Published online Sep 28, 2018. doi: 10.4329/wjr.v10.i9.108
Peer-review started: April 27, 2018
First decision: June 14, 2018
Revised: August 6, 2018
Accepted: August 23, 2018
Article in press: August 24, 2018
Published online: September 28, 2018
Processing time: 154 Days and 6.6 Hours
To report 17-mo experience of femoral artery puncture site closure during angiographic procedures using ExoSeal vascular closure devices (VCDs).
Between November 2015 and April 2017, we performed 179 diagnostic and interventional angiographic procedures via a common femoral arterial access. The ExoSeal VCD was used at the puncture site to achieve hemostasis in 125 patients. We evaluated the technical and procedural success rates, the complications, and the factors affecting the hemostasis time of the ExoSeal VCDs.
Technical and procedural successes were achieved in 176 cases (98.0%) and 128 cases (71.5%), respectively. Device failure occurred in 3 (1.7%) cases. In 1 case (0.6%) a small hematoma developed, but there were no major complications. Among the hemostasis-relevant variables, a history of drinking alcohol, low platelet (PLT) count, and high prothrombin time-international normalized ratio (commonly known as PT-INR) values were the statistically significant predictors of the need for longer manual compression (MC). There was no difference in the success rates between the repeat and single ExoSeal procedure groups, and repeated use of the ExoSeal did not affect hemostasis time.
The ExoSeal VCD effectively achieves hemostasis, with few complications. Longer light MC may be needed with alcohol drinkers, low PLT count, and high PT-INR values.
Core tip: This study aimed to report the experience of closure of the femoral artery over a 17-mo period using ExoSeal vascular closure devices (VCDs). Technical and procedural successes were achieved in 176 cases (98.0%) and 128 cases (71.5%), respectively, while device failure occurred in only 3 (1.7%) cases. Repeated use of the ExoSeal did not affect hemostasis time or success rate. Thus, ExoSeal VCD is a simple, safe and effective device for achieving hemostasis with few complications. However, longer light manual compression may be needed with alcohol drinkers, low platelet counts, and high prothrombin time-international normalized ratio values.
- Citation: Han Y, Kwon JH, Park S. Korean single-center experience with femoral access closure using the ExoSeal device. World J Radiol 2018; 10(9): 108-115
- URL: https://www.wjgnet.com/1949-8470/full/v10/i9/108.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i9.108
Femoral arterial puncture using the Seldinger technique is the most common method of gaining vascular access for diagnostic and interventional arterial catheterization procedures. After the procedure, the gold standard method for achieving long-term hemostasis at the femoral arterial puncture site has been manual compression (MC). However, MC has limitations, such as prolonged hemostasis time, patient pain and discomfort, and a requirement for prolonged bed rest. Therefore, various hemostatic devices have been developed to improve the efficacy and safety of percutaneous intravascular treatment[1].
Vascular closure devices (VCDs) can be categorized into two main types, passive and active, according to their mechanisms of action. Passive VCDs include hemostasis pads and compression devices, while active VCDs include suture devices, collagen plug devices, and clips. Schwartz et al[2] have reviewed several different types of VCDs. Among these, the AngioSeal-which has been widely applied as an active VCD using collagen plugs such as ExoSeal-reduces hemostasis and ambulation time compared to MC; in addition, it significantly reduced the incidence of major complications.
The ExoSeal VCD (Cordis Corporation, Miami Lakes, FL, United States) is a collagen plug device of the active VCD category. It places a bioabsorbable polyglycolic acid (PGA) plug in the extravascular space at the top of the arterial incision. As far as we know, ExoSeal is the most recently developed VCD that has been approved by the United States Federal Drug Administration. The Ensure’s Vascular Closure Device Speeds Hemostasis Trial (ECLIPSE) studies were conducted to compare the safety and effectiveness of MC performed to promote hemostasis and early ambulation of patients experiencing percutaneous arterial, diagnostic or interventional procedures. In the ECLIPSE study, the success rate of the ExoSeal device was 94%. After an average of 2.5 h, ambulation was possible, and there was no significant difference between MC and the device in the incidence rate of complications[3]. The purpose of the present study was to report the experience of closure of the femoral artery using ExoSeal VCDs over a 17-mo period.
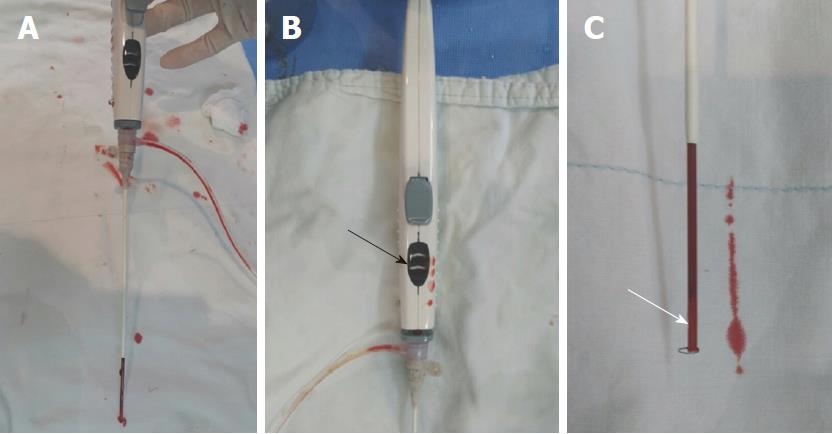
The ExoSeal VCD is an active VCD, collagen plug device. It consists of a plug applicator and a bioabsorbable PGA plug. The applicator places the plug on the external surface of the arteriotomy site of the femoral artery via the previously inserted vascular sheath. When the plug is placed on the arterial incision site, hemostasis is achieved. To ensure that the plug is positioned properly in the extravascular space, the ExoSeal delivery system has two markers that allow the operator to visually identify the location of the plug. These markers allow the operator to determine the position of the plug relative to the vessel wall before dropping the plug. The first indicator of the plug position is leakage of the pulsatile blood flow, and the second is a visual display on the handle.
When the VCD is inserted into and attached to the vascular sheath that has been pre-inserted into the artery, the indicator wire automatically forms a loop in the lumen of the blood vessel. When the VCD/sheath assembly is pulled back slightly at a 45° angle, the nontraumatic loop at the distal end of the indicator wire (Figure 1) contacts the inner surface of the vessel, causing the shape of the loop to change longitudinally. The predetermined displacement of the distal loop of the indicator wire indicates when the plug is placed just above the arterial incision. When the plug is in the correct position, the visual indicator on the handle then changes from white to black, indicating that it is ready for the plug to be dropped (Figure 2). Pulsatile blood leakage is significantly reduced or stopped at this point.
When the button on the handle is pressed, the plug is deployed in the proper position on the arteriotomy site. Then, the VCD and introducer sheath are removed, and the puncture site is lightly hand-pressed for a few minutes. The plug is absorbed considerably over 30 d after implantation and completely absorbed between 60 and 90 d.
In our institution, two interventional radiologists with at least 10 years of experience performed a total of 179 diagnostic and interventional angiographic procedures between November 2015 and April 2017. The radiologists used a common femoral arterial access with the ExoSeal VCD at the puncture site, in addition to angiographic control of hemostasis, in 125 patients (91 men, 34 women; mean age: 59.4 years, range: 20-87 years).
A total of 171 interventional angiographic procedures were performed in 117 patients. Among these patients, 24 underwent consecutive interventional procedures, with 23 treated with 76 transarterial chemoembolization (TACE) for hepatocellular carcinoma, ranging from two to six procedures per person. Nine patients received TACE twice, five patients were treated three times, three patients were treated four times, five patients were treated five times, and one patient was treated six times. One of the 24 consecutively treated patients underwent visceral arterial embolization for lower gastrointestinal bleeding twice. The rest of the 93 patients underwent interventional angiographic procedures. Eight patients underwent eight pure diagnostic angiographies. Five patients received interventions with antegrade femoral puncture. Ten patients were on anticoagulation therapy. One patient was taking warfarin, seven patients were taking aspirin, and two patients received 4000 IU heparin peri-interventionally.
Table 1 shows the patient and procedure characteristics. A total of 172 5-Fr ExoSeals were used in 118 patients. Six patients underwent peripheral interventional procedures requiring 6-Fr vascular sheaths, so that six 6-Fr ExoSeals were used. A 7-Fr ExoSeal was used in 1 patient who underwent percutaneous transluminal angioplasty and stenting in the common iliac artery.
| Characteristic | n = 179 |
| Age (yr) | 59.4 (20–87) |
| Men | 145 (81) |
| Hypertension | 59 (33) |
| Diabetes | 52 (29.1) |
| Smoking status | |
| Current smoker | 37 (20.7) |
| Ex-smoker | 53 (29.6) |
| Nonsmoker | 89 (49.7) |
| Alcohol intake | 57 (31.8) |
| Anticoagulation | 10 (5.6) |
| Aspirin | 7 (3.9) |
| Warfarin | 1 (0.6) |
| Heparin | 2 (1.1) |
| Interventional procedure | 171 (95.5) |
| Consecutive | 78 (43.6) |
| TACE for HCC | 76 (42.5) |
| Visceral embolization of gastrointestinal bleeding | 2 (1.1) |
| Single | 93 (51.9) |
| Diagnostic procedure | 8 (4.5) |
| Antegrade puncture | 5 (2.8) |
| ExoSeal | |
| 5-Fr | 172 (96.1) |
| 6-Fr | 6 (3.4) |
| 7-Fr | 1 (0.5) |
After completion of the procedure, the puncture site was closed using the ExoSeal VCD according to the manufacturer’s instructions for use, with light MC applied for 3 min. Three minutes later, if any blood leaked after the release of pressure then additional MC was performed for 2 min. If hemostasis was successful after 5 min of MC, compression bandages were not used. Otherwise, the puncture site was pressed by hand until the bleeding stopped completely (at least 10 min), followed by the application of a 2-h compressive bandage. All patients were prescribed 2 h of immobilization.
Technical success of the device was defined as successful placement of the plug in the puncture site without immediate major vascular complications associated with the VCD. Procedural success was defined as successful hemostasis without major vascular complications, and only 3 min of mild MC after successful deployment of the plug.
Complications were classified as either major or minor, as defined in the ECLIPSE trial[2]. Major complications were defined as: (1) needing vascular repair by surgical or nonsurgical techniques; (2) bleeding requiring a blood transfusion; (3) infection requiring antibiotics, extended hospitalization, or both; (4) new-onset ischemia of the ipsilateral lower extremity; (5) need for surgical repair of access-site-related nerve injury; and (6) permanent access-site-related nerve injury. Minor post-procedural complications were defined as: (1) recurrent local bleeding requiring hemostatic interventions, or hematomas ≤ 6 cm; (2) development of pseudoaneurysms, arteriovenous fistulae, vascular lacerations, or retroperitoneal bleeding; (3) ipsilateral manifestations of vascular insufficiency or embolization, including loss of distal pulse, total arterial occlusion, or deep vein thrombosis; (4) infection; and (5) nerve injury.
We recorded hemostasis-relevant patient history, access size, pre- or intraprocedural anticoagulation[4,5], laboratory findings [activated partial thromboplastin time (aPTT), platelet (PLT) count, and prothrombin time-international normalized ratio (PT-INR)], procedure characteristics, time to hemostasis, and any other complications. We reported the data as mean and standard deviation for continuous variables, and frequency (percentage) for categorical variables.
We analyzed the variables (patient age, sex, history of diabetes, hypertension, smoking or alcohol use, access size, and anticoagulation) using a logistic regression model to determine which variables require more than 3 min of MC to obtain hemostasis after using ExoSeal VCDs. Hemostasis-relevant laboratory tests (aPTT, PLT count, and PT-INR) were performed to determine the interrelation between the need for more than 3-min MC to achieve hemostasis, and the hemostasis-relevant parameters. We evaluated these parameters using the Mann-Whitney U test, also known as the Wilcoxon rank sum test, to evaluate the differences between the two groups based on a single, ordinal variable with no specific distribution. The Student's t-test and chi-square test were used to evaluate whether the frequency of ExoSeal use affected the time to hemostasis in patients on who ExoSeal was used several times. Results were considered statistically significant if a P-value < 0.05 was reached. All statistical analyses were performed using SPSS statistical software (version 20.0; IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY, United States).
Device failure occurred in 3 (1.7%) patients. One patient had hypodermal fat so thin that the deployed PGA plug was pushed out of the skin. In 2 patients, plug deployment failed due to product defects (Figure 3). In those 3 patients, hemostasis required MC for 10 to 30 min. The overall technical device success rate was 98.3%. There were no cases of intraluminal plug deployment.
Hemostasis after successful PGA plug deployment and MC < 3 min was achieved in 128 cases. Therefore, as defined above, the procedural success rate was 71.5%. In 3 cases (1.7%), the aforementioned technical failures did not result in procedural success. In 44 cases (24.6%), hemostasis was achieved with MC for an additional 2 min. So, in 172 cases (96.1%) hemostasis was achieved after successful plug deployment in less than 5 min. There were 7 (3.9%) cases that needed MC for > 5 min. The mean compression time for these 7 cases was 10.2 min. In 2 cases hemostasis was unsuccessful, despite prolonged MC. In 1 patient, the blood oozing continued after prolonged MC for 13 min. The other patient’s PLT count was 45 (× 103/μL) and blood oozing continued after plug deployment followed by MC for 10 min. Thus, compressive bandages were applied in these 2 cases and successful hemostasis was achieved after compression bandaging for 2 h.
Among the antegrade femoral punctures performed in 5 patients, one procedural failure occurred. The patient was on warfarin due to heart disease and was admitted due to bleeding from the right deep femoral artery. He underwent embolization of the right deep femoral artery with antegrade puncture of the right superficial femoral artery. Subsequent to embolization, it took 10 min to achieve hemostasis after successful ExoSeal plug deployment. The patient’s PLT count (170 × 109/L) was in the normal range but his PT-INR value (1.43) was slightly high. There were no major complications.
Of the 179 ExoSeal closure procedures, one (0.6%) minor complication (a 3-cm or smaller hematoma) occurred. The patient with the hematoma, who did not use an anticoagulant, underwent angiography through a 5-Fr vascular sheath. The laboratory results were in the normal ranges.
Table 2 shows the comparison of success rates between the repeated-procedure group and the single-procedure group. There were 78 cases of repeat procedures and 101 cases of single procedures. Successful plug deployment was achieved in 77 patients in the repeated-procedure group and in 99 patients in the single-procedure group, with technical success rates of 98.7% and 98.0%, respectively. Successful hemostasis with MC for 3 min was achieved in 52 cases (66.7%) in the repeated-procedure group and in 76 cases (75.2%) in the single-procedure group. Successful hemostasis with 5 min of MC occurred in 76 cases in the repeated-procedure group and in 96 cases in the single-procedure group. The success rates of hemostasis after 5 min of MC were 97.4% in the repeated-procedure group and 95.0% in the single-procedure group. The P-values of the Student’s t-test (Table 3) and chi-square test (Table 4), assessing whether the frequency of ExoSeal use affected hemostasis times for patients with repeated ExoSeal applications, were 0.606 and 0.137, respectively.
| Technical success | Procedural success | Success within 5 min | |
| Repeated-procedure, n = 78 | 77 (98.7) | 52 (66.7) | 76 (97.4) |
| Single-procedure, n = 101 | 99 (98.0) | 76 (75.2) | 96 (95.0) |
| Overall, n = 179 | 176 (98.3) | 128 (71.5) | 172 (96.1) |
| ExoSeal | n | Mean ± SD | P-value | |
| Hemostasis time | Repeat use | 78 | 4.79 ± 4.32 | 0.606 |
| Single use | 101 | 4.46 ± 4.38 |
| Cross table of hemostasis time and ExoSeal repeatability, P-value = 0.137 | |||||
| ExoSeal repeatability | Total | ||||
| Repeat use | Single use | ||||
| Hemostasis time | < 3 min | Frequency | 52 | 76 | 128 |
| % of hemostasis time | 40.6 | 59.4 | 100 | ||
| % of ExoSeal repeated use | 66.7 | 75.2 | 71.5 | ||
| % of total | 29.1 | 42.5 | 71.5 | ||
| > 3 min | Frequency | 26 | 25 | 51 | |
| % of hemostasis time | 51 | 49 | 100 | ||
| % of ExoSeal repeated use | 33.3 | 24.8 | 28.5 | ||
| % of total | 14.5 | 14 | 28.5 | ||
| Total | Frequency | 78 | 101 | 179 | |
| % of hemostasis time | 43.6 | 56.4 | 100 | ||
| % of ExoSeal repeated use | 100 | 100 | 100 | ||
| % of total | 43.6 | 56.4 | 100 | ||
Table 5 shows the multiple logistic regression analysis of the continuous variables. History of drinking alcohol (P = 0.01) and PT-INR (P = 0.001) were statistically significant predictors of a need for > 3 min of MC. The odds ratios for alcohol intake and PT-INR were 3.370 [95% confidence interval (CI): 1.345-8.440] and 0.065 (95%CI: 0.012-0.343), respectively. Table 6 shows the Wilcoxon rank sum test of hemostasis-relevant laboratory findings, yielding statistically significant correlations between the need for > 3 min of MC and PLT count (P = 0.032) and PT-INR (P = 0.037).
| B | P < 0.05 | OR | 95%CI for OR | ||
| Lower limit | Upper limit | ||||
| Women | 0.186 | 0.707 | 1.204 | 0.457 | 3.176 |
| Age | 0.013 | 0.354 | 1.013 | 0.985 | 1.042 |
| DM, yes | 0.666 | 0.135 | 1.946 | 0.813 | 4.662 |
| HTN, yes | -0.500 | 0.251 | 0.606 | 0.258 | 1.424 |
| Smoking | 0.000 | 0.241 | 0.000 | 0.000 | 0.000 |
| Ex-smoker | -0.752 | 0.094 | 0.471 | 0.196 | 1.137 |
| Current smoker | -0.280 | 0.599 | 0.756 | 0.266 | 2.149 |
| Alcohol use | 1.215 | 0.010 | 3.370 | 1.345 | 8.440 |
| 5-Fr | 0.000 | 0.559 | 0.000 | 0.000 | 0.000 |
| 6-Fr | -0.992 | 0.281 | 0.371 | 0.061 | 2.252 |
| 7-Fr | -21.226 | 1.000 | 0.000 | 0.000 | 0.000 |
| Anticoagulation | -0.447 | 0.599 | 0.640 | 0.121 | 3.386 |
| PT-INR | -2.732 | 0.001 | 0.065 | 0.012 | 0.343 |
| Variable | < 3 min | > 3 min | P-value | ||||
| n | mean ± SD | Median (min, max) | n | mean ± SD | Median (min, max) | ||
| aPTT | 100 | 36.54 ± 8.53 | 34.6 (22, 77.4) | 40 | 39.53 ± 10.13 | 36.25 (24.9, 71.7) | 0.108 |
| PLT count | 128 | 171.27 ± 93.64 | 172.5 (27, 706) | 51 | 140.37 ± 86.87 | 155.5 (28, 347) | 0.032a |
| PT-INR | 128 | 1.17 ± 0.18 | 1.11 (0.91, 1.93) | 51 | 1.26 ± 0.27 | 1.14 (0.97, 2.34) | 0.037a |
This study was conducted to report our experiences with the use of the ExoSeal VCD for femoral artery closure. Until recently, compressive bandages and MC have mainly been used in our intervention unit. Compression bandages have fewer limitations compared to MC, but they do have limitations, such as prolonged hemostasis time, patient pain or discomfort, and prolonged bed rest. Therefore, other VCDs were considered. Several studies have shown that various types of VCDs can achieve generally safe and effective hemostasis[1,6]. In addition, VCDs allow for early ambulation, thereby relieving patient discomfort and improving satisfaction and quality of life[6,7]. Among the VCDs, the ExoSeal is a collagen plug device belonging to the category of active VCDs. The ExoSeal VCD is an extravascular device that acts as a sealant to prevent blood from leaking by placing a bioabsorbable PGA plug directly into the vascular puncture site[8]. Several studies have been conducted on the efficacy of the ExoSeal VCDs in retrograde femoral artery closure as well as antegrade femoral artery closure[3,8-11].
Over a period of 17 mo, two interventional radiologists performed a total of 179 diagnostic and interventional angiographic procedures using ExoSeal VCDs to close 175 retrograde common femoral artery and 5 antegrade superficial femoral artery access sites. The manufacturer recommends a light MC of 2 or 3 min. We positioned the plugs, followed by MC for 3 min. Then, when MC pressure was released, if even scant blood seepage was observed, MC was performed for an additional 2 min. The overall technical success rate was 98.3%, but the procedural success rate was slightly disappointing at 71.5%. However, if we define the procedural success as 5 min instead of 3 min of MC, as recommended by the manufacturer, the procedural success rate will increases to 96.1%. Boschewitz et al[9] defined procedural success as successful plug deployment and MC for 5 min or less[12,13]. Their procedural success rate was 97.3%. Also, in the ECLIPSE study[3], that first introduced the ExoSeal in 2009, and in the 7-Fr ECLIPSE study[8], the use of a 7-Fr ExoSeal required only mild MC after plug placement and hemostasis occurred at about 4 min. None of the six major complications defined by the ECLIPSE trial[3] occurred in our study, and there were no minor complications other than a small groin hematoma > 3 cm in size.
Our study was a retrograde study, and we did not make any special exceptions when using the ExoSeal VCD. Therefore, we evaluated the factors affecting hemostasis time in cases of hemostasis obtained with > 3 min of MC in these 179 cases. In this study, the ExoSeal was used many times in the same patient. TACE was performed 76 times in 26 patients with liver cancer, and 1 patient underwent embolization twice because of lower gastrointestinal bleeding. When comparing the success rate of hemostasis between the repeated-procedure group (n = 78) and the single-procedure group (n = 101), the procedural success rates were 67.9% and 74.3%, respectively. This was not different from the overall procedural success rate of 71.5%.
If the definition of procedural success is broadened to 5 min of manual pressure, the success rates of the repeated-procedure group and the single-procedure group become 97.4% and 95.0%, respectively. This was not significantly different from the overall success rate of 96.1%. There was also no difference from the results of Boschewitz et al[13] (95.5% procedural success rate), in the report of their experiences using ExoSeal VCDs in 404 repeated closures. The P-values of the Student’s t-test and chi-square test for assessing whether the ExoSeal use frequency affected hemostasis time after multiple ExoSeal uses were 0.606 and 0.137, respectively. This indicated that repeated use of the ExoSeal did not affect the hemostasis times[14].
According to the data from the multiple logistic regressions of the hemostasis-relevant variables (patient age, sex, diabetes, hypertension, smoking, and alcohol history, access size, and anticoagulation), only history of drinking alcohol and PT-INR were statistically significant predictors of the need for > 3 min of MC (Table 5). Anticoagulation or access size did not affect hemostasis time. Also, the PLT count (P = 0.032) and PT-INR (P = 0.037), among the hemostasis-relevant laboratory findings (aPTT, PLT count, and PT-INR), exhibited statistically significant correlations with the need for > 3 min of MC by the Wilcoxon rank sum test. Therefore, it is predictable that history of drinking alcohol, low PLT counts, and high PT-INR values can lead to a requirement for > 3 min of MC. Schelp et al[15] reported the experience of using ExoSeal VCDs in 801 patients who underwent coronary angiography and interventions through the femoral artery. In that article, lengthy procedures, percutaneous coronary interventions, use of GP IIb/IIIa inhibitors, and elderly age of patients were the strongest independent predictors of bleeding/vascular complications and device failure.
Our study was limited by the fact that the number of antegrade femoral punctures (n = 5) was too small to compare to the number of retrograde femoral punctures (n = 174). Among the 5 patients who underwent the antegrade femoral puncture, 1 procedural failure occurred. In the remaining 4 patients, hemostasis was achieved with a 3-min MC. There were no complications among these 5 cases. According to several other articles on the use of ExoSeal in antegrade femoral punctures, ExoSeal VCDs were found to be safe and effective, with high technical success rates and acceptable complication rates in antegrade procedures[9-11,16,17]. Another limitation was that we did not analyze the failure rate according to the operator’s learning curve because the ExoSeal was so easy to use.
In summary, the ExoSeal VCD is a very simple, safe and effective hemostasis device for both antegrade and retrograde femoral punctures, and is associated with few complications. In addition, the ExoSeal VCD is an instrument that can effectively obtain hemostasis even when used repeatedly in the same patient. However, longer light MC may be needed in patients with history of drinking alcohol, low PLT counts, and high PT-INR values.
The gold standard method for achieving hemostasis at the femoral arterial puncture site has been manual compression (MC). However, MC has limitations, such as prolonged hemostasis time, patient pain and discomfort, and a requirement for prolonged bed rest. Therefore, various hemostatic devices have been developed to improve the efficacy and safety of percutaneous intravascular treatment and one of those hemostasis devices is ExoSeal.
We have used MC or other compressive devices (such as sand bags or balloon compressive devices) before introducing ExoSeal into our clinical practice. When we first started to use ExoSeal, we began studying the cases in which it was applied.
To report the experience of closure of the femoral artery using ExoSeal vascular closure devices (VCDs) over a 17-mo period.
We evaluated the technical and procedural success rates, complications, and factors affecting the hemostasis time of the ExoSeal VCDs.
Technical and procedural successes were achieved in 176 cases (98.0%) and 128 cases (71.5%), respectively. In one case (0.6%), a small hematoma developed, but there were no major complications. A history of drinking alcohol, low platelet (PLT) count, and high prothrombin time-international normalized ratio (PT-INR) values were the statistically significant predictors of the need for longer MC.
The ExoSeal VCD is a simple, safe and effective device for hemostasis of femoral punctures. In addition, the ExoSeal VCD is an instrument that effectively achieves hemostasis with few complications, even when used repeatedly in the same patient. However, longer light MC may be needed in patients with a history of drinking alcohol, low PLT count, and high PT-INR values.
Based on this and other studies, ExoSeal is expected to improve further and other similar VCDs will be developed.
Manuscript source: Unsolicited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gao BL, Paraskevas KII S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Eggebrecht H, Haude M, Woertgen U, Schmermund A, von Birgelen C, Naber C, Baumgart D, Kaiser C, Oldenburg O, Bartel T. Systematic use of a collagen-based vascular closure device immediately after cardiac catheterization procedures in 1,317 consecutive patients. Catheter Cardiovasc Interv. 2002;57:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Schwartz BG, Burstein S, Economides C, Kloner RA, Shavelle DM, Mayeda GS. Review of vascular closure devices. J Invasive Cardiol. 2010;22:599-607. [PubMed] |
| 3. | Wong SC, Bachinsky W, Cambier P, Stoler R, Aji J, Rogers JH, Hermiller J, Nair R, Hutman H, Wang H; ECLIPSE Trial Investigators. A randomized comparison of a novel bioabsorbable vascular closure device versus manual compression in the achievement of hemostasis after percutaneous femoral procedures: the ECLIPSE (Ensure’s Vascular Closure Device Speeds Hemostasis Trial). JACC Cardiovasc Interv. 2009;2:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Kim HY, Choo SW, Roh HG, Han H, Kim SS, Lee JY, Park YR, Lee SH, Shin SW, Park KB. Efficacy of femoral vascular closure devices in patients treated with anticoagulant, abciximab or thrombolytics during percutaneous endovascular procedures. Korean J Radiol. 2006;7:35-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Resnic FS, Blake GJ, Ohno-Machado L, Selwyn AP, Popma JJ, Rogers C. Vascular closure devices and the risk of vascular complications after percutaneous coronary intervention in patients receiving glycoprotein IIb-IIIa inhibitors. Am J Cardiol. 2001;88:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Robertson L, Andras A, Colgan F, Jackson R. Vascular closure devices for femoral arterial puncture site haemostasis. Cochrane Database Syst Rev. 2016;3:CD009541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Duffin DC, Muhlestein JB, Allisson SB, Horne BD, Fowles RE, Sorensen SG, Revenaugh JR, Bair TL, Lappe DL. Femoral arterial puncture management after percutaneous coronary procedures: a comparison of clinical outcomes and patient satisfaction between manual compression and two different vascular closure devices. J Invasive Cardiol. 2001;13:354-362. [PubMed] |
| 8. | Wiemer M, Langer C, Fichtlscherer S, Firschke C, Hofbauer F, Lins M, Haude M, Debèfve C, Stoll HP, Hanefeld C. First-in-man experience with a new 7F vascular closure device (EXOSEAL™): the 7F ECLIPSE study. J Interv Cardiol. 2012;25:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Boschewitz JM, Pieper CC, Andersson M, Nadal J, Schild HH, Meyer C. Efficacy and time-to-hemostasis of antegrade femoral access closure using the ExoSeal vascular closure device: a retrospective single-center study. Eur J Vasc Endovasc Surg. 2014;48:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Schmelter C, Liebl A, Poullos N, Ruppert V, Vorwerk D. Suitability of Exoseal vascular closure device for antegrade femoral artery puncture site closure. Cardiovasc Intervent Radiol. 2013;36:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Maxien D, Behrends B, Eberhardt KM, Saam T, Thieme SF, Reiser MF, Treitl M. Evaluation of the 6-F ExoSeal vascular closure device in antegrade femoral artery punctures. J Endovasc Ther. 2012;19:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Biancari F, D’Andrea V, Di Marco C, Savino G, Tiozzo V, Catania A. Meta-analysis of randomized trials on the efficacy of vascular closure devices after diagnostic angiography and angioplasty. Am Heart J. 2010;159:518-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Boschewitz JM, Andersson M, Naehle CP, Schild HH, Wilhelm K, Meyer C. Retrospective evaluation of safety and effectiveness of the EXOSEAL vascular closure device for single vascular closure and closure after repeat puncture in diagnostic and interventional radiology: single-center experience. J Vasc Interv Radiol. 2013;24:698-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hieb RA, Neisen MJ, Hohenwalter EJ, Molnar JA, Rilling WS. Safety and effectiveness of repeat arterial closure using the AngioSeal device in patients with hepatic malignancy. J Vasc Interv Radiol. 2008;19:1704-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Schelp V, Freitag-Wolf S, Hinzmann D, Bramlage P, Frey N, Frank D. Large-scale experience with an anchorless vascular closure device in a real-life clinical setting. Clin Res Cardiol. 2015;104:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Hackl G, Gary T, Belaj K, Hafner F, Rief P, Deutschmann H, Brodmann M. Exoseal for puncture site closure after antegrade procedures in peripheral arterial disease patients. Diagn Interv Radiol. 2014;20:426-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Rimon U, Khaitovich B, Yakubovich D, Bensaid P, Golan G, Silverberg D. The Use of ExoSeal Vascular Closure Device for Direct Antegrade Superficial Femoral Artery Puncture Site Hemostasis. Cardiovasc Intervent Radiol. 2015;38:560-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |