Revised: December 17, 2009
Accepted: December 21, 2009
Published online: December 31, 2009
Patients with liver cirrhosis are at increased risk of hepatocellular carcinoma (HCC). Conventional or baseline ultrasound (BUS) is often used as the first-line tool for HCC surveillance or detection, but the accuracy of BUS in HCC detection or differentiation from other focal liver lesions (FLLs) is limited. Contrast-enhanced ultrasound (CEUS) represents a recent revolution in the field of ultrasonography and it has become increasingly important in the detection and evaluation of FLLs. In CEUS, HCC typically exhibits arterial hyper-enhancement and portal-venous washout represented by hypo-enhanced lesions in the portal venous and late phases. The detection rate of HCC was significantly higher with CEUS compared with BUS. Even regenerative or some dysplastic nodules may exhibit arterial hyper-enhancement as they are differentiated from HCC by its iso-enhancing pattern in portal and late phases. The contrast-enhancement patterns of other different types of benign and malignant FLLs, as well as their detection rates with CEUS, were also discussed.
- Citation: Wong GLH, Xu HX, Xie XY. Detection Of Focal Liver Lesions In Cirrhotic Liver Using Contrast-Enhanced Ultrasound. World J Radiol 2009; 1(1): 25-36
- URL: https://www.wjgnet.com/1949-8470/full/v1/i1/25.htm
- DOI: https://dx.doi.org/10.4329/wjr.v1.i1.25
Liver cirrhosis is a potentially life-threatening condition, as it may be complicated by hepatic decompensation and hepatocellular carcinoma (HCC)[1]. Therefore HCC surveillance has been recommended by expert associations to improve survival of patients by identifying tumors in earlier stages[2-4]. Conventional or baseline ultrasound (BUS) is often used as the first-line tool for HCC surveillance, as well as for the detection of HCC or other focal liver lesions (FLLs) because of its efficiency, availability, non-invasiveness, and relatively low cost[5]. However, in view of the low ability of BUS to demonstrate tumor vascularity, it is sometimes difficult to differentiate benign FLLs (generally having a preferential portal venous blood supply) from malignant ones (generally having a preferential hepatic arterial supply) using BUS alone[6]. This is particularly relevant in the setting of liver cirrhosis, as in the presence of nodular liver parenchyma it can be difficult to differentiate HCC from regenerative, dysplastic nodules or other FLLs.
Contrast-enhanced ultrasound (CEUS) represents a recent breakthrough in the field of ultrasonography and it has becoming increasingly important in the evaluation of FLLs. CEUS involves the use of microbubble contrast agents and specialized imaging techniques such as harmonic and pulse inversion imaging to show sensitive blood flow and tissue perfusion information. The introduction of new generation microbubble contrast agents allows real-time imaging, which further improves the characterization and detection of FLLs[7]. CEUS significantly improved the diagnostic performance in small FLLs compared to BUS[8]. Several other studies also provided evidence that CEUS has sensitivity and specificity similar to computed tomography (CT) and magnetic resonance imaging (MRI) in terms of detection and characterization of FLLs[9-12]. In this article, the application of CEUS for the detection of different benign and malignant FLLs in liver cirrhosis will be described and discussed on the basis of our experience and latest literature data.
Microbubbles smaller than 8 μm in diameter have been proved to pass through capillary vessels, and an ultrasound pulse with a frequency of 2 MHz and a negative pressure of about 700 kPa has the ability to disrupt the microbubbles and generate echo signals[13]. Thus, contrast agents with transpulmonary stability, which are administered intravenously into peripheral veins, have become commercially available for use in sonographic enhancement studies. Levovist, Definity, SonoVue, and the latest Sonazoid, are the four UCAs most commonly studied in the liver (Table 1).
| Agent | Manufacturer | Resonance range (MHz) | Chemical composition | MI level |
| Levovist® | Schering | 2-3 | Lipid | High > 0.6 |
| Air (galactose-based) | ||||
| Definity® | BMS | 1.5-4 | Liposome | Low < 0.4 |
| Perfluorocarbon | ||||
| SonoVue® | Bracco | 1.8-3.2 | Phospholipid | Low < 0.4 |
| Sulfur hexafluoride | ||||
| Sonazoid® | GE healthcare | 2-8 | Lipid | Low < 0.4 |
| Perfluorocarbon |
The first generation UCAs, such as Levovist, produce a very weak signal when submitted to a low mechanical index ultrasound beam owing to the fragility of the microbubbles containing air with galactose/palmitic acid surfactant[14]. One of the second-generation UCAs, SonoVue, is the most commonly used UCA in China and Europe. Bubbles of SonoVue contain sulfur hexafluoride with a phospholipid shell[15]. The microbubbles are isotonic to human plasma and stable and resistant to pressure. SonoVue improves the display of focal tumor vascularity and normal parenchymal liver vascularity[16]. Sonazoid is another second-generation UCA solely available in Japan. Sonazoid consists of perfluorobutane microbubbles with a median diameter of 2 to 3 μm[17]. A pharmacokinetic study of Sonazoid showed that blood concentrations of perfluorobutane declined biphasically with a distribution half-life of 2 to 3 min and an elimination half-life of 30 to 45 min[18]. The special feature of Sonazoid is the Kupffer imaging in the post-vascular phase, which is stable for at least 60 min post-injection and tolerable for multiple scanning and can be obtained with low acoustic power, because Sonazoid microbubbles are phagocytosed by Kupffer cells[19]. In general, UCAs are very safe with a low incidence of side effects. Serious adverse events in abdominal applications have been reported with a rate of 0.0086%[20]. The common adverse events include pruritus, nonspecific malaise, numbness of limb and dyspnea; while serious adverse events include hypotension and bronchospasm[20].
Contrast-specific techniques suppress linear ultrasound signals coming from tissues and use the non-linear response of microbubbles to enhance signals from UCAs over the background. The advent of second-generation agents has been significant in improving the ease and the reproducibility of the examination, since low solubility gases offer improved stability and more favorable resonance behavior than air at low acoustic pressure[21]. Hence, contrast-specific imaging can be performed at a low mechanical index (usually less than 0.20), thus preventing microbubble disruption and enabling visualization of the dynamic enhancement pattern in real time over several minutes.
The hepatic artery supply usually starts 10 to 20 s post-injection into a peripheral vein and lasts for approximately 10-15 s. This is followed by the portal venous phase, which usually lasts 2 min after UCA injection. The late phase lasts until clearance of UCA from the liver parenchyma, up to approximately 4-6 min for SonoVue[22]. Another post-vascular or Kupffer phase, up to at least 60 min, is present for Sonazoid (Table 2)[19].
The UCA reaches the liver first via the hepatic artery and provides information on the degree and pattern of vascularity. Tumors with substantial arterial blood supply show hyper-enhancement during this phase. This phase is usually defined as the period from 0 to 30 s after UCA injection.
After the arterial phase, the UCA has passed through the circulation and spreads through the liver via the portal branches. This phase is usually defined as the period from 31 to 120 s after UCA injection.
The late or parenchymal phase follows the portal phase, in which UCA is slowly distributed throughout the entire liver parenchyma. The origin of the late phase is the subject of ongoing scientific discussion, and suggested mechanisms include sinusoid pooling and reticulo-endothelial system or Kupffer cell uptake[23]. Both the portal and late phases provide information regarding the washout of contrast agent from the lesion compared with normal liver tissue. In the case of hemangiomas, a progressive filling can be observed during these phases. The portal and late phase enhancement can provide important information regarding the character of the lesions. Most malignant lesions are hypo-enhanced, while the majority of solid benign lesions are iso- or hyper-enhanced[24,25]. This phase is usually defined as the period from 121 to 360 s after UCA injection.
This is an extra phase when Sonazoid is used. Kupffer imaging in the post-vascular phase is stable for at least 60 min post-injection and tolerable for multiple scanning. This can be obtained with low acoustic power, because Sonazoid microbubbles are phagocytosed by Kupffer cells[19].
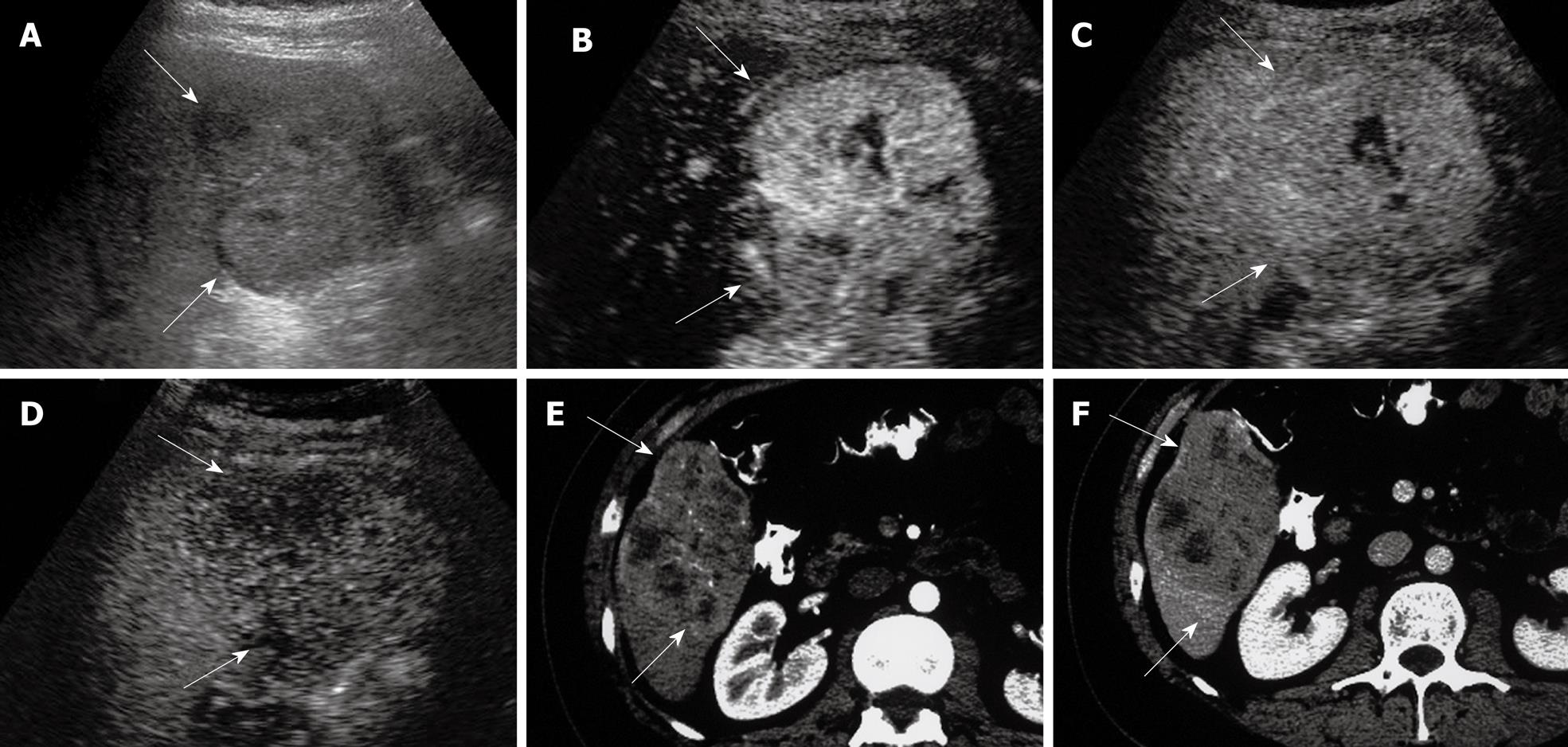
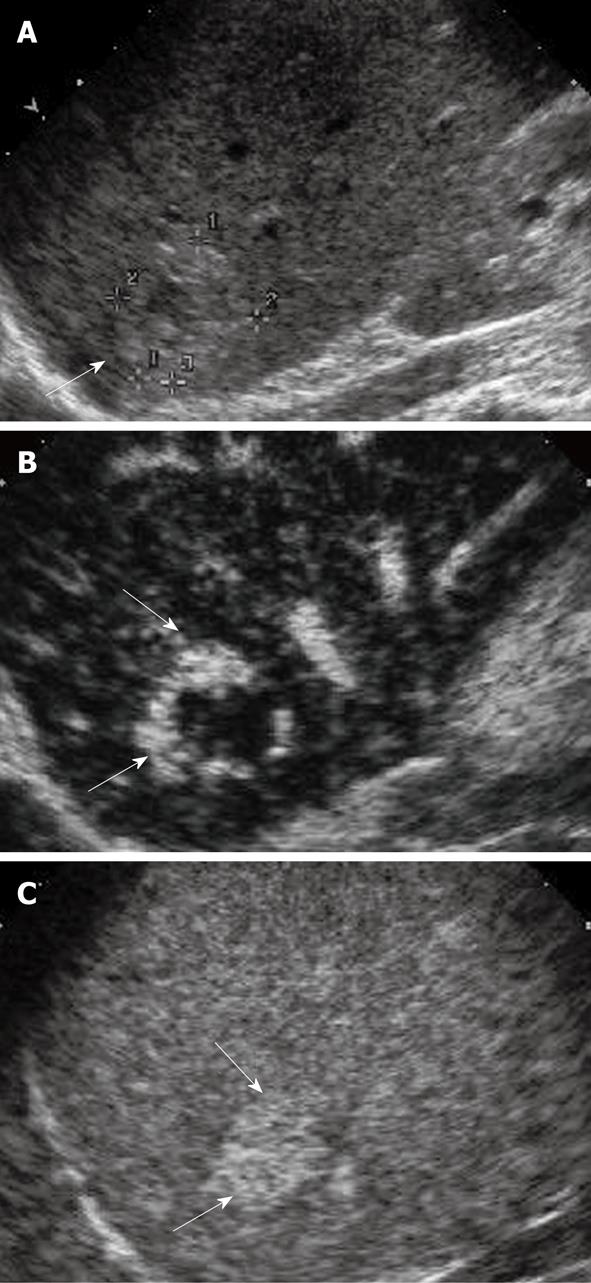
HCC is the most common primary malignancy of the liver. It usually occurs in patients with chronic liver diseases such as chronic hepatitis B and C, which are highly prevalent in the Asian-Pacific region, Japan and America, respectively. The risk of HCC is substantially increased to 10 times or even more in the presence of cirrhosis when compared to non-cirrhotic patients[1]. The annual detection incidence of HCC ranges from 2% to 5% when cirrhosis is established[26]. On a histopathologic basis, evolving malignant change in a cirrhotic nodular lesion shows that the arterial flow supply progressively increases to the lesion and the portal flow progressively decreases[27]. This progressive neo-angiogenesis provides the clue for clinical diagnosis with imaging techniques[28]. HCC typically exhibits arterial hyper-perfusion compared with the surrounding liver tissue at the time when in the surrounding liver parenchyma no contrast effect is as yet discernible (Figure 1)[29]. Uneven arterial hyper-enhancement of HCC may be noted, because the tumors may have septa, different cell differentiation and arterio-venous shunting patterns among the neo-formed vessels[30]. In the portal and late phase, HCC are usually hypo-enhanced, as the UCA will wash out from HCC to the liver parenchyma rapidly, and the tumor may appear hypo-enhanced with respect to the surrounding liver in the late phase[24,25,29]. As some regenerative nodules may also exhibit additional arterial enrichment, analysis of the portal venous phase makes the differentiation of these iso-enhanced nodules from hypo-enhanced HCC possible.
The detection rates of FLLs are dependent on the sizes of the lesions. While the detection rates are generally higher than 90% with CEUS[9,28]. A summary of CEUS findings of common FLLs was shown in Table 3. The characterization of a hepatic nodule smaller than 2 cm by imaging is more difficult. Detection of small HCCs in the cirrhotic liver is always a great challenge in cirrhotic liver, as multistage processes including regenerative nodules, dysplastic nodules and HCC may co-exist. In general, CEUS improved the sensitivity, negative predictive value, and overall accuracy of detection of HCC ≤ 2 cm from 29% to 80%, 60% to 91%, and 64% to 87%, respectively, when compared with BUS[31]. A recent investigation has shown that in the setting of cirrhotic patients undergoing HCC surveillance, the sole imaging finding of arterial hyper-vascularization in small solitary nodules of 2 cm or less has a specificity of 86% and a positive predictive value of 92% for the diagnosis of HCC[32]. Small HCCs in cirrhotic liver may be detected as areas of hyper-enhancement in the arterial phase. However, the short duration of the arterial phase does not allow scanning of the whole liver. Hence the portal and late phases may provide more information in the detection of small lesions in cirrhotic liver[24,25]. Evaluation of all three vascular phases has been shown to be superior to the evaluation of enhancement in the late phase alone: the sensitivity increased from 78% to 98%, and the accuracy from 81% to 93%[33]. In a study of 41 cirrhotic patients with small monofocal lesions smaller than 3 cm in diameter using contrast-enhanced Doppler ultrasound, the intra-tumoral arterial blood flow was detected in 95% of HCCs, compared with 28% of nonmalignant nodules[34]. All false-positive findings were noted either in high-grade dysplastic nodules or evolving HCCs[34]. CEUS was also found to improve the detection of HCC in patients with chronic hepatitis C related cirrhosis, and it was useful to rule out malignancy in many cases where BUS findings were indeterminate[35]. To increase the specificity of imaging diagnosis, it is mandatory to evaluate contrast washout during the portal venous and the late phases, as recently recommended by the EASL panel of experts on HCC and the AASLD practice guideline[36,37]. These latest guidelines from various panel experts also recommended that the diagnosis of nodules of sizes 1 to 2 cm must be confirmed by two different imaging techniques[36,37].
| Arterial | Portal | Late vascular phase | Parenchymal phase | |
| Malignant focal liver lesions | ||||
| Hepatocellular carcinoma | Hyperechoic | Isoechoic | Hypo- or isoechoic (30%) | Hypoechoic |
| Metastases | Hypo- or hyperechoic (hypervascular) | Hypoechoic with or without rim enhancement | Hypoechoic | Hypoechoic |
| Cholangiocarcinoma | Hypo- or hyperechoic (15%) | Iso- or hypoechoic | Hypoechoic | Hypoechoic |
| Benign focal liver lesions | ||||
| Regenerating nodules | Iso- or hypoechoic | Isoechoic | Isoechoic | Isoechoic |
| Dysplastic nodules | Hyper, iso- or hypoechoic | Isoechoic | Hypo- or isoechoic | Hypo- or isoechoic |
| Hemangiomas | Hyperechoic (peripheral nodular enhancement) | Centripetal filling | Isoechoic if filling is complete (intralesional hypoechoic areas in 50%) | Isoechoic (intralesional hypo-echoic areas in 50%) |
| Homogenous hyperechoic in 20% of small hemangiomas | Hyper- or isoechoic | Hyper- or isoechoic | Isoechoic | |
| Focal nodular hyperplasia | Hyperechoic | Hyper- or isoechoic; non-enhancing central scar in 45% | Hyper- or isoechoic; non-enhancing central scar in 45% | Hyper- or isoechoic |
| Hepatocellular adenoma | Hyperechoic | Isoechoic | Isoechoic | Isoechoic |
| Fatty changes or focal fatty sparing | Isoechoic | Isoechoic | Isoechoic | Isoechoic |
| Noncomplicated cysts | Absence of enhancement | Absence of enhancement | Absence of enhancement | Absence of enhancement |
Despite these promising results and recommendations, detection of small lesions is still a great challenge. A small study involving 30 cirrhotic patients with 30 small FLLs (1 to 2 cm) showed that a combination of characteristics of arterial phase enhancement and absence of delayed phase enhancement in CEUS had a high specificity of 92%, with a relatively low sensitivity of 56%, for detecting small HCC in cirrhosis patients[38]. Another recent study showed that the detection rate by coincident arterial hyper-vascularity at CEUS and CT was 44% in nodules of 1 to 2 cm, compared with 84% in larger nodules of sizes 2 to 3 cm in cirrhotic liver[39]. Relying on imaging techniques in nodules of 1 to 2 cm, the missed diagnosis of HCC was up to 38%[39]. This evidence showed that the diagnosis of nodules of 1 to 2 cm in cirrhotic patients is not satisfactory even with arterial hyper-vascularity shown by CEUS and CT. Late-phase pulse-inversion CEUS improved diagnostic sensitivity from 85% to 100% and specificity from 30% to 63% compared with BUS, and with lower inter-observer variability, for the discrimination of malignant versus benign liver lesions[40]. The new UCA Sonazoid provides Kupffer imaging, which is extremely stable and tolerable for multiple scanning at least up to 60 min in the post-vascular phase, and may further improve the detection rate of HCC with CEUS[41]. The analysis of Kupffer function provides essential information compared with other contrast agents[17,42]. CEUS with Sonazoid detects liver malignancy as defects on the sinusoidal phase with a high sensitivity of 95%, specificity of 93%, positive predictive value of 99%, and negative predictive value of 97%[43].
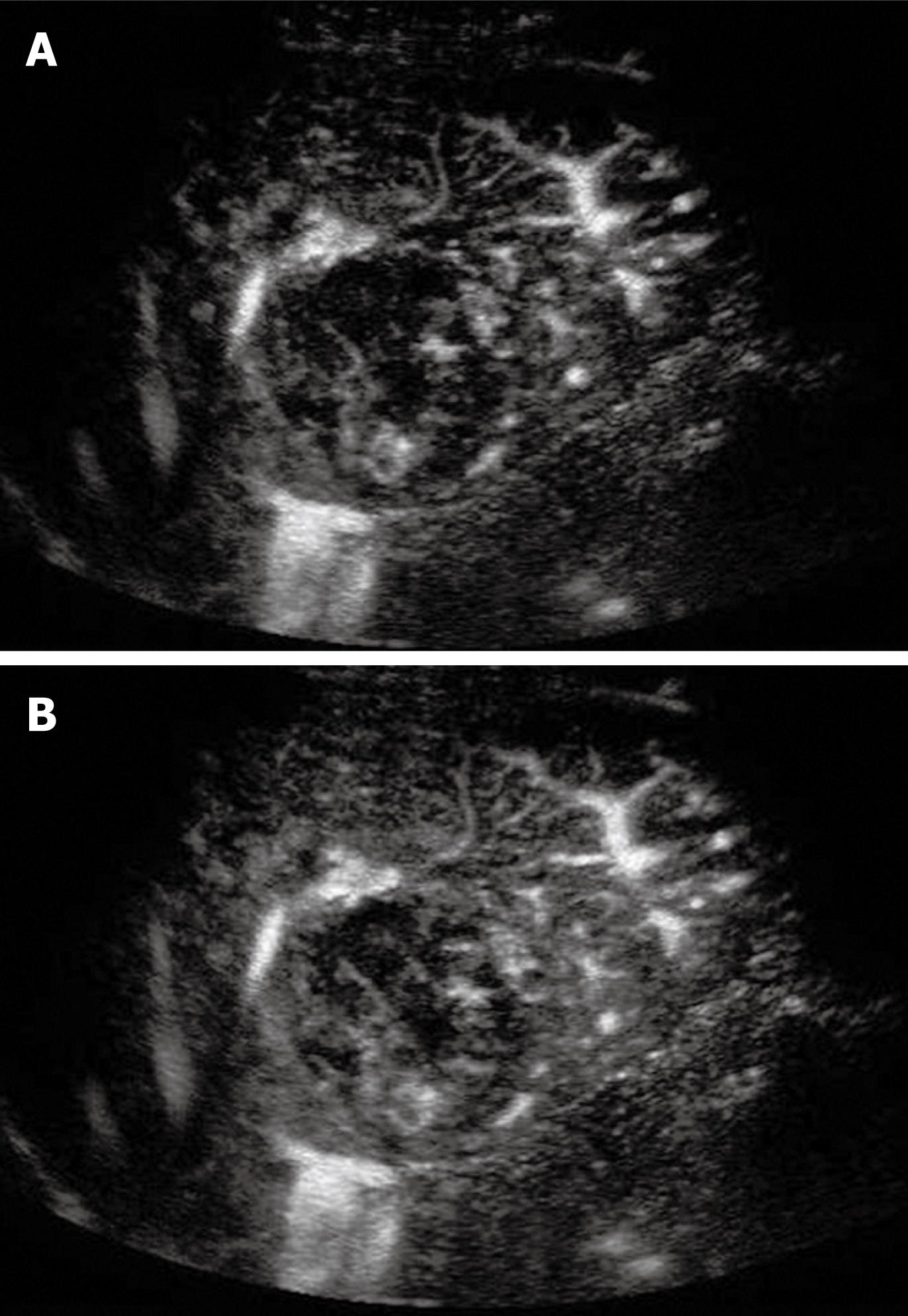
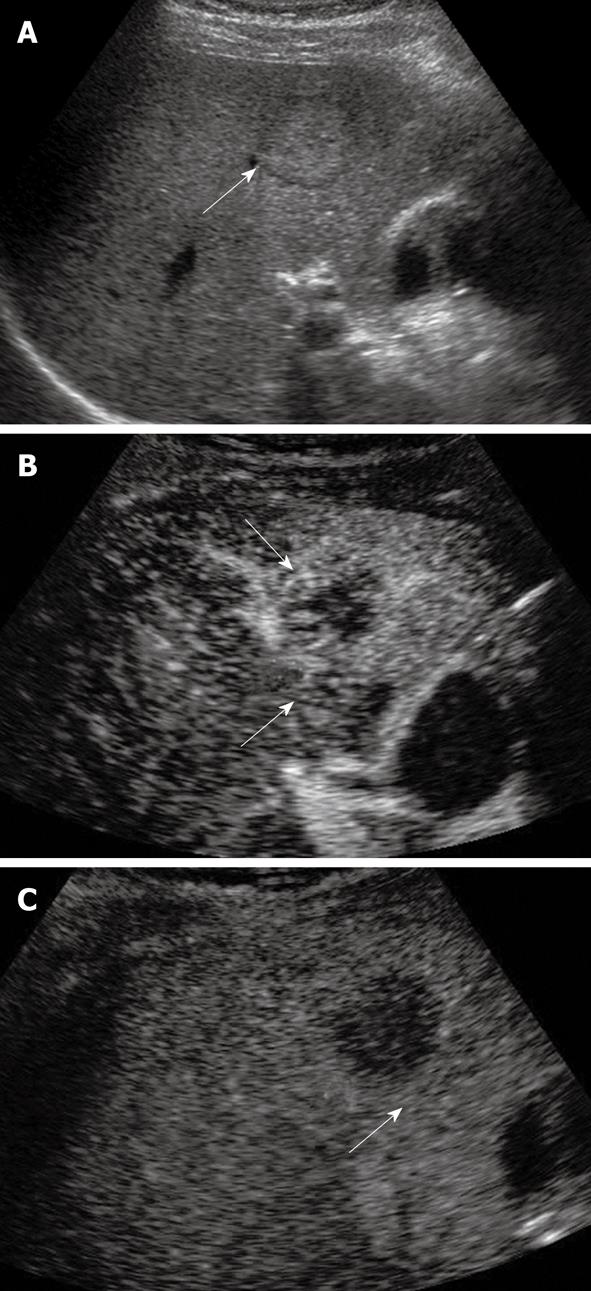
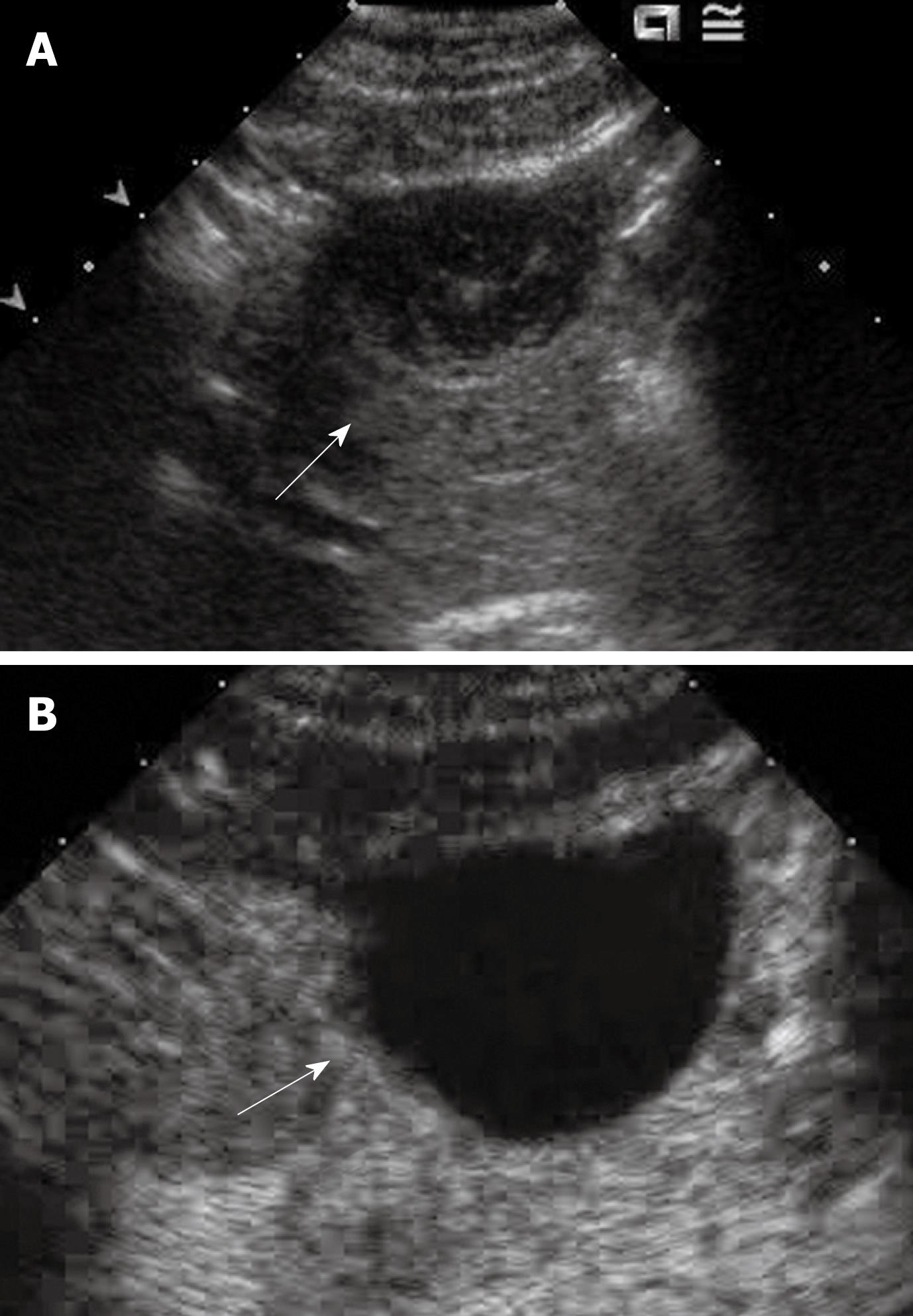
Tumor differentiation was found to be correlated with the pattern of enhancement in the portal and late phases[44]. The timing of HCC becoming hypo-enhanced on CEUS is correlated with tumor cell differentiation; well-differentiated tumors wash out more slowly than poorly differentiated ones[44,45]. The micro flow imaging technique was found to be effective in depicting the intra-tumoral vascular architecture which correlated with pathologic differentiation of HCC (Figure 2)[46]. A minority of HCCs may exhibit sustained hyper-enhancement in the late phase, especially for the small or well-differentiated ones (Figure 3)[30]. This makes the differentiation from other benign lesions such as focal nodular hyperplasia (FNH) or hemangioma impossible. In this scenario, other imaging modalities or biopsy of the nodule may be needed to confirm the diagnosis.
Even though the incidence of MLC and cholangiocarcinoma is not increased in the presence of liver cirrhosis, detection of MLC may become more difficult in the presence of the nodular background of liver parenchyma. In the presence of liver cirrhosis, the detection rate of MLC was reported to be 33% in an autopsy series[47], while no data is available for cholangiocarcinoma as it is relatively uncommon. Detection of MLC has an important role in optimizing the therapeutic strategy, particularly in patients suffering from colorectal carcinoma[48,49]. Histologically, metastatic tumors have intratumoral hypo-vascularity and peripheral hyper-vascularity. BUS may miss iso-echoic lesions and lesions smaller than 1 cm[50]. Many studies have confirmed the improvement in accuracy of CEUS in diagnosing MLC[40,51,52]. CEUS also improved the detection of miliary metastases (0.5-1 cm) [53].
In the arterial phase, hypovascular metastases appear as hypo-enhanced lesions, with a typical rim enhancement of varying size (“halo sign”, “rim sign”), whereas hypervascular metastases appear as hyper-enhanced and homogeneous lesions[28]. Rapid washout of arterial enhancement is found in the late arterial and portal phases[54]. Therefore, at the beginning of the portal phase, the arterial enhancement fades and the entire hypovascular lesion becomes hypo-echoic. In the late phase, both hypovascular and hypervascular metastases invariably appear dark compared with the enhanced background of normal liver parenchyma (“black-hole sign”)[52]. During this late phase, both portal and late-phase imaging markedly increase the contrast between the enhanced normal liver (Figure 3). In the late portal phase very small metastases stand out better, because the artifacts are then less pronounced than directly after the injection of the signal enhancer. Thus, non-enhancing metastases improve detection, especially of small lesions smaller than 1 cm and lesions iso-echoic at BUS[55]. A high diagnostic accuracy in the differentiation between metastases and benign FLLs in the late phase has been reported[56]. A study showed that the addition of CEUS to BUS improved sensitivity for the detection of individual metastases from 71% to 87%. On a per-patient basis, sensitivity improved from 94% to 98% and specificity improved from 60% to 88%[57].
It is often difficult to use BUS alone to differentiate cholangiocarcinoma from other FLLs because its sonographic findings are non-specific. CEUS has much improved the detection rate of this tumor when compared to BUS, and it was found to have the same accuracy as contrast-enhanced CT in diagnosing intrahepatic cholangiocarcinoma (ICC)[58]. ICC usually appeared in inhomogeneous hyper-enhancement of different patterns in arterial phase: peripheral irregular rim-like hyper-enhancement; diffused heterogeneous hyper-enhancement; diffuse homogeneous hyper-enhancement and diffuse heterogeneous hypo-enhancement[58,59]. In the portal phase, most of the ICC was hypo-enhanced and appeared as punched-out defects (Figure 4)[58,59]. CEUS is also useful for differentiating ICC from HCC based on the enhancement pattern[60].
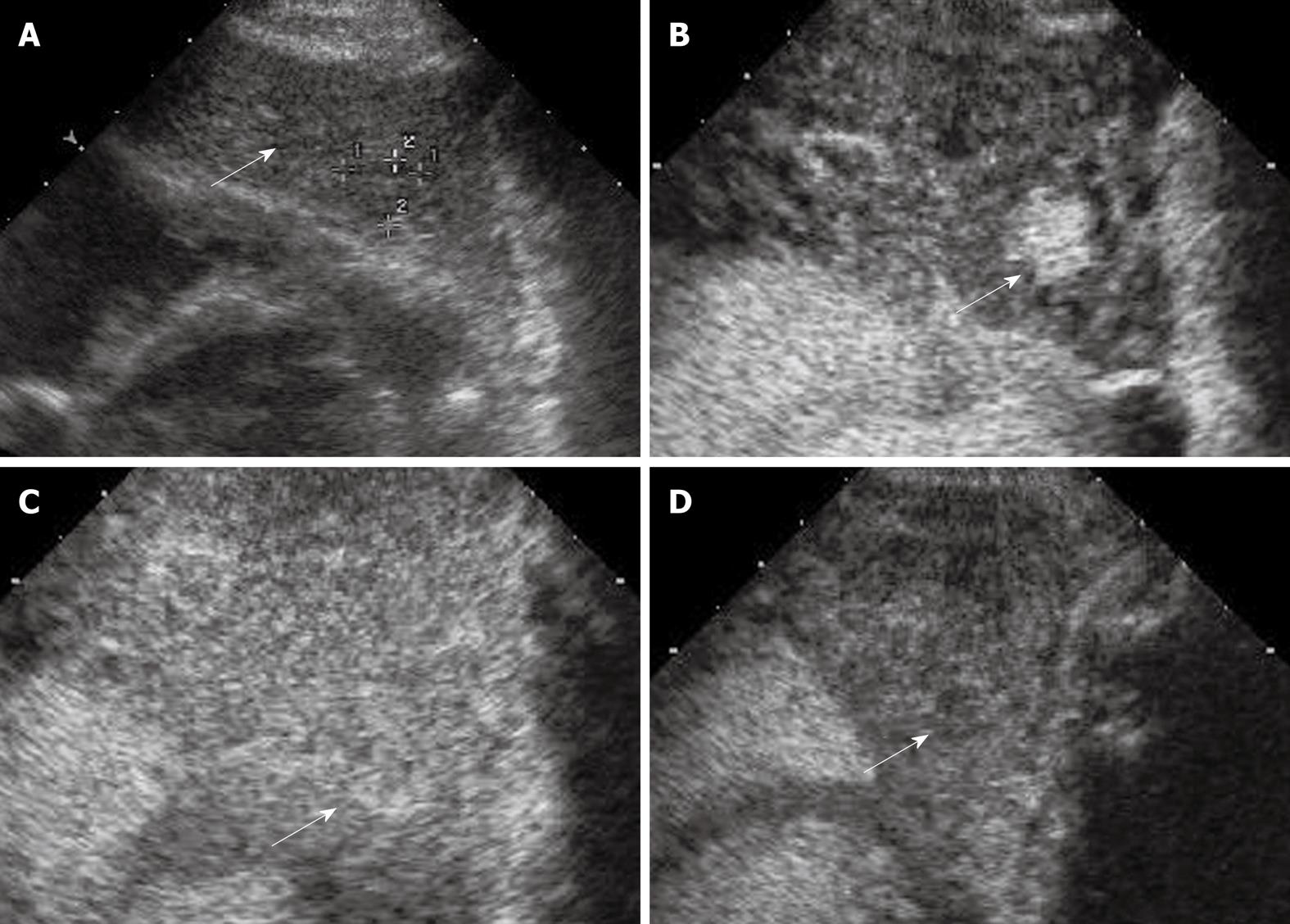
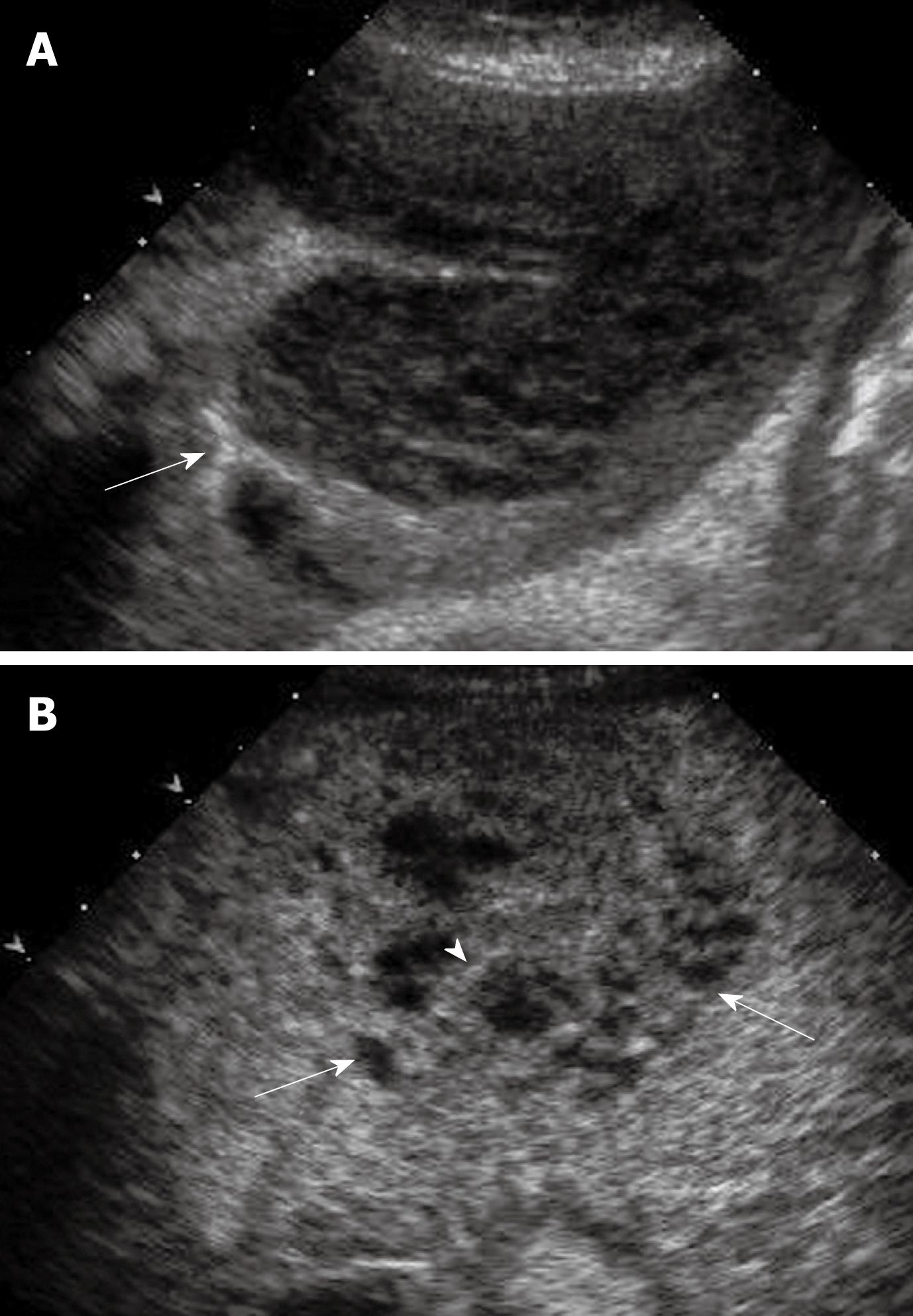
A stepwise carcinogenesis for HCC has been proposed from regenerative nodules, through low-grade dysplastic nodules and high-grade dysplastic nodules, to overt HCC[61]. Occasionally cancerous foci of very well differentiated HCC are encountered within dysplastic nodules, which are called nodule-in-nodule lesions or dysplastic nodules with a focus of HCC[62]. Differentiation between HCC and these nodules is always a major concern in cirrhotic liver, as the appearance in BUS may be similar but their prognosis is substantially different from each other: regenerative nodules are considered as benign lesions; dysplastic nodules are considered as pre-cancerous lesions; while nodule-in-nodule lesions are considered malignant[62]. As definite differentiation among these nodules is almost impossible with BUS, CEUS plays an important role in differentiating these lesions because of its ability to demonstrate the vascularity of the lesions[63-65]. Regenerative nodules and dysplastic nodules are differentiated from HCC as the former lesions usually do not show enhancement in the arterial phase and are iso-enhanced similar to the surrounding liver parenchyma in the portal and late phases (Figure 5)[64,65]. Nonetheless, regenerative nodules and some dysplastic nodules may also exhibit additional arterial hyper-enhancement; by analysis of the portal venous and late phases it may be possible to differentiate these iso-enhancing nodules from hypo-enhancing HCC[64,65].
CEUS with Sonazoid is more sensitive for detecting arterial vascularity of target nodules than contrast-enhanced CT and MRI. CEUS with Sonazoid provides the additional post-vascular or Kupffer phase, such that it allows an assumption of the degree of malignancy based on Kupffer function. When uptake of contrast agent is reduced in the Kupffer phase of CEUS with Sonazoid in nodules not depicted as hypervascular lesions by CT or MRI, these lesions should basically be treated as malignant and a biopsy is not indispensable[62]. However, only a part of the liver can be evaluated in the short arterial phase of 20 to 30 s after administration of the contrast agent. As multiple nodules are often present in cirrhotic liver, this is a major limitation of CEUS. This may be overcome by using several doses of contrast agent to scan different segments of the liver. Another way is using CEUS in combination with CT, which was found to increase the accuracy of detecting intra-nodular arterial vascularity, compared to that by a single method[62].
Hemangiomas are the most frequent benign tumor found in the liver. Therefore it is also commonly found in cirrhotic liver, and the presence of hemangioma may be misdiagnosed as HCC. On the other hand, with progressive cirrhosis, hemangiomas are likely to decrease in size and become more fibrotic and difficult to diagnose radiologically[63]. The typical findings of hemangioma in CEUS are peripheral nodular contrast-enhancement and centripetal fill in (60% to 80%). However, atypical findings are found in 20% of hemangiomas if thrombosed areas and calcifications are present[66]. In small hemangiomas of diameter less than 2 cm, the arterial phase may show diffuse enhancement, which may occur in hypervascular malignant tumors such as HCC or metastases. Hemangiomas can be differentiated from those malignant lesions as they usually have hyper- or iso-enhancement with respect to the surrounding liver tissue in the portal and late phases, while malignant lesions become hypo-enhanced (Figure 6)[67]. CEUS can be used in the diagnosis of hemangioma, when centripetal fill-in enhancement is a positive finding in hemangioma, and the sensitivity and specificity are 96% and 98%, respectively[68].
FNH is the second most common benign, hyperplastic liver neoplasm composed of all the components of normal liver tissue, and about 45% of cases have a central stellate fibrous scar[69]. FNH was present in 3.4% of cirrhotic patients who received liver transplantation[70]. The findings of FNH in CEUS are rapid arterial hyper-enhancement with atypical centrifugal radiating or “spoke-wheel” pattern, then homogeneous hyper-enhancement in the late arterial phase[71,72]. The spoke-wheel pattern represents a central feeding artery and centrifugal blood supply from the center of the lesion to the periphery. FNH gradually changes to iso-enhanced in portal and late phases as FNH contains all the components of normal liver tissue, while some lesions even show as slightly hyper-enhanced with respect to the surrounding normal liver[40]. A central scar is another characteristic feature of FNH in CEUS due to the central stellate fibrosis scar. With the characteristic features in CEUS, the sensitivity and specificity of diagnosing FNH were 88% to 97% and 95%, respectively[71,73].
Hepatocellular adenoma is a relatively rare benign focal liver lesion, and is mainly found in young women with a history of oral contraceptive use, androgen steroid therapy and glycogen storage disease[74]. Histologically, hepatocellular adenoma is composed of cords of tumor cells, which closely resemble hepatocytes and contain fat and glycogen. In the arterial phase of CEUS, early and homogeneous hyperechoic enhancement is found in most cases. However, no enhancement will be seen if the tumor contains hemorrhage or necrosis[72]. In the portal and late phases, the enhancement of hepatocellular adenoma is almost the same as that of liver parenchyma and remains slightly hypo-enhanced in relation to the adjacent liver tissue in later stages because of varying numbers and activity of Kupffer cells[75]. Studies regarding the detection rate of CEUS in hepatocellular adenoma are not available.
Focal fatty change and focal spared areas are usually demonstrated adjacent to the right main portal vein, the gallbladder bed or the falciform ligament. However, a single well-demarcated nodule can be found anywhere in the liver. Because this type of lesion has normal liver components, CEUS shows the same enhancement pattern with respect to the normal liver in all phases and remains iso-enhanced[52]. In the arterial and venous phase the supplying and draining vessels can be imaged[76].
Liver cysts are a common ultrasonographic finding and readily diagnosed with BUS features of typical cyst appearance of echo free, round, well-defined borders with lateral shadowing and posterior echo enhancement. Blood vessels have to be excluded by color Doppler imaging ruling out arterio-porto-venous malformations with a cystic appearance. Simple liver cysts typically show no contrast enhancement at all the phases in CEUS[77]. CEUS is usually not necessary to diagnose simple liver cysts. Sometimes, it can play an useful role in the presence of complex, septated cysts to exclude a malignant cystic lesion (Figure 7)[78].
CEUS enables a better delineation of liver abscesses, which usually show a peripheral rim of contrast enhancement, surrounding an inner, generally necrotic, hypoechoic non-enhancing area, when compared to BUS. Septa may also enhance after contrast agent administration and show a honeycomb-like appearance (Figure 8)[79]. A recent study of CEUS in infective liver lesions showed that most liver abscesses were irregularly rim-enhanced with non-enhanced central necrotic areas; whereas infected granulomas and inflammatory pseudotumors exhibit variable CEUS patterns[80]. Biopsy may be required in these lesions as most infected FLLs showed more rapid contrast wash-out than the surrounding liver parenchyma, which is similar to malignant lesions[80].
Some other unusual lesions including intrahepatic biliary cystadenoma, angiomyolipoma, lipoma, biliary epithelial dysplasia, a fungal inflammatory mass, tuberculoma, sarcoidosis, solitary necrotic nodules, peliosis hepatis, and focal fibrosis after surgery were demonstrated in CEUS. The benign nature of some of these lesions was shown as hyper-enhancement during the arterial phase and sustained enhancement during the portal or late phase in CEUS. But some benign lesions (e.g. intrahepatic biliary cystadenoma, sarcoidosis) may have various enhancing patterns during the arterial phase and even hypo-enhancement during the late phase[81].
Similar to BUS, CEUS is a dynamic examination that depends on the skill of the sonographers and/or sonologists, hence the accuracy is often operator-dependent[82]. Limited access to certain parts of the liver (e.g. near dome of diaphragm or far from the abdominal wall), especially in obese patients, remains a similar problem as in BUS. As a general rule, if BUS is suboptimal, results from CEUS may be disappointing[21]. Only a part of the liver can be evaluated in the short arterial phase after administration of the contrast agent. In the presence of multiple FLLs, which are particularly common in cirrhotic liver, this may be overcome by using several doses of contrast agent to scan different segments of the liver[83]. The value of scanning in the late phase after contrast administration in cirrhotic patients is limited: while the detection of an hypo-enhanced lesion in the late phase in a cirrhotic patient is very suggestive of HCC, iso-enhanced HCC is not easily detected. Furthermore, due to hemodynamic changes in cirrhotic patients with hyperdynamic circulation and shunting, the parenchymal enhancement in the late phase may appear heterogeneous and less intense than in normal livers, making evaluation difficult[83]. Another major limitation is the inability to evaluate the extrahepatic extension of HCC or other malignant diseases. All these insufficiencies may be overcome by combining CEUS with another dynamic test, such as contrast-enhanced CT or MRI[83].
CEUS can clearly demonstrate the vascular pattern and parenchymal contrast in FLLs, hence with CEUS the detection rates of different types of FLLs are much improved compared to BUS, and comparable to CT and MRI. CEUS also improves the diagnostic accuracy of FLLs, even for those as small as 1 to 2 cm. The significantly improved detection and diagnostic values of CEUS is particularly relevant in the setting of liver cirrhosis because of the nodularity of the liver parenchyma and the coexistence of benign regenerative nodules and malignant HCC. This safe, convenient, low cost and non-invasive diagnostic modality should be promoted in routine clinical practice, especially in cirrhotic patients. Further research should explore the role of CEUS in different clinical applications, such as in the HCC surveillance program.
Peer reviewers: Hadi Rokni Yazdi, MD, Central Radiology, Imam Khomeini Hospital, Keshavarz Blvd, Tehran, 1419733141, Iran; Roberto Miraglia, MD, Adjunct Associate Professor of Radiology, Department of Diagnostic and Interventional Radiology, Mediterranean Institute for Transplantation and Advanced Specialized Therapies (IsMeTT), University of Pittsburgh, Via Tricomi 1, Palermo, 90100, Italy
S- Editor Wang JL L- Editor O’Neill M E- Editor Zheng XM
| 1. | Chan HL, Tse CH, Mo F, Koh J, Wong VW, Wong GL, Chan SL, Yeo W, Sung JJ, Mok TS. High Viral Load and Hepatitis B Virus Subgenotype Ce Are Associated With Increased Risk of Hepatocellular Carcinoma. J Clin Onco. 2008;26:177-182. |
| 2. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. |
| 3. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. |
| 4. | Wong GL, Wong VW, Tan GM, Ip KI, Lai WK, Li YW, Mak MS, Lai PB, Sung JJ, Chan HL. Surveillance programme for hepatocellular carcinoma improves the survival of patients with chronic viral hepatitis. Liver Int. 2008;28:79-87. |
| 5. | Ariff B, Lloyd CR, Khan S, Shariff M, Thillainayagam AV, Bansi DS, Khan SA, Taylor-Robinson SD, Lim AK. Imaging of liver cancer. World J Gastroenterol. 2009;15:1289-1300. |
| 6. | Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749-761. |
| 7. | Morin SH, Lim AK, Cobbold JF, Taylor-Robinson SD. Use of second generation contrast-enhanced ultrasound in the assessment of focal liver lesions. World J Gastroenterol. 2007;13:5963-5670. |
| 8. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of small focal liver lesions using real-time contrast-enhanced sonography: diagnostic performance analysis in 200 patients. J Ultrasound Med. 2006;25:349-361. |
| 9. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. |
| 10. | Catala V, Nicolau C, Vilana R, Pages M, Bianchi L, Sanchez M, Bru C. Characterization of focal liver lesions: comparative study of contrast-enhanced ultrasound versus spiral computed tomography. Eur Radiol. 2007;17:1066-1073. |
| 11. | Li R, Guo Y, Hua X, He Y, Ding J, Guo A, Luo M. Characterization of focal liver lesions: comparison of pulse-inversion harmonic contrast-enhanced sonography with contrast-enhanced CT. J Clin Ultrasound. 2007;35:109-117. |
| 12. | Larsen LP, Rosenkilde M, Christensen H, Bang N, Bolvig L, Christiansen T, Laurberg S. The value of contrast enhanced ultrasonography in detection of liver metastases from colorectal cancer: a prospective double-blinded study. Eur J Radiol. 2007;62:302-327. |
| 13. | Holland CK, Apfel RE. An improved theory for the prediction of microcavitation thresholds. IEEE Trans Ultrason Ferroelectr Freq Control. 1989;36:204-208. |
| 14. | Youk JH, Kim CS, Lee JM. Contrast-enhanced agent detection imaging: value in the characterization of focal hepatic lesions. J Ultrasound Med. 2003;22:897-910. |
| 15. | Schneider M, Arditi M, Barrau MB, Brochot J, Broillet A, Ventrone R, Yan F. BR1: a new ultrasonographic contrast agent based on sulfur hexafluoride-filled microbubbles. Invest Radiol. 1995;30:451-457. |
| 16. | Leen E, Angerson WJ, Yarmenitis S, Bongartz G, Blomley M, Del Maschio A, Summaria V, Maresca G, Pezzoli C, Llull JB. Multi-centre clinical study evaluating the efficacy of SonoVue (BR1), a new ultrasound contrast agent in Doppler investigation of focal hepatic lesions. Eur J Radiol. 2002;41:200-206. |
| 17. | Hatanaka K, Kudo M, Minami Y, Ueda T, Tatsumi C, Kitai S, Takahashi S, Inoue T, Hagiwara S, Chung H. Differential diagnosis of hepatic tumors: value of contrast-enhanced harmonic sonography using the newly developed contrast agent, Sonazoid. Intervirology. 2008;51 Suppl 1:61-69. |
| 18. | Landmark KE, Johansen PW, Johnson JA, Johansen B, Uran S, Skotland T. Pharmacokinetics of perfluorobutane following intravenous bolus injection and continuous infusion of sonazoid in healthy volunteers and in patients with reduced pulmonary diffusing capacity. Ultrasound Med Biol. 2008;34:494-501. |
| 19. | Yanagisawa K, Moriyasu F, Miyahara T, Yuki M, Iijima H. Phagocytosis of ultrasound contrast agent microbubbles by Kupffer cells. Ultrasound Med Biol. 2007;33:318-325. |
| 20. | Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369-1375. |
| 21. | Lencioni R, Cioni D, Crocetti L, Donati F, Franchini C, Giusti S, Bartolozzi C. Ultrasound imaging of focal liver lesions with a second-generation contrast agent. Acad Radiol. 2002;9 Suppl 2:S371-S374. |
| 22. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D'Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. |
| 23. | Kono Y, Steinbach GC, Peterson T, Schmid-Schönbein GW, Mattrey RF. Mechanism of parenchymal enhancement of the liver with a microbubble-based US contrast medium: an intravital microscopy study in rats. Radiology. 2002;224:253-257. |
| 24. | Dietrich CF, Ignee A, Trojan J, Fellbaum C, Schuessler G. Improved characterisation of histologically proven liver tumours by contrast enhanced ultrasonography during the portal venous and specific late phase of SHU 508A. Gut. 2004;53:401-405. |
| 25. | Bryant TH, Blomley MJ, Albrecht T, Sidhu PS, Leen EL, Basilico R, Pilcher JM, Bushby LH, Hoffmann CW, Harvey CJ. Improved characterization of liver lesions with liver-phase uptake of liver-specific microbubbles: prospective multicenter study. Radiology. 2004;232:799-809. |
| 26. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. |
| 27. | Roncalli M, Roz E, Coggi G, Di Rocco MG, Bossi P, Minola E, Gambacorta M, Borzio M. The vascular profile of regenerative and dysplastic nodules of the cirrhotic liver: implications for diagnosis and classification. Hepatology. 1999;30:1174-1178. |
| 28. | Gaiani S, Celli N, Piscaglia F, Cecilioni L, Losinno F, Giangregorio F, Mancini M, Pini P, Fornari F, Bolondi L. Usefulness of contrast-enhanced perfusional sonography in the assessment of hepatocellular carcinoma hypervascular at spiral computed tomography. J Hepatol. 2004;41:421-426. |
| 29. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261-272. |
| 30. | Kim TK, Kim AY, Choi BI. Hepatocellular carcinoma: harmonic ultrasound and contrast agent. Abdom Imaging. 2002;27:129-138. |
| 31. | Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Zheng YL, Liang JY, Chen LD. Contrast-enhanced sonography in the diagnosis of small hepatocellular carcinoma < or =2 cm. J Clin Ultrasound. 2008;36:257-266. |
| 32. | Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, Boix L, Sala M, Varela M, Llovet JM. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97-104. |
| 33. | Nicolau C, Vilana R, Catalá V, Bianchi L, Gilabert R, García A, Brú C. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol. 2006;186:158-167. |
| 34. | Fracanzani AL, Burdick L, Borzio M, Roncalli M, Bonelli N, Borzio F, Maraschi A, Fiorelli G, Fargion S. Contrast-enhanced Doppler ultrasonography in the diagnosis of hepatocellular carcinoma and premalignant lesions in patients with cirrhosis. Hepatology. 2001;34:1109-1112. |
| 35. | Rahbin N, Siösteen AK, Elvin A, Blomqvist L, Hagen K, Hultcrantz R, Aleman S. Detection and characterization of focal liver lesions with contrast-enhanced ultrasonography in patients with hepatitis C-induced liver cirrhosis. Acta Radiol. 2008;49:251-257. |
| 36. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. |
| 37. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. |
| 38. | Wang JH, Lu SN, Hung CH, Chen TY, Chen CH, Changchien CS, Lee CM. Small hepatic nodules (< or =2 cm) in cirrhosis patients: characterization with contrast-enhanced ultrasonography. Liver Int. 2006;26:928-934. |
| 39. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. |
| 40. | von Herbay A, Vogt C, Häussinger D. Late-phase pulse-inversion sonography using the contrast agent levovist: differentiation between benign and malignant focal lesions of the liver. AJR Am J Roentgenol. 2002;179:1273-1279. |
| 41. | Kudo M, Hatanaka K, Maekawa K. Sonazoid-enhanced Ultrasound in the Diagnosis and Treatment of Hepatic Tumors. J Med Ultrasound. 2008;16:130-139. |
| 42. | Kudo M, Okanoue T. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice manual proposed by the Japan Society of Hepatology. Oncology. 2007;72 Suppl 1:2-15. |
| 43. | Sontum PC, Ostensen J, Dyrstad K, Hoff L. Acoustic properties of NC100100 and their relation with the microbubble size distribution. Invest Radiol. 1999;34:268-275. |
| 44. | Nicolau C, Catalá V, Vilana R, Gilabert R, Bianchi L, Solé M, Pagés M, Brú C. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092-1099. |
| 45. | Liu GJ, Xu HX, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Correlation between enhancement pattern of hepatocellular carcinoma on real-time contrast-enhanced ultrasound and tumour cellular differentiation on histopathology. Br J Radiol. 2007;80:321-330. |
| 46. | Yang H, Liu GJ, Lu MD, Xu HX, Xie XY. Evaluation of the vascular architecture of hepatocellular carcinoma by micro flow imaging: pathologic correlation. J Ultrasound Med. 2007;26:461-467. |
| 47. | Vanbockrijck M, Klöppel G. Incidence and morphology of liver metastasis from extrahepatic malignancies to cirrhotic livers. Zentralbl Pathol. 1992;138:91-96. |
| 48. | Gillams A. Minimally invasive treatment for liver and lung metastases in colorectal cancer. BMJ. 2007;334:1056-1057. |
| 49. | Garden OJ, Rees M, Poston GJ, Mirza D, Saunders M, Ledermann J, Primrose JN, Parks RW. Guidelines for resection of colorectal cancer liver metastases. Gut. 2006;55 Suppl 3:iii1-iii8. |
| 50. | Oldenburg A, Hohmann J, Foert E, Skrok J, Hoffmann CW, Frericks B, Wolf KJ, Albrecht T. Detection of hepatic metastases with low MI real time contrast enhanced sonography and SonoVue. Ultraschall Med. 2005;26:277-284. |
| 51. | Harvey CJ, Blomley MJ, Eckersley RJ, Cosgrove DO, Patel N, Heckemann RA, Butler-Barnes J. Hepatic malignancies: improved detection with pulse-inversion US in late phase of enhancement with SH U 508A-early experience. Radiology. 2000;216:903-908. |
| 52. | Tanaka S, Ioka T, Oshikawa O, Hamada Y, Yoshioka F. Dynamic sonography of hepatic tumors. AJR Am J Roentgenol. 2001;177:799-805. |
| 53. | Solbiati L, Tonolini M, Cova L, Goldberg SN. The role of contrast-enhanced ultrasound in the detection of focal liver leasions. Eur Radiol. 2001;11 Suppl 3:E15-E26. |
| 54. | Hohmann J, Albrecht T, Hoffmann CW, Wolf KJ. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147-159. |
| 55. | Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515-1526. |
| 56. | Quaia E, Bertolotto M, Forgács B, Rimondini A, Locatelli M, Mucelli RP. Detection of liver metastases by pulse inversion harmonic imaging during Levovist late phase: comparison with conventional ultrasound and helical CT in 160 patients. Eur Radiol. 2003;13:475-483. |
| 57. | Albrecht T, Blomley MJ, Burns PN, Wilson S, Harvey CJ, Leen E, Claudon M, Calliada F, Correas JM, LaFortune M. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology. 2003;227:361-370. |
| 58. | Chen LD, Xu HX, Xie XY, Lu MD, Xu ZF, Liu GJ, Liang JY, Lin MX. Enhancement patterns of intrahepatic cholangiocarcinoma: comparison between contrast-enhanced ultrasound and contrast-enhanced CT. Br J Radiol. 2008;81:881-889. |
| 59. | Xu HX, Lu MD, Liu GJ, Xie XY, Xu ZF, Zheng YL, Liang JY. Imaging of peripheral cholangiocarcinoma with low-mechanical index contrast-enhanced sonography and SonoVue: initial experience. J Ultrasound Med. 2006;25:23-33. |
| 60. | Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2009;Epub ahead of print. |
| 61. | Hussain SM, Zondervan PE, IJzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023-1036; discussion 1037-1039. |
| 62. | Kudo M. Multistep human hepatocarcinogenesis: correlation of imaging with pathology. J Gastroenterol. 2009;44 Suppl 19:112-118. |
| 63. | Brancatelli G, Federle MP, Blachar A, Grazioli L. Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology. 2001;219:69-74. |
| 64. | Catalano O, Lobianco R, Cusati B, Siani A. Hepatocellular carcinoma: spectrum of contrast-enhanced gray-scale harmonic sonography findings. Abdom Imaging. 2004;29:341-347. |
| 65. | Kudo M. Early detection and characterization of hepatocellular carcinoma: value of imaging multistep human hepatocarcinogenesis. Intervirology. 2006;49:64-69. |
| 66. | Lee JY, Choi BI, Han JK, Kim AY, Shin SH, Moon SG. Improved sonographic imaging of hepatic hemangioma with contrast-enhanced coded harmonic angiography: comparison with MR imaging. Ultrasound Med Biol. 2002;28:287-295. |
| 67. | Konopke R, Bunk A, Kersting S. The role of contrast-enhanced ultrasound for focal liver lesion detection: an overview. Ultrasound Med Biol. 2007;33:1515-1526. |
| 68. | Ding H, Wang WP, Huang BJ, Wei RX, He NA, Qi Q, Li CL. Imaging of focal liver lesions: low-mechanical-index real-time ultrasonography with SonoVue. J Ultrasound Med. 2005;24:285-297. |
| 69. | Wanless IR, Mawdsley C, Adams R. On the pathogenesis of focal nodular hyperplasia of the liver. Hepatology. 1985;5:1194-1200. |
| 70. | Quaglia A, Tibballs J, Grasso A, Prasad N, Nozza P, Davies SE, Burroughs AK, Watkinson A, Dhillon AP. Focal nodular hyperplasia-like areas in cirrhosis. Histopathology. 2003;42:14-21. |
| 71. | Di Stasi M, Caturelli E, De Sio I, Salmi A, Buscarini E, Buscarini L. Natural history of focal nodular hyperplasia of the liver: an ultrasound study. J Clin Ultrasound. 1996;24:345-350. |
| 72. | Nicolau C, Brú C. Focal liver lesions: evaluation with contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:348-359. |
| 73. | Yen YH, Wang JH, Lu SN, Chen TY, Changchien CS, Chen CH, Hung CH, Lee CM. Contrast-enhanced ultrasonographic spoke-wheel sign in hepatic focal nodular hyperplasia. Eur J Radiol. 2006;60:439-444. |
| 74. | Petrovic LM. Benign hepatocellular tumors and tumor-like lesions. Pathology (Phila). 1994;3:119-139. |
| 75. | Lim AK, Patel N, Gedroyc WM, Blomley MJ, Hamilton G, Taylor-Robinson SD. Hepatocellular adenoma: diagnostic difficulties and novel imaging techniques. Br J Radiol. 2002;75:695-699. |
| 76. | Solbiati L, Kirn V, Cova L. Other benign lesions and pseudolesions. Contrast-enhanced ultrasound of liver diseases. Rome: Springer-Verlag 2003; 60-61. |
| 77. | Dietrich CF. Characterisation of focal liver lesions with contrast enhanced ultrasonography. Eur J Radiol. 2004;51 Suppl:S9-S17. |
| 78. | Lin MX, Xu HX, Lu MD, Xie XY, Chen LD, Xu ZF, Liu GJ, Xie XH, Liang JY, Wang Z. Diagnostic performance of contrast-enhanced ultrasound for complex cystic focal liver lesions: blinded reader study. Eur Radiol. 2009;19:358-369. |
| 79. | Catalano O, Sandomenico F, Raso MM, Siani A. Low mechanical index contrast-enhanced sonographic findings of pyogenic hepatic abscesses. AJR Am J Roentgenol. 2004;182:447-450. |
| 80. | Liu GJ, Lu MD, Xie XY, Xu HX, Xu ZF, Zheng YL, Liang JY, Wang W. Real-time contrast-enhanced ultrasound imaging of infected focal liver lesions. J Ultrasound Med. 2008;27:657-666. |
| 81. | Xu HX, Xie XY, Lu MD, Liu GJ, Xu ZF, Liang JY, Chen LD. Unusual benign focal liver lesions: findings on real-time contrast-enhanced sonography. J Ultrasound Med. 2008;27:243-254. |
| 82. | Wilson SR, Greenbaum LD, Goldberg BB. Contrast-enhanced ultrasound: what is the evidence and what are the obstacles? AJR Am J Roentgenol. 2009;193:55-60. |
| 83. | Nicolau C, Vilana R, Brú C. The use of contrast-enhanced ultrasound in the management of the cirrhotic patient and for detection of HCC. Eur Radiol. 2004;14 Suppl 8:P63-P71. |