-DWI.
Compared to FB-DWI, both PACE-DWI and SMS-DWI provide better image quality and decreased quantitative variability in the measurement of ADC values of liver lesions.
Core tip: We compared 3 diffusion-weighted imaging (DWI) techniques for liver imaging: The free-breathing (FB), simultaneous multi-slice (SMS) and prospective acquisition correction (PACE) sequences. Three radiologists independently scored the images. We evaluated the image quality. We also compare the calculated apparent diffusion coefficient (ADC) for liver lesions and variability of ADC histogram for liver lesions for each sequence. The PACE and SMS provide better image quality and less variability in ADC values compared to FB-DWI.
- Citation: Szklaruk J, Son JB, Wei W, Bhosale P, Javadi S, Ma J. Comparison of free breathing and respiratory triggered diffusion-weighted imaging sequences for liver imaging. World J Radiol 2019; 11(11): 134-143
- URL: https://www.wjgnet.com/1949-8470/full/v11/i11/134.htm
- DOI: https://dx.doi.org/10.4329/wjr.v11.i11.134
Diffusion-weighted imaging (DWI) is sensitive to the Brownian motion of intracellular and extracellular water molecules. Because it can reveal changes in the tissue microenvironment, DWI has been found useful in imaging several different diseases. Although its first successful application was in the setting of acute stroke[1,2], DWI has become a useful tool in the detection, characterization, and evaluation of response to treatment of many cancers[3], including malignant liver lesions[4-9]. DWI offers higher image contrast between lesions and normal liver tissue than other sequences. This is achieved through bright signal suppression from free water fluid in the abdomen, bile ducts, and vessels. Additionally, DWI images acquired at two or more b-values can be used to derive an apparent diffusion coefficient (ADC). The quantitative ADC removes T2 “shine-through” artifacts, improves image interpretation, and strengthens liver lesion characterization[5,10]. ADC values are quantitative and changes in ADC have been used to evaluate lesions’ response to treatment[7,9].
Although it is widely used, DWI in the body has several technical challenges. The large diffusion weighting gradients that make the technique sensitive to microscopic diffusion also make DWI sequences very susceptible to bulk macroscopic motion. Because of this motion sensitivity, the most successful and widely used DWI sequence is based on single-shot (ss) echo planar imaging (EPI), which acquires all the k-space data for an image after a single radiofrequency excitation and thus freezes the motion. ss-EPI sequences are prone to image ghosts and distortion in the presence of magnetic field inhomogeneity and uncompensated eddy currents. ss-EPI–based DWI images are also limited by their low signal-to-noise ratio (SNR) and often require averaging multiple signals to improve image quality. The calculation of the quantitative ADC using DW images acquired with multiple b-values may pose additional challenges because mis-registration of DW images of different b-values from different motion sources (e.g., respiratory or cardiac) can occur[11,12]. The resulting errors in the ADC values of a tumor region of interest (ROI) directly affect the usefulness of DWI for evaluating response to treatment in liver lesions[13,14]. High-quality DW images with an increased SNR and diminished motion artifacts yield increased confidence in assessing the mean ADC value and changes in the ADC caused by the underlying biological effects of treatment (such as tumor necrosis).
Respiratory-triggered acquisition, based either on external respiratory signals or internally placed navigators, is effective in reducing respiratory motion artifacts and mis-registration of DW images. Respiratory triggering, that are not breath hold techniques, increases the total scan time by as much as 3-fold, depending on the respiratory pattern of the patient. As a result, many institutions, including ours, use free breathing (FB) for abdominal DWI. One way to overcome these time limitations is the use of simultaneous multi-slice (SMS) acceleration, a novel technique that can speed DWI data acquisition by many times without incurring the typical SNR penalty found with other acceleration techniques such as parallel imaging[15,16]. The SMS technique is based on simultaneous multiband radiofrequency excitation and the acquisition of multiple slices in a shared readout time[15-17]. SMS is compatible with many different pulse sequences; it has recently been implemented with DWI, enabling respiratory-triggered DWI acquisition of the abdomen in a reasonable scan time. Prospective acquisition correction (PACE) is an internal navigator-based technique for respiratory signal monitoring and correction. The PACE navigator is typically placed at the diaphragm to detect its displacement as a monitoring signal of the respiratory cycles. The PACE navigator signal is used to acquire images only during a pre-defined triggering window. The PACE DWI allows the synchronization of the DWI acquisition with respiratory cycles without the need to place external monitoring devices on the patient[18].
The purpose of this study was to evaluate and compare qualitative assessments of image quality and quantitative measurements of ADC from 3 different DWI sequences for liver imaging: (1) Conventional ss-EPI–based DWI with free breathing (FB-DWI); (2) SMS DWI with FB (SMS-DWI); and (3) SMS DWI using PACE triggering (PACE-DWI).
This prospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Patients with documented liver lesions on prior magnetic resonance imaging (MRI) studies who were scheduled for MRI scanning of the abdomen using DWI were eligible for participation in this study. Informed consent was obtained from all patients prior to their participation. The statistical methods of this study were reviewed by Wei W from The University of Texas MD Anderson Cancer Center, Department of Biostatistics.
All patients included in this study underwent a standard abdominal MRI protocol, with and without contrast. In addition, the 3 DWI sequences (FB-DWI, SMS-DWI, and PACE-DWI) were acquired in random orders (Table 1). All the sequences were obtained with b-values of 50, 400 and 800 s/mm2. ADC maps were generated for each of the 3 sequences. The average scan times for FB-DWI, SMS-DWI, and PACE-DWI were 4 min 56 s (4 min 44 s-6 min), 3 min 8 s (3 min 4 s-3 min 38 s), and 5 min 40 s (3 min 40 s-12 min). The readout bandwidth was kept the same at 2.44 kHz/pixel for all 3 DWI sequences. PACE DWI enabled a similar acquisition time for a respiratory triggered acquisition as that of FB DWI because of the scan time savings from SMS. Breath holds were not employed in any of the three DWI techniques.
| Sequence | TE (ms) | TR (ms) | Slice Thickness/0-mm gap (mm) | Time | Matrix size |
| T1-gradient dual echo | 2.1 and 4.2 | 170 | 5 | 2 s × 19 s BH | 320 × 219 |
| T2 fast-spin echo | 93 | 5331 | 5 | 5 min | 256 × 135 |
| Dynamic pre-Gd and post-Gd VIBE | 2.65 | 2.65 | 3 | 16 s BH | 288 × 192 |
| FB-DWI | 67 | 7900 | 5 | 4 min 44 s | 128 × 104 |
| SMS-DWI | 56 | 5000 | 5 | 3 min 4 s | 128 × 104 |
| PACE-DWI | 56 | 2600 | 5 | 5 min 58 s | 128 × 104 |
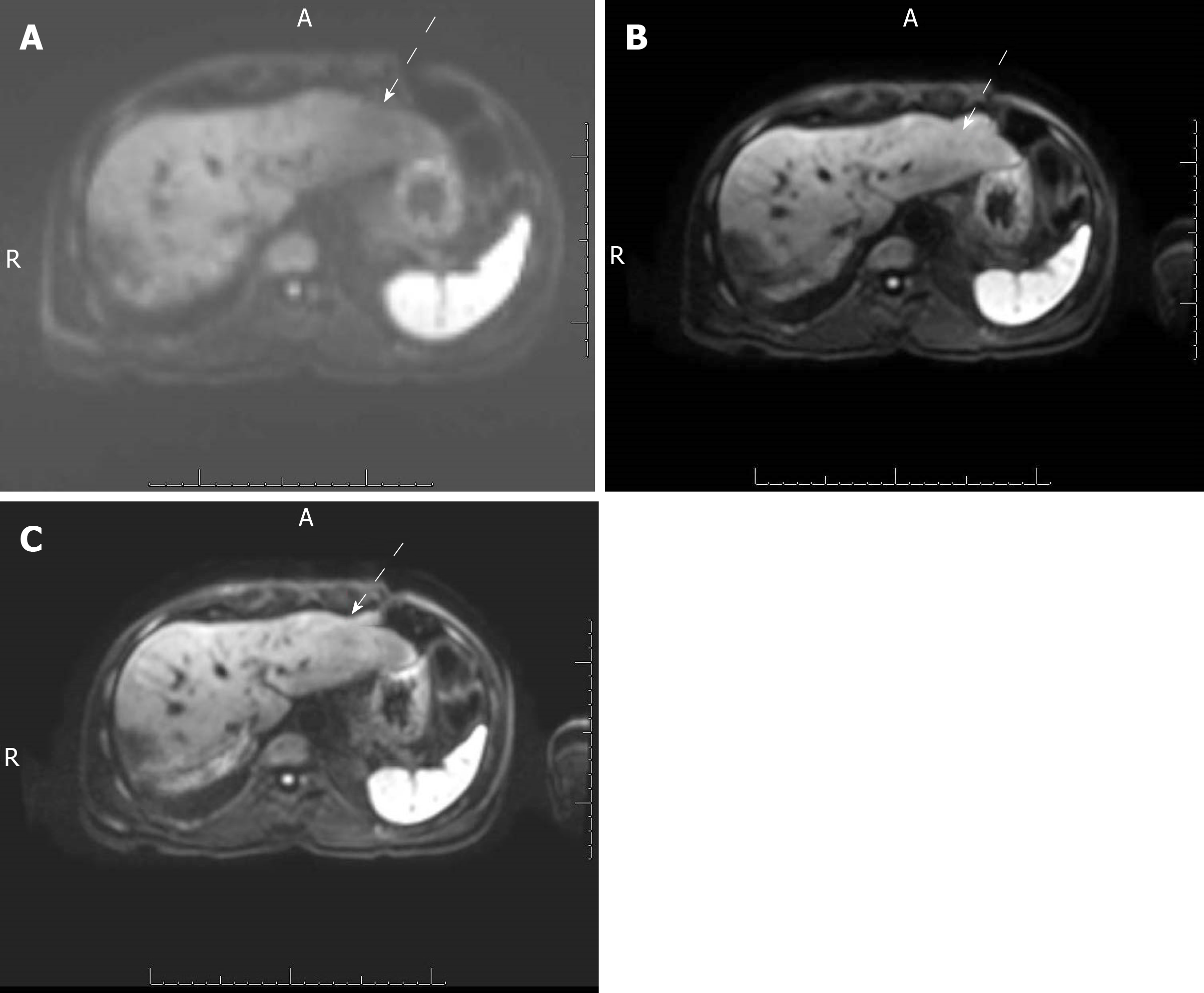
A qualitative analysis of the DWI images was performed independently by 3 radiologists with 4, 13 and 20 years of experience, respectively. The radiologists provided a qualitative score on a 5-point Likert scale (with 5 = excellent, 4 = good, 3 = moderate, 2 = poor, 1 = non-diagnostic) for each of the 3 DWI series for all 3 b-values (50, 400 and 800 s/mm2). In addition, 1 radiologist (JS) selected an ROI for each liver lesion and placed it on all the DWI images. The average size of the ROIs was 4.09 cm2.
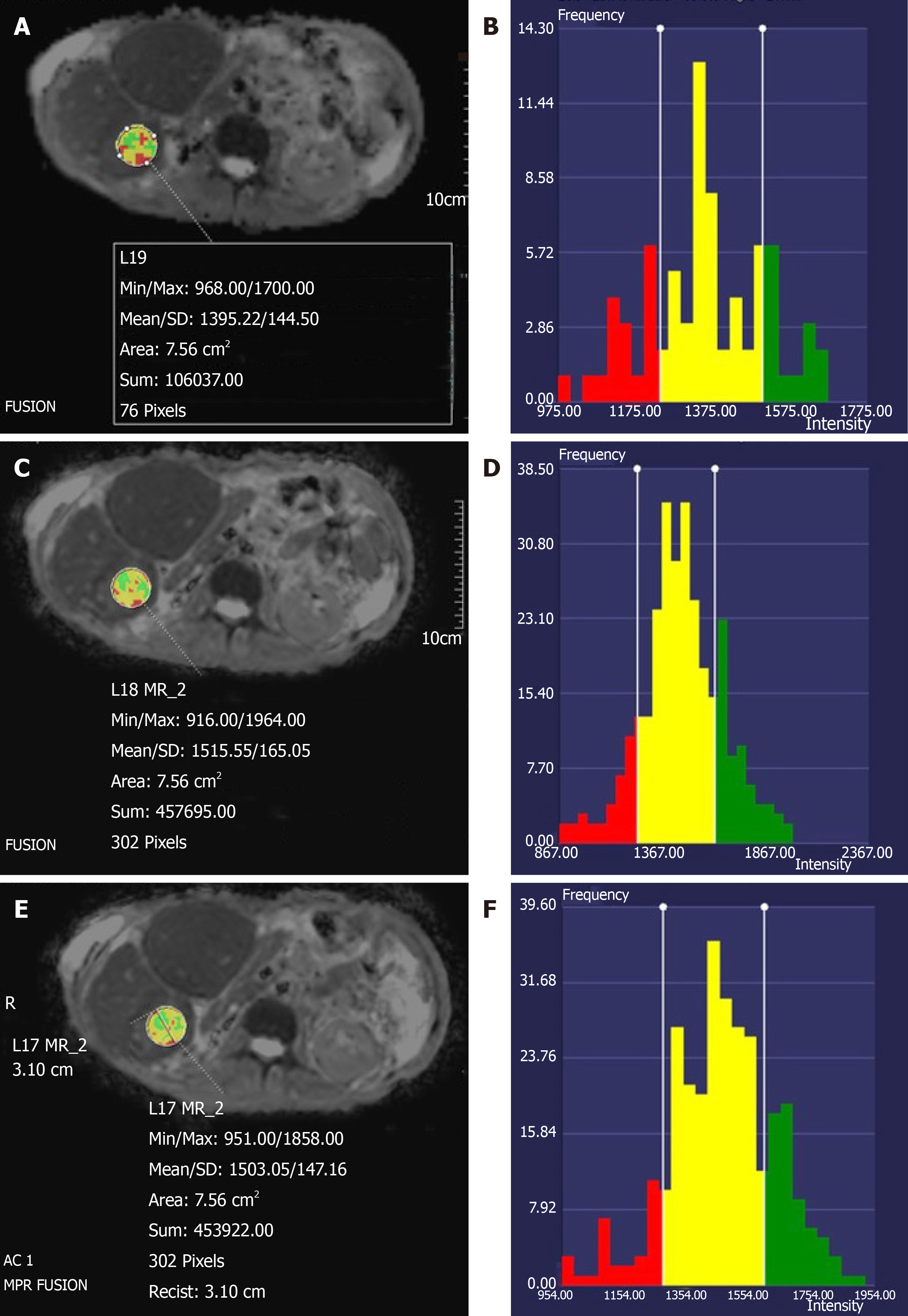
For each ROI, ADC histograms were generated using the vendor-provided software (Syngo.via, Siemens Healthineers). Two readers independently and qualitatively compared the ADC histograms from all 3 DWI sequences side-by-side on the basis of the ADC histogram distribution that reflected the tumor heterogeneity (e.g., “bell shape” vs irregular distributions). The histogram with the most usable pixels was considered the superior one; the readers were blinded to the type of DWI sequence during the histogram comparison.
For the quantitative analysis, the same radiologist (JS) placed an ROI for each liver lesion. For each ROI, the calculated mean ADC value was recorded and compared between sequences. The standard deviation (SD) of the ADC was also recorded and compared.
The image quality scores for each DWI sequence were summarized as frequencies and percentages. The image quality scores for each sequence were divided into 2 groups: Scores of 1 to 3 and scores of 4 or 5. The Wilcoxon signed-rank test was used to compare image quality scores between all pairs of 3 DWI sequences (e.g., PACE vs FB; PACE vs SMS; SMS vs FB).
A linear mixed model was used to estimate and compare the means and SDs of ADC values among the sequences. The SDs of ADC values were transformed to a logarithmic scale before analysis. The image quality scores were compared using estimates from a linear mixed model with patient and reader as random effects. Pairwise comparisons between sequences were conducted and 95% confidence intervals were determined. Agreement between the 3 readers was assessed for image quality score and ADC histogram preference. All tests were 2-sided, and P values of 0.05 or less were considered statistically significant. The statistical analysis was conducted using SAS software version 9.4 (SAS Institute, Cary, NC, United States).
A total of 20 patients were included in the study. Their liver lesion diagnoses are listed in Table 2. Of the 56 total liver lesions, 50 were malignant. The mean qualitative image quality score of PACE-DWI (4.48) was significantly better than that of SMS-DWI (4.22) and FB-DWI (3.15) (P < 0.05, Table 3 and Figure 1). For PACE-DWI, all readers agreed on a score of 4 or 5 in 80% (16/20) of patients. For SMS-DWI, all readers agreed on a score of 4 or 5 in 55% (11/20) of patients. For FB-DWI, all readers agreed on a score of 1 to 3 in 55% (11/20) of patients. The quality of the ADC histograms was considered the best for PACE-DWI in 28 of 56 lesions by Reader 1 and 35 of 56 lesions by Reader 2 (Figure 2). In general, the histogram with more pixels per ADC value was considered superior quality. The FB-DWI ADC histogram was considered superior for 2 of 56 lesions by Reader 1 and no lesions by Reader 2. There was no difference between Reader 1 and Reader 2 regarding the quality of the PACE-DWI or SMS-DWI histograms.
| Lesion type | No. of patients | No. of lesions |
| Liver cyst | 1 | 1 |
| Treated metastasis | 1 | 1 |
| Desmoid tumor | 2 | 2 |
| Hemangioma | 1 | 2 |
| Metastatic thyroid cancer | 1 | 3 |
| Metastatic pancreatic cancer | 1 | 4 |
| Metastatic colon cancer | 2 | 9 |
| Hepatocellular carcinoma | 5 | 11 |
| Metastatic neuroendocrine tumor | 6 | 23 |
| Total | 20 | 56 |
| Sequence | Mean Score1 | 95% LCL | 95% UCL |
| FB-DWI | 3.15 | 2.79 | 3.51 |
| PACE-DWI | 4.48 | 4.12 | 4.85 |
| SMS-DWI | 4.22 | 3.85 | 4.58 |
Quantitatively, the mean ADC values from the 3 different sequences did not significantly differ (Table 4). FB-DWI had a markedly higher variation in the SD of the ADC values than did SMS-DWI and PACE-DWI. We found statistically significant differences in the SDs of the ADC values for FB-DWI vs PACE-DWI (P < 0.0001) and for FB-DWI vs SMS-DWI (P = 0.03). For PACE-DWI vs SMS-DWI, the SD of the ADC value pair was -0.119 (P = 0.18).
| Sequence1 | N | Mean (mm2/s) | SD (mm2/s) | Min (mm2/s) | Median (mm2/s) | Max (mm2/s) |
| FB | 56 | 1313.51 | 387.44 | 654.00 | 1290.38 | 2506.33 |
| PACE | 56 | 1273.28 | 403.30 | 453.71 | 1216.42 | 2401.55 |
| SMS | 56 | 1269.14 | 442.84 | 425.12 | 1155.53 | 2457.30 |
| All | 168 | 1285.31 | 409.87 | 425.12 | 1211.17 | 2506.33 |
In this study, we found that the PACE-DWI and SMS-DWI techniques produced qualitatively better image quality than conventional FB-DWI. This was partially due to the presence of fewer artifacts, which in turn was likely a result of the shorter scan time for SMS-DWI[17,19] and the better respiration-triggering technique for PACE-DWI. We also note that TE for SMS-DWI and PACE-DWI is 11ms shorter than that of FB-DWI. The decrease in TE was enabled by the higher overall acceleration of SMS than parallel imaging alone and was likely helpful in improving the image SNR.
We noted that no patients in this study experienced a markedly irregular breathing pattern or motion during FB-DWI, which minimized the number of FB-DWI images that were substantially affected by artifacts. If the study population had included patients with irregular breathing patterns, we may have seen an even larger difference between the FB and SMS or PACE DWI sequences.
Qualitatively, all lesions were detected in all sequences. PACE-DWI had the best image quality according to all readers. PACE-DWI also had the highest level of agreement among the readers, with readers rating its image quality as 4 or 5 in 80% of cases. Kappa calculations of the level of inter-observer agreement were not performed because of the small sample size, so we believe that the percentage of agreement is an accurate representation of the results.
We also evaluated the mean ADC value for each lesion and evaluated ADC histograms instead of the SNR. The mean ADC values for each lesion did not significantly differ among the 3 sequences, suggesting that although the ADC maps were acquired using different techniques, they produced similar results and can be used interchangeably to characterize lesions and treatment response. We observed a trend towards similar mean ADC values for PACE-DWI, SMS-DWI and FB-DWI, which ranged from 1269 to 1313. Taouli et al[18] compared the ADC values of PACE-DWI and breath-hold-DWI for malignant lesions and found no statistically significant difference between the techniques[20].
We also compared the SDs of the ADC values. A larger SD may suggest that the technique produces more increased variability and decreased precision among ADC values. The SD of the ADC value was significantly smaller for PACE-DWI than for the other DWI techniques, suggesting that this technique has lower inherent variability. The variation in ADC values may also be due to the inherent heterogeneity of the liver lesions.
Similarly, Taouli et al[18] also concluded that the PACE-DWI sequence provides a more precise ADC value. However, they did not compare ADC histograms and used different b-values from those used in our study (0, 50 and 500 s/mm2). Boss et al[15] compared SMS-DWI and EPI-DWI for liver imaging[8], and Taron et al[17] evaluated SMS-DWI, both in healthy volunteers. Neither study compared the ADC histograms. Thus, an advantage of our study is that we were able to compare ADC values, and evaluate the precision of the ADC calculations.
It is well known that the source DWI images contain valuable information, especially for lesion detection. In our review, the ability of the sequences to detect lesions was similar. This may have been due to several factors. For instance, FB-DWI showed fewer artifacts, no lesions in our study population were located in the left liver (inferior to the heart), and the patients all breathed regularly. While ADC values are useful in characterizing liver lesions, in our patient population, most lesions were malignant; therefore, we could not determine the usefulness of ADC values for lesion characterization.
The ADC histogram provides additional data that can help in image interpretation. For example, a shift in the ADC histogram may be seen in treated liver lesions. In our study, the ADC histograms for each lesion were, by qualitative assessments, inferior for FB-DWI and superior for SMS-DWI and PACE-DWI. Recently, ADC histograms and calculated percentiles of the histograms have been used to differentiate types of metastases in the liver, tumor types[21], and tumor genomic profiles[22]. We found that the ADC histogram of PACE-DWI was superior to that of SMS-DWI, probably because of the improved SNR that results from longer scan time and better motion management through respiratory triggering. Because ADC histogram parameters ranging from the 10th through the 95th percentile of ADC have been used and are considered useful for assessment of tumors, it is imperative that the histogram have enough pixels to assess the tumor characteristics. Moreover, because a large SD indicates more variability than does a smaller SD, the smaller SD of the ADC values for PACE-DWI would be expected to provide a more robust tumor assessment, as it will produce similar and reliable values for different readers. To our knowledge, our study is the first to combine the SD value of ADC and ADC histogram for a comparison of these 3 different DWI sequences in malignant liver lesions.
There are some limitations to our study. We had a limited study population, with only 20 patients and 56 lesions. Most of the lesions were malignant. One lesion had been treated with local therapy, likely resulting in a heterogeneous ADC value for the ROI evaluated. Also, tumor necrosis, not the MRI technique alone, may have been responsible for some of the variability in ADC values. In addition, the studies were all performed on the 1.5-T scanners of a single vendor and using a single hardware and software platform. It is possible that the techniques may differ on different scanners and with different software. We were not able to use the kappa measure of inter-reader variability because of the small study population. However, we believe that the percentage of agreement provides a good representation of the results.
In conclusion, our study found that PACE-DWI provides a robust ADC histogram of liver lesions with less variability than the free breathing DWI techniques and a larger number of pixels for quantitative analysis. Therefore, this technique may provide a better characterization of the intrinsic diffusion characteristics of the tumor than that provided by FB-DWI and SMS-DWI. However, both PACE-DWI and SMS-DWI provided better image quality with fewer artifacts and less variability in the ADC values. These are valuable in assessing tumor treatment response and comparably better than the FB-DWI technique.
Diffusion-weighted imaging (DWI) in the body has several technical challenges. This include ghosting artifacts, mis-registration and susceptibility artifacts.
New DWI sequences have been developed to overcome some of these challenges. Our goal is to evaluate 3 new DWI sequences for liver imaging.
To compare the image quality and quantitative apparent diffusion coefficients (ADC) of 3 DWI sequences for in vivo liver imaging: Free-breathing (FB)-DWI, simultaneous multislice (SMS)-DWI, and prospective acquisition correction (PACE)-DWI.
Magnetic resonance imaging (MRI) of the abdomen was performed at 1.5 T on 20 patients with liver lesions in this Institutional Review Board-approved prospective study. The MR study included 3 separate DWI sequences: FB-DWI, SMS-DWI, and PACE-DWI. The image quality, mean ADC values, standard deviations (SD) of the ADC, and quality of the ADC histogram were compared. Wilcoxon signed-rank tests were used to compare qualitative image quality scores. A linear mixed model was used to compare the mean ADC values and the SDs of the ADC values. All tests were 2-sided and P values of 0.05 or less were considered statistically significant.
PACE-DWI had the highest mean image quality score (4.48), followed by SMS-DWI (4.22) and FB-DWI (3.15). The image quality of PACE-DWI was rated superior to that of SMS-DWI and FB- DWI (P < 0.03). The quality of the PACE-DWI ADC histograms were better than the SMS-DWI and FB-DWI. The SD of the ADC values was not statistically significant in terms of difference for PACE-DWI and SMS-DWI (P = 0.18), whereas FB-DWI had significantly more variation in the SD of its ADC.
PACE-DWI and SMS-DWI are equivalent in their ability to measure ADC. Compared to FB-DWI, both PACE-DWI and SMS-DWI provide better image quality and decreased variability in the quantitative diffusion measurement of liver lesions.
Therefore, this technique may provide a better characterization of the intrinsic diffusion characteristics of the tumor than that provided by FB-DWI and SMS-DWI. However, both PACE-DWI and SMS-DWI provided better image quality with fewer artifacts and less variability in the ADC values. These are valuable in assessing tumor treatment response and comparably better than the FB-DWI technique.
We thank Ms. Amy Ninetto of the Department of Scientific Publications, The University of MD Anderson Cancer Center, Mr. Bryce P. Starr and Ms. Palencia Lewis for their help in the preparation of documents.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bazeed MF, Gao BL, Kwok WE S-Editor: Ma RY L-Editor: A E-Editor: Qi LL
| 1. | Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, Rordorf G, Buonanno FS, Schaefer PW, Gonzalez RG. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268-2276. [PubMed] |
| 2. | Johnston KC, Wagner DP, Wang XQ, Newman GC, Thijs V, Sen S, Warach S; GAIN, Citicoline, and ASAP Investigators. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D, Hammoud DA, Rustin GJ, Taouli B, Choyke PL. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102-125. [PubMed] |
| 4. | Kim JH, Joo I, Kim TY, Han SW, Kim YJ, Lee JM, Han JK. Diffusion-Related MRI Parameters for Assessing Early Treatment Response of Liver Metastases to Cytotoxic Therapy in Colorectal Cancer. AJR Am J Roentgenol. 2016;207:W26-W32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Calistri L, Castellani A, Matteuzzi B, Mazzoni E, Pradella S, Colagrande S. Focal Liver Lesions Classification and Characterization: What Value Do DWI and ADC Have? J Comput Assist Tomogr. 2016;40:701-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Colagrande S, Castellani A, Nardi C, Lorini C, Calistri L, Filippone A. The role of diffusion-weighted imaging in the detection of hepatic metastases from colorectal cancer: A comparison with unenhanced and Gd-EOB-DTPA enhanced MRI. Eur J Radiol. 2016;85:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Willems SM, Koekkoek PS, Kwee TC, van den Bosch MA. Diffusion-weighted MRI of the liver for early tumor response assessment: Promising technique but evidence is still lacking. Acta Oncol. 2010;49:252-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Nakamura Y, Higaki T, Akiyama Y, Fukumoto W, Kajiwara K, Kaichi Y, Honda Y, Komoto D, Tatsugami F, Iida M, Ohmoto T, Date S, Awai K. Diffusion-weighted MR imaging of non-complicated hepatic cysts: Value of 3T computed diffusion-weighted imaging. Eur J Radiol Open. 2016;3:138-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Donati F, Boraschi P, Pacciardi F, Cervelli R, Castagna M, Urbani L, Falaschi F, Caramella D. 3T diffusion-weighted MRI in the response assessment of colorectal liver metastases after chemotherapy: Correlation between ADC value and histological tumour regression grading. Eur J Radiol. 2017;91:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Duran R, Ronot M, Kerbaol A, Van Beers B, Vilgrain V. Hepatic hemangiomas: factors associated with T2 shine-through effect on diffusion-weighted MR sequences. Eur J Radiol. 2014;83:468-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kwee TC, Takahara T, Niwa T, Ivancevic MK, Herigault G, Van Cauteren M, Luijten PR. Influence of cardiac motion on diffusion-weighted magnetic resonance imaging of the liver. MAGMA. 2009;22:319-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Mazaheri Y, Do RK, Shukla-Dave A, Deasy JO, Lu Y, Akin O. Motion correction of multi-b-value diffusion-weighted imaging in the liver. Acad Radiol. 2012;19:1573-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Bonekamp D, Bonekamp S, Halappa VG, Geschwind JF, Eng J, Corona-Villalobos CP, Pawlik TM, Kamel IR. Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol. 2014;83:487-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Corona-Villalobos CP, Halappa VG, Bonekamp S, Eng J, Reyes D, Cosgrove D, Rastegar N, Pan L, Pawlik TM, Kamel IR. Functional magnetic resonance imaging response of targeted tumor burden and its impact on survival in patients with hepatocellular carcinoma. Invest Radiol. 2015;50:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Boss A, Barth B, Filli L, Kenkel D, Wurnig MC, Piccirelli M, Reiner CS. Simultaneous multi-slice echo planar diffusion weighted imaging of the liver and the pancreas: Optimization of signal-to-noise ratio and acquisition time and application to intravoxel incoherent motion analysis. Eur J Radiol. 2016;85:1948-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Filli L, Ghafoor S, Kenkel D, Liu W, Weiland E, Andreisek G, Frauenfelder T, Runge VM, Boss A. Simultaneous multi-slice readout-segmented echo planar imaging for accelerated diffusion-weighted imaging of the breast. Eur J Radiol. 2016;85:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Taron J, Martirosian P, Erb M, Kuestner T, Schwenzer NF, Schmidt H, Honndorf VS, Weiβ J, Notohamiprodjo M, Nikolaou K, Schraml C. Simultaneous multislice diffusion-weighted MRI of the liver: Analysis of different breathing schemes in comparison to standard sequences. J Magn Reson Imaging. 2016;44:865-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Taouli B, Sandberg A, Stemmer A, Parikh T, Wong S, Xu J, Lee VS. Diffusion-weighted imaging of the liver: comparison of navigator triggered and breathhold acquisitions. J Magn Reson Imaging. 2009;30:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Kenkel D, Barth BK, Piccirelli M, Filli L, Finkenstädt T, Reiner CS, Boss A. Simultaneous Multislice Diffusion-Weighted Imaging of the Kidney: A Systematic Analysis of Image Quality. Invest Radiol. 2017;52:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Li Q, Wu X, Qiu L, Zhang P, Zhang M, Yan F. Diffusion-weighted MRI in the assessment of split renal function: comparison of navigator-triggered prospective acquisition correction and breath-hold acquisition. AJR Am J Roentgenol. 2013;200:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Payabvash S, Tihan T, Cha S. Volumetric voxelwise apparent diffusion coefficient histogram analysis for differentiation of the fourth ventricular tumors. Neuroradiol J. 2018;31:554-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Drevelegas K, Nikiforaki K, Constantinides M, Papanikolaou N, Papalavrentios L, Stoikou I, Zarogoulidis P, Pitsiou G, Pataka A, Organtzis J, Papadaki E, Porpodis K, Kougioumtzi I, Kioumis I, Kouskouras C, Akriviadis E, Drevelegas A. Apparent Diffusion Coefficient Quantification in Determining the Histological Diagnosis of Malignant Liver Lesions. J Cancer. 2016;7:730-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |