INTRODUCTION
Gastrointestinal tumors (GTs) are common tumors of the digestive system and are among the leading causes of cancer death worldwide[1]. It was estimated that there will be 176960 new cases and 62880 deaths in United States in 2018[2]. The current therapeutic methods for GTs are surgical resection and chemotherapy and/or chemoradiotherapy (CRT)[3]. However, surgery inflicts substantial trauma to both the body and mind. For advanced stage patients, systemic chemotherapy is the better choice. In this situation, imaging monitoring for the chemotherapeutic response and evaluation is essential. Magnetic resonance imaging (MRI) is a promising modality for tumor detection, diagnosis and evaluation, because of its many advantages, including absence of radiation, multiplane and multiple-parameter imaging. Diffusion weighted imaging (DWI) is an important imaging sequence, based on the Brownian motion of water molecules[4]. The apparent diffusion coefficient (ADC) is sensitive to water molecules in the tissues and reflects the microenvironmental changes of tumors[5]. It is used to differentiate malignant from benign tumors and to evaluate tumor responses after chemotherapy[6,7]. However, ADC values of the tumor are not consistent after treatment because treatment may cause cell swelling or fibrosis that decreases ADC values[8]. Based on the ADC reflection of the tissue diffusion and perfusion, Le Bihan et al[9] proposed the intravoxel incoherent motion (IVIM) model to depict perfusion and diffusion effects. It is a new dual exponential imaging mode with multiple b values that is applied in oncologic imaging and related studies[10,11]. With multiple b values, IVIM-DWI quantifies microvascular perfusion effects with smaller b-values (0-200 s/mm2), and quantifies tissue water molecular diffusion with higher b-values (> 200 s/mm2). Therefore, IVIM-DWI MRI differentiates microvascular perfusion activity from diffusion. Consequently, the following parameters derived from IVIM DW-MRI can be calculated without the contrast: (Slow) diffusion coefficient (D), pseudo-perfusion (fast) diffusion coefficient (D*) and perfusion fractions, (f)[9,12]. The f and D* parameters have the potential to reflect tumor angiogenesis activity noninvasively and are significantly correlated with microvessel density (MVD) scores[13]. D is generally thought to be the pure diffusion coefficient that depicts extracellular and extravascular tissue water molecular motion[14]. Since IVIM-DWI was introduced, this technique showed great potential in tumor evaluation and grading[15,16]. In recent years, IVIM-DWI has also been used to distinguish benign from malignant tumors[17] and to evaluate chemotherapy therapeutic responses in various tumors[10,18]. However, the application of IVIM-DWI in the gastrointestinal tract tumors may be challenged: first, gastrointestinal tract tumors are relatively small and thin, and imaging may be affected by motion artifact; second, the exact nature of the IVIM signal is not well-understood, and there are heterogeneous patterns of signal attenuation on a voxelwise basis in normal tissues and tumors[19,20]; third, the sensitivity and specificity of these parameters used in the evaluation of GTs vary and the results of the IVIM in the GTs are discrepant[15,18]; finally, the variability of IVIM measurements in response to chemotherapy and/or radiotherapy in these studies remains controversial[10]. Nevertheless, the following findings in GTs may dispel our worries to some extent and may provide hope for the use of IVIM for GTs.
GASTRIC TUMORS
Few studies reported the IVIM-DWI was used in the gastric cancers, because gastric cancer can be diagnosed by contrast computed tomography (CT)/MRI[21] and may be confirmed by endoscopy and biopsy[22]. Nevertheless, IVIM has been used to evaluate the biological behavior of gastric cancer, including cell proliferation, differentiation, invasion, metastasis and survival[23-25], as well as to monitor chemotherapeutic responses[10,26]. D* and f values correlated with MVD in tumor tissues[13], suggesting that D* and f might serve as imaging markers for the noninvasive evaluation of MVD of tumor grading and treatment effectiveness.
In fifty-three patients with gastric cancer reported by Ji et al[25], the D value positively correlated with human epidermal growth factor receptor-2 (HER2) scores (r = 0.481, P < 0.001), and the D values of HER2(+) gastric cancers were substantially higher than those of HER2(-) tumors (P = 0.007). With the cut-off value of 1.123 × 10-3 mm2/s, the D value differentiated HER2(+) from HER2(-) gastric cancers with an area under the curve of 0.762 (P = 0.011). Therefore, the IVIM-DWI is feasible for preoperative assessment of HER2 status of gastric cancers and could be a potential biomarker in evaluating HER2 status of gastric cancers.
As for chemotherapeutic responses, IVIM-DWI and derived parameters are useful for predicting the early efficacy of chemotherapy and are more sensitive imaging biomarkers for gastric cancer. In mouse models bearing two kinds of human gastric cancer xenografts, and in human gastric adenocarcinoma AGS cells models (baseline: day 0), when the mouse received 5-fluorouracil (5-FU) (15 mg/kg)/calcium folinate (5 mg/kg) treatment, mean D values in the treated groups (ΔD%: 17.12% ± 8.20%, 24.16% ± 16.87%, 38.54% ± 19.36%) were significantly higher than those of the controls (ΔD%: -0.13% ± 4.23%, 5.89% ± 4.56%, 5.54% ± 4.44%) at days 1, 3, 5 and 7. The f values were significantly lower than those of the control group (-34.13% ± 16.61% vs 1.68% ± 3.40%; -50.64% ± 6.82% vs 3.01% ± 6.50%; -49.93% ± 6.05% vs 0.97% ± 4.38% and -46.22% ± 7.75% vs 8.14% ± 6.75%). The D* values were also significantly lower than those of the control group at all-time points (-32.10% ± 12.22% vs 1.85% ± 5.54%; -44.14% ± 14.83% vs 2.29% ± 10.38%; -59.06% ± 19.10% vs 3.86% ± 5.10% and -47.20% ± 20.48% vs 7.13% ± 9.88%). Furthermore, the histopathologic findings showed that D positively correlated with tumor necrosis and cellular apoptosis. Values of f and D* correlated positively with MVD and negatively correlated with cellular apoptosis[26]. In MKN-45 human gastric adenocarcinoma xenograft mouse models, after fluorouracil and calcium folinate treatment, D* values in the treated group decreased markedly (ΔD*% = -30%, -34% and -20%, P < 0.05) and f values increased dramatically (Δf % = 93%, 113% and 181%, P < 0.05) on days 3, 7 and 14. D* and f values correlated well with histopathological changes demonstrating the reduction of cell proliferation and MVD and the increase in tumor apoptosis and necrosis[10]. These findings indicated that IVIM-DWI and derived parameters could be potentially useful for early evaluation of chemotherapy response and may provide additional pivotal information for the evaluation of therapeutic effect in gastric tumors (Figure 1).
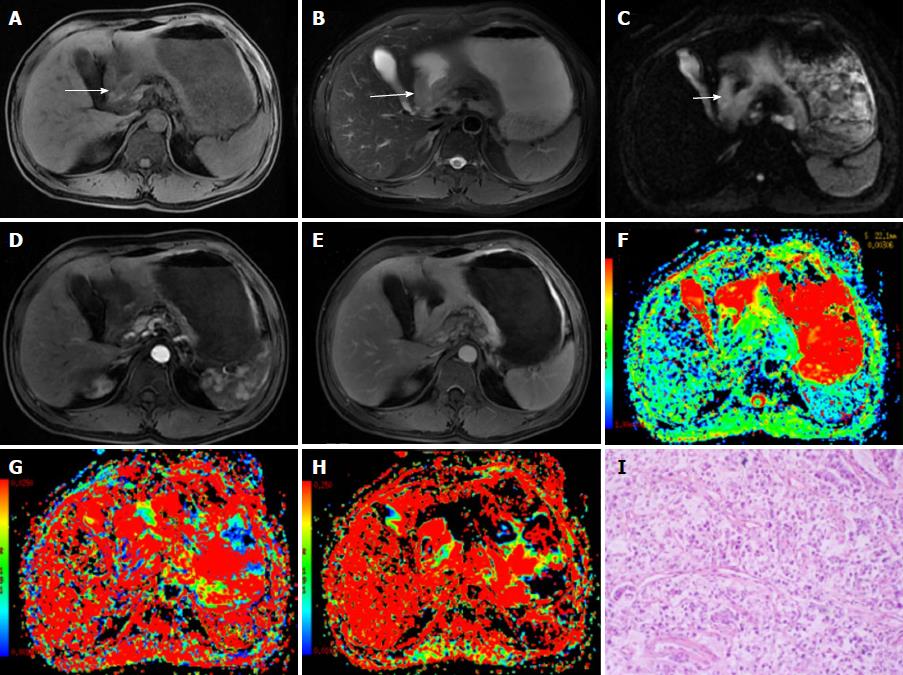
Figure 1 A 48-year-old male diagnosed with malignant gastric carcinoma (signet ring cell cancer).
A, B: The lesion has slightly low signal on T1-weighted image (A) and slightly high signal intensity on T2-weighted image (B); C: On DWI, the cancer shows hyperintensity (white arrows); D, E: After contrast agent injection, the lesion shows mild-to-moderate enhancement in arterial and portal venous phases; F-H: The pseudocolor maps of D, D* and f derived from IVIM were displayed, the values of the D, D* and f are 0.92 ± 0.11 × 10-3 mm2/s, 26.75 ± 13.61 × 10-3 mm2/s and 17.24% ± 4.8%, respectively; I: The HE staining of the tissues (100 ×).
COLORECTAL TUMORS
Many studies focusing on the new techniques for the evaluation of the colorectal tumors have been published[27,28]. A newly proposed modified VARiable PRO jection (VARPRO) algorithm specifically tailored for fitting the IVIM to DWI showed better performance than did the Levenberg-Marquardt (LM) algorithm in 64% of cases and stronger “segmented” methods in 100% of cases in locally advanced rectal cancer (LARC). Therefore, VARPRO algorithm is a better fit for the IVIM model than is LARC DWI[28].
In colorectal tumors, IVIM and the parameters were used to evaluate tumor histopathology, Kirsten rat sarcoma viral oncogene homologue (KRAS) mutation status, CRT response, pathological response to neoadjuvant chemotherapy (NACT) and relationships with tumor prognostic markers[11,29-32].
IVIM-DWI has been explored in genomic expression experiments to predict the genotype of rectal cancer (KRAS mutant/wild type)[29]. D values were significantly lower and D* values were significantly higher in the KRAS mutant group than in the KRAS wild-type group. According to the ROC curve, D* values displayed moderate diagnostic significance with the area under curve (AUC) values of 0.710. The cut-off value of D* was 26.58 × 10-3 mm2/s. The findings suggest a relatively high tumor cellularity and hypervascularity[13] caused by mutation of the KRAS oncogene[29].
IVIM parameters correlated with histopathology of rectal tumor tissues. D values were more likely to correlate with cell count, Ki-67 index and total nucleic area. The f values showed good correlation with stained vessel area, total vessel area and vessel count. D* values correlated with mean vessel diameter[33]. These findings confirmed that D reflected cell structure and water motion, and D* and f values reflect the vessel microenvironment[11,13].
In rectal non-mucinous carcinoma and mucinous carcinoma, D, D* and f distinguished rectal tumor tissues from normal rectal wall, reflecting tumor tissue cellularity and microenvironment changes. Furthermore, lower f values were observed in poorly differentiated non-mucinous carcinoma and there were significant positive correlations with differentiation degree. This may be related to the fewer glands and glandular architecture in poorly differentiated tumors. D values were higher and D* values were lower in mucinous carcinomas than in non-mucinous carcinomas. Interestingly, correlation analysis showed D and D* had significant correlations with histological type. D was more likely to be related to cellular microstructure than to tumor cellularity[11], and D* actually reflected blood flow and was affected by flow velocity and vascular geometry[34]. IVIM-DWI–derived parameters were also useful for describing rectal tumor aggressiveness and prognosis[31]. D* and f tended to increase with greater tumor differentiation, and D and D* decreased with advanced tumor stages. The f is the partial volume of the whole capillary vascular fraction[9] and the proportion of the arterial blood is greater than the venous component for f at low b values[35]. This phenomenon is reflected in well-differentiated tumors and poorly differentiated tumors: the capillary vascular network is relatively mature in the former, and there is poor structure of luminal vessels leading to low perfusion of the microcirculation in the latter[31]. This could be confirmed by the correlation between the D*, f and MVD in colorectal tumors[13]. Another important finding is that tumor invading the vascular wall had lower D* than did the group with no vascular wall invasion. This suggested that D* may be related to tumor stage. IVIM parameters were associated with some critical clinical indices, such as carcinoembryonic antigen (CEA) and CA199, which are related with prognosis[31].
Another important application of IVIM-DWI is monitoring the therapeutic response. Sun et al[36] reported repeatability coefficients for 3.0T MRI in rectal cancer: correlations for D, f, and D* were 11.1%, 55.4%, and 40.3%, for intraobserver analysis, respectively, and were 41.6%, 134.0%, and 177.6%, for interobserver analysis, respectively. The test-retest repeatability coefficients for D, f, and D* were 24.5%, 126.3%, and 197.4%, respectively, larger than the intraobserver values. Therefore, D value showed better short-term test-retest reproducibility than did f or D*. The authors concluded that f and D* variance should be understood prudently in longitudinal studies on rectal cancer in which treatment response is monitored[36]. In a report including 25 consecutive patients with advanced rectal carcinoma, D values were highest in the rectum (1.29 × 10-3 mm2/s), then the tumor (0.96 × 10-3 mm2/s) and fat (0.37 × 10-3 mm2/s), and the f values were lower notably in tumor (9.12%) than in fat (16.05%) in patients not receiving neoadjuvant CRT. In patients receiving neoadjuvant CRT, D was higher in tumor (1.10 × 10-3 mm2/s) and the rectum (1.26 × 10-3 mm2/s) than in fat (0.33 × 10-3 mm2/s).
For patients not receiving CRT, the vascular area fraction negatively correlated with D and positively correlated with f. For the rectum, D negatively correlated with cellularity C in patients after CRT[11]. The findings implied that D is related to tumor tissue activity that is frequently strongly vascularized[37] and indicate the heterogeneous tumor tissue microenvironment. Furthermore, the correlation between D and cellularity C reflects the cellular microenvironment in the tumor, adjacent rectal wall and fat that affects water molecule Brownian motion directly[11]. In another report with 31 patients with rectal cancer[18], median D values increased remarkably pre- and post-CRT and were much higher in good responders to CRT. The median D was lower than the median ADC before and after CRT. The relative change was significantly greater in the good responders than in poor responders. Median D values showed higher AUCs than did ADC values for treatment response evaluation. This was because perfusion contributes to ADC in rectal cancer[38] and microcirculation or perfusion effects can be identified by true tissue diffusion with sufficient b value sampling and bi-exponential curve fit analysis with IVIM[9]. Nevertheless, median f and D* values change before and after CRT were not consistent with the degree of tumor response. This may be the limitation of D*, due to its high uncertainty and poor reproducibility[39]. Similar results were reported by Lu et al[40] and Xu et al[41]. In these two studies, the IVIM-derived D value was a promising tool for predicting and identifying the pCR status prior to therapy. The D percentage changing values after therapy may be helpful and more accurate than traditional DWI for assessing pCR status.
Interestingly, in LARC before and after NACT, high tumor f was found to be useful for predicting better tumor response (tumor regression grade, TRG1-2) and the sensitivity and specificity was 69% and 100%, respectively. More importantly, f combined with tumor volume (fpre/Vpre) offered the best prediction of poor tumor response with a sensitivity of 88% and specificity of 91%, as well as 5-year progression-free survival (PFS) (P < 0.01)[30]. These findings indicated that high f suggests tumor tissues with good vascular structures, and low f indicates poor vascular structures; and high f has been shown to be related to pathologic complete response (pCR)[30] (Figure 2).
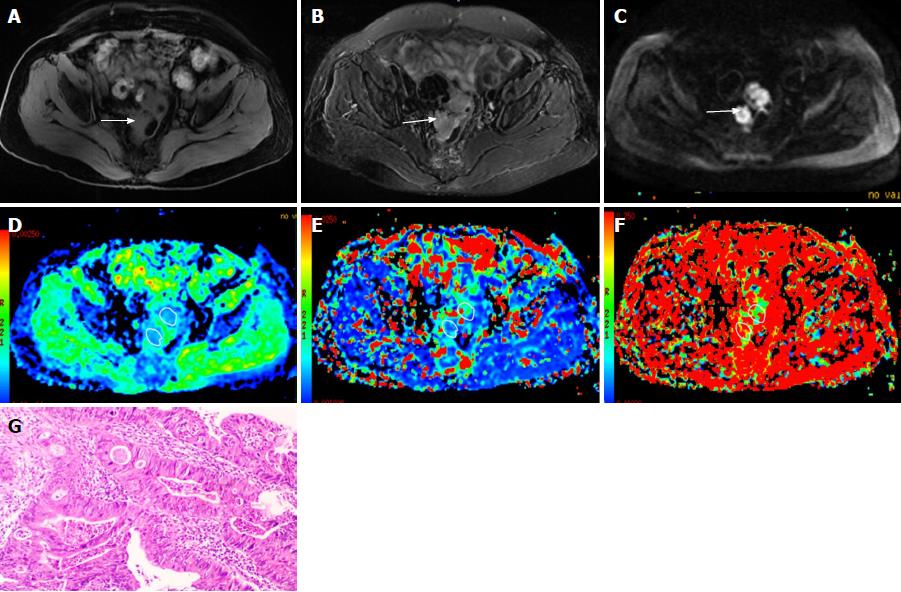
Figure 2 A 67-year-old female diagnosed as rectal cancer (poorly differentiated adenocarcinoma).
A, B: The rectal cancer is isointense on T1-weighted image (A) with slightly high signal intensity on T2-weighted image (B); C: On diffusion weighted imaging, the cancer shows hyperintensity (white arrows); D-F: The pseudocolor maps of D, D* and f derived from intravoxel incoherent motion are displayed, the values of the D, D* and f were 1.03 ± 0.12 × 10-3mm2/s, 50.35 ± 24.96 × 10-3mm2/s and 20.37% ± 5.9%, respectively; G: HE staining of the tissues (100 ×).
METASTATIC LESIONS
The parameters derived from IVIM-DWI are currently used for diagnosis of metastases, intra-tumor changes and therapeutic responses.
In metastasis diagnosis, IVIM-DWI may be useful in differentiating metastatic and non-metastatic lymph nodes in patients with rectal carcinoma. In metastatic lymph nodes, because of the increased water molecular diffusion and microperfusion, reduced cellular density and increased tumor-related blood vessels within the metastatic lymph node, mean D and f values increased significantly, whereas mean D* values were lower than those of normal lymph nodes. The lower D* values may be due to the low blood velocity and MVD of tumor tissues at low b value (< 200 s/mm2)[34]. Among the parameters, D values and D values combined with the short-axis diameter had the highest AUC, and D* values had the lowest[34,42], suggesting that D is more sensitive and has the highest diagnostic efficacy in distinguishing normal from lymphatic metastasis[15].
In assessment of therapeutic responses of metastatic lesions, parameters derived from IVIM-DWI changes are thought to be surrogate markers of tumor therapeutic responses. IVIM-DWI is usually sensitive to tumor necrosis after chemotherapy, because ADC in CRC metastases change along with specific increases in free molecular diffusion D that correlates with tumor necrosis[43]. In another report, distant metastasis lesions had higher D* and relative perfusion (fD*) values, suggesting that IVIM parameters might reflect different clinical and histopathological features in rectal cancer[33], although there were no significant differences between other IVIM parameters.
In patients with liver metastases from CRC treated with cytotoxic chemotherapy reported by Kim et al[44], after the first cycle of chemotherapy, ADC values increased (1191.9 ± 232.2 × 10-3 mm2/s vs 1263.5 ± 266.4 × 10-3 mm2/s; P = 0.012) and D (1085.9 ± 232.9 × 10−3 mm2/s vs 1173.5 ± 248.9 × 10-3 mm2/s; P = 0.012), while f values decreased (173.7% ± 39.8% vs 133.5% ± 28.3%; P = 0.017) in eight responding patients. In 24 responding metastatic lesions and 12 non-responding lesions after neoadjuvant FOLFIRI (5-FU, leucovorin, irinotecan) plus bevacizumab therapy, f values showed statistically significant differences between responder and non-responder lesions, and the f variation sensitivity and specificity were 62% and 93%, respectively[45]. All findings indicated that IVIM-DWI and the parameters were useful for the prediction of therapeutic response after chemotherapy for metastases in CRC.
GASTROINTESTINAL STROMAL TUMORS
IVIM-DWI in gastrointestinal stromal tumors (GISTs) was also investigated to evaluate therapeutic responses to treatment with imatinib. In mice with xenografts bearing GIST-T1 cells, ADC values increased in the treated group. D* values in the treated group decreased significantly (ΔD* = -41%, -49%, and -49%), and f increased significantly (Δf = 79%, 82% and 110%) on days 1, 3 and 7 after treatment. D* and f did not show significant changes in the control group. The parameters from IVIM-DWI showed good correlation with histopathology with a decrease in cell proliferation and MVD and an increase in apoptosis and tumor necrosis in the treated group[46]. Therefore, IVIM-DWI may serve as an effective imaging biomarker to assess GIST response to treatment.
CONCLUSION
It is a great challenge to evaluate and predict histopathological and therapeutic responses after GTs chemotherapy. IVIM, a new sequence derived from DWI, is a potentially useful tool for evaluation of GTs. The derived parameters D, D* and f reflect the microenvironment, microcirculation and blood flow changes in tumor tissues[34], endowing them with the ability to predict tumor pathology and to monitor therapeutic responses. Therefore, the IVIM could offer a potentially accurate evaluation of chemotherapy efficacy, possibly facilitating individualized treatment planning in patients with GTs.
Encouragingly, based on the IVIM technique, more precise and effective parameters emerged for GTs, including α. The parameter α, derived from stretched-exponential model, appears to be more suitable for colorectal tumors in evaluating pCR after CRT because of the superior diagnostic performance of D, D* and f[47] and their better reliability than ADC for assessing pCR after CRT.
In conclusion, with the technical assistance of IVIM, IVIM-DWI will be considerably more useful in evaluating GTs, reflecting histopathological changes and therapeutic responses before and after chemotherapy. Much deeper investigations and applications of IVIM-DWI in GTs are on the horizon.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Cao D, Ciocalteu A, Hori T, Mastoraki A S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ