Copyright
©The Author(s) 2015.
World J Radiol. Sep 28, 2015; 7(9): 220-235
Published online Sep 28, 2015. doi: 10.4329/wjr.v7.i9.220
Published online Sep 28, 2015. doi: 10.4329/wjr.v7.i9.220
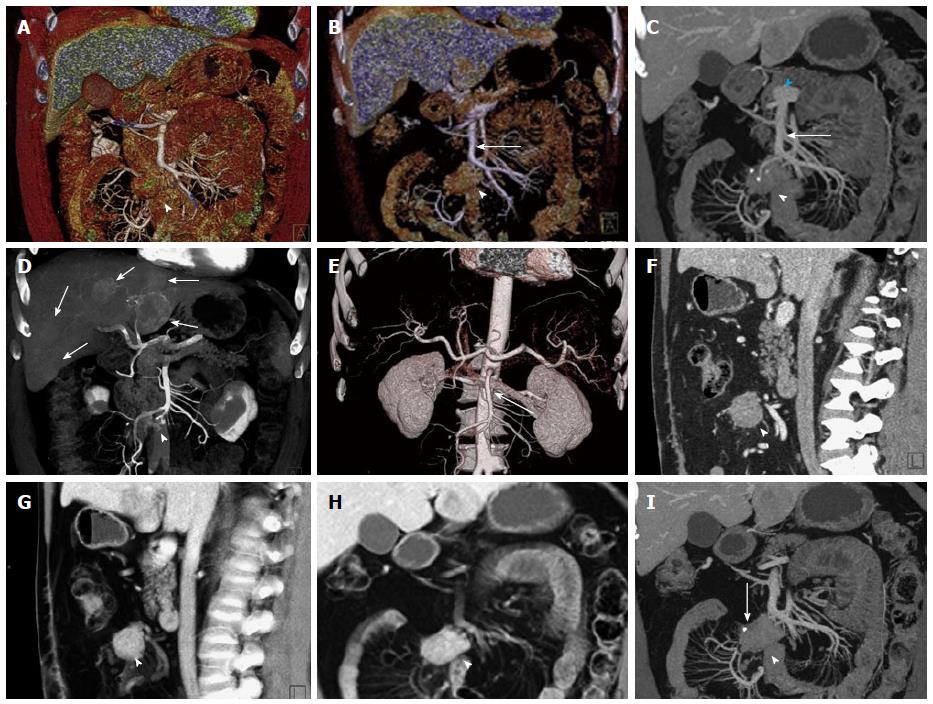
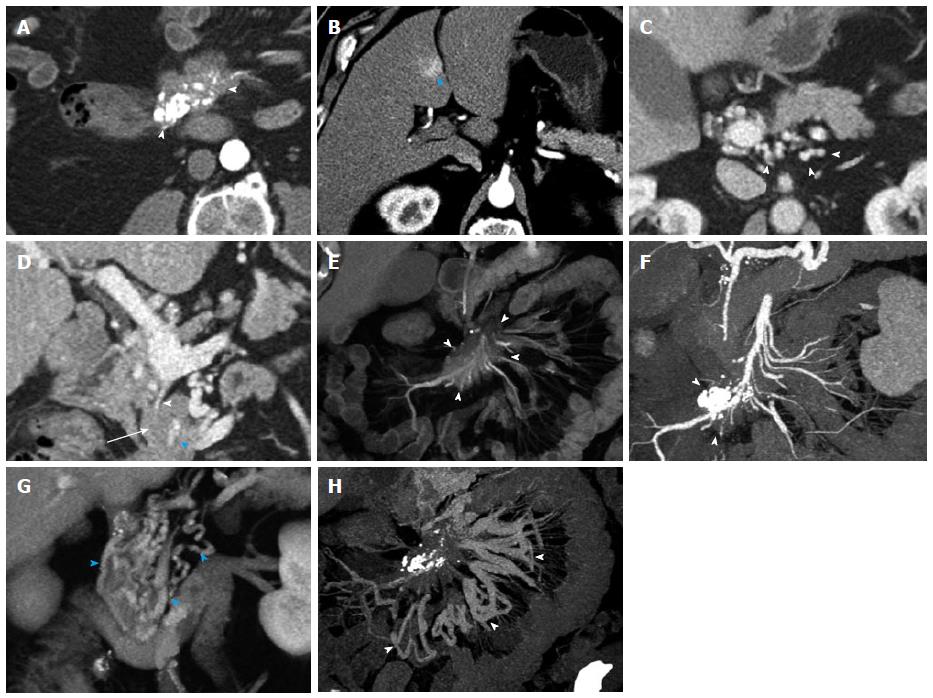
Figure 1 General use of maximum intensity projections, multiplanar reconstructions, volume rendered technique and surface shading.
A, B: Coronal surface rendering technique with two different window settings allows to obtain a 3D understanding of a mesenteric mass (arrowhead) and its relationship to the SMV (arrow). The lower window setting (A) includes more soft tissue detail, and better depicts the mesenteric structures and adjacent bowel loops, while the higher window setting (B) excludes structures with lower attenuation and focuses the view on vascular morphology; C: Coronal venous phase VRT image showing the mesenteric mass with calcifications (arrowhead), and the relationship to the SMV (arrow), which can be visualized in its entire course to the portosplenic confluence (blue arrowhead); D: Volume rendering of arterial phase CTA data simultaneously depicts hypervascular liver metastases (arrows) and the relationship of the mesenteric mass to the SMA with vascular encasement and rarefaction of SMA branches (arrowhead); E: 3D surface shading has its major advantage in the depiction the vascular anatomy (SMA, arrow), while the chosen windowing reduces the conspicuity of the mesenteric mass (arrowhead) and removes the voxels pertaining to the liver metastases and the hepatic parenchyma for depiction of the hepatic arteries; F, G: Comparison of sagittal venous phase slice (F) and VRT postprocessed image (G). The mesenteric mass (arrowheads) and its relationship to the adjacent vessels is better defined with VRT (G); H, I: Comparison of coronal VRT (H) to coronal thick slab MPR (I). While VRT makes the spatial relationships more apparent, MPR better demonstrates vascular anatomical detail and the relationship of vessels to the mesenteric mass (arrowheads). Also, a small calcification in the mesenteric mass (arrow) is only visualized with MPR, while the VRT algorithm hides this important detail. VRT: Volume rendered technique; MPR: Multiplanar reconstructions; MIP: Maximum intensity projections; SMV: Superior mesenteric vein; CTA: Computed tomography angiography; SMA: Superior mesenteric artery; 3D: Three-dimensional.
Figure 2 Duodenal carcinoid.
A 80-year-old male with duodenal carcinoid. Upper endoscopy for screening showed 1.5 cm mass in the 1st portion of the duodenum. An attempt at endoscopic resection failed, as the mass was too deeply located and invaded the muscularis propria. This case well demonstrates the imaging appearance of the intramural primary tumor as hypervascular mass and the choice of 3D postprocessing methods for optimal evaluation. A: On axial arterial phase imaging, the mass in the wall of the 1st portion of the duodenum is quite subtle (arrowhead). The mass is much better depicted on a coronal VRT image (B) or a thin slab coronal MPR (C), improving diagnostic confidence, while coronal MIP (D) does not provide good contrast in this case. VRT: Volume rendered technique; MPR: Multiplanar reconstructions; 3D: Three-dimensional; MIP: Maximum intensity projections.
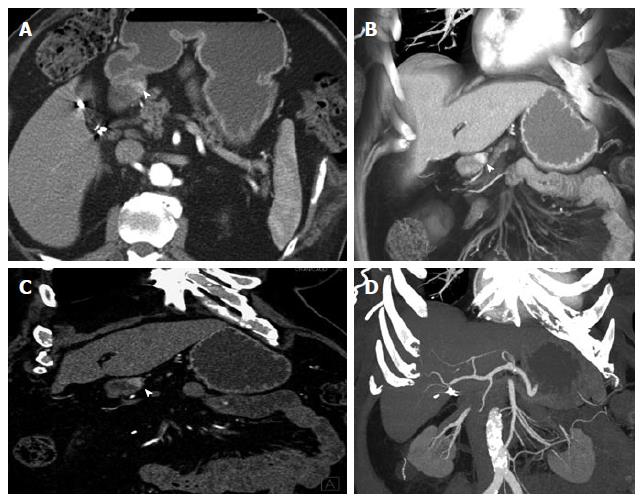
Figure 3 Ampullary neuroendocrine tumor.
A 49-year-old female with 1.2 cm neuroendocrine neoplasm of the ampulla, metastatic to the liver. The tumor was incidentally found due to dilated biliary and pancreatic ducts on a renal protocol computed tomography (CT). The patient underwent classic pancreaticoduodenectomy and liver wedge resection, confirming a low grade (G1) 1.2 cm neuroendocrine neoplasm of the ampulla with invasion of the pancreas and peripancreatic tissues. 1 of 19 lymph nodes was involved and lymphovascular invasion was present. This case demonstrates the typical imaging appearance of a hypervascular intramural primary carcinoid, with additional specific imaging features of a periampullary mass. The case also illustrates important aspects of 3D postprocessing. Axial arterial phase (A) and venous phase (B) CT angiography (CTA) images of the 2nd portion of the duodenum show a hypervascular mass in the ampulla (arrowheads). C: Coronal arterial phase image shows the hypervascular mass extending outside the duodenal wall, with adjacent hypervascular peripancreatic lymph nodes (arrowhead). Intrahepatic biliary ductal dilation is noted (arrow); D: Pancreatic ductal dilation results from the obstructing ampullary mass (arrowhead); E, F: Thick slab MPR (E) and VRT (F) images better depict the spatial relationships: The ampullary mass (blue arrowheads) causes moderate extrahepatic and intrahepatic biliary ductal dilation (arrowheads). Peripancreatic hypervascular lymph node metastases (arrows) are present. Note the advantage of MPR and VRT over axial slices in making the mass much more apparent. VRT: Volume rendered technique; MPR: Multiplanar reconstructions; 3D: Three-dimensional.
Figure 4 Duodenal Carcinoid.
A 61-year-old male with duodenal carcinoid, status-post exploratory laparotomy for a mesenteric mass at an outside institution. Subsequent Whipple procedure demonstrated a 1.8 cm unifocal duodenal carcinoid (pT3, invading pancreas), with 6 of 15 regional lymph node metastases (pN1). This case illustrates the distinction of the primary mass from adjacent nodal metastatic disease and poses an example for nodal metastatic disease having a much larger volume than the primary tumor. A: Venous phase axial computed tomography image shows a 1.8 cm mass in the medial wall of the duodenum, extending into the moderately fatty replaced pancreas, compatible with the primary tumor (arrowheads); B: Arterial phase axial image (magnified, similar slice position) demonstrates heterogenous hypervascularity in the primary tumor (arrowheads). Multiple metastatic hypervascular peripancreatic lymph nodes are present (blue arrowheads); C: Arterial phase axial image shows a large hypervascular nodal metastasis in the inferior pancreatic groove (blue arrowhead); D: Axial venous phase image shows an enlarged portocaval lymph node (arrowhead); E: Note that in the same node on arterial phase axial image a left-sided hypervascular metastasis can be identified, which was not discernible on the venous phase (D) (arrow).
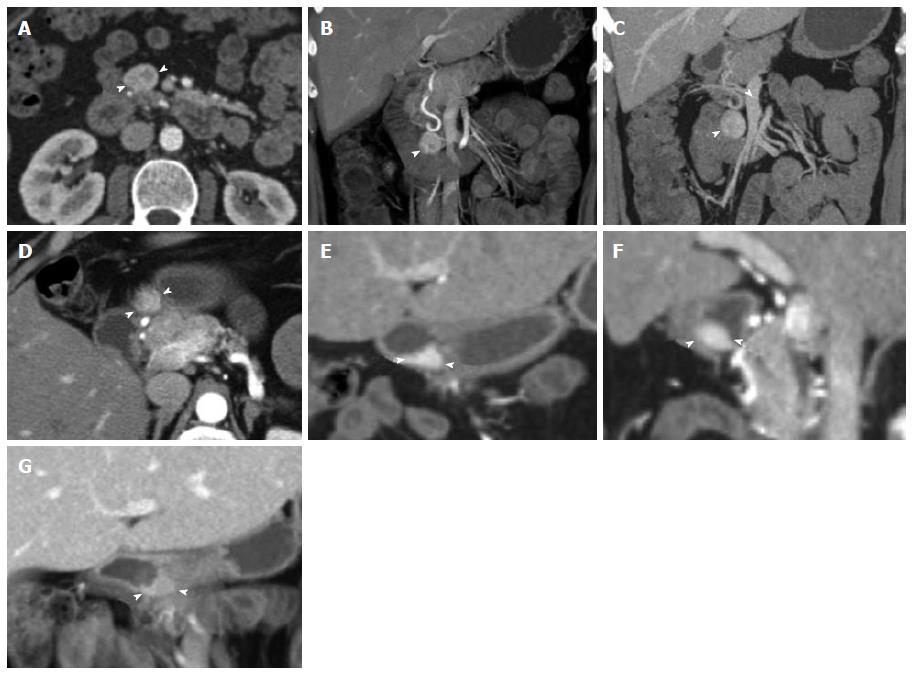
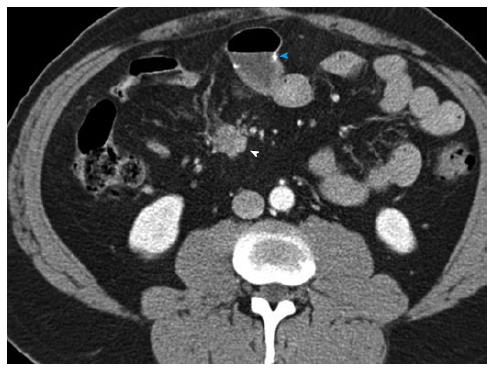
Figure 5 Duodenal carcinoid.
Duodenal carcinoid tumor illustrated at two distinct time points. 46-year-old female status-post surgery for removal of an omental carcinoid metastasis, at the first time point presenting with a peripancreatic hypervascular nodal metastasis, which was subsequently surgically removed. A: Axial arterial phase computed tomography (CT) image shows a hypervascular peripancreatic nodal carcinoid metastasis (arrowheads); B: Coronal arterial phase VRT image well demonstrates the location of the hypervascular nodal mass (arrowhead) in the pancreatic groove, abutting the second portion of the duodenum; C: Coronal thick slab MIP image delineates the hypervascular nodal metastasis (arrowhead) in relationship to the SMV (arrow). One year later, at the second time point, a follow-up CT showed appearance of the primary tumor in the duodenal bulb, which was subsequently treated with pancreaticoduodenectomy (Whipple procedure); D, E: Axial (D) and coronal (E) arterial phase CT images show a hyperenhancing mural mass (arrowheads) in the duodenal bulb, just distal to the pylorus, compatible with the patient’s primary carcinoid tumor; F: Sagittal arterial phase CT image shows the hyperenhancing mural mass (arrowheads) in the duodenal bulb; G: Coronal arterial phase VRT image demonstrates the intramural hyperenhancing mass (arrowheads). This case illustrates that while the primary tumor may not be initially apparent, it may demarcate itself in the course of the disease, and it also shows the typical imaging appearance of the primary tumor in the form of a hypervascular intramural mass. SMV: Superior mesenteric vein; VRT: Volume rendered technique; MIP: Maximum intensity projections.
Figure 6 Ileal Carcinoid.
A 53-year-old male with stage IV ileal carcinoid tumor, who presented acutely with small bowel obstruction and a history of several months of weight loss, crampy abdominal pain and distention. The patient underwent exploratory laparotomy with surgical resection of an ileal mass (biopsy-proven carcinoid) with primary anastomosis. A large mesenteric mass involving the main superior mesenteric vein and bilateral liver metastases were not resectable. A: Coronal venous phase computed tomography (CT) image shows a hypervascular mass in the wall of a distal ileal loop (arrow) representing the primary carcinoid tumor; B: Coronal venous phase CT image slightly more anterior shows dilated small bowel (arrowhead) with mural thickening and hypoenhancement, compatible with small bowel obstruction and suggestive of hypoperfusion. A portion of a mesenteric nodal mass (arrow) representing regional metastatic disease is shown (arrow); C: Serial axial CT images from the venous phase acquisition; Left: a dilated small bowel loop proximal to the obstruction demonstrates normal bowel wall enhancement and thickness; Middle: Compare the wall thickness and enhancement of the bowel wall at the site of the primary tumor (circumferential mass, invading the mesentery on the left side, arrowhead); Right: The next slice shows the hyperenhancing bowel wall mass (arrowhead) directly abutting the mesenteric lymph nodal metastatic conglomerate mass (arrow); D: Axial arterial phase computed tomography angiography (CTA) image shows the hypervascular mesenteric mass (arrow) reflecting regional lymph node metastatic disease; E: Coronal arterial phase CTA image shows the hypervascular mesenteric mass (arrowhead) reflecting regional lymph node metastatic disease. Hypervascular liver metastases (arrows) are depicted.
Figure 7 Small bowel carcinoid.
A 74-year-old male with small bowel carcinoid (Stage IV), incidentally detected during computed tomography (CT) for presumed kidney stones. He subsequently described a history of flushing and diarrhea, and his urine 5-HIAA level was found elevated. In this case the primary tumor could not be detected on CT. Surgery and pathology demonstrated a polypoid 2 cm mass in the small bowel, likely not detected on CT due to bowel ischemia of the bowel segment involving the tumor. Local nodal metastatic and liver metastatic disease were present. A, B: Axial arterial phase (A) and venous phase (B) images show a hyperenhancing mesenteric mass surrounded by spiculations (arrows) with a small focal calcification (arrowheads), compatible with local nodal metastatic disease with desmoplastic reaction. Multiple dilated small bowel loops with hypoenhancing walls surround the mesenteric mass compatible with the surgical finding of bowel ischemia; C, D: Axial (C) and coronal (D) thick slab MPR images allow the depiction of the relationship of the mesenteric mass (arrows) to the SMA (arrowhead) and the adjacent bowel. The SMA appears irregular and is partially encased by the mass; E: Coronal VRT image allows good visualization of spatial relationships of mesenteric mass (arrow) with calcification, ischemic dilated small bowel loops (arrowhead) and a necrotic liver metastasis in the liver dome (blue arrowhead). VRT: Volume rendered technique; MPR: Multiplanar reconstructions; SMA: Superior mesenteric artery.
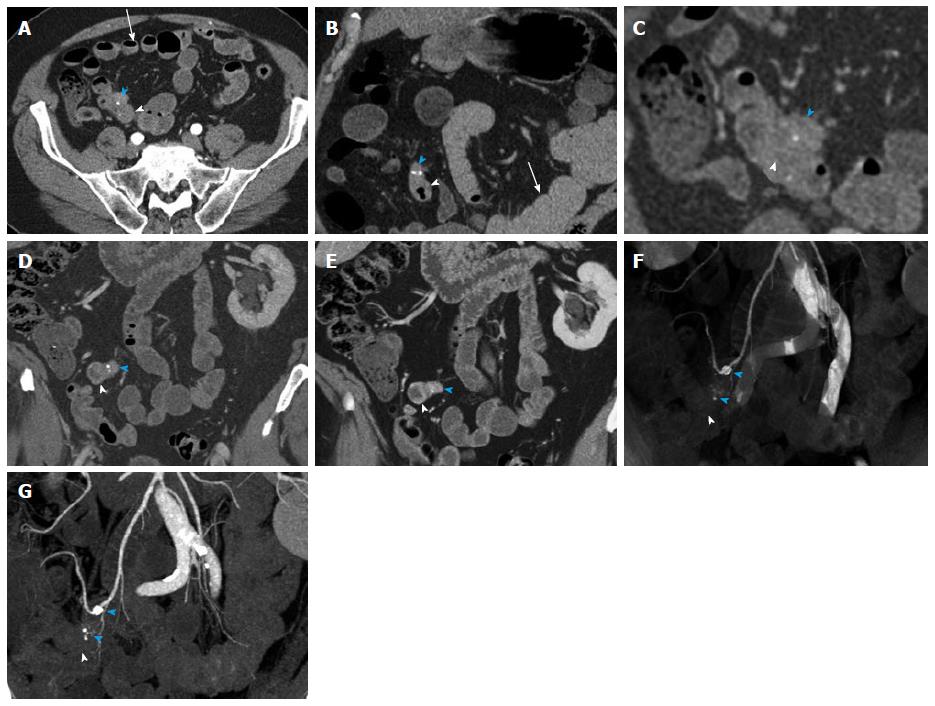
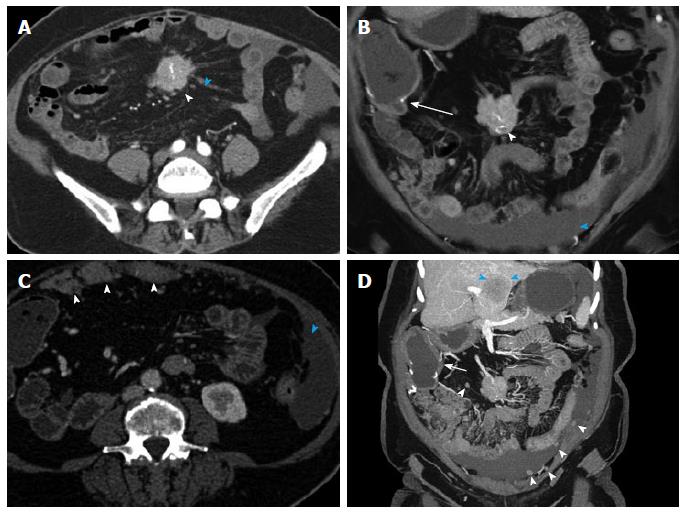
Figure 8 Small bowel carcinoid.
A 71-year-old male with small bowel carcinoid, who presented with intermittent abdominal pain and small bowel obstruction. He underwent a 40 cm distal ileal resection, demonstrating a 1.5 cm carcinoid tumor (pT3, invading into subserosal tissue), with regional nodal metastases (2 of 21). After 6 mo of available follow up, there is currently no evidence of metastatic or residual disease. This case illustrates the evaluation of a primary tumor in an underdistended small bowel segment in the setting of small bowel obstruction caused by the mass. Depiction of locoregional metastatic disease. A: Axial arterial phase CTA image shows a small focal area of asymmetric wall thickening in an underdistended small bowel loop (arrowhead), representative of the primary tumor. There is directly adjacent metastatic adenopathy with small calcifications (blue arrowhead). Note that these findings are rather subtle on the axial images. Multiple proximal small bowel loops with fluid levels are compatible with partial small bowel obstruction (arrow). The presence of SBO limits the ability of oral contrast to aid in adequate distention of the pathological small bowel segment; B: Coronal arterial phase image better demonstrates asymmetric wall thickening (arrowhead) and extramural mesenteric extension of tumor with calcifications (blue arrowhead). Multiple dilated proximal small bowel loops are compatible with partial small bowel obstruction (arrow); C: Thin slice reconstructions (0.75 mm) of the venous phase data allow better evaluation of the small bowel mass (arrowhead) and the adjacent calcified mesenteric mass (blue arrowhead); D, E: Coronal thin (D) and thick (E) slab MIP images of the venous phase data demonstrate the small bowel mass (arrowheads) and the adjacent metastatic calcified adenopathy (blue arrowheads) to better advantage; F, G: Coronal VRT (F) and MIP (G) of the CTA data demonstrate the mass (arrowheads) and associated calcified nodal masses (blue arrowheads) in relation to the mesenteric vessels. VRT: Volume rendered technique; MIP: Maximum intensity projections; CTA: Computed tomography angiography. SBO: Small bowel obstruction.
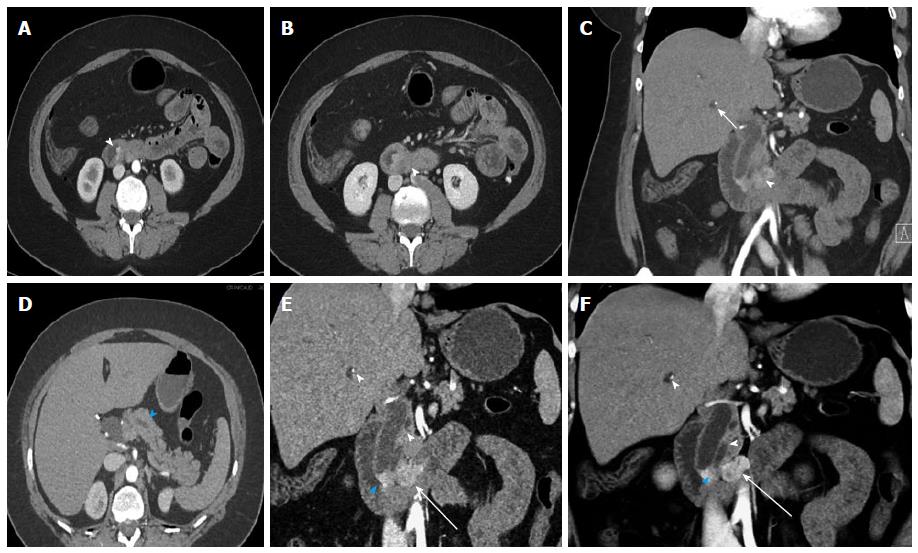
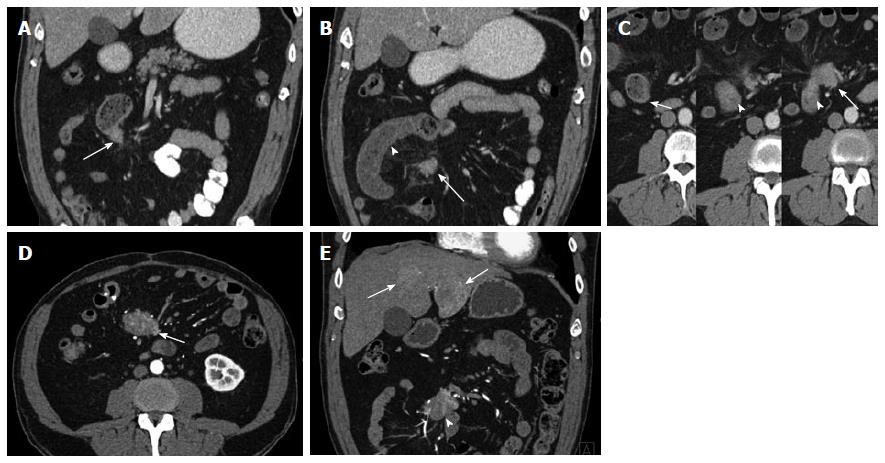
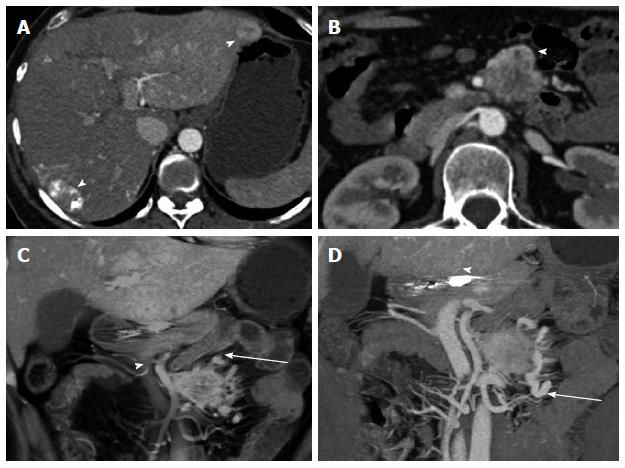
Figure 9 Metastatic small bowel carcinoid.
A 66-year-old male with metastatic small bowel carcinoid. The tumor was found unresectable with large mesenteric nodal metastatic disease encasing SMA and SMV, and with liver metastases. In this case the primary tumor location is unknown (and possibly very small), in the presence of bulky regional metastatic disease and liver metastatic disease. It also demonstrates the evaluation of vascular complications (SMV occlusion, encasement of SMV and SMA) using 3D imaging. It is not unusual that the primary tumor is much smaller than the nodal metastatic disease. A: Arterial phase axial image demonstrates a large mesenteric mass with surrounding desmoplastic reaction (arrowheads), compatible with regional nodal metastatic disease from carcinoid tumor. The primary tumor was not identified on the exam; B: Arterial phase axial image demonstrates a hypervascular metastasis in segment IV B of the liver; C: Axial venous phase image shows extensive peripancreatic collateral vessels (arrowheads) as a result of SMV occlusion by tumor encasement; D: Encasement of the SMV (arrowhead) and SMA (blue arrowhead) by the tumor. The distal SMV is severely narrowed (arrowhead), while the more proximal SMV is completely occluded by the tumor (arrow); E: Coronal VRT arterial phase image demonstrates the large mesenteric mass (arrowheads), which encases the SMA and its branches; F: Coronal thick slab MIP arterial phase image demonstrates the calcified mesenteric mass (arrowheads), encasing SMA branches; G: Coronal VRT venous phase image shows multiple peripancreatic collateral vessels (blue arrowheads) to better advantage than axial or multiplanar imaging; H: Coronal thick slab venous phase MIP image shows moderately dilated mesenteric veins (arrowheads) resulting from SMV occlusion. VRT: Volume rendered technique; MIP: Maximum intensity projections; SMA: Superior mesenteric artery; SMV: Superior mesenteric vein.
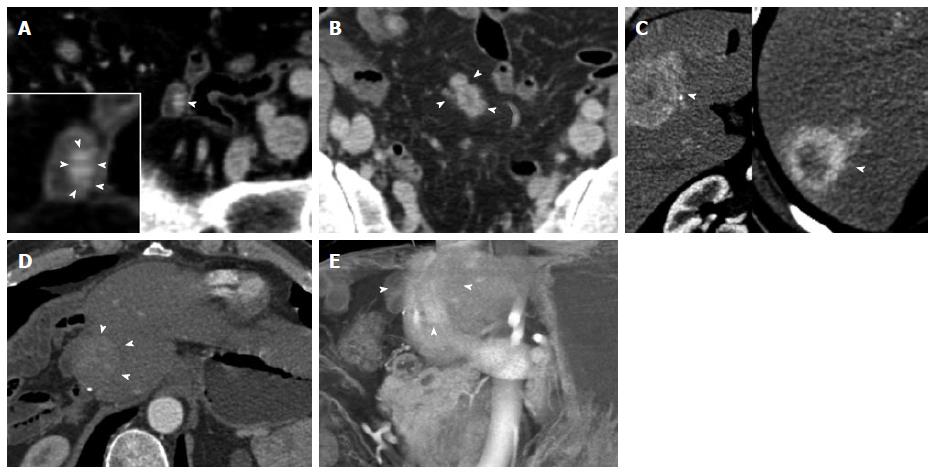
Figure 10 Metastatic terminal ileal carcinoid.
Terminal ileal small bowel carcinoid tumor with liver metastases. A 57-year-old male with longstanding history of flushing, diarrhea and sweating. Three hypervascular hepatic metastases were found during a computed tomography (CT) evaluation performed after he presented with a diverticular perforation to an outside institution. A Hartmann procedure was performed. After diagnosis, he underwent an extended right hemihepatectomy. An ileal resection proved a terminal ileal 1.5 cm primary carcinoid tumor. Four years later, he presented with a recurrence at the liver resection margin. This case illustrates the imaging appearance of the primary tumor as a hypervascular mural mass and extensive locoregional nodal metastatic and liver metastatic disease despite a small (1.5 cm) primary tumor. A: Axial venous phase CECT demonstrates a small bowel loop (terminal ileum) with a small hyperenhancing mural mass (arrowhead). A detail view better demonstrates the mass (inset, arrowheads), which represents the primary tumor and was confirmed at surgery. The primary tumor is subtle and easily missed on the axial CT slices; B: Axial venous phases CECT image slightly superiorly demonstrates matted hypervascular mesenteric lymph node metastases with minimal surrounding desmoplastic reaction (arrowheads), typical for carcinoid tumor local metastases; C: Axial arterial phase images demonstrate segment VIII/V (left) and segment VII (right) hypervascular, centrally necrotic liver metastases (arrowheads). Note that this advanced disease is associated with the small (1.5 cm) primary tumor. It is not uncommon that metastatic disease is significantly larger than the primary carcinoid tumor; D, E: Follow-up exam 4 years after extended right hepatectomy shows recurrence at the resection margin (arrowheads), shown on the axial venous phase image (D) and a coronal volume rendered technique image (E). CETC: Contrast enhanced computed tomography.
Figure 11 Carcinoid of unknown primary.
Functional carcinoid tumor of unknown primary, metastatic to liver and mesenteric root. A 43-year-old female with history of postprandial nausea. Ultrasound exam detected bilobar liver metastases. Subsequent computed tomography (CT) demonstrated hypervascularity of the liver metastases. Liver biopsy confirmed carcinoid metastases. She underwent systemic chemotherapy with VP-16 and carboplatin and locoregional therapy to the liver metastases (Yttrium-90 microsphere embolization). This case illustrates the evaluation of and appearance of locoregional nodal metastatic and liver metastatic disease. A: Axial arterial phase CT shows multiple hypervascular liver metastases with treatment changes after chemoembolization (arrowheads) and diffuse perfusion change in the left liver lobe related to prior locoregional therapy; B: Axial arterial phase CT image shows a hypervascular mesenteric mass (arrowhead) compatible with metastatic nodal disease; C: Coronal thick slab MIP image demonstrates the hypervascular mesenteric mass (arrow) and its relationship to the SMA and portal vein (arrowhead); D: Coronal VRT image demonstrates hypervascular liver metastasis (arrowhead) and mesenteric mass (arrow). The extensive desmoplastic reaction surrounding the mesenteric mass is well shown. VRT: Volume rendered technique; MIP: Maximum intensity projections; SMA: Superior mesenteric artery.
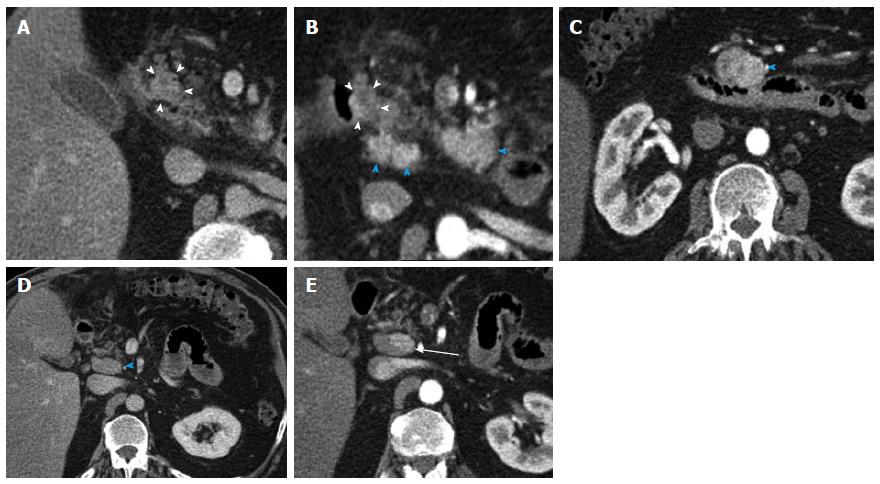
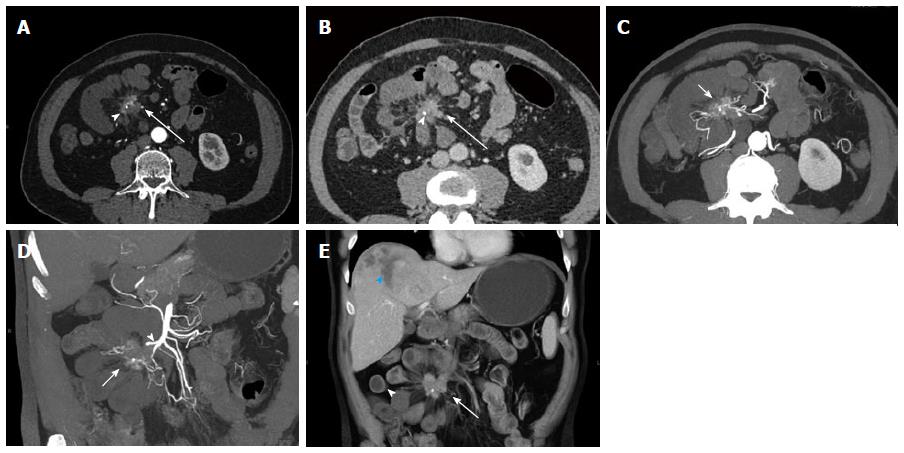
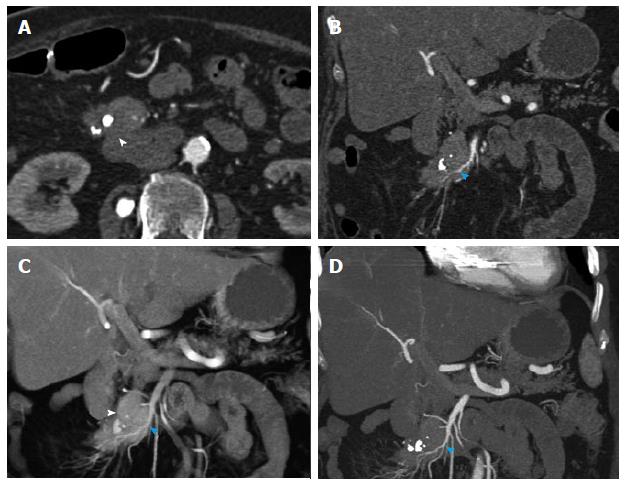
Figure 12 Recurrent metastatic small bowel carcinoid.
A 72-year-old female with metastatic small bowel carcinoid. She underwent right hemicolectomy and partial small bowel resection 10 years ago, and is currently on long-acting octreotide. This case illustrates the imaging appearance and evaluation of mesenteric locoregional nodal metastatic disease recurrence with 3D imaging. A: Axial arterial phase imaging shows a calcified mass in the mesentery (arrowhead) compatible with recurrent metastatic carcinoid tumor; B: Coronal CTA image shows encasement of branches of superior mesenteric artery (blue arrowhead), with associated luminal irregularities and narrowing of the artery; C: Coronal VRT image depicts the calcified mass (arrowhead) and allows analysis of the morphology of the SMA branches within and distal to the tumor. Encased SMA branches demonstrate luminal narrowing and irregularity (blue arrowhead); D: Thick slab coronal MIP is of high diagnostic value in this case, as it benefits from the excellent contrast between vascular lumen and tumor tissue, probably providing the best assessment of the morphology of the encased SMA branches (blue arrowhead). VRT: Volume rendered technique; MIP: Maximum intensity projections; SMA: Superior mesenteric artery.
Figure 13 Recurrent metastatic small bowel carcinoid.
52-year-old male with metastatic small bowel carcinoid. He presented for follow-up one year after small bowel resection for carcinoid tumor. This case illustrates the imaging appearance of recurrent locoregional nodal metastatic disease. Axial arterial phase axial images show a small mass in the small bowel mesentery (arrowhead) with surrounding mild desmoplastic reaction, compatible with recurrence of metastatic carcinoid tumor. A surgical small bowel anastomosis is noted (blue arrowhead).
Figure 14 Recurrent metastatic small bowel carcinoid.
71-year-old female with metastatic small bowel carcinoid. Twelve years ago she had undergone a segmental distal ileal resection and liver segmental resection for metastatic carcinoid. This case illustrates advanced recurrent metastatic carcinoid with extensive abdominal and pelvic peritoneal implants and hepatic metastatic disease. A: Axial arterial phase axial image shows a calcified mass in the small bowel mesentery (arrowhead) with surrounding linear desmoplastic reaction (blue arrowhead), compatible with carcinoid tumor metastasis; B: Coronal arterial phase VRT image shows the calcified mesenteric mass (arrowhead). The patient is status post terminal ilial and proximal colonic resection with ileocolic anastomosis (arrow). Moderate ascites is present (blue arrowhead); C: Axial arterial phase axial image shows multiple metastatic omental implants (arrowheads). Ascites is present (open arrowhead); D: Many additional omental implants are noted (arrowheads) on this coronal thick slab MIP image. Ileocolic anastomosis and colonic thickening (arrow) in the setting of ascites are noted. Large segment IVB liver metastasis (blue arrowheads). VRT: Volume rendered technique; MIP: Maximum intensity projections.
- Citation: Bonekamp D, Raman SP, Horton KM, Fishman EK. Role of computed tomography angiography in detection and staging of small bowel carcinoid tumors. World J Radiol 2015; 7(9): 220-235
- URL: https://www.wjgnet.com/1949-8470/full/v7/i9/220.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i9.220