Copyright
©The Author(s) 2016.
World J Radiol. Mar 28, 2016; 8(3): 226-239
Published online Mar 28, 2016. doi: 10.4329/wjr.v8.i3.226
Published online Mar 28, 2016. doi: 10.4329/wjr.v8.i3.226
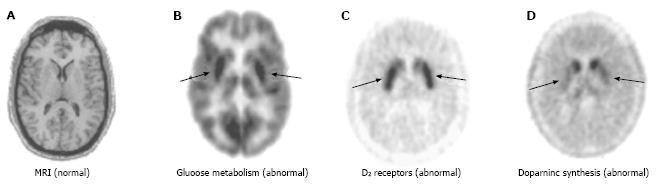
Figure 1 Imaging of early Parkinson’s disease.
A: MRI of the striatum does not indicate any significant anatomical abnormalities; B: PET reveals selective regional alterations in diverse neurochemical processes: FDG PET shows putaminal hypermetabolism relative to the caudate (approximately 10%); C: [18F]fluoroethylspiperone PET shows approximately 15% greater D2 receptor density in the putamen as compared to the caudate; D: FDOPA PET shows significant reduction in dopamine synthetic capacity (80%) in the putamen but not in the caudate. Arrows in B, C, and D indicate putamen. MRI: Magnetic resonance imaging; PET: Positron emission tomography; FDG: 2-[18F]-fluoro-2-deoxy-D-glucose; FDOPA: [18F]-labeled L-3,4-dihydroxiphenylalanine. (Originally published in the JNM. Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med 2000; 41: 661-681. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
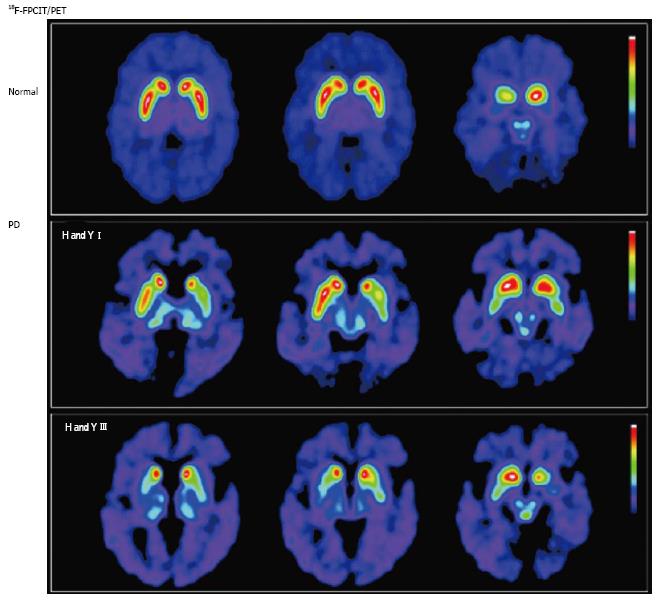
Figure 2 Nigrostriatal dopaminergic system degeneration in Parkinson’s disease.
PET images show striatal uptake of [18F]FPCIT, a ligand with high affinity for pre-synaptic dopamine transporters, for a normal volunteer (top row), and 2 patients with Parkinson’s disease (PD) [middle row: Hoehn and Yahr (H and Y) stage I; bottom row: H and Y stage III]. Striatal [18F]FPCIT uptake is mainly reduced in the putamen contralateral to the most symptomatic side in early PD (middle row), progressing to bilateral reduction in a caudal-to-rostral pattern (bottom row). (Originally published in the JNM. Katzumata K, Dhawan V, Chaly T, et al. Dopamine transporter imaging with fluorine-18-FPCIT and PET. J Nucl Med 1998; 39: 1521-1530. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
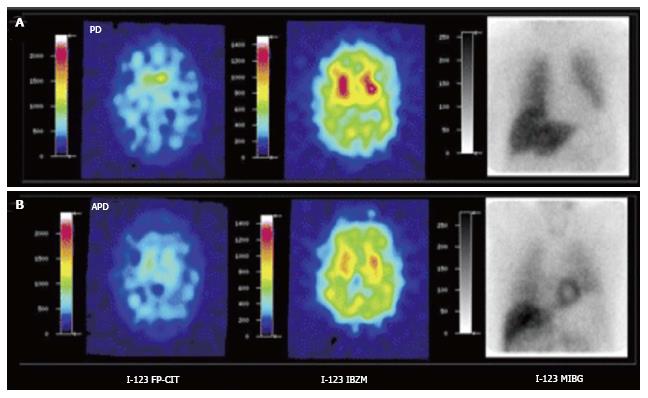
Figure 3 [123I]FPCIT, [123I]IBZM and cardiac SPECT imaging [[123I]MIBG] of Parkinson’s disease (A) and atypical parkinsonism (B).
Striatal [123I]FPCIT is decreased in both PD and APD. Striatal D2 receptor binding is normal in PD but decreased in APD. Myocardial sympathetic SPECT signal is decreased in PD but normal in APD. PD: Parkinson’s disease; APD: Atypical parkinsonism; SPECT: Single photon emission computerized tomography. (Originally published in the JNM. Südmeyer M, Antke C, Zizek T, et al. J Nucl Med 2011; 52: 733-740. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
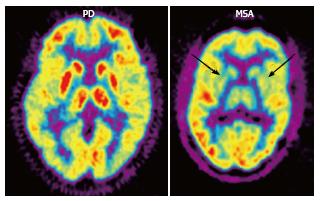
Figure 4 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG PET) in Parkinson’s disease (PD) and multiple system atrophy (MSA).
FDG PET shows hypometabolism in bilateral parieto-occipital and prefrontal cortices, as well as hypermetabolism in the basal ganglia and thalamus in PD, as opposed to bilateral striatal and thalamic hypometabolism in MSA (arrows). (Originally published in the JNM. Brooks DJ. J Nucl Med 2010; 51: 596-609. © by the Society of Nuclear Medicine and Molecular Imaging, Inc.)
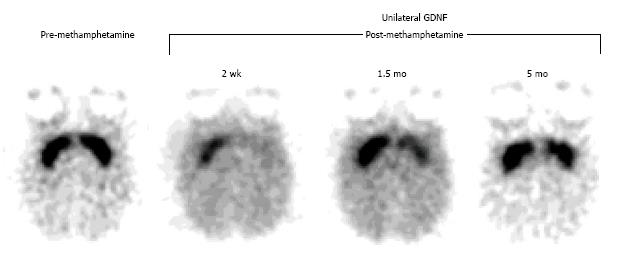
Figure 5 Positron emission tomography assessment of a neuroprotective strategy for methamphetamine-induced neurotoxicity with [11C]WIN 35,428, a selective dopamine transporter ligand.
Prior to drug treatment, symmetrical and selective striatal uptake was demonstrated (left, pre-methamphetamine). Glial cell-line derived growth factor (GDNF) was then unilaterally injected into the striatum (left-sided striatum on the images shown). One week later, a neurotoxic methamphetamine (METH) dosage was systemically administered. Subsequent studies showed that the GDNF-pretreated striatum was partially protected from the METH-induced neurotoxicity and that it recovered at a faster rate relative to the contralateral striatum (right, post-methamphetamine). (Originally published in Synapse. Melega WP, Lacan G, Desalles AA, Phelps ME. Long-term methamphetamine-induced decreases of [(11)C]WIN 35,428 binding in striatum are reduced by GDNF: PET studies in the vervet monkey. Synapse 2000; 35: 243-249. © by John Wiley & Sons publications, Inc.)
- Citation: Lizarraga KJ, Gorgulho A, Chen W, De Salles AA. Molecular imaging of movement disorders. World J Radiol 2016; 8(3): 226-239
- URL: https://www.wjgnet.com/1949-8470/full/v8/i3/226.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i3.226