Published online Sep 26, 2017. doi: 10.4330/wjc.v9.i9.749
Peer-review started: January 16, 2017
First decision: May 11, 2017
Revised: July 20, 2017
Accepted: August 2, 2017
Article in press: August 2, 2017
Published online: September 26, 2017
Processing time: 255 Days and 15.5 Hours
To assess utility and correlation of known anticoagulation parameters in the management of pediatric ventricular assist device (VAD).
Retrospective study of pediatric patients supported with a Berlin EXCOR VAD at a single pediatric tertiary care center during a single year.
We demonstrated associations between activated thromboplastin time (aPTT) and R-thromboelastography (R-TEG) values (rs = 0.65, P < 0.001) and between anti-Xa assay and R-TEG values (rs = 0.54, P < 0.001). The strongest correlation was seen between aPTT and anti-Xa assays (rs = 0.71, P < 0.001). There was also a statistically significant correlation between platelet counts and the maximum amplitude of TEG (rs = 0.71, P < 0.001). Importantly, there was no association between dose of unfractionated heparin and either measure of anticoagulation (aPTT, anti-Xa or R-TEG value).
This study suggests that while there is strong correlation between aPTT, anti-Xa assay and R-TEG values for patients requiring VAD support, there is a lack of relevant correlation between heparin dose and degree of effect. This raises concern as various guidelines continue to recommend using these parameters to titrate heparin therapy.
Core tip: This study suggests that while there is strong correlation between activated thromboplastin time, anti-Xa assay and R-thromboelastography values for patients requiring ventricular assist device support, there is a lack of relevant correlation between heparin dose and degree of effect. This raises concern as various guidelines continue to recommend using these parameters to titrate heparin therapy. A comprehensive strategy for appropriate anticoagulation may therefore warrant a combination of parameter monitoring and warrants further study.
- Citation: Bhatia AK, Yabrodi M, Carroll M, Bunting S, Kanter K, Maher KO, Deshpande SR. Utility and correlation of known anticoagulation parameters in the management of pediatric ventricular assist devices. World J Cardiol 2017; 9(9): 749-756
- URL: https://www.wjgnet.com/1949-8462/full/v9/i9/749.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i9.749
Appropriate anticoagulation continues to be a significant challenge in pediatric patients supported with ventricular assist devices (VADs). VAD implantation leads to dysregulation of hemostasis through contact of blood with foreign materials and introduction of shear forces that activate vascular endothelium, platelets, leukocytes and the coagulation cascade. This constellation of events increases the generation of thrombin and thus greatly increases the risk of thrombosis. Clinicians attempt to address the resultant imbalance between the pro- and anti-thrombotic states through the administration of anticoagulation and antiplatelet therapy. However, appropriate titration of these therapies in the pediatric population is challenging and resulting in various complications related to either a pro-thrombotic state leading to embolic complications or an overly anti-thrombotic state presenting as post-operative bleeding, gastrointestinal bleeding or hemorrhagic stroke.
Despite technological advances in VAD design and development of new methods of anticoagulation, complication rates remain significant. Adults on VAD support have bleeding rate of 15%-50% while the risk of stroke has been reported at 5%[1,2]. Unfortunately the overall incidence of these complications in children with VAD appears to be higher[3-5]. While the VAD technology and anticoagulation agents are the same as those for adult patients, there are marked differences in dosing of medications, device performance characteristics in children and intrinsic differences in the maturity of the hemostatic system in children as they develop[6-9]. One retrospective study of 28 pediatric patients with various types of VAD demonstrated major bleeding in 29% and stroke in 25%. Given that there are several types of VAD that can be used in the pediatric population, and technology is constantly evolving, interpretation of older studies is challenging. The Berlin Heart EXCOR Pediatric VAD, a pulsatile extracorporeal device, is currently the most commonly used in pediatrics as it can accommodate a wide range of patient sizes and can support both the right and left heart as necessary. A prospective study comparing the Berlin Heart Pediatric EXCOR device to extracorporeal membrane oxygenation (ECMO) as bridge-to-transplantation demonstrated bleeding in 50% of patients and stroke in 29%[3] in the setting of a prescribed anticoagulation protocol with high degree of adherence.
The major obstacle to achieving adequate anticoagulation while minimizing the risk of hemorrhagic complications revolves around ineffective monitoring strategies and the lack of evidence-based pediatric guidelines to assist clinicians in modifying therapy. Various laboratory tests exist that measure specific components of the hemostatic system, including anti-Xa, activated thromboplastin time (aPTT), prothrombin time (PT), and international normalized ratio (INR), but none of these gives a complete picture of hemostasis[7,10-12]. Thromboelastography (TEG) has been proposed to more accurately demonstrate the in vivo state of hemostasis[13,14]. Specifically the R-value is thought to reflect the anticoagulant effect of heparin. Current VAD anticoagulation guidelines, including those adopted for clinical trials, lack standardization to guide heparin therapy[15]. In addition, there is very limited data on coagulation parameters in pediatric patients supported on VADs. This disconnect may explain why, in many cases, using target lab values to indicate degree of anticoagulation does not prevent poor clinical outcomes. This study attempts to assess the utility and correlation between various measures of anti-coagulation, including the value of TEG, in a cohort of patients who received the Berlin Heart Pediatric EXCOR VAD.
Anticoagulation parameters from four patients supported with a Berlin Heart EXCOR VAD at a single center during 2013 were studied retrospectively. The study was approved by the institutional review board. Standard anticoagulation therapy was initiated for all of these patients after the implantation of the Berlin EXCOR VAD in accordance with the published guidelines[15]. All management decisions for anticoagulation and anti-platelet therapy were made by the VAD team, again with target levels for various parameters consistent with the published protocol. Briefly, our standard regimen included unfractionated heparin initiated typically about 12 h post-operative, followed by initiation of anti-platelet therapy with aspirin and dipyridamole typically, 48 h post-operative in the setting of good surgical hemostasis. This was followed by dose adjustments as needed based on monitoring parameters. Patients were monitored closely by assessing various anticoagulation parameters such as PT, aPTT, anti-Xa assay, complete blood count, fibrinogen level daily. TEG was performed using a TEG® 5000 Thrombelastograph® Hemostasis Analyzer system (Haemonetics Corporation, MA, United States). Kaolin TEG as well as heparinase TEG were both performed as part of a standard approach to assess whole blood anticoagulation related to heparin as well as the health of coagulation system without the heparin effect. Additionally, TEG was also used to perform platelet-mapping studies using the platelet agonists arachidonic acid (AA) and adenosine diphosphate (ADP) to study platelet inhibition achieved by aspirin and dipyridamole. We tabulated all laboratory tests that were ordered to both assess their coagulation system and to direct their anticoagulation therapy. Additionally, we tabulated incidental heparin dose at time of laboratory collection, as well as clinical data reflecting outcomes, adverse events, morbidities and mortality. Statistical analysis was performed using SPSS 21 software (SPSS Inc., Chicago, IL, United States). Continuous data are reported as mean ± SD, categorical data are reported as frequency (%). Continuous data was compared using student t-test while χ2 test was used for categorical variables. Spearman’s Correlation was used to assess correlation between various tests. Statistical significance was defined as P < 0.05.
Chart review of anticoagulation parameters from a total of four patients who were supported with the Berlin EXCOR Pediatric VAD during a single year yielded nearly 100 data points for every test. Three of the four patients had the primary diagnosis of dilated cardiomyopathy while the fourth patient carried a diagnosis of congenital heart block and developed pacemaker induced cardiomyopathy (Table 1). No other significant comorbidities, genetic syndrome or coagulation disorders noted prior to the implants. Indications for VAD placement were heart failure non-responsive to standard inotropic therapy with milrinone and need for a second agent (dobutamine), along with evidence of end-organ injury. The later was extremely poor tolerance of enteral feeds in 3 patients while it was increased need for respiratory support (including intubation) in one patient. Berlin EXCOR VAD implantation was performed in the standard fashion per manufacturer’s detailed instructions. There were no intraoperative complications.
| Patient 1 K | Patient 2 S | Patient 3 P | Patient 4 N | |
| Diagnosis | DCM | DCM | CHB, DCM | DCM |
| Age | 13 mo | 5 mo | 8 mo | 10 yr |
| Weight | 8.4 kg | 7.2 kg | 8.1 kg | 24.5 kg |
| Gender | F | M | M | F |
| Type of VAD | LVAD | LVAD | LVAD | LVAD |
| Days on VAD | 141 | 69 | 13 | 54 |
| Outcome | OHT | OHT | OHT | OHT |
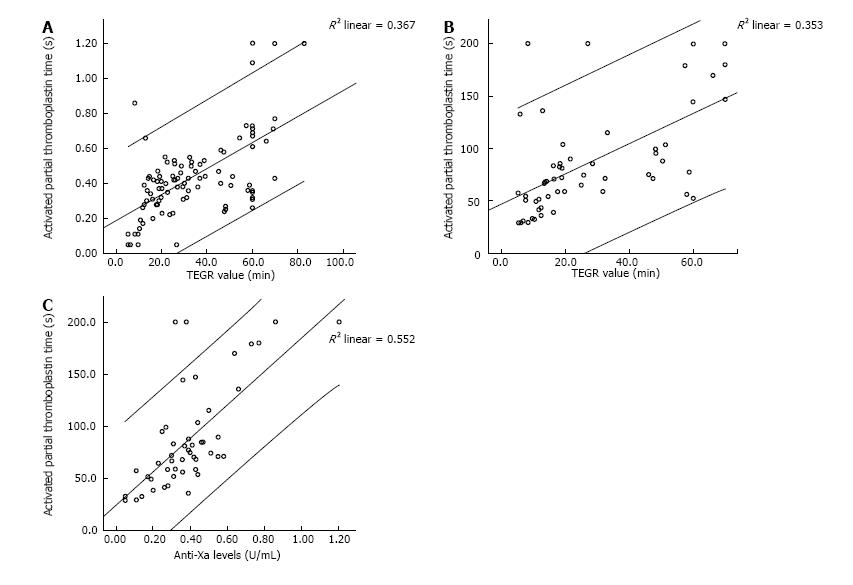
The results for the various tests are shown in Table 2. As noted, there was wide variation in the degree of anticoagulation achieved. We performed Spearman correlation testing to assess the relationship between individual tests measuring degree of anticoagulation, namely, aPTT, anti-Xa assay and R value on TEG. There was a strong and statistically significant correlation between all of these three parameters, with the strongest correlation existing between the aPTT value and anti-Xa assays (R2 = 0.55, Spearman correlation coefficient of 0.71, P < 0.001). R-TEG had correlation coefficients of 0.54 and 0.65 with anti-Xa and aPTT, respectively. These correlations are summarized in Table 3 and demonstrated in Figure 1.
| Test | n | Minimum | Maximum | mean | SD |
| Prothrombin time | 97 | 12.5 | 30.8 | 14.542 | 2.34 |
| Activated partial thromboplastin time (s) | 98 | 26.1 | 200 | 79.779 | 44.62 |
| INR | 98 | 0.9 | 3 | 1.132 | 0.25 |
| Anti-Xa levels (U/mL) | 97 | 0.05 | 1.2 | 0.4381 | 0.24 |
| TEG-R (min) | 102 | 5.2 | 82.8 | 32.464 | 19.77 |
| TEG-alpha angle | 99 | 5.9 | 71.8 | 28.83 | 18 |
| TEG-MA | 98 | 10 | 75 | 46.002 | 18.08 |
| TEG R (heparinase) (min) | 102 | 5.1 | 34.5 | 8.455 | 3.2 |
| TEG-K (heparinase) | 102 | 0.8 | 12 | 2.029 | 1.12 |
| TEG-α angle (heparinase) | 102 | 17.8 | 74.4 | 63.306 | 7.3 |
| TEG-MA (heparinase) | 102 | 45.1 | 73 | 59.696 | 6.03 |
| TEG-G (heparinase) | 100 | 4.1 | 13.5 | 7.712 | 2.01 |
| Platelet inhibition-ADP (%) | 90 | 0 | 100 | 41.113 | 33.73 |
| Platelet inhibition-AA (%) | 91 | 0 | 100 | 43.69 | 37.05 |
| Platelet count (k/μL) | 131 | 77 | 451 | 267.05 | 89.6 |
| Platelet volume | 121 | 6.8 | 10.1 | 8.46 | 0.69 |
| Heparin dose (units/kg per hour) | 131 | 15 | 46 | 33.66 | 7.15 |
| aPTTcorrelationcoefficient | P | Anti-Xacorrelationcoefficient | P | R-TEGcorrelationcoefficient | P | |
| aPTT | 1 | 0.71 | < 0.001 | 0.65 | < 0.001 | |
| Anti Xa | 0.71 | < 0.001 | 1 | 0.54 | < 0.001 | |
| R-TEG | 0.65 | < 0.001 | 0.54 | < 0.001 | 1 |
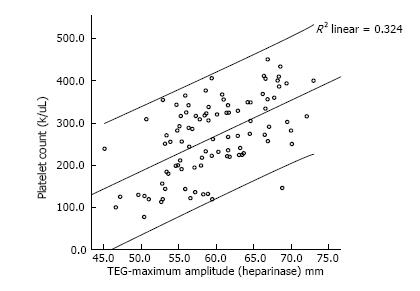
We also assessed the correlation between platelet count (PLT) and the maximum amplitude (MA) on TEG with heparinase added to nullify the heparin effect. We demonstrated that there was a strong and statistically significant correlation between the two values (Spearman correlation coefficient of 0.71, P < 0.001) (Figure 2).
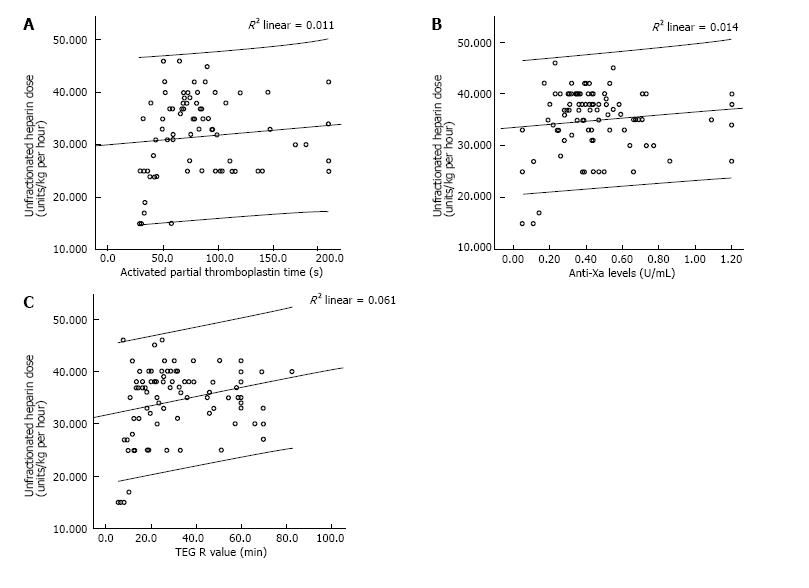
Similar to previous studies, we found no clinically relevant association between heparin dose and the degree of anticoagulation measured by the tests. There was no relationship between aPTT and Heparin dose (Figure 3A) giving a Spearman’s rho correlation coefficient of 0.152 and a P value of 0.168. Similarly, there was no correlation between the heparin dose and anti-Xa levels (Spearman’s rho of -0.004, P = 0.971). There was weak correlation between heparin dose and TEG-R values (Spearman’s rho of 0.24, P = 0.015). To account for patient variation in response to heparin as well as inherent differences in the coagulation system in pediatric patients, we also performed a correlation analyses by patient. In this case, there was a large variation in correlation between heparin dosing and aPTT or anti-Xa levels for a given patient. The R2 value ranged from 0.0318 to 0.108 with a P value between 0.01 to 0.18 giving a statistically unstable model.
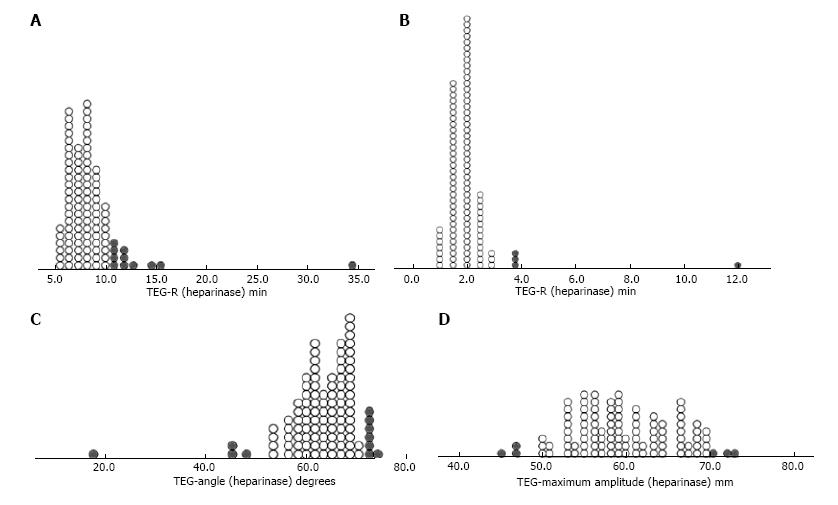
It has been widely hypothesized that the circulatory support devices themselves induce a coagulopathic state beyond that induced by anti-coagulant therapy[7,16]. The degree and nature of coagulopathy was assessed using heparinase-TEG to neutralize the heparin effect. Figure 4 shows dot-density plots of the distribution of individual values for individual parameters such as R, K, Angle, Maximum Amplitude value obtained on heparinase TEG. As demonstrated in the panel, we found a wide variation in the health of the underlying coagulation system with variable demonstration of factor deficiencies as well as clot strength. Four point nine to 13.72% of all values for individual parameters were out of the normal range (represented by solid grey circles in the plots) suggesting significant coagulopathy or factor deficiencies. These findings were used to guide therapy for correcting the coagulopathy by administering appropriate factors in the form of cryoprecipitate or fresh frozen plasma.
The mean duration of VAD support was 69.25 d (range 13 to 141 d). Two patients suffered stroke. One patient suffered an ischemic stroke with hemorrhagic conversion, a second patient suffered an ischemic stroke diagnosed by computed tomography (CT). Although the exact timing of the strokes could not be ascertained, the degree of anticoagulation was within prescribed ranges for the 12 h before the CT scan and or clinical detection of the event. Both these patients made complete clinical recovery. A secondary endpoint was need for VAD pump change-out. A total of 8 pump exchanges were performed. The indications for pump change were made by the VAD team based on rate of clot growth, visualization of a dark clot measuring greater than 4 mm and subjective mobility of the clots. White clots and fibrin deposits in the blood chamber did not initiate pump exchanges, per manufacturer guidelines. Pump exchanges were well tolerated and did not result in any procedural complication. We were unable to identify predictors, such as degree of anticoagulation, fibrinogen levels, heparin dosing and the occurrence of either stroke or need for pump change. There were no mortalities in the cohort. All four patients underwent successful heart transplantation and at follow-up are alive and well.
Managing anticoagulation in the pediatric VAD patient remains a challenging task. Failure to provide adequate anticoagulation results in thromboembolic events. Unfortunately, if the balance is tipped too far, devastating hemorrhagic complications may ensue. Clinicians are further stymied by the lack of evidence-based guidelines to direct therapy based on available laboratory data. The current study provides a direct comparison of these laboratory tests to determine their degree of correlation with one another as well as with anticoagulant effect. In a robust comparison sample of greater than 100 individual data points from four patients, our study showed very strong correlations between aPTT, anti-Xa assay and R-TEG (Figure 1). This is not unexpected, but demonstrates that these tests segregate together and may be substituted for one another, especially in the clinically relevant ranges.
The role of TEG in routine monitoring remains controversial[17]. While TEG has limitations, including difficulty with reproducibility, the utilization of TEG may be beneficial when employed routinely by experienced practitioners within a single center. Interestingly, we found a significant and clinically important correlation between platelet count and MA-TEG (Figure 2). This supports the importance of maintaining a normal platelet count and need for increased anti-platelet agents in the setting of thrombocytosis to manage the strength of clot formation. Additionally, TEG with- and without heparinase is important for diagnosing coagulopathy on VAD and guiding therapy. Furthermore, the presence of a wide range of values suggests a significant underlying coagulopathy that would otherwise be under-appreciated. This may be secondary to multiple factor deficiencies, prothrombotic microparticles or activation of coagulation factors. TEG may enable clinicians to monitor underlying VAD-induced coagulopathy and thereby explain how complications of anticoagulation therapy arise despite achievement of target levels for other coagulation parameters. We are currently validating this hypothesis using a larger cohort of patients that includes those dependent upon mechanical circulatory support devices as well as those requiring extracorporeal membrane oxygenation support.
One important observation from our investigation is the lack of relevant correlation between unfractionated heparin (UNFH) dose and degree of effect as measured by aPTT, anti-Xa or R value (Figure 3). This potentially reflects a significant variation in response to heparin by patient as well as by the coagulation milieu at any given time. These results differ somewhat from data that suggests good correlation between aPTT and UNFH levels in adults on extracorporeal life support (ECLS)[18] as well as a recent study in a small cohort of pediatric patients on ECLS[19]. These discrepancies may reflect variations in heparin response amongst patients due to developmental differences in hemostasis and genetic variability[20,21]. Lastly, none of these tests are specific in their assessment of the effect of unfractionated heparin in vivo. A lack of correlation between heparin dose and PTT or anti-Xa assay has also been noted in other settings, including a cohort of critically ill children[10]. This is extremely relevant as various guidelines continue to recommend use of these monitoring parameters to titrate heparin therapy.
We also noted timing and significance of thrombotic or hemorrhagic events in our patient cohort. Three patients experienced significant morbidities. Two had an ischemic stroke and one had a hemorrhagic stroke. The older patient had an uneventful course. All patients eventually underwent successful bridge to transplantation and were discharged to home. At follow-up, all of them are alive. The patients with ischemic strokes have made a complete functional recovery, albeit after extensive rehabilitation. The patient who had hemorrhagic stroke still has speech delay and motor delay, but no deficits. Unfortunately, due to a small number of events, predictive modeling could not be performed to analyze further risk factors. Correlation of TEG and anticoagulation values with thromboembolic or hemorrhagic events in a larger patient cohort will provide valuable data as to the predictive ability of these tests. This study was also limited only to a single type of VAD. Future studies will include non-pulsatile and implantable devices such as Heartmate II or HeartWare HVAD in an effort to not only provide device-specific information, but also to determine if standards can be applied across all devices.
This study provides valuable data regarding the utility of common laboratories to monitor the state of hemostasis in VAD patients, as suggested by existing guidelines. It also highlights the imprecise nature of current means of monitoring and demonstrates that multiple targets in the hemostatic pathway need to be targeted in order to achieve the desired balance between prevention of device thrombosis and hemorrhagic consequences.
Appropriate anticoagulation continues to be a significant challenge in pediatric patients supported with ventricular assist devices (VADs) resulting in high rate of complications related to bleeding or clotting related complications. Clinicians attempt to address the imbalance between the pro- and anti-thrombotic states through the administration of anticoagulation and antiplatelet therapy. However, the data regarding monitoring parameters is largely an extension of adult experience with very little data to support any pediatric monitoring strategies.
There is therefore an immediate need for improving our understanding of coagulation and anticoagulation parameters in pediatric patients on VAD as well as for studies that validated anticoagulation strategies.
The current study provides a direct comparison of various laboratory tests to determine their degree of correlation with one another as well as with anticoagulant effect. In a robust comparison sample, this study showed very strong correlations between activated thromboplastin time (aPTT), anti-Xa assay and R-thromboelastography (R-TEG). Additionally, the authors show that the dose-response relationship between heparin and these monitoring parameters in very weak, underscoring the authors’ presumption that current guidelines for dose-titration based on anti-Xa levels may not be appropriate. Lastly, for the first time, the authors show the degree of underlying coagulopathy that can be assessed using TEG and underline the utility of the same.
The study underscores the need for continued research in pediatric coagulation system especially within hitherto unexplored world of pediatric VAD and hopefully improves understanding of monitoring and management parameters to improve the morbidity and mortality associated with VADs.
VAD: Ventricular assist device, mechanical support as a circulatory assist; TEG: Thromboelastogram - a whole blood test for assessing the coagulation system in real time.
This is an interesting manuscript about the utility and correlation of anticoagulation parameters such as aPTT, anti-Xa, and R-TEG in the management of pediatric VADs.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ueda H S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Slaughter MS. Hematologic effects of continuous flow left ventricular assist devices. J Cardiovasc Transl Res. 2010;3:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Adzic A, Patel SR, Maybaum S. Impact of adverse events on ventricular assist device outcomes. Curr Heart Fail Rep. 2013;10:89-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Fraser CD Jr, Jaquiss RD, Rosenthal DN, Humpl T, Canter CE, Blackstone EH, Naftel DC, Ichord RN, Bomgaars L, Tweddell JS, Massicotte MP, Turrentine MW, Cohen GA, Devaney EJ, Pearce FB, Carberry KE, Kroslowitz R, Almond CS; Berlin Heart Study Investigators. Prospective trial of a pediatric ventricular assist device. N Engl J Med. 2012;367:532-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 367] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 4. | Almond CS, Morales DL, Blackstone EH, Turrentine MW, Imamura M, Massicotte MP, Jordan LC, Devaney EJ, Ravishankar C, Kanter KR. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation. 2013;127:1702-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 5. | Adachi I, Fraser CD Jr. Berlin Heart EXCOR Food and Drug Administration Investigational Device Exemption Trial. Semin Thorac Cardiovasc Surg. 2013;25:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Monagle P. Anticoagulation in the young. Heart. 2004;90:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Annich G, Adachi I. Anticoagulation for pediatric mechanical circulatory support. Pediatr Crit Care Med. 2013;14:S37-S42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med. 2013;14:e77-e84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 305] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 9. | Monagle P, Chan AKC, Goldenberg NA, Ichord RN, Journeycake JM, Nowak-Göttl U, Vesely SK. Antithrombotic therapy in neonates and children: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e737S-e801S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 1028] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 10. | Kuhle S, Eulmesekian P, Kavanagh B, Massicotte P, Vegh P, Lau A, Mitchell LG. Lack of correlation between heparin dose and standard clinical monitoring tests in treatment with unfractionated heparin in critically ill children. Haematologica. 2007;92:554-557. [PubMed] |
| 11. | Miller BE, Bailey JM, Mancuso TJ, Weinstein MS, Holbrook GW, Silvey EM, Tosone SR, Levy JH. Functional maturity of the coagulation system in children: an evaluation using thrombelastography. Anesth Analg. 1997;84:745-748. [PubMed] |
| 12. | Alexander DC, Butt WW, Best JD, Donath SM, Monagle PT, Shekerdemian LS. Correlation of thromboelastography with standard tests of anticoagulation in paediatric patients receiving extracorporeal life support. Thromb Res. 2010;125:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Spiess BD, Tuman KJ, McCarthy RJ, DeLaria GA, Schillo R, Ivankovich AD. Thromboelastography as an indicator of post-cardiopulmonary bypass coagulopathies. J Clin Monit. 1987;3:25-30. [PubMed] |
| 14. | Miller BE, Guzzetta NA, Tosone SR, Levy JH. Rapid evaluation of coagulopathies after cardiopulmonary bypass in children using modified thromboelastography. Anesth Analg. 2000;90:1324-1330. [PubMed] |
| 15. | Almond CS, Buchholz H, Massicotte P, Ichord R, Rosenthal DN, Uzark K, Jaquiss RD, Kroslowitz R, Kepler MB, Lobbestael A. Berlin Heart EXCOR Pediatric ventricular assist device Investigational Device Exemption study: study design and rationale. Am Heart J. 2011;162:425-435.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Achneck HE, Sileshi B, Parikh A, Milano CA, Welsby IJ, Lawson JH. Pathophysiology of bleeding and clotting in the cardiac surgery patient: from vascular endothelium to circulatory assist device surface. Circulation. 2010;122:2068-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Chitlur M, Lusher J. Standardization of thromboelastography: values and challenges. Semin Thromb Hemost. 2010;36:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Brill-Edwards P, Ginsberg JS, Johnston M, Hirsh J. Establishing a therapeutic range for heparin therapy. Ann Intern Med. 1993;119:104-109. [PubMed] |
| 19. | Liveris A, Bello RA, Friedmann P, Duffy MA, Manwani D, Killinger JS, Rodriquez D, Weinstein S. Anti-factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2014;15:e72-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | Chan KL, Summerhayes RG, Ignjatovic V, Horton SB, Monagle PT. Reference values for kaolin-activated thromboelastography in healthy children. Anesth Analg. 2007;105:1610-1613, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Newall F, Johnston L, Ignjatovic V, Monagle P. Unfractionated heparin therapy in infants and children. Pediatrics. 2009;123:e510-e518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |